Abstract
Background
Quantitative flow cytometry (QFCM) is being applied in the clinical flow cytometry laboratory. Quantitative normal T-cell CD4 expression represents a biologic standard and quality control agent. However, low levels of CD4 expression were detected in normal T-cells in Hairy Cell Leukemia (HCL) samples.
Methods
The QuantiBrite System® was used to determine the level of CD4 expression (mean antibody bound per cell, ABC) in fresh and shipped HCL blood and fresh normal donor blood (NDB). The effects of shipping, lysing reagent, cell preparation method and antibody lot were evaluated.
Results
Shipped HCL specimens (n = 69) had a significantly lower mean CD4 ABC of 38,788 (CV = 9.1%) compared to fresh specimens (n = 105) CD4 value of 40,330 (CV = 8.4%) (p < 0 .05). In NDB, significant differences were seen for fresh versus shipped specimens using the stain/lyse method but not for lyse/stain method. Consistent differences in CD4 ABC based upon antibody lot were observed in fresh HCL and NDB samples. Stain/lyse and lyse/stain methods using NH4Cl lyse were compared in NDB using identical samples and antibodies. The NDB CD4 ABC values obtained with the lyse (NH4Cl )/stain method (45,562, 3.7% CV) were lower than those obtained with the stain/lyse (NH4Cl) method (49,955, 3.3% CV) with p<0.001.
Conclusions
CD4 expression in HCL patient samples is not inherently different from that observed in NDB and therefore may serve as a biological control in clinical QFCM. Technical variables impact significantly on QFCM of CD4.
Introduction
Quantitation of antigen expression has demonstrated utility in the clinical flow cytometry laboratory (1–4). Flow cytometric antigen quantitation is typically accomplished by measuring antibody binding. Quantitative flow cytometry (QFCM) determines the number of molecules of bound fluorescent antibodies (5). When saturating concentrations of antibodies and optimal conditions are used, QFCM provides an objective measurement of the molecules of antigen on the cell surface. The baseline separation of positive from negative CD4 distributions, tight distribution in terms of its coefficient of variation (CV) and known low interpersonal variation of CD4 expression by normal T cells have allowed for the standardization of CD4 expression (6–8). Furthermore as the normal level of CD4 expression is known, CD4 QFCM has been used as a biological control in its own right (9).
Several approaches have been taken to quantitate the actual amount of CD4 antigen expressed on the surface of the CD4 lymphocyte (10–14). Molecular equivalents of soluble flurochrome (MESF), as developed by Schwartz and colleagues and made more universal by the National Institute of Standards and Technology (NIST), represents one approach to the quantification of CD4 expression (12, 13). Prior to this Poncelet and coworkers developed a method using radio-labeled antibodies for the determination of CD4 expression (11). The latest approach using 1:1 PE conjugates of the anti-CD4 antibody was developed and tested in a series of papers by Davis and colleagues (14–16). During the course of immunophenotyping blood samples from patients with hairy cell leukemia (HCL), one of us (MS) noticed that the level of CD4 expression was decreased compared to normal published values. This brief technical report describes and reviews experiments conducted to define the technical variables affecting CD4 quantitation.
Materials and Methods
Patient samples
Peripheral blood specimens from a total of 174 patients with a confirmed diagnosis of hairy cell leukemia were submitted to the Flow Cytometry Unit, Laboratory of Pathology, National Cancer Institute (Bethesda, MD, USA) for evaluation by FCM of the numbers of malignant B cells prior to and post therapy. 105 specimens were received fresh within 3 hours of collection while 69 specimens were shipped to the laboratory by overnight express and were at least 24 hours old upon receipt. Specimens were submitted for evaluation by QFCM of cell surface antigen expression by malignant and normal lymphoid cells. Patients were undergoing eligibility evaluation for a research protocol studying the efficacy of novel therapies in hairy cell leukemia. All patients signed IRB-approved informed consent to be screened for eligibility.
NCI Sample Immunophenotyping Preparation of HCL Samples
Cell surface expression of CD4 by normal T-cells was evaluated in these specimens. Specimens were stained within 48 hours of collection with a panel of antibodies (fresh specimens stained in less than 12 hours, shipped specimens stained within 24–48 hours of collection). Erythrocytes were lysed by incubating with lysing solution (150 mM NH4Cl, 10 mM KHCO3, 0.1 mM EDTA) for 10 minutes at room temperature at a ratio of 1:9 (volume of sample: volume of lysing solution). Specimens were then washed with phosphate buffered saline (PBS) to remove cytophilic antibodies before determining cell number (17). Cellularity was manually determined by a hemocytometer and viability by trypan blue uptake. In evaluating levels of CD4 expression, specimens were stained for 30 minutes at room temperature with a cocktail of the following three antibodies: CD4PE (1:1 conjugate), CD45PerCP, CD3APC (antibody concentration for CD4 at the saturation level and the concentrations of other two antibodies used according to manufacturer’s recommendations). After incubation, cells were pelleted by centrifugation (500 x g for 15 minutes at room temperature), the media was aspirated, and the cells washed twice in a PBS solution containing 0.1% NaN3 and 0.5% albumin. All cells were fixed in 1.0% paraformaldehyde and stored at 4°C for up to 12 h before acquisition.
NCI QFCM Analysis of HCL Samples
Three-color flowcytometry was performed using a BD Biosciences FACS Calibur flow cytometer. The sensitivity of fluorescent detectors was monitored using Calibrite beads (BD Biosciences, San Jose, CA) according to the manufacturer’s recommendations. Data (collected in list mode) were analyzed with CellQuest Pro software (BD Bioscience) and FCS Express (De Novo Software). At least 5,000 lymphocytes were acquired per tube. For analysis, relevant cell populations were analyzed by gating on forward scatter (FSC), side scatter (SSC), CD45, CD3, and CD4. QuantiBRITE beads were run through a FACS Calibur flow cytometer on the same day at the same instrument settings as the individual patient specimens. QuantiBRITE PE Beads (BD Bioscience) are pre-calibrated standard beads containing known levels of PE molecules. A standard curve comparing the geometric mean of fluorescence to known PE content of the QuantiBRITE beads was constructed using QuantiCALC software. The regression analysis, slope, intercept and correlation coefficient were determined. Analysis gates were drawn based upon immunophenotype and cell size to include only the T cells for determination of the geometric mean fluorescence of CD4 staining. The ABC values were generated from the measured geometric mean fluorescence of cells in analysis gates containing only the normal T cells using the QuantiBRITE standard curve.
Evaluation Using Normal Blood Donor Samples
Experimental Design
A series of experiments were conducted. In the NCI method of lyse and stain (L/S), whole blood was lysed with NH4Cl and then washed twice. A hemocytometer based cell count was applied for the NH4Cl lysed sample. Using this protocol, two normal donors, #1 and #2 were stained with single reagent CD PE 1:1 (lot 1 FDA and lot 2 NCI), and a third tube contained an NCI premixed cocktail (with lot 2 CD4 PE combined with CD45 PerCp, CD3 APC) to evaluate the potential effect of other reagents in the panel. In the FDA protocol, heparin anti-coagulated blood was washed twice with phosphate buffer saline (PBS) to remove cytophilic antibody (17). The washed blood was stained followed by applying the NH4Cl lyzing reagent (stain and lyse, S/L). Thus in this first set of experiments, the lysing reagent NH4Cl was fixed and the order of lysing and staining was varied (L/S versus S/L) as well as the antibody lot and single antibody versus three color cocktail. In the second experiment, a direct comparison was carried out between two lysing reagents using a single normal donor #3, specifically: the NCI NH4Cl (L/S) and the FDA FACSLyse (S/L). In the third experiment, normal donors, #4 and #5 were processed precisely following NCI protocol of L/S with NH4Cl and FDA protocol of S/L with FACSLyze, and their results were compared. In the fourth experiment, peripheral blood form normal donors 6, 7 and 8 were split, with half processed immediately and half shipped overnight express to the laboratory to expose the specimens to shipping conditions and a delayed processing time. Both the immediately processed and shipped specimens were processed identically with NH4Cl lysing reagent and fixation. The order of lysing and staining was varied (L/S versus S/L). We performed a three-way factorial repeated measures analysis of variance on the raw data. The model included the three main effects – Method (L/S, S/L), CD4 (Alone, Cocktail), and Treatment (Fresh, Shipped) -- and all interaction effects. Samples (n=8) taken from blood donors (n=3) were modeled as the repeated measures. The standard errors were adjusted using a sandwich estimator or the empirical covariance matrix estimator. P-values were corrected for multiple comparisons by a stepdown Bonferroni method (18).
Sample Preparation
In the first experiment, either 100 μl (1×106 cells) of washed whole blood or lysed whole blood was added to each tube with pre-aliquoted reagents. Tubes were vortexed gently and incubated for 30 minutes at room temperature in the dark. For the S/L NH4Cl protocol, the stained whole blood samples were treated with the NH4Cl lysing solution for ten minutes at room temperature, and then washed twice and resuspended in 0.5 ml PBS for acquisition. For the second experiment, a single normal donor #3 was employed. While using the S/L method, one set of tubes was lysed with 2 ml of FACSLyse solution (BD Biosciences) and the second set was lysed with 2 ml of NH4Cl from NCI laboratory. Both sets were incubated for 10 minutes in the dark at room temperature. Sample tubes were centrifuged for 10 minutes and supernatant was decanted. Cell pellet was vortexed gently and washed with PBS. Cells were then resuspended in 0.5 ml of PBS for acquisition. In the third experiment, the NCI L/S NH4Cl protocol was unchanged and the FACSLyse was selected as the lysing reagent in the FDA S/L method as it is routinely performed in this FDA laboratory.
FDA QFCM Analysis of Normal Donor Blood Samples
FACSCantoII flow cytometer performance and setup was monitored using cytometer set up and tracking (CS&T) microbeads (BD Biosciences). For each experiment, PE Quantibrite beads (BD) and Ultra Rainbow beads (URB, Spherotec) were acquired prior to the acquisition of cellular samples for quantitation. The number of events was 50,000 per tube. Data was analyzed using FACSDiva software.
Results
CD4 Expression in HCL Samples
The initial observation was that the level of CD4 expression on T cells from both fresh and overnight shipped samples from patients with HCL was lower than consensus values for normal T cells (14–16,19). The CD4 ABC values determined in fresh, in house, peripheral blood from HCL patients were compared to those determined in specimens shipped by overnight express. Thirty-nine percent (39%) of the peripheral blood specimens studied were shipped by overnight express and therefore were at least 24 hours old and possibly exposed to extreme temperatures. The shipped peripheral blood (n = 69) had a significantly lower mean CD4 ABC of 38,788 (CV = 9.1%) compared to the fresh, in house specimens (n = 105) CD4 ABC mean value of 40,330 (CV = 8.4%) (p < 0 .05).
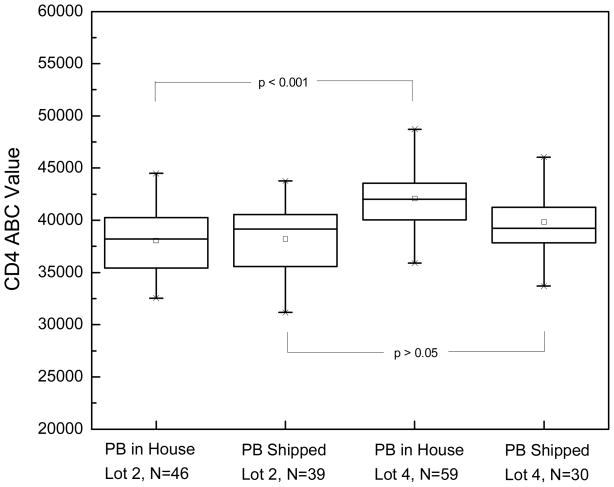
The effect of lot to lot variation in CD4 antibody was also studied in the fresh, in house and shipped hairy cell leukemia patient specimens. There was a significant difference in mean CD4 ABC between lots 2 and 4 of anti-CD4 antibody in the fresh in house specimens (Figure 1), even though both lots were from the same manufacturer and were certified as 1:1 for the fluorochrome: antibody ratio. With the sub-optimal shipped specimens, the difference between Lot 2 and lot 4 was not significant (Fig. 1). It is important to note that the magnitude of the CD4 difference between fresh, in house samples and the overnight shipped samples was dependent upon the lot being used.
Figure 1.
Comparison of two different antibody lots in determining the mean CD4 ABC values for normal T cells in fresh peripheral blood and overnight shipped peripheral blood from Hairy Cell Leukemia patients.
CD4 Expression in Normal Donors
Five normal donors were used to define the variables, one at a time, in attempt to explain the observations from HCL samples in terms of its technical and/or biological origin. Variables studied included anti-CD4 1:1 lot, staining protocols, single antibody versus cocktails, and lysing ragents.
Two lots of anti-CD4 antibody (lots 1 and 2) were compared using a single antibody (anti-CD4 1:1) among 2 normal donors, using both the stain-then-lyse (S/L) and lyse-then-stain (L/S) methods. The CD4 ABC values obtained with Lot 1 were consistently lower than those obtained with Lot 2 (Table 1).
Table 1.
Comparison of Two Different Antibody Lots in Determining Mean CD4 ABC Values for T Cells in Fresh Blood from Normal Donors
| S/L, CD4 PE 1:1, NH4Cl | Unstained | Lot 1 | Lot 2 |
|---|---|---|---|
| Normal Donor 1 | 70 | 48,159 | 52,417 |
| Normal Donor 2 | 68 | 46,570 | 51,469 |
| L/S, CD4 PE 1:1, NH4Cl | |||
| Normal Donor 1 | 71 | 43,718 | 47,257 |
| Normal Donor 2 | 70 | 43,491 | 47,510 |
The stain/lyse and lyse/stain methods, both using the NH4Cl lyse, were compared using identical normal donor cells and antibodies. The CD4 ABC values obtained with the lyse with NH4Cl and then stain were consistently lower than those obtained with the stain and then lyse with NH4Cl method (Table 2) regardless as to whether the CD4 PE was the sole reagent or present in the cocktail.
Table 2.
Comparison of NH4Cl Reagent L/S and S/L Methods in Determining the Mean CD4 ABC Values for T cells in Fresh Blood from Normal Donors
| Normal Donor | Antibody / Lot | L/S | S/L |
|---|---|---|---|
| 1 | CD4 PE 1:1 / Lot 1 | 43,718 | 48,159 |
| 1 | CD4 PE 1:1 / Lot 2 | 47,257 | 52,417 |
| 2 | CD4 PE 1:1 / Lot 1 | 43,491 | 46,570 |
| 2 | CD4 PE 1:1 / Lot 2 | 47,510 | 51,469 |
| 3 | CD4 PE 1:1 / Lot 3 | 47,308 | 50,901 |
| 3 | CD4 PE 1:1 / Lot 4 | 47,149 | 51,043 |
| 1 | Cocktail / Lot 2 | 45,242 | 49,895 |
| 1 | Cocktail / Lot 2 | 45,399 | 49,811 |
| 2 | Cocktail / Lot 2 | 44,650 | 50,450 |
| 2 | Cocktail / Lot 2 | 42,142 | 50,193 |
| 3 | Cocktail / Lot 4 | 44,874 | 48,598 |
The CD4 results of the single anti-CD4 antibody vs. a cocktail are summarized in Table 3. The CD4 ABC values obtained with a single anti-CD4 antibody were consistently higher but not statistically significant than those obtained with a cocktail containing CD4 PE, CD45 PerCP and CD3 APC.
Table 3.
Comparison of Single Antibody versus Cocktails in Determining Mean CD4 ABC Values for T Cells in Fresh Blood from Normal Donors
| Normal Donor | Protocol, Lyse | CD4 PE 1:1 Lot | CD4 PE 1:1 | Cocktail |
|---|---|---|---|---|
| 1 | L/S, NH4Cl | 2 | 47,257 | 45,242 |
| 1 | S/L, NH4Cl | 2 | 52,417 | 49,895 |
| 2 | L/S, NH4Cl | 2 | 47,510 | 44,650 |
| 2 | S/L, NH4Cl | 2 | 51,469 | 50,450 |
| 3 | L/S, NH4Cl | 4 | 47,149 | 44,874 |
| 3 | S/L, NH4Cl | 4 | 51,043 | 48,598 |
| 3 | S/L, FACS Lyse | 4 | 46,753 | 45,156 |
| 4 | S/L, FACS Lyse | 2 | 48,272 | 45,317 |
| 4 | L/S, NH4Cl | 2 | 47,119 | 44,592 |
| 5 | L/S, NH4Cl | 2 | 48,137 | 45,508 |
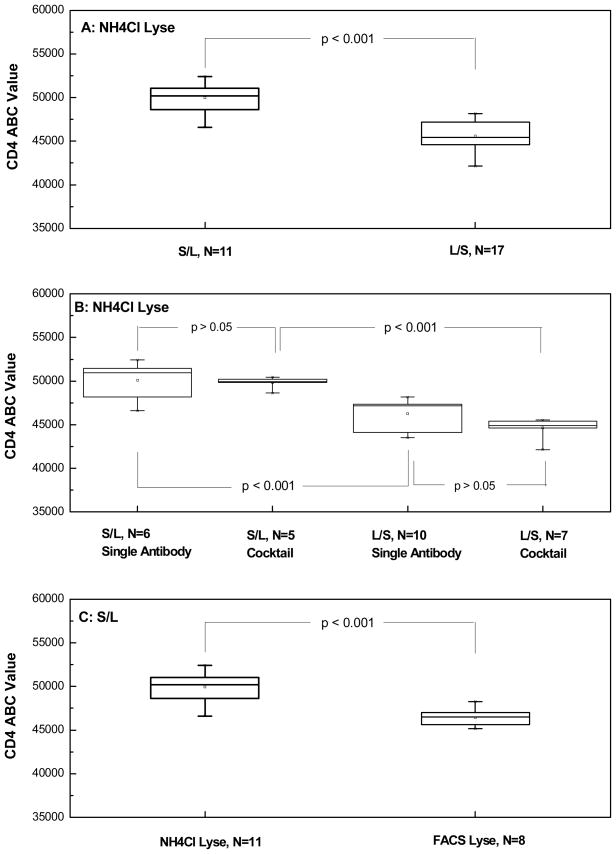
Comparison of NH4Cl and FACS Lyse: CD4 ABC values were compared in the same normal donor (normal donor 3) using the stain-then-lyse method. The values obtained with FACS Lyse were consistently lower than those obtained with NH4Cl lyse (Table 4). The data in tables 1–4 and the statistical significance of the observed differences is summarized in Figure 2.
Table 4.
Comparison of NH4Cl and FACS Lyse in Determining Mean CD4 ABC Values for Normal T Cells in Fresh Blood from Normal Donors
| Antibody / Lot | NH4Cl Lyse | FACS Lyse |
|---|---|---|
| CD4 PE 1:1, Lot 3 | 50,901 | 47,174 |
| CD4 PE 1:1, Lot 4 | 51,043 | 46,753 |
| Cocktail, Lot 4 | 48,598 | 45,156 |
Figure 2.
A summary of the experimental data conducted on a total of five normal blood donors for the evaluation of sample preparation protocol, lysing reagent, and antibody addition format on CD4 QFCM. Multiple measurements were carried out on each normal donor samples under different experimental conditions/designs. (A) effect of the sample preparation protocol using the same lysing reagent NH4Cl; (B) effects of the sample preparation protocol and antibody addition format (single and cocktail) using the sole lysing reagent NH4Cl; (C) effect of the lysing reagent with the use of only S/L sample preparation protocol. These data summarized here include those listed in Tables 1–4 in addition to data obtained for the same donors carried out with different lots of CD4 PE unimolar conjugate and cocktail reagents.
The effect of overnight shipment of normal donor peripheral bloods on CD4 ABC values was investigated (Table 5). The three way interaction effect of shipped versus fresh cell preparation (lyse then stain or stain and then lyse) and CD4 antibody (CD4 antibody alone or in cocktail) was significant (P=0.044). For the lyse then stain method and CD4 single antibody or CD4 in cocktail there was no significant difference between the shipped and fresh specimens (P=0.81). However, for the stain and then lyse method there was a significant difference between the shipped and fresh specimens (P<0.020). Since the CD4 single antibody and CD4 cocktail appeared to yield similar results the possibility of combining the different CD4 groups was examined. The adjusted P-values for the differences for single antibody CD4 versus cocktail were both >0.05 (0.34 and 0.057, for lyse-then-stain and stain-then-lyse, respectively). Thus, we dropped the three-way interaction effect from the model and further examined the method of specimen processing and shipment of specimens (i.e., the data was pooled over the CD4 groups). Table 5B shows the summary statistics and adjusted P-values for the treatment difference within each method of specimen preparation. For the lyse-then-stain method, there was not a significant difference between shipped and fresh groups (P=0.46), but for the stain-then-lyse method, there was a significant difference between shipped and fresh groups (P=0.008).
Table 5.
Effect of Overnight Shipping in Determining Mean CD4 ABC Values for Normal T cells in Blood from Normal Donors
| A. Single Antibody versus Cocktail | |||||
|---|---|---|---|---|---|
| Method | CD4 | Shipped vs. Fresh | Adjusted Mean | SEM* | P-value |
| L/S | Single Antibody | Fresh | 38,300 | 1,930 | 0.81 |
| L/S | Single Antibody | Shipped | 41,000 | 1,410 | |
| L/S | Cocktail | Fresh | 36,100 | 1,680 | 0.81 |
| L/S | Cocktail | Shipped | 39,200 | 1,340 | |
| S/L | Single Antibody | Fresh | 44,800 | 1,170 | 0.020 |
| S/L | Single Antibody | Shipped | 41,500 | 1,380 | |
| S/L | Cocktail | Fresh | 42,400 | 1,100 | 0.011 |
| S/L | Cocktail | Shipped | 38,400 | 1,160 | |
| B. All Antibodies Studied | ||||
|---|---|---|---|---|
| Method | Shipped vs. Fresh | Adjusted Mean | SEM* | P-value |
| L/S | Fresh | 37,200 | 1,800 | 0.46 |
| L/S | Shipped | 40,100 | 1,370 | |
| S/L | Fresh | 43,600 | 1,130 | 0.008 |
| S/L | Shipped | 40,000 | 1,270 | |
SEM is standard error of the mean.
Discussion
QFCM is being implemented as a diagnostic and prognostic aid in the clinical flow cytometry laboratory. CD4 expression on normal T cells, as measured by QFCM in normal volunteers, has low interpersonal variation and has a known value that ranges from 46,000–49,000, depending upon the quantification methodology and monoclonal reagent utilized (12, 14–16). These qualities have led to the use of CD4 QFCM to study the comparability of different QFCM methods, technical concerns and inter-laboratory variability in performing this test. CD4 expression on normal T cells has even been used as a biological calibrator in the place of fluorescent bead standards because it mimics biological cell samples better than the artificial fluorescent beads (9). The consistency of CD4 ABC values for normal T cells led to the inclusion of CD4 quantitation for quality control in QFCM of clinical samples in some laboratories.
The observation of significantly lower CD4 ABC values for the normal T cells in peripheral blood from patients with hairy cell leukemia (Fig. 1) than those reported in literature for normal volunteers was therefore unexpected. This prompted a search for a possible technical/biological source for this discrepancy. Significant reagent lot to lot variation on CD4 expression was observed (Fig. 1) on fresh, in house specimens though the reagents showed no impact on overnight shipped samples.
As the reference values for CD4 QFCM were determined in normal control samples, the effects of specific technical aspects, including sample preparation protocol, staining reagent (individual or cocktail reagent, and different reagent lots), lysing reagents and overnight shipment were evaluated in normal controls. The two sample preparation procedures evaluated here are NCI’s protocol (L/S) and FDA’s (S/L). Using only NH4Cl lyse and regardless of the staining reagent, the S/L method produces statistically higher ABC values for CD4 expression (mean value, 49,955 and CV of 3.3%) than the L/S method (mean value, 45,562 and CV of 3.7%) as shown in Figure 2A (Box 1 vs. Box 2, p <0.001). This mean ABC value of 49,955 by the S/L method is consistent with our previous reported value and the literature (14–16, 19).
The use of single and cocktail staining reagents produced no significant difference (p>0.05) in CD4 expression level under the single lysing reagent of NH4Cl and a single sample staining protocol (Fig. 2B, Box 1 vs. Box 2: p>0.05; Box 3 vs. Box 4: p>0.05). The result suggests that the interference between three different antibody reagents in the cocktail is negligible statistically.
When two lots of anti-CD4 antibody (lots 1 and 2) were compared among 2 normal blood donors using both the S/L and L/S methods with the single lysing reagent NH4Cl, the CD4 ABC values obtained with lot 1 were consistently lower than those obtained with lot 2 (Table 1). This observation is consistent with the fresh, in house hairy cell leukemia patient specimens where there was a significant difference in mean CD4 ABC values between lots 2 and 4 of anti-CD4 antibody (Fig. 1), even though both lots were from the same manufacturer and were certified as 1:1 for fluorochrome:antibody ratio. This indicates the impact of subtle differences in antibody quality on QFCM measurements. It’s also consistent with our results that the use of FACS Lyse in both S/L and L/S methods resulted in a significant decrease in CD4 expression (Fig. 2C). This result further supports the use of NH4Cl lyse with clinical sample preparation protocol (L/S). However, higher CD4 ABC values might be seen using NH4Cl in the stain and lyse method.
Thirty-nine percent (39%) of the HCL peripheral blood specimens studied were shipped by overnight express and therefore were at least 24 hours old and possibly exposed to extremes of temperature. The shipped peripheral blood had a significantly lower mean CD4 ABC compared to the fresh, in house specimens (Fig. 1, Lot 4). This lower ABC value may be secondary to greater age, exposure to an extreme environment, disease specific or all three. We therefore studied the effect of overnight shipment on CD4 ABC values in normal control blood (Table 5) and found that the effect of overnight shipment was process dependent. For the stain and then lyse procedure, overnight shipment resulted in lower CD4 ABC values. For the lyse and then stain method of processing, overnight shipment of peripheral blood resulted in no significant difference in CD4 ABC values. The results indicate that QFCM of shipped and older specimens requires validation of methodology.
Consistent lower CD4 expression levels in HCL samples led us to compare the clinical staining protocol (L/S) and research laboratory protocol (S/L) side by side. The mean CD4 expression levels on both the fresh prepared and shipped normal blood samples under the identical fixation condition using the L/S method (37,200 and 40,100, respectively, Table 5B) are generally in agreement with the results of fresh and shipped HCL samples (mean 40,330 and 38,788 respectively) (Fig. 1). The results suggest that CD4 expression in HCL patient samples is not inherently different from that observed in normal blood donors, and therefore may serve as a biological control in clinical QFCM of HCL patients. Our results also point out the significance of the quality of the CD4 PE unimolar conjugate on CD4 QFCM. Because of the importance of CD4 serving as a biological control in clinical QFCM, the quality control of this reagent is essential. At the same time, this study raises the need to continuously evaluate the effects of sample preparation methods for each reagent within cocktails (20).
Acknowledgments
This research was supported in part by the Intramural Research Programs of the NIH, NCI and CBER, FDA.
Footnotes
Disclaimer: Certain commercial equipment, instruments, and materials are identified in this paper to specify adequately the experimental procedure. In no case does such identification imply recommendation or endorsement by the National Institute of Standards and Technology, nor does it imply that the materials or equipment are necessarily the best available for the purpose.
References
- 1.Mandy F, Janossy G, Bergeron M, Pilon R, Faucher S. Affordable CD4 T-Cell Enumeration for Resource-Limited Regions: A Status Report for 2008. Cytometry Part B. 2008;74B (Suppl 1):S27–S39. doi: 10.1002/cyto.b.20414. [DOI] [PubMed] [Google Scholar]
- 2.Bae SY, Park HC, Oh JS, Yoon S-Y, Park DW, Choi IK, Kim HJ, Oh JH, Hur DS, Chung C, Chang JK, Robinson JP, Lim CS. Absolute CD41 Cell Count Using a Plastic Microchip and a Microscopic Cell Counter. Cytometry Part B. 2009;76B:345–353. doi: 10.1002/cyto.b.20470. [DOI] [PubMed] [Google Scholar]
- 3.National Committee for Clinical Laboratory Standards. NCCLS Document. H42. 1992. Clinical Applications of Flow Cytometry: Quality Assurance and Immunophenotyping of Peripheral Blood Lymphocytes. [Google Scholar]
- 4.Clinical and Laboratory Standards Institute H43A2. Clinical Flow Cytometric Analysis of Neoplastic Hematolymphoid Cells; Approved Guideline. 2. pp. A43–A25. [Google Scholar]
- 5.CLSI/NCCLS Documemt I/LA24-A: Fluoresence Calibration and Quantitative Measurement of Flurorescence Intensity; Approved Guideline, 2004.
- 6.Marti GE, Schuette W, Magruder L, Vail M, Gralnick HR. A Method to Average Immunofluorescent Histograms. Cytometry. 1986;7:450–452. doi: 10.1002/cyto.990070510. [DOI] [PubMed] [Google Scholar]
- 7.Nicholson JK, Jones BM, Hubbard M. CD4 T-Lymphocyte Determinations on Whole Blood Specimens Using a Single-Tube Three-Color Assay. Cytometry. 1993;14:685–689. doi: 10.1002/cyto.990140614. [DOI] [PubMed] [Google Scholar]
- 8.Nicholson JKP, Mandy F, Livnat D, Kagan J. Three-Color Supplement to the NIAID DAIDS Guideline for Flow Cytometric Immunophenotyping. Cytometry (Communications in Clinical Cytometry) 1996;26:227–230. doi: 10.1002/(SICI)1097-0320(19960915)26:3<227::AID-CYTO8>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 9.Giorgi J, Cheng H, Margolick J, et al. Quality Control in the Flow Cytometric Measurement of T Lymphocyte Subsets. Clin Immunol Immunopathol. 1990;55:173–186. doi: 10.1016/0090-1229(90)90096-9. [DOI] [PubMed] [Google Scholar]
- 10.Hultin LE, Matud JL, Giorgi JV. Quantitation of CD38 Activation Antigen Expression on CD81 T Cells in HIV-1 Infection Using CD4 Expression on CD41 T Lymphocytes as a Biological Calibrator. Cytometry. 1998;33:123–132. doi: 10.1002/(sici)1097-0320(19981001)33:2<123::aid-cyto6>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 11.Poncelet P, Carayon P. Cytofluorometric Quantification of Cell Surface Antigens by Indirect Immunofluorescence Using Monoclonal Antibodies. J Immunol Methods. 1985;85:65–74. doi: 10.1016/0022-1759(85)90274-1. [DOI] [PubMed] [Google Scholar]
- 12.Schwartz A, Fernandez-Repollet E, Vogt R, Gratama J. Standardizing Flow Cytometry: Construction of a Standardized Fluorescence Calibration Plot Using Matching Spectral Calibrators. Commun Clin Cytometry. 1996;26:22–31. doi: 10.1002/(SICI)1097-0320(19960315)26:1<22::AID-CYTO4>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 13.Gaigalas AK, Wang L, Schwartz A, Marti GE, Vogt RF. Quantitating Fluorescence Intensity from Fluorophore: Assignment of MESF Values. J Res Natl Inst Stand Technol. 2005;110:101–114. doi: 10.6028/jres.110.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davis KA, Abrams B, Iyer SB, Hoffman RA, Bishop JE. Determination of CD4 Antigen Density on Cells: Role of Antibody Valency, Avidity, Clones, and Conjugation. Cytometry. 1998;33:197–205. doi: 10.1002/(sici)1097-0320(19981001)33:2<197::aid-cyto14>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 15.Iyer SB, Hultin LE, Zawadzki JA, Davis KA, Giorgi JV. Quantitation of CD38 Expression Using QuantiBRITE Beads. Cytometry. 1998;33:206–212. doi: 10.1002/(sici)1097-0320(19981001)33:2<206::aid-cyto15>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 16.Pannu KK, Joe ET, Iyer SB. Performance Evaluation of QuantiBRITE Phycoerythrin Beads. Cytometry. 2001;45:250–258. doi: 10.1002/1097-0320(20011201)45:4<250::aid-cyto10021>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 17.Fukushima PI, Nguyen PK, O’Grady P, Stetler-Stevenson M. Flow Cytometric Analysis of kappa and lambda Light Chain Expression in Evaluation of Specimens for B-cell Neoplasia. Cytometry. 1996;26(4):243–52. doi: 10.1002/(SICI)1097-0320(19961215)26:4<243::AID-CYTO2>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 18.Huber PJ. The Behavior of Maximum Likelihood Estimates Under Nonstandard Conditions. Proc Fifth Berkeley Symp Math Statist Prob. 1967;1:221–233. [Google Scholar]
- 19.Wang LAF, Gaigalas AK, Hoffman RA, Flagler D, Marti GE. Discrepancy in Measuring CD4 Expression on T-lymphocytes Using Fluorescein Conjugates in Comparison with Unimolar CD4-phycoerythrin Conjugates. Cytometry Part B (Clin Cytometry) 2007;72B(6):442–449. doi: 10.1002/cyto.b.20354. [DOI] [PubMed] [Google Scholar]
- 20.Carter PH, Resto-Ruiz S, Washington GC, Ethridge S, Palini A, Vogt R, Waxdal M, Fleisher T, Noguchi P, Marti GE. Whole Blood Lysis: a Flow Cytometric Analysis of Three Anticoagulants and Five Cell Preparations. Cytometry. 1992;13:68–74. doi: 10.1002/cyto.990130111. [DOI] [PubMed] [Google Scholar]