Abstract
Hydrogel materials are promising vehicles for the delivery of protein therapeutics. Proteins can impart physical interactions, both steric and electrostatic in nature, that influence their release from a given gel network. Here, model proteins of varying hydrodynamic diameter and charge are directly encapsulated and their release studied from electropositive fibrillar hydrogels prepared from the self assembling peptide, MAX8. Hydrogelation of MAX8 can be triggered in the presence of proteins for their direct encapsulation with no effect on protein structure nor the hydrogel's mechanical properties. Bulk release of the encapsulated proteins from the hydrogels was assessed for a month time period at 37°C before and after syringe delivery of the loaded gels to determine the influence of protein structure on release. Release of positively charged and neutral proteins was largely governed by the sterics imposed by the network. Conversely, negatively charged proteins interacted strongly with the positively charged fibrillar network, greatly restricting their release to <10% of the initial protein load. Partition and retention studies indicated that electrostatic interactions dictate the amount of protein available for release. Importantly, when protein encapsulated gels were delivered via syringe, the release profiles of the macromolecules showed similar trends as those observed for non-sheared gels. This study demonstrates that proteins can be directly encapsulated in self assembled MAX8 hydrogels, which can then be syringe delivered to a site where subsequent release is controlled by protein structure.
Keywords: peptide, hydrogel, delivery, protein, syringe
Introduction
There is intense interest in developing small protein and antibody therapeutics. (1–7) Protein therapeutics are complex macromolecules typically characterized by a high molecular weight, compositional variety, and for some, an amphipathic nature. (8) These attributes not only define the native structure of the protein, but also provide therapeutic advantages over their smaller drug counterparts. Benefits include high specificity to native targets, less interference with normal biological processes, and a decreased potential for eliciting an immune response. (2, 5)
Drug delivery vehicles, such as hydrogels, provide an effective avenue for protein delivery.(9–15) They can improve therapeutic efficacy by providing a solubilizing environment in which proteins can be encapsulated and protected from degradation. In addition, the hydrogel network can be tailored to control the release rate and profile of the encapsulated macromolecule.(9, 10, 13, 15) Hydrogel pore sizes can be tailored to be on the order of protein diameters. This allows the slow diffusion of proteins within the sterically imposing network and the subsequent release from the gel with temporal control. Gels can also be designed to immobilize the proteins and only release the therapeutic as the polymer network degrades. (16–19)
Another promising mechanism for hydrogel design is to take advantage of the electrostatic nature of a protein to optimize its controlled release. (14, 20) Proteins and antibodies have distinct surface compositions that impart solubility and charge to the macromolecule. (8, 21) Depending on the protein's isoelectric point (pI) and the pH of the solution, a given protein will have a net positive (pI > pH), a net negative (pI < pH), or neutral (pI ~ pH) charge displayed from its surface. Hydrogel networks can be designed to form electrostatic interactions with the diffusing protein as a means to control their release.(14, 20–28) Attractive interactions retard release whereas repulsive interactions can hasten release. Only a few examples exist where charged hydrogels are used for protein delivery; these gels contain ionic polymers, such as carrageenan (20), gelatin (23), alginate (24), and agarose (25), or are modified with amino (22), sulfonyl (26), or phosphate (27, 28) functional groups. Most recently, peptide hydrogels with charged residues were also capable of protein release. (29, 30)
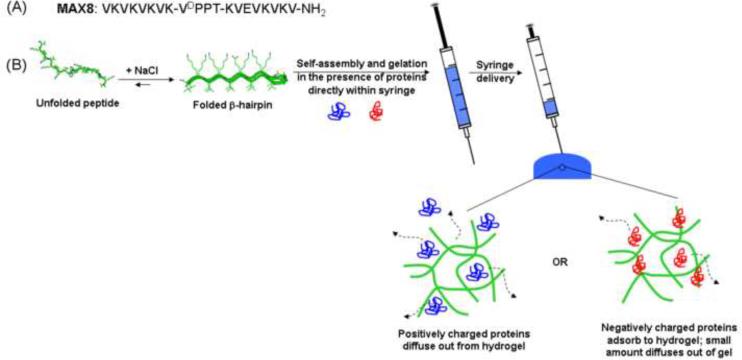
We have developed a self-assembling peptide that can undergo triggered hydrogelation in response to physiological conditions (pH 7.4, 150 mM salt, and 37°C) to afford a mechanically rigid, viscoelastic gel with a net positive charge. (31–34) These gels display shear-thin recovery behavior that allows them to be syringe delivered. (32) As will be shown, hydrogelation can be triggered in the presence of protein to directly encapsulate the macromolecule within the network for subsequent delivery of the loaded gel via syringe. MAX8 is a small, de novo designed β-hairpin peptide that is comprised of two β-strands of alternating valines and lysines with a glutamic acid at position 15 (Figure 1A). A tetrapeptide (-VDPPT-) sequence with a high propensity to form a type II' β-turn centrally connects the two strands. In low ionic strength, aqueous solutions, MAX8 is freely soluble and remains unfolded due to electrostatic repulsions between the positively charged lysines. Increasing the ionic strength of the solution by adjusting the NaCl concentration to 150 mM (physiologically relevant salt concentration) screens some charge on the lysine side chains and promotes the folding of the peptide into a facially amphiphilic β-hairpin structure, see Figure 1B. The folded hairpin subsequently self assembles to form a physically crosslinked network of fibrils with solvent exposed, positive point charges. Each molecule of MAX8 displays seven lysine side chains and one N-terminal amine that contribute to a highly electropositive network. Previously, FITC-labeled dextrans were used as models to investigate the ability of this gel to release polysaccharides. (31) Here, we study how protein structure, in particular hydrodynamic diameter and surface charge, dictates the temporal release of model proteins from 1 wt% MAX8 peptide hydrogels for a month period at 37°C. In addition, the effect of syringe delivering the loaded gels on protein release is also accessed. Model proteins include lysozyme, α-lactalbumin, myoglobin, lactoferrin, bovine serum albumin (BSA), and human immunoglobin G (IgG), which vary in hydrodynamic diameters and isoelectric points. (Table 1) (21, 35–38)
Figure 1.
(A) Sequence of MAX8. (B) Self assembly mechanism of MAX8 into hydrogel directly in a syringe, subsequent delivery, and expected interactions between differently charged proteins and positively charged MAX8 hydrogel network.
Table 1.
Name, molecular weight (kD), hydrodynamic diameter (nm), reported diffusion coefficient in water at 37°C (Daq), reported isoelectric point (pI), and the net charge at pH 7.4 of model proteins.
| Protein | Molecular Weight (kD) | Hydrodynamic diameter (nm) | Daq 37°C) (10−8 cm/sec) | Isoelectric point, pI | Charge at pH 7.4 |
|---|---|---|---|---|---|
| α-lactalbumin | 14.1 | 3.2 | 142 | 42–45 | − |
| Lysozyme | 14.7 | 4.1 | 111 | 11.0 | + |
| Myoglobin | 17.4 | 3.9 | 113 | 7.0 | Ø |
| Bovine Serum Albumin (BSA) | 66 | 7.2 | 63 | 4.6–4,8 | − |
| Lactoferrin | 77 | 6.1 | 74 | 8.4–9.0 | + |
| Human lgG | 146 | 10.7 | 41 | 5.8–8.0 | Ø |
Materials and Methods
Materials
Bis-tris propane (BTP) and sodium chloride (NaCl) were purchased from Sigma-Aldrich. The proteins, α-lactalbumin (bovine milk, Type I), lysozyme (chicken egg white), myoglobin (equine skeletal muscle), albumin (bovine serum), lactoferrin (bovine milk), and IgG (human serum) were also purchased from Sigma-Aldrich. MAX8 peptide was synthesized according to a previously published protocol. (31, 32) Briefly, peptide was synthesized on an automated ABI 433A peptide synthesizer on RINK amide resin using standard Fmoc-protocol with HCTU activation. Cleavage and side-chain deprotection of the dry resin-bound peptide was performed with a trifluoroacetic acid (TFA): thioanisole: ethanedithiol: anisole (90:5:3:2) cocktail for 2 hr under low N2 atmosphere. Filtration followed by diethyl ether precipitation yielded crude MAX8 peptide. Purification of the crude peptide was performed by RP-HPLC using a preparative Vydac C18 peptide column with a flow rate of 8 mL/min. Peptide is injected onto the column under isocratic conditions. MAX8 gradient: 0% B for 2 min, then a linear gradient from 0 to 24% B over 4 min, then 24% to 100% B over 152 min. Peptide elutes at 30 min. MS (ESI) m/z: 1115.9 [(M+2H)2+, calculated: 1116.5]. Elutants for RPHPLC consisted of Standard A (0.1% TFA in water) and Standard B (90% acetonitrile, 9.9% water, 0.1% TFA). Analytical HPLC chromatograph and ESI (+) mass spectra for the pure peptide are located in the supporting information. Lyophilized purified peptide was dissolved in water at 1 mg/mL and re-lyophilized twice before using for hydrogelation.
Dynamic Oscillatory Rheology
Oscillatory rheology experiments were performed on a Paar Physica MCR 500 rheometer using 25 mm diameter stainless steel parallel plate geometry. For the rheological measurements, gels were prepared directly on the rheometer plate in the following manner. MAX8 peptide stock solutions were first prepared in glass vials by dissolving 4 mg of peptide in 200 μL of sterile, chilled water. To this solution, 200 μL of chilled BTP buffer (100 mM, pH 7.4) containing 300 mM NaCl was added. To prepare gels that directly encapsulate the proteins, 200 μL of chilled BTP buffer (100 mM, pH 7.4) containing 300 mM NaCl and 2 mg/mL of the respective protein was added to the peptide solution. This results in 1.0 wt % gel of a final total volume of 400 μL. Then, 300 μL of the resulting solution was quickly added to the rheometer plate, which was pre-equilibrated at 5°C. The parallel plate tool was then lowered to a gap height of 0.5 mm and the temperature was ramped linearly to 37°C to initiate gelation.
For the gelation experiment, a dynamic time sweep was performed to measure the storage (G') and loss (G”) modulus at a frequency of 6 rad/sec and 0.2% strain as a function of time for 1 hour. A dynamic frequency sweep (0.1 to 100 rad/sec at constant 0.2% strain) was then performed, followed by a dynamic strain sweep (0.1 to 1000% strain at constant 6 rad/sec), which was used to verify that the former measurements were within the linear viscoelastic regime. For the shear-thinning and recovery experiment, a dynamic time sweep was performed at a frequency of 6 rad/sec and 0.2% strain for 1 hour. This was immediately followed by a 30 second period, in which 1000% strain at a frequency of 6 rad/sec was applied to the sample. This was then followed by another one hour dynamic time sweep (6 rad/sec, 0.2% strain) to measure the sample's moduli recovery after shear. A dynamic frequency sweep (0.1 to 100 rad/sec at constant 0.2% strain) and a dynamic strain sweep (0.1 to 1000% strain at constant 6 rad/sec) were also performed for these rheology experiments.
Bulk Release Studies
For the bulk release studies, MAX8 peptide stock solutions were first prepared in glass vials by dissolving 4 mg of each peptide in 200 μL of sterile, chilled water. Then, 150 μL of each stock was added to a separate glass vial. 150 μL of chilled, fresh BTP buffer (100 mM, pH 7.4) containing 300 mM NaCl and 2 mg/mL of the respective protein was added to the stock solution. Samples were carefully shaken to initiate hydrogelation, resulting in 1.0 wt% gels of a final total volume of 300 μL containing 1 mg/mL protein. Samples were placed in an incubator at 37°C for 3 hours. To mimic syringe delivery, a mixture of peptide solution and buffer was drawn into a 1 mL syringe and allowed to gel directly in the syringe for 1.5 h in an incubator at 37°C. The sample was then sheared through a 26-3/8 gauge needle into a separate glass vial. The sample was then placed in the incubator at 37°C for an additional 1.5 h prior to the release experiment.
All hydrogels were made in cylindrical glass vials and had only the top surface exposed for release. The gels had approximate heights of 0.35 cm from the bottom of the vial. After 3 hrs, 1 mL of pH 7.4, 50 mM BTP, 150 mM NaCl buffer was added to the top of gel. At scheduled time points, the entire volume of buffer above the gel was removed and replaced with fresh buffer. Probe concentration was determined for each removed aliquot as a function of time. Each time point was performed in triplicate and experiments were carried out for 28 days. The concentration of protein in the stock solutions and in the supernatant was determined from the absorbance at 280 nm by UV-Vis spectroscopy, which were compared to a calibration curve.
The bulk release profiles were then fit to a 1D nonsteady-state Fickian diffusion model for a probe with a constant diffusion coefficient, D, diffusing through a thin slab with a height, h (39, 40).
| [1] |
where Mt is the mass of protein released at time t, M0 is the mass of protein initially loaded into the gel, and Mt=28days is the mass of protein released at the end of the 28 day experiment. The 95% confidence intervals of the fitted diffusion coefficients were also calculated.
Circular Dichroism
Circular dichroism (CD) spectra were collected on a Jasco model J-810 spectropolarimeter. The supernatant from the bulk protein release experiments at the 24 hour time point was analyzed by CD to determine the secondary structure of the proteins after release. CD spectra were also measured for 0.5 mg/mL fresh solutions of native protein for comparison with the released protein samples. Wavelength spectra from 260 to 205 nm were obtained for 150 μL aliquots of both the native samples and the released samples in a 0.1 cm path length quartz cell at 25°C. The mean residue ellipticity, θ, was calculated from the equation [θ] = (θobs/10lc)/r, where θobs is the measure ellipticity in millidegrees, l is the length of the cell (centimeters), c is the molar concentration, and r is the number of residues.
Adsorption Studies
For the bulk adsorption studies, MAX8 peptide stock solutions were first prepared in glass vials by dissolving 4 mg of each peptide in 200 μL of sterile, chilled water. Then, 150 μL of each stock was added to a separate glass vial. 150 μL of chilled, fresh BTP buffer (100 mM, pH 7.4) containing 300 mM NaCl was added to the stock solution. Samples were carefully shaken to initiate hydrogelation, resulting in 1.0 wt% gels of a final total volume of 300 μL. Samples were placed in an incubator at 37°C for 3 hours. After 3 hrs, 300 μL of pH 7.4, 50 mM BTP, 150 mM NaCl buffer containing 1 mg/mL of the respective protein was added to the top of gel. The hydrogels were then placed in the incubator at 37°C. After 48 hours, the entire volume of buffer above the gel was then removed and replaced with 1 mL of fresh buffer. This buffer wash was repeated after 48 hours twice more for a total of three washes. The experiment was performed in triplicate. The concentration of protein in the stock solutions and in the supernatants was determined from the absorbance at 280 nm by UV-Vis spectroscopy.
Results and Discussion
Direct Encapsulation of Protein during Hydrogelation
The most efficient method to incorporate proteins into a hydrogel is their direct loading during gel formation. (9–11, 13, 14, 41) This ensures that precise concentrations of therapeutics can be directly incorporated within the network with homogeneous distribution. However, certain gel crosslinking strategies may involve toxic agents or can degrade the therapeutic during loading. Materials formed by self assembly offer the opportunity to trigger hydrogelation under aqueous conditions in the presence of the therapeutic. (9) Consequently, exact quantities of the proteins can be encapsulated within the hydrogel network without the use of harsh crosslinking agents.
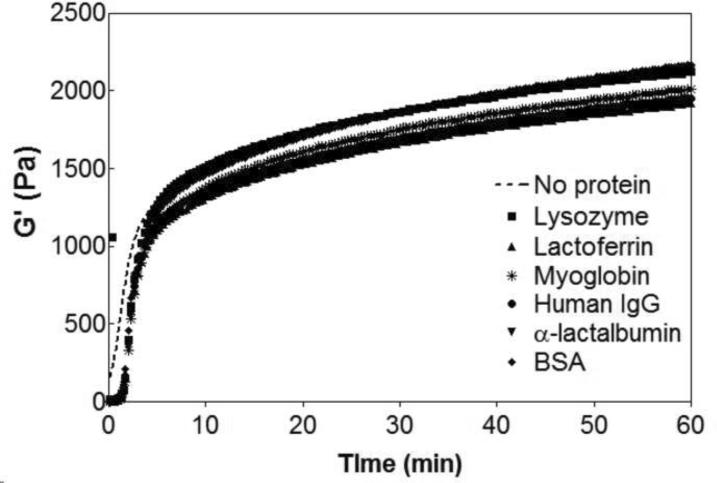
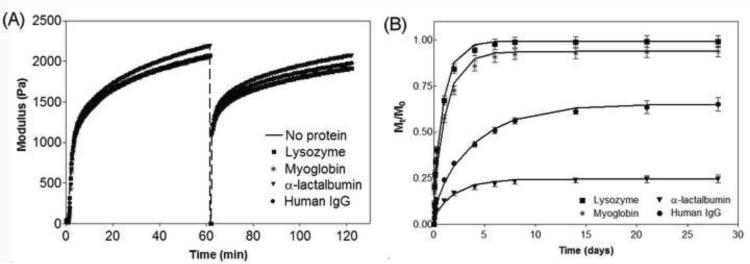
Figure 2 shows rheological data that monitors the triggered hydrogelation of 1 wt% MAX8 after a saline buffered aqueous solution is added to the aqueous peptide solution. The experiment measures the storage modulus (G'), a quantity of the material's mechanical rigidity, as a function of time. At the end of the hour experiment, a 1 wt% MAX8 gel has a storage modulus of 2200 ± 250 Pa. Proteins of varying size and charge (Table 1) were incorporated in the saline buffered solution and then added to the aqueous MAX8 solution to trigger self assembly. Figure 2 shows that there is a small lag phase for the gels containing protein but within approximately a minute, hydrogelation takes place, affording materials having nearly equivalent storage moduli (~2000 Pa) as the protein-free gel. Frequency and strain sweep rheological data are provided in the Supporting Information. Visual assessment of the materials confirms that these hydrogels are rigid, and self supporting (not shown). These rheological experiments demonstrate that including the proteins during gelation does not influence the material forming ability of MAX8 and provides a convenient means to load the gel with protein.
Figure 2.
Dynamic oscillatory time sweep monitoring the formation of 1 wt% MAX8 in pH 7.4, 50 mM BTP, 150 mM NaCl at 37°C alone and in the presence of the model proteins: lysozyme, lactoferrrin, myoglobin, human IgG, α-lactalbumin and bovine serum albumin (BSA). Dashed line represents dynamic time sweep of peptide hydrogel alone.
Bulk Protein Release from Peptide Hydrogels
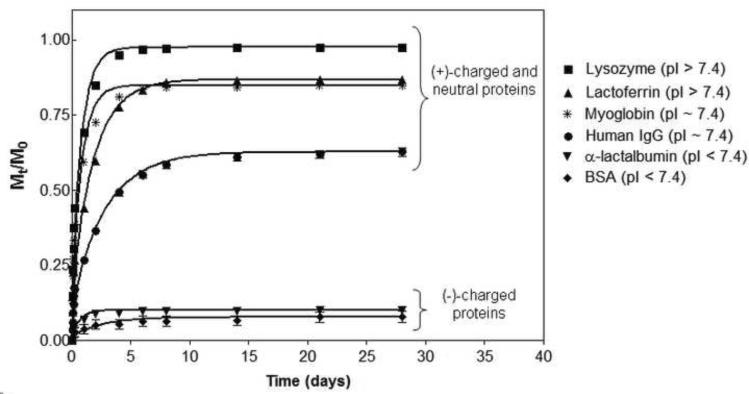
The value of the storage modulus, G', provides insight to the network's mesh size. The Mackintosh theory for semiflexible, crosslinked polymer networks was used to calculate a mesh size of 22 – 23 nm for the MAX8 gel. (31, 42, 43) This mesh size is on the order of the hydrodynamic diameter of the proteins used in this study and therefore, interactions between the network and protein, both steric and electrostatic in nature, should influence macromolecular diffusion. The bulk release of the encapsulated proteins from the 1 wt% MAX8 gel was monitored for a 28 day period. In these initial studies, the loaded gels have not been shear-thinned delivered to first investigate the native network's influence on delivery. Figure 3 shows the amount of protein released from the gels (Mt) normalized to the initial protein loaded (M0) as a function of time. During the experiment, no hydrogel swelling or degradation was observed, suggesting that the release of the proteins is primarily diffusion controlled and will be dependent on any physical interactions between the macromolecules and the peptide network.
Figure 3.
Cumulative release profiles (Mt/M0) of proteins from 1 wt% MAX8 hydrogels with pH 7.4, 50 mM BTP, 150 mM NaCl at 37°C over a month period. Lines represent diffusion coefficient fits with Equation 1. Error bars are smaller than symbols when not visible.
Figure 3 shows that the amount and rate of protein release varies as a function of protein size and electrostatic nature. The positively charged proteins are gradually released over a period of days from the positively charged peptide hydrogel with little retention of the protein in the network. The fastest release is observed for lysozyme, a small (14kD, dH = 4.1 nm) protein with a net positive charge at pH 7.4. At the end of ten days, nearly 100% of the protein is released from the peptide hydrogel network. Lactoferrin, a medium sized (dH = 6.1 nm), positively charged protein, is released more slowly, with 85% released after 10 days, after which time no additional protein is released. The neutral proteins, myoglobin and human IgG, are also gradually released from the 1 wt% MAX8 network, but a greater quantity of protein is retained in the gel, even after one month. Myoglobin is a neutral protein with an equivalent molecular weight (17.4 kD) to lysozyme. This protein has a similar release profile as lysozyme for the first few days but only 85% of the macromolecule is released at the end of the study. Human IgG is actually a pool of proteins that are the largest in the study with an average dH of 10.7 nm. Human IgG shows the most hindered diffusion with 40% of the antibody still retained in the gel at the end of the experiment. α-lactalbumin (dH = 3.2 nm) and BSA (dH = 7.2 nm) have similar hydrodynamic diameters as lysozyme and lactoferrin, respectively, but display a net negative surface charge at pH 7.4 (Table 1). The release of these proteins is greatly impeded by the positively charged peptide fibrillar network. At the end of 28 days, only 10% of these proteins have been released with the remaining amount retained within the gel. Therefore, it is evident from Figure 3 that the size and charge state of the protein play important roles in their release. The data in Figure 3 also suggests that there are possibly two distinct populations of proteins encapsulated in the network, a mobile fraction capable of being released in an early time regime (~1 week to a month) and an immobile fraction that is retained in the gel. The relative amounts of mobile and immobile fractions seem to be dictated by the charge state of the protein.
The apparent diffusion coefficients of these proteins were calculated using a 1D non-steady-state Fickian diffusion model (Equation 1) and are listed in Table 2. In this model, diffusion coefficients are calculated for mobile proteins capable of being released within the one month time period. Appropriately, protein involved in solute-material interactions and thus, retained in the network (immobile fraction) are not accounted for in this model. (29) When compared to the diffusion coefficients of the proteins in an aqueous solution (Table 1), the diffusivities of the proteins are reduced to 20 – 30% of the value (Table 2), making evident the strong influence of the 1.0 wt% MAX8 hydrogel on the mobility of the proteins. The smallest protein, α-lactalbumin, has the largest diffusion coefficient of approximately 40 × 10−8 cm2/sec, whereas the largest protein, human IgG antibody, has the smallest diffusivity of 10 × 10−8 cm2/sec. This is expected because as the protein's hydrodynamic diameter approaches the mesh size of the gel, the mobility of the protein becomes more restricted, resulting in a diffusion coefficient that is smaller in value. Also, the calculated values of the diffusion coefficients are equivalent for proteins of the same size, independent of the protein charge state at pH 7.4. Lysozyme, myoglobin, and α-lactalbumin are all small proteins with similar average molecular weights (14 – 17 kD), but vary in their net charge. Each have a diffusivity of 30 – 40 × 10−8 cm2/sec. BSA also has a diffusion coefficient that is equivalent within error to its positively charged protein counterpart, lactoferrin. Thus, this confirms that the rate of release for the mobile proteins is dependent on the protein size, not its charge state, even when the protein's hydrodynamic radius is on the order of the network's mesh size.
Table 2.
Diffusion coefficients (Dgel ×10−8 cm2/sec) of model proteins released from 1 wt% MAX8 hydrogels at pH 7.4, 50 mM BTP, 150 mM NaCl at 37°C determined by Equation 1. Dgel/Daq represents the ratio of the diffusion coefficients in the gel to the diffusion coefficient of the protein in water at 37°C. The percent of unreleased protein remaining in hydrogels after 1 month release. The percent of protein adsorbed to the network determined from an independent partition/retention experiment.
| Protein | Diffusion Coefficient (10−8 cm/sec) | Dget/Daq | Protein Retained (%) | Protein Adsorbed (%) |
|---|---|---|---|---|
| Lysozyme | 35 ± 6 | 0.32 | 3 ± 1 | 0 ± 6 |
| Lactoferrin | 16 ± 2 | 0.22 | 14 ± 0 | 6 ± 2 |
| Myoglobin | 35 ± 7 | 0.31 | 15 ± 1 | 3 ± 3 |
| Human lgG | 10 ± 1 | 0.24 | 41 ± 1 | 30 ± 3 |
| α-lactalbumin | 41 ± 15 | 0.29 | 91 ± 0 | 84 ± 3 |
| BSA | 11 ± 9 | 0.17 | 94 ± 0 | 80 ± 2 |
The amount of protein retained, i.e, the immobile fraction in a gel after 1 month, however, is largely governed by electrostatic interactions between the proteins and the peptide network, Table 2. Positively charged lysozyme is fully released from the gel. Myoglobin, the neutral, small protein, retains about 15% of initial load within the gel, and 90% of the initial load of negatively charged α-lactalbumin remains in the hydrogel. As the net negative charge of the protein increases, a greater amount of protein is retained in the network. This indicates that attractive electrostatic interactions between the negatively charged proteins and the peptide matrix greatly retard release and trap the protein within the network. Conversely, repulsive electrostatic interactions between positively charged proteins and the peptide network promote release and disfavor any retention of proteins in the network, which may explain the difference in retention between electropositive lysozyme and neutral myoglobin.
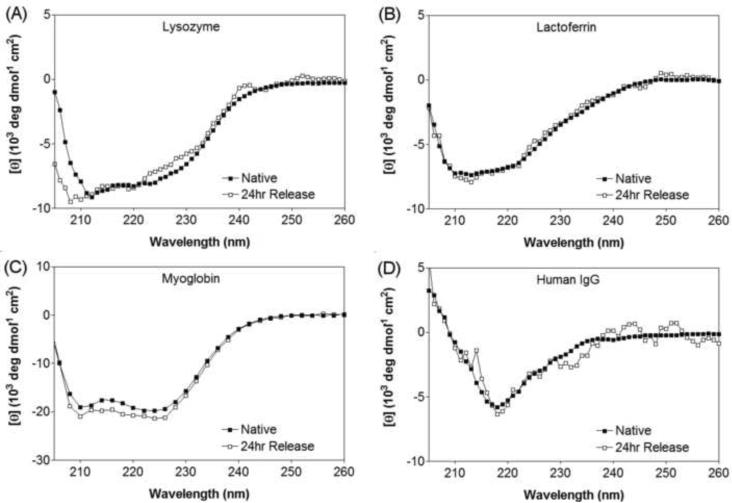
In addition, circular dichroism studies of released protein at the 24 hour time point were conducted to observe any changes to the protein's secondary structure as a result of encapsulation and release. Figure 4 shows that the proteins, lysozyme, myoglobin, lactoferrin, and human IgG, retain their native secondary conformation after release, suggesting that the encapsulation and release process did not adversely affect the proteins' structure. It was not possible to collect CD spectra for α-lactalbumin and BSA due to the minimal amount of protein released to the supernatant.
Figure 4.
Circular dichroism wavelength scans of (A) lysozyme, (B) lactoferrin, (C) myoglobin, and (D) Human IgG in pH 7.4, 50 mM BTP, 150 mM NaCl at 25°C. Native state (■) corresponds to a 1 mg/mL solution of protein in buffer that was never encapsulated or released. 24 hour Release (□) corresponds to encapsulated protein that has been released at the 24 hr time point.
Partition and Retention Studies
The nature of protein retention in the hydrogel network was investigated by measuring the ability of differently charged proteins to partition into the network and become irreversibly retained. In this experiment, protein solutions (1 mg/mL) were introduced above separate 1 wt% MAX8 hydrogels. Proteins were allowed to diffuse into their respective gels over 48 hrs and the amount of protein that had partitioned into the network was measured by difference. Next, the amount of protein that diffused into the network and becomes irreversibly retained was quantified by repeatedly washing the gels with buffer over a week period to remove mobile protein capable of diffusion. The amount of protein irreversibly retained in the hydrogel network was measured by difference.
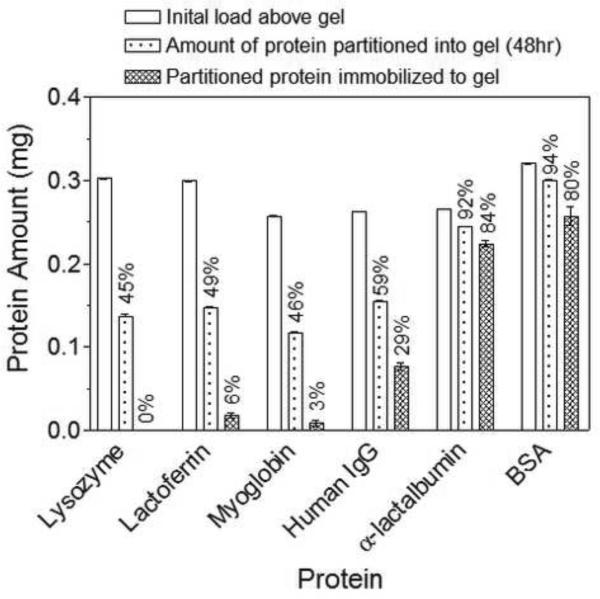
For the initial partition study, all proteins, independent of size or charge, partition into the hydrogel network. 50% of the positively charged proteins, lysozyme and lactoferrin, and neutral myoglobin diffuse into the 1.0 wt% MAX8 hydrogels; the other 50% remains in the supernatant above the gel. The large antibody, which has a hydrodynamic diameter approaching the mesh size of the gel, also diffuses into the gel after 2 days with only 40% remaining in the supernatant above the gel. The greatest uptake into the gel is observed for α-lactalbumin and BSA, the negatively charged proteins. More than 90% of the proteins partitioned into the gel, indicating the preferential affinity of these proteins to the positively charged network.
The retention studies also indicate that the charge state of the protein strongly influences its potential to become irreverisibly retained within the gel network. After washing for a week, most of the positively charged proteins diffuse out from the network with less than 6% of lysozyme and lactoferrin remaining in the peptide hydrogel. Less than 3% of the small, neutral myoglobin is retained in the gel after washing. Both the partition and retention analyses indicate that positively charged and neutral proteins are free to diffuse into, within, and out of the hydrogel network and that their mobility is largely governed by sterics. Approximately half of the amount of antibody that partitions into the gel is still sequestered in the hydrogel. This may be due to its larger size, which limits it mobility out of the network. As for the negatively charged proteins, these macromolecules preferentially partition into the network and have the potential to be irreversibly retained with 80% of the initial load still associated within the network after extensive washing. Taken together, the data suggest that the electrostatic nature of protein strongly influences its mobility in the material network.
Interestingly, if one compares the amount of protein that is retained in the hydrogel in the partition/retention studies (Figure 5) to the amount that is not released in the bulk release experiments (Figure 3), the values are near equivalent, (comparison in Table 2). This observation further supports that the charge state of the macromolecules greatly affects the interactions that the proteins have with the peptide network, independent of how the protein is introduced to the network, either by partitioning the protein into pre-fabricated gels or by directly encapsulating them during self assembly. Also, this similarity between the two experiments indicates that the protein that remains in the hydrogel in the release experiments may be tightly associated with fibril surfaces as opposed to being physically integrated within the self assembled fibril network, which may occur during the self assembly process. This physical association to the network provides another opportunity for controlled release that is dependent on material degradation in addition to diffusion. For example, when the material is introduced into a host where proteolytic enzymes are present, the mobile fraction can be first released by diffusion and upon the degradation of the peptide hydrogel, the remaining load that is adsorbed to the network can then be released into the surrounding tissues. This will be the subject of a future study.
Figure 5.
White bar: Initial amount of protein added above 1 wt% MAX8 gels in pH 7.4, 50 mM BTP, 150 mM NaCl at 37°C; dotted bar: protein amount that partitioned into gel after 48hr incubation; hashed bar: amount of protein retained in network after 1 week wash period. Percentages correspond to protein concentrations relative to the initial protein load above the gel.
Effect of Syringe Delivery on Hydrogel Rigidity and Protein Release
The ideal drug delivery vehicle should be easily administered with little discomfort to the patient. (9, 10) A minimally invasive method of delivery is via syringe injection. MAX8 hydrogels shear-thin under applied stress, but quickly recover upon the cessation of stress, making it a suitable injectable material. (32) The ability of the 1 wt% MAX8 hydrogel to shear-thin and recover in the absence and presence of protein was assessed by oscillatory rheology. Figure 6A shows a rheological experiment where hydrogelation of MAX8 is triggered at physiological buffer conditions in the absence of protein and the storage modulus is monitored over an hour, identical to Figure 2. To model the delivery of the gel through a syringe needle, a strain of 1000% is applied to the material for 30 seconds to convert it to a low viscosity gel. When the high strain is removed, the material rapidly recovers its mechanical integrity (~60% within the first few seconds) and after an hour, the gel has a storage modulus that is 90% of the value prior to the shearing event.
Figure 6.
(A) Dynamic oscillatory time sweep of 1 wt% MAX8 in pH 7.4, 50 mM BTP, 150 mM NaCl at 37°C in the absence and presence of lysozyme, myoglobin, α-lactalbumin, and Human IgG. Hydrogel was allowed to form for one hour at 6 rad/sec, 0.2% strain, followed by application of 1000% strain for 30 seconds, and then allowed to recover for another hour at 6 rad/sec, 0.2% strain. (B) Cumulative release profiles (Mt/M0) of proteins from 1 wt% MAX8 hydrogels in pH 7.4, 50 mM BTP, 150 mM NaCl at 37°C that were sheared through a syringe needle into a glass vial. Lines represent diffusion coefficient fits with Equation 1. Error bars are smaller than symbols when not visible.
This experiment was also conducted in the presence of lysozyme, myoglobin, α-lactalbumin, and human IgG to assess if the proteins affect not only the initial material formation but importantly, the shear-thinning and recovery capability of the MAX8 gel. Figure 5a verifies that the proteins do not influence the peptide's ability to self assemble and gel. In addition, the loaded gels are still capable of being thinned in response to shear. Importantly, the data demonstrate that the incorporated proteins do not influence the restructuring of the physically crosslinked material after shear is removed, irrespective of the protein size or electrostatic character. The gels display near quantitative recovery of their storage modulus (>90%) after one hour, similar to MAX8 hydrogel alone. Also, when a material is disrupted by the shearing event, it may be expected that the network, i.e., the mesh size, will change as a result. However, since the modulus before and after the shearing event is equivalent, this suggests that there was no gross change in mesh size and that that nanostructure of the network is not perturbed.
In addition to the hydrogel's mechanical properties, the effect of syringe delivery on the release profiles of the proteins was also studied. During the reorganization of the network during recovery after shear thinning, protein may leak from the gel, resulting in a burst release, or protein may become physically entrapped within the network, retarding its mobility. To investigate these possibilities, MAX8 hydrogels in the presence of lysozyme, myoglobin, α-lactalbumin, and human IgG were each formed directly in a syringe and allowed to cure for 1.5 hours. The gels were then syringe-delivered to a glass vial and allowed to recover for 1.5 hours. After which, buffer was added above the gels and the bulk release of the proteins was measured as a function of time. The release profiles of the three proteins are shown in Figure 6B and display the same release trends observed for the gels that had not been shear-thinned. The greatest amount released is observed for lysozyme (99%), followed by myoglobin (94%), and then human IgG (65%). Approximately 75% of the α-lactalbumin is retained in the hydrogel after 1 month.
Data in Table 3 shows only a minor change in the release characteristics after syringe delivery, consistent with the negligible changes in rheology and hence, hydrogel topology upon shear thinning and recovery. There is a systematic but small increase in the amount released, accompanied by a small decrease in the overall diffusion coefficient. Shear may act to rearrange regions in the gel, so as to enable more of the protein to diffuse out of the hydrogel, but at a slightly reduced net rate. However, the effects of shear thinning and reformation of the gel on the release characteristics are small and the trends with protein charge and size are preserved.
Table 3.
Comparison of diffusion coefficients (×10−8 cm2/sec) for proteins in a native gel versus a shear-thin recovered gel. (1 wt% MAX8 gels, pH 7.4, 50 mM BTP, 150 mM NaCl, 37°C) Also shown is the percent of protein remaining in hydrogels after 1 month release.
| Non Shear-Thinned Gels | Syringe-Delivered Gels | |||
|---|---|---|---|---|
| Protein | Diffusion Coefficient (×10−8 cm2/sec) | Unreleased Amount (%) | Diffusion Coefficient (×10−8 cm2/sec) | Unreleased Amount (%) |
| Lysozyme | 35 ± 6 | 3 ± 1 | 29 ± 4 | 1 ± 3 |
| Myoglobin | 35 ± 7 | 15 ± 1 | 21 ± 3 | 7 ± 3 |
| α-lactalbumin | 41 ± 15 | 91 ± 0 | 14 ± 2 | 75 ± 2 |
| Human lgG | 10 ± 1 | 41 ± 1 | 7 ± 1 | 35 ± 4 |
Conclusion
Proteins were directly encapsulated into MAX8 hydrogels during peptide self assembly, and the effect of protein size and charge on macromolecular release was studied before and after syringe delivery. Release of positively charged and neutral proteins was largely governed by steric interactions between the network and the protein. Repulsive electrostatic interactions between positively charged proteins and the peptide network enhanced release and disfavored any adsorption of the proteins to the network. Conversely, negatively charged proteins were strongly attracted to the positively charged peptide network, which greatly restricted their release to less than 10% of the initial protein load. Here, attractive electrostatic interactions between the negatively charged proteins and the peptide matrix greatly retarded release by trapping the protein within the network. Partition and retention studies confirmed that encapsulated protein exists in two distinct populations. The first is a mobile phase, whose release is largely dictated by steric interactions between the protein and the network. The second is an immobile phase that is trapped within the network. Lastly, when protein encapsulated gels are delivered via syringe, the release profiles of the macromolecules had similar trends as observed for non-sheared gels, indicating that this method of delivery does not significantly alter their bulk release profiles. These studies indicate that the MAX8 hydrogel is an injectable delivery system with potential for the easy, effective formulation and administration of a variety of hydrophilic proteins.
Supplementary Material
Acknowledgements
This work was supported by National Institutes of Health Grant R01 DE016386-01, while Schneider was at the University of Delaware.
Abbreviations
- (BSA)
bovine serum albumin
- (IgG)
immunoglobin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nieri P, Donadio D, Rossi S, Adinolfi B, Podesta A. Antibodies for Therapeutic Uses and the Evolution of Biotechniques. Curr Med Chem. 2009;16:753–79. doi: 10.2174/092986709787458380. [DOI] [PubMed] [Google Scholar]
- 2.Krejsa C, Rogge M, Sadee W. Protein therapeutics: new applications for pharmacogenetics. Nat Rev Drug Discovery. 2006;5(6):507–21. doi: 10.1038/nrd2039. [DOI] [PubMed] [Google Scholar]
- 3.Lawrence S. Biotech drug market steadily expands. Nat Biotechnol. 2005;23(12):1466. doi: 10.1038/nbt1205-1466. [DOI] [PubMed] [Google Scholar]
- 4.Lawrence S. Billion dollar babies - biotech drugs as blockbusters. Nat Biotechnol. 2007;25(6):687. doi: 10.1038/nbt0407-380. [DOI] [PubMed] [Google Scholar]
- 5.Leader B, Baca QJ, Golan DE. Protein therapeutics: a summary and pharmacological classification. Nat Rev Drug Discov. 2008;7(1):21–39. doi: 10.1038/nrd2399. [DOI] [PubMed] [Google Scholar]
- 6.Dipak SP, Matthew PK, Sathy VB., I Delivery of therapeutic proteins. J Pharm Sci. 2010;99(6):2557–75. doi: 10.1002/jps.22054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Woodnutt G, Violand B, North M. Advances in protein therapeutics. Curr Opin Drug Discov Devel. 2008;11(6):754–61. [PubMed] [Google Scholar]
- 8.Crommelin DJA, Storm G, Verrijk R, de Leede L, Jiskoot W, Hennink WE. Shifting paradigms: biopharmaceuticals versus low molecular weight drugs. Int J Pharm. 2003;266(1–2):3–16. doi: 10.1016/s0378-5173(03)00376-4. [DOI] [PubMed] [Google Scholar]
- 9.Branco MC, Schneider JP. Self-assembling materials for therapeutic delivery. Acta Biomater. 2009;5(3):817–31. doi: 10.1016/j.actbio.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoare TR, Kohane DS. Hydrogels in drug delivery: progress and challenges. Polymer. 2008;49(8):1993–2007. [Google Scholar]
- 11.Kobsa S, Saltzman WM. Bioengineering approaches to controlled protein delivery. Pediatr Res. 2008;63(5):513–9. doi: 10.1203/PDR.0b013e318165f14d. [DOI] [PubMed] [Google Scholar]
- 12.Pawar R, Ben-Ari A, Domb AJ. Protein and peptide parenteral controlled delivery. Exp Opin Bio Ther. 2004:1203–12. doi: 10.1517/14712598.4.8.1203. [DOI] [PubMed] [Google Scholar]
- 13.Peppas NA, Hilt JZ, Khademhosseini A, Langer R. Hydrogels in biology and medicine: from molecular principles to bionanotechnology. Advanced Materials. 2006;18(11):1345–60. [Google Scholar]
- 14.Lee M, Chen TT, Iruela-Arispe ML, Wu BM, Dunn JCY. Modulation of protein delivery from modular polymer scaffolds. Biomaterials. 2007;28(10):1862–70. doi: 10.1016/j.biomaterials.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 15.Lin CC, Metters AT. Hydrogels in controlled release formulations: network design and mathematical modeling. Adv Drug Delivery Rev. 2006;58(12–13):1379–408. doi: 10.1016/j.addr.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 16.Brandl F, Hammer N, Blunk T, Tessmar J, Goepferich A. Biodegradable hydrogels for time-controlled release of tethered peptides or proteins. Biomacromolecules. 2010;11(2):496–504. doi: 10.1021/bm901235g. [DOI] [PubMed] [Google Scholar]
- 17.Yamamoto M, Ikada Y, Tabata Y. Controlled release of growth factors based on biodegradation of gelatin hydrogel. J Biomater Sci. 2001;12:77–88. doi: 10.1163/156856201744461. Polym Ed. [DOI] [PubMed] [Google Scholar]
- 18.Meyvis T, De Smedt S, Stubbe B, Hennink W, Demeester J. On the release of proteins from degrading dextran methacrylate hydrogels and the correlation with the rheologic properties of the hydrogels. Pharm Res. 2001;18(11):1593–9. doi: 10.1023/a:1013038716373. [DOI] [PubMed] [Google Scholar]
- 19.Jo YS, Gantz J, Hubbell JA, Lutolf MP. Tailoring hydrogel degradation and drug release via neighboring amino acid controlled ester hydrolysis. Soft Matter. 2009;5(2):440–6. [Google Scholar]
- 20.Hirota N, Kumaki Y, Narita T, Gong JP, Osada Y. Effect of charge on protein diffusion in hydrogels. Journal of Physical Chemistry B. 2000;104(42):9898–903. [Google Scholar]
- 21.Nystrom M, Aimar P, Luque S, Kulovaara M, Metsamuuronen S. Fractionation of model proteins using their physiochemical properties. Colloids and Surfaces A: Physicochemical and Engineering Aspects. 1998;138:185–205. [Google Scholar]
- 22.Hua ZD, Chen ZY, Li YZ, Zhao MP. Thermosensitive and salt-sensitive molecularly imprinted hydrogel for bovine serum albumin. Langmuir. 2008;24(11):5773–80. doi: 10.1021/la703963f. [DOI] [PubMed] [Google Scholar]
- 23.Yamamoto M, Tabata Y, Ikada Y. Growth factor release from gelatin hydrogel for tissue engineering. J Bioact Compat Polym. 1999;14(6):474–89. [Google Scholar]
- 24.Inukai M, Yonese M. Effects of charge density on drug permeability through alginate gel membranes. Chem Pharm Bull. 1999;47(8):1059–63. [Google Scholar]
- 25.Johnson EM, Berk DA, Jain RK, Deen WM. Diffusion and partitioning of proteins in charged agarose gels. Biophysical Journal. 1995;68(4):1561–8. doi: 10.1016/S0006-3495(95)80328-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cal CF, Bakowsky U, Rytting E, Schaper AK, Kissel T. Charged nanoparticles as protein delivery systems: a feasibility study using lysozyme as model protein. Eur J Pharm Biopharm. 2008;69(1):31–42. doi: 10.1016/j.ejpb.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 27.Sato T, Uchida R, Tanigawa H, Uno K, Murakami A. Application of polymer gels containing side-chain phosphate groups to drug-delivery contact lenses. J Appl Polym Sci. 2005;98(2):731–5. [Google Scholar]
- 28.Andrade-Vivero P, Fernandez-Gabriel E, Alvarez-Lorenzo C, Concheiro A. Improving the loading and release of NSAIDs from pHEMA hydrogels by copolymerization with functionalized monomers. J Pharm Sci. 2007;96(4):802–13. doi: 10.1002/jps.20761. [DOI] [PubMed] [Google Scholar]
- 29.Gelain F, Unsworth LD, Zhang S. Slow and sustained release of active cytokines from self-assembling peptide scaffolds. J Control Release. 2010;145(3):231–9. doi: 10.1016/j.jconrel.2010.04.026. [DOI] [PubMed] [Google Scholar]
- 30.Koutsopoulos S, Unsworth LD, Nagaia Y, Zhang SG. Controlled release of functional proteins through designer self-assembling peptide nanofiber hydrogel scaffold. Proc Natl Acad Sci U S A. 2009;106(12):4623–8. doi: 10.1073/pnas.0807506106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Branco MC, Pochan DJ, Wagner NJ, Schneider JP. Macromolecular diffusion and release from self-assembled beta-hairpin peptide hydrogels. Biomaterials. 2009;30(7):1339–47. doi: 10.1016/j.biomaterials.2008.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haines-Butterick L, Rajagopal K, Branco M, Salick D, Rughani R, Pilarz M, et al. Controlling hydrogelation kinetics by peptide design for three-dimensional encapsulation and injectable delivery of cells. Proc Natl Acad Sci U S A. 2007;104(19):7791–6. doi: 10.1073/pnas.0701980104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ozbas B, Kretsinger J, Rajagopal K, Schneider JP, Pochan DJ. Salt-triggered peptide folding and consequent self-assembly into hydrogels with tunable modulus. Macromolecules. 2004;37(19):7331–7. [Google Scholar]
- 34.Schneider JP, Pochan DJ, Ozbas B, Rajagopal K, Pakstis L, Kretsinger J. Responsive hydrogels from the intramolecular folding and self-assembly of a designed peptide. J Am Chem Soc. 2002;124(50):15030–7. doi: 10.1021/ja027993g. [DOI] [PubMed] [Google Scholar]
- 35.Farnaud S, Evans RW. Lactoferrin - a multifunctional protein with antimicrobial properties. Mol Immunol. 2003;40(7):395–405. doi: 10.1016/s0161-5890(03)00152-4. [DOI] [PubMed] [Google Scholar]
- 36.Hong GY, Chappey O, Niel E, Scherrmann JM. Enhanced cellular uptake and transport of polyclonal immunoglobulin G and Fab after their cationization. J Drug Target. 2000;8(2):67–77. doi: 10.3109/10611860008996853. [DOI] [PubMed] [Google Scholar]
- 37.Merrill EW, Dennison KA, Sung C. Partitioning and diffusion of solutes in hydrogels of poly(ethylene oxide) Biomaterials. 1993;14(15):1117–26. doi: 10.1016/0142-9612(93)90154-t. [DOI] [PubMed] [Google Scholar]
- 38.Permyakov EA, Berliner LJ. alpha-Lactalbumin: structure and function. FEBS Lett. 2000;473(3):269–74. doi: 10.1016/s0014-5793(00)01546-5. [DOI] [PubMed] [Google Scholar]
- 39.Jason AC, Peters GR. Analysis of bimodal diffusion of water in fish muscle. J Phys D-Appl Phys. 1973;6(4):512–21. [Google Scholar]
- 40.Crank J. The mathematics of diffusion. second edition Oxford University Press; 1975. [Google Scholar]
- 41.Tessmar JK, Gopferich AM. Matrices and scaffolds for protein delivery in tissue engineering. Adv Drug Delivery Rev. 2007;59(4–5):274–91. doi: 10.1016/j.addr.2007.03.020. [DOI] [PubMed] [Google Scholar]
- 42.Mackintosh FC, Kas J, Janmey PA. Elasticity of semiflexible biopolymer networks. Phys Rev Lett. 1995;75(24):4425–8. doi: 10.1103/PhysRevLett.75.4425. [DOI] [PubMed] [Google Scholar]
- 43.Ozbas B, Rajagopal K, Schneider JP, Pochan DJ. Semiflexible chain networks formed via self-assembly of beta-hairpin molecules. Phys Rev Lett. 2004;93(26) doi: 10.1103/PhysRevLett.93.268106. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.