Abstract
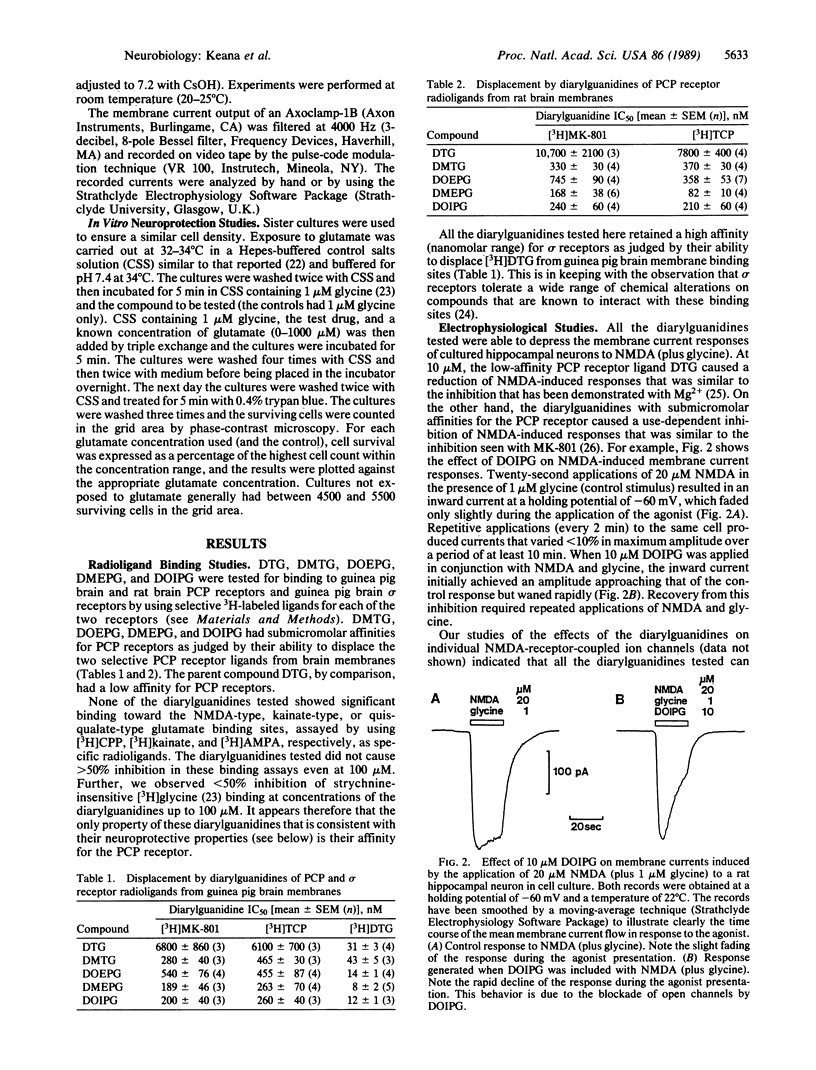
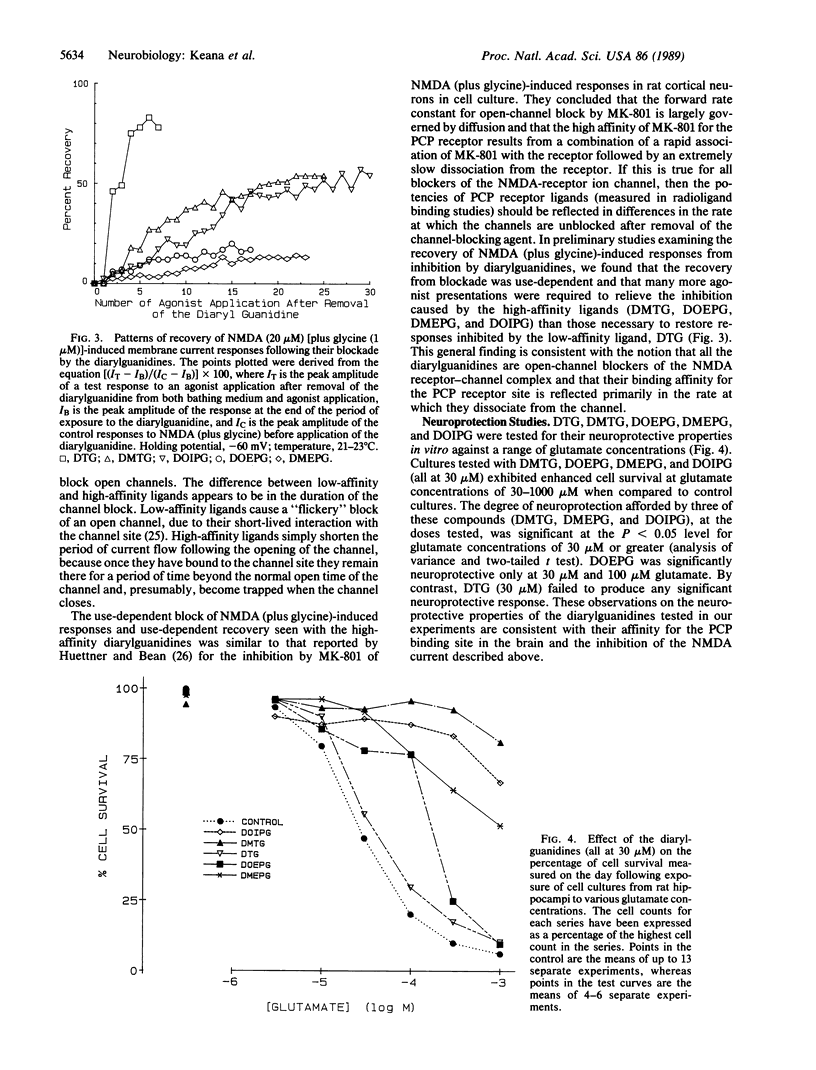
Four diarylguanidine derivatives were synthesized. These compounds were found to displace, at submicromolar concentrations, 3H-labeled 1-[1-(2-thienyl)cyclohexyl]piperidine and (+)-[3H]MK-801 from phencyclidine receptors in brain membrane preparations. In electrophysiological experiments the diarylguanidines blocked N-methyl-D-aspartate (NMDA)-activated ion channels. These diarylguanidines also protected rat hippocampal neurons in vitro from glutamate-induced cell death. Our results show that some diarylguanidines are noncompetitive antagonists of NMDA receptor-mediated responses and have the neuroprotective property that is commonly associated with blockers of the NMDA receptor-gated cation channel. Diarylguanidines are structurally unrelated to known blockers of NMDA channels and, therefore, represent a new compound series for the development of neuroprotective agents with therapeutic value in patients suffering from stroke, from brain or spinal cord trauma, from hypoglycemia, and possibly from brain ischemia due to heart attack.
Full text
PDF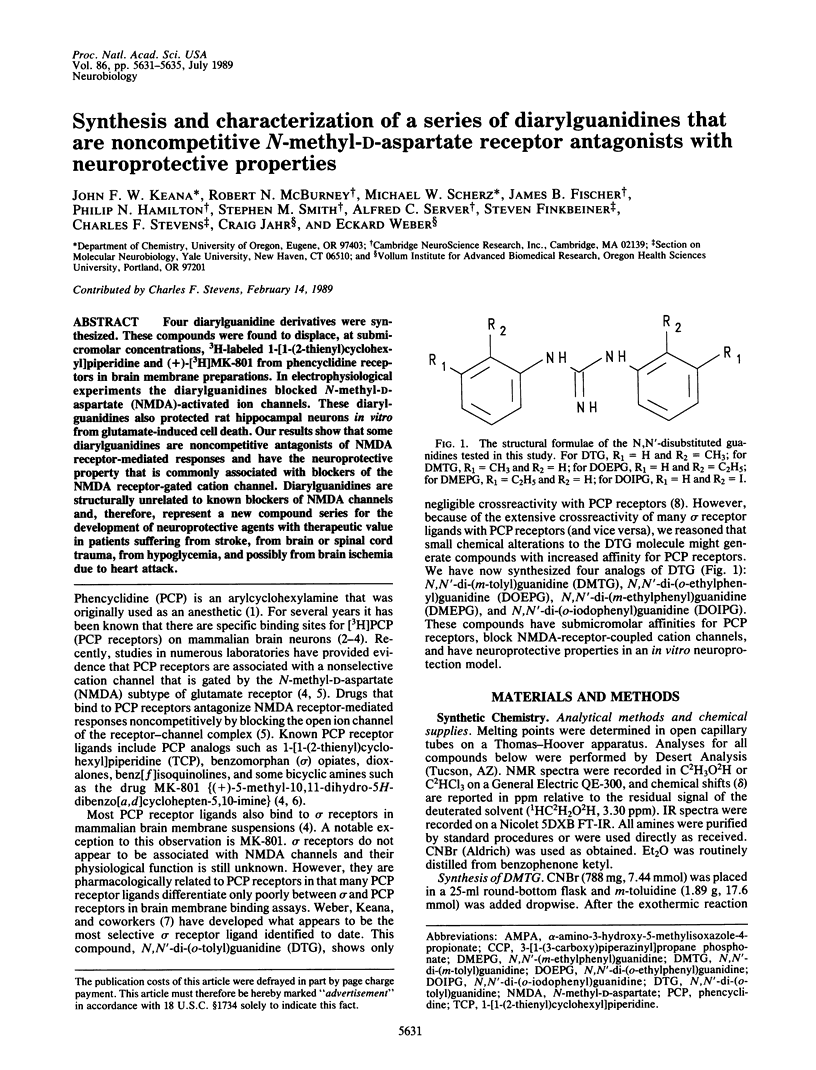


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anis N. A., Berry S. C., Burton N. R., Lodge D. The dissociative anaesthetics, ketamine and phencyclidine, selectively reduce excitation of central mammalian neurones by N-methyl-aspartate. Br J Pharmacol. 1983 Jun;79(2):565–575. doi: 10.1111/j.1476-5381.1983.tb11031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beal M. F., Kowall N. W., Ellison D. W., Mazurek M. F., Swartz K. J., Martin J. B. Replication of the neurochemical characteristics of Huntington's disease by quinolinic acid. Nature. 1986 May 8;321(6066):168–171. doi: 10.1038/321168a0. [DOI] [PubMed] [Google Scholar]
- Bristow D. R., Bowery N. G., Woodruff G. N. Light microscopic autoradiographic localisation of [3H]glycine and [3H]strychnine binding sites in rat brain. Eur J Pharmacol. 1986 Jul 31;126(3):303–307. doi: 10.1016/0014-2999(86)90062-2. [DOI] [PubMed] [Google Scholar]
- Choi D. W. Ionic dependence of glutamate neurotoxicity. J Neurosci. 1987 Feb;7(2):369–379. doi: 10.1523/JNEUROSCI.07-02-00369.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi D. W., Maulucci-Gedde M., Kriegstein A. R. Glutamate neurotoxicity in cortical cell culture. J Neurosci. 1987 Feb;7(2):357–368. doi: 10.1523/JNEUROSCI.07-02-00357.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenwick E. M., Marty A., Neher E. A patch-clamp study of bovine chromaffin cells and of their sensitivity to acetylcholine. J Physiol. 1982 Oct;331:577–597. doi: 10.1113/jphysiol.1982.sp014393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkbeiner S., Stevens C. F. Applications of quantitative measurements for assessing glutamate neurotoxicity. Proc Natl Acad Sci U S A. 1988 Jun;85(11):4071–4074. doi: 10.1073/pnas.85.11.4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREIFENSTEIN F. E., DEVAULT M., YOSHITAKE J., GAJEWSKI J. E. A study of a 1-aryl cyclo hexyl amine for anesthesia. Anesth Analg. 1958 Sep-Oct;37(5):283–294. [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Honoré T., Drejer J., Nielsen M. Calcium discriminates two [3H]kainate binding sites with different molecular target sizes in rat cortex. Neurosci Lett. 1986 Mar 28;65(1):47–52. doi: 10.1016/0304-3940(86)90118-7. [DOI] [PubMed] [Google Scholar]
- Huettner J. E., Baughman R. W. Primary culture of identified neurons from the visual cortex of postnatal rats. J Neurosci. 1986 Oct;6(10):3044–3060. doi: 10.1523/JNEUROSCI.06-10-03044.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huettner J. E., Bean B. P. Block of N-methyl-D-aspartate-activated current by the anticonvulsant MK-801: selective binding to open channels. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1307–1311. doi: 10.1073/pnas.85.4.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J. W., Ascher P. Glycine potentiates the NMDA response in cultured mouse brain neurons. Nature. 1987 Feb 5;325(6104):529–531. doi: 10.1038/325529a0. [DOI] [PubMed] [Google Scholar]
- Keana J. F., Scherz M. W., Quarum M., Sonders M. S., Weber E. Synthesis and characterization of a radiolabelled derivative of the phencyclidine/N-methyl-D-aspartate receptor ligand (+) MK-801 with high specific radioactivity. Life Sci. 1988;43(12):965–973. doi: 10.1016/0024-3205(88)90541-3. [DOI] [PubMed] [Google Scholar]
- Kurland L. T. Amyotrophic lateral sclerosis and Parkinson's disease complex on Guam linked to an environmental neurotoxin. Trends Neurosci. 1988 Feb;11(2):51–54. doi: 10.1016/0166-2236(88)90163-4. [DOI] [PubMed] [Google Scholar]
- Largent B. L., Wikström H., Gundlach A. L., Snyder S. H. Structural determinants of sigma receptor affinity. Mol Pharmacol. 1987 Dec;32(6):772–784. [PubMed] [Google Scholar]
- McBurney R. N., Neering I. R. The measurement of changes in intracellular free calcium during action potentials in mammalian neurones. J Neurosci Methods. 1985 Mar;13(1):65–76. doi: 10.1016/0165-0270(85)90044-5. [DOI] [PubMed] [Google Scholar]
- Murphy D. E., Schneider J., Boehm C., Lehmann J., Williams M. Binding of [3H]3-(2-carboxypiperazin-4-yl)propyl-1-phosphonic acid to rat brain membranes: a selective, high-affinity ligand for N-methyl-D-aspartate receptors. J Pharmacol Exp Ther. 1987 Mar;240(3):778–784. [PubMed] [Google Scholar]
- Murphy D. E., Snowhill E. W., Williams M. Characterization of quisqualate recognition sites in rat brain tissue using DL-[3H]alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA) and a filtration assay. Neurochem Res. 1987 Sep;12(9):775–781. doi: 10.1007/BF00971514. [DOI] [PubMed] [Google Scholar]
- Nowak L., Bregestovski P., Ascher P., Herbet A., Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984 Feb 2;307(5950):462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- Sonders M. S., Keana J. F., Weber E. Phencyclidine and psychotomimetic sigma opiates: recent insights into their biochemical and physiological sites of action. Trends Neurosci. 1988 Jan;11(1):37–40. doi: 10.1016/0166-2236(88)90048-3. [DOI] [PubMed] [Google Scholar]
- Vincent J. P., Kartalovski B., Geneste P., Kamenka J. M., Lazdunski M. Interaction of phencyclidine ("angel dust") with a specific receptor in rat brain membranes. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4678–4682. doi: 10.1073/pnas.76.9.4678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber E., Sonders M., Quarum M., McLean S., Pou S., Keana J. F. 1,3-Di(2-[5-3H]tolyl)guanidine: a selective ligand that labels sigma-type receptors for psychotomimetic opiates and antipsychotic drugs. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8784–8788. doi: 10.1073/pnas.83.22.8784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong E. H., Kemp J. A., Priestley T., Knight A. R., Woodruff G. N., Iversen L. L. The anticonvulsant MK-801 is a potent N-methyl-D-aspartate antagonist. Proc Natl Acad Sci U S A. 1986 Sep;83(18):7104–7108. doi: 10.1073/pnas.83.18.7104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zukin S. R., Zukin R. S. Specific [3H]phencyclidine binding in rat central nervous system. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5372–5376. doi: 10.1073/pnas.76.10.5372. [DOI] [PMC free article] [PubMed] [Google Scholar]


