Abstract
Actin and tubulin cytoskeletal components are studied extensively in chondrocytes, but less is known about vimentin intermediate filaments. In other cell types, vimentin is a determinant of cell stiffness and disruption of vimentin networks weakens the mechanical integrity of cells. Changes in vimentin organization correlate with osteoarthritis progression, but the functional consequences of these changes remain undetermined in chondrocytes. The objective of this study was to compare the contribution of vimentin to the mechanical stiffness of primary human chondrocytes isolated from normal versus osteoarthritic cartilage.
Chondrocytes were embedded in alginate and vimentin networks disrupted with acrylamide. Constructs were imaged while subjected to 20% nominal strain on a confocal microscope stage, and the aspect ratios of approximately 1900 cells were measured. Cytosolic stiffness was estimated with a finite element model, and live-cell imaging of GFP-vimentin was used to further analyze the nature of vimentin disruption.
Vimentin in normal chondrocytes formed an inner cage-like network that was substantially stiffer than the rest of the cytosol and contributed significantly to overall cellular stiffness. Disruption of vimentin reduced stiffness approximately 2.8-fold in normal chondrocytes. In contrast, osteoarthritic chondrocytes were less stiff and less affected by vimentin disruption. This 3D experimental system revealed contributions of vimentin to chondrocyte stiffness previously not apparent, and correlated changes in vimentin-based chondrocyte stiffness with osteoarthritis.
Suggested Keywords: vimentin, cytoskeleton, chondrocyte stiffness, osteoarthritis, mechanotransduction
Introduction
The chondrocyte cytoskeleton consists mainly of actin microfilaments, tubulin microtubules, and vimentin intermediate filaments. Actin and tubulin have been studied extensively in chondrocytes (1–4). Extensive intermediate filament networks in chondrocytes by electron microscopy were reported over forty years ago(5), but the function of the vimentin networks of chondrocytes remains unknown. Descriptive studies show that vimentin is more abundant in chondrocytes than in other cells (6). Vimentin production is increased upon transfer from monolayer to suspension culture systems, which more closely mimic the native chondrocyte environment (3, 7–8).
In osteoarthritic chondrocytes the protein expression level and filament network organization of vimentin are altered (9–11). At the protein level, increased vimentin fragments were observed in a rat osteoarthritis model(9). Vimentin fragments were also found in human osteoarthritic cartilage(10) and this correlated with a disrupted vimentin network. Cells with elongated vimentin-rich processes were found more often in osteoarthritic than normal cartilage (11). Experimental disruption of vimentin filaments with acrylamide decreased extracellular matrix synthesis in chondrocyte cultures, suggesting that cartilage homeostasis requires an intact vimentin network and that altered vimentin might contribute to cartilage pathology (12). Vimentin network rearrangements were observed in a single-impact cartilage injury model, and correlated with pro-teoglycan loss (13). Similarly, vimentin was identified in a proteomics analysis of proteins released from cartilage explants after injurious compression (14).
While a mechanical role for vimentin in chondrocytes remains unknown, the vimentin cytoskeleton is important for maintenance of the mechanical stability in other cell types. The viscoelastic properties of vimentin filaments are non-linear; at slow strain rates they deform easily but at high strain rates they exhibit greater rigidity and resist breakage more than actin or tubulin filaments(15). In fibroblasts, vimentin is a major contributor to cell stiffness, and experimental disruption of the vimentin network with acrylamide weakens the mechanical integrity of these cells(16). Studies of vasculature from a vimentin knockout mouse led to the conclusions that vimentin participates in mechanotransduction of shear stresses(17).
Clues that vimentin might also have a mechanical role in chondrocytes come from the observations that vimentin expression is increased in chondrocytes of weight-bearing cartilage(18) and in regions of cartilage that undergo greater levels of cellular deformation(4). In cartilage explant cultures vimentin filaments initially depolymerized, but were subsequently re-established in the presence of compressive forces(2). In annulus fibrosus cells, static compression increased both the amount of vimentin mRNA as well as its incorporation into filaments(19). Based on confocal immunofluorescence microscopy of vimentin in agarose-embedded chondrocytes, it was postulated that the vimentin network is also ideally situated to transmit mechanical forces from to cytosol the nucleus during mechanotransduction(20). Recently it was shown that disruption of the vimentin network resulted in chondrocytes becoming incompressible(21).
Our hypothesis is that vimentin has a mechanical function in maintaining chondrocyte stiffness, and that this function of vimentin is altered or impaired in chondrocytes from arthritic cartilage. We embedded unpassaged human chondrocytes from healthy and OA cartilage in hydrogel of known stiffness, then measured the cellular deformation using live-cell imaging upon application of a 20% nominal strain. To determine the contribution of vimentin to stiffness, parallel experiments were performed on cells in which the vimentin networks were disrupted. Our results demonstrate for the first time that vimentin significantly contributed to the stiffness of healthy chondrocyte, while osteoarthritic chondrocytes were not as stiff and also less affected by vimentin disruption.
Methods
Cartilage
Osteoarthritic human articular cartilage was obtained from femoral and tibial condyles resected during total knee arthroplasty (TKA) surgeries performed at Scripps Hospital, healthy human cartilage was obtained from tissue banks, both in accordance with HIPPA guidelines and with IRB approval. Samples were obtained from 8 TKA patients. Fibrillated and diseased cartilage (OA group) was dissected away from cartilage with normal appearance (Normal group). In addition, healthy cartilage was obtained from 1 male and 2 female tissue donor without cartilage pathology (Healthy group). The average TKA donor age was 62 (±14 range 40–84), the average healthy tissue donor was 24 (Supplemental Table 1).
Cell culture
Unpassaged primary human articular chondrocytes were isolated and cast into 6mm diameter discs of 2% alginate as follows: chondrocytes were released from the cartilage by overnight digestion with type IV collagenase as described previously(22). Chondrocytes were counted, centrifuged, and resuspended at 5×105 cells/ml in a 2% solution of sterile-filtered and endotoxin-free alginate (FMC BioPolymer, Sandvika Norway) in HBSS. The alginate-cell suspension was pipetted into a polysulfone casting frame 1cm × 4cm × 2.4mm thick sandwiched between Whatman 3mm filter paper held in place by stainless steel mesh and clamps (modified from Ragan et al (23)). The assembly was submerged in 102mM CaCl2 for 45 minutes to polymerize the alginate. Four discs were created using 6mm dermal biopsy punches as shown in Figure 1A, and rinsed several times in culture media (DMEM with 10% calf serum, 1% penicillin/streptomycin, and 30μg/ml ascorbic acid).
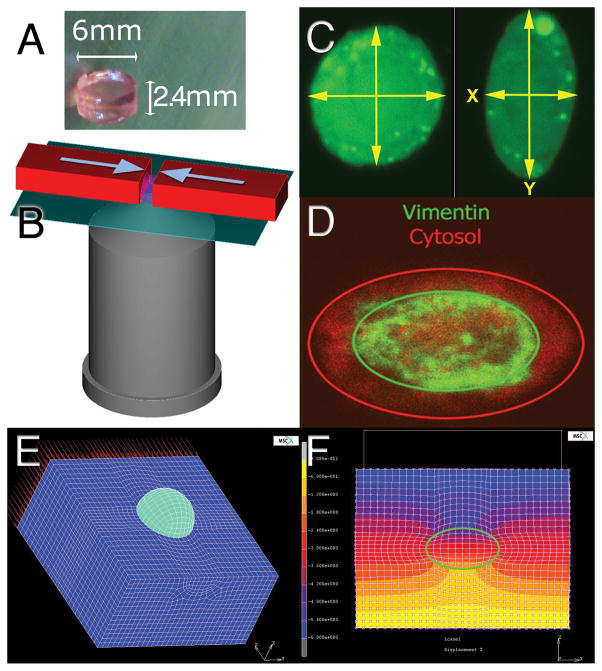
Figure 1.
A) Chondrocytes in alginate gel. These gels were cut in half radially for compression and confocal imaging. B) Schematic of the compression setup depicting the microscope objective, and the direction of compression (arrows) on the gel half. C) Typical images of uncompressed and compressed cells obtained with the confocal microscope. The green fluorescence is from the calcein-AM component of the live-dead stain. The yellow arrows indicate the measurements taken for analysis. D) GFP-Vimentin and cytosolic RFP staining of a transduced chondrocyte demonstrating the inner vimentin cage structure. E) Finite element mesh of a single alginate-embedded chondrocyte. The mesh (dark blue) has been sectioned to show the spherical cell (light blue) before and after 20% nominal strain is applied. F) Contour map of the displacement field of a cross-section of the cell-gel construct in the direction of compression, with the cell boundary outlined in green (units = micrometers).
To disrupt vimentin, monomeric acrylamide (Sigma) was added at a final concentration of 4mM to the culture media of two alginate discs, control discs were cultured without acrylamide. At this concentration, acrylamide causes collapse of intermediate filament structures but does not noticeably affect either microtubules or actin filaments (24–26). In preliminary studies we observed that 40mM acrylamide was cytotoxic within 12 hours, but that 4mM acrylamide monomer caused a collapse of vimentin filaments without significantly affecting chondrocyte viability up to 72 hours when compared to untreated control cells from the same donors.
Mechanical Loading
Mechanical loading was performed 24 hours after cell seeding in alginate. Pilot studies indicated that at this timepoint the primary chondrocytes do not produced sufficient pericellular matrix to affect the cytosolic stiffness measurements. Gels were cut in half radially and labeled for 30 minutes with calcein-AM and 10 minutes with ethidium homodimer (Live-dead stain, Invitrogen). Gels were placed onto a coverslip on the stage of a Zeiss LSM510 inverted confocal microscope custom-fitted with a linear actuator with 1μm resolution (SMAC, Carlsbad CA) as shown diagrammatically in Figure 1B. The actuator was used to measure the exact dimensions of each gel and then compress them to 80% of their original thickness (20% nominal strain). Strain was applied in the Y-axis of the gels relative to the microscope stage. Strain was applied at a rate of 50 microns/sec. While strained, at least 50 cells were imaged in each gel by taking a single confocal z-slice of the cell at the widest diameter. Images were acquired after stress relaxation was complete: approximately 10 minutes after compression. The calcein fluorescence in the cytosol of living cells was imaged with a 63x water immersion lens and saved as 512x512 pixel tiff files.
Image Processing and Statistical Analysis
A Matlab (MathWorks, Natick MA) subroutine was developed to automate measurements of the maximum cell diameter in the X and Y direction and calculate the ratios of the Y and X axes, based conceptually on the techniques described by Lee et al(27). Whenever possible, at least 50 cells were imaged from each condition, and 4 conditions were measured for each TKA patient (untreated normal cells, untreated OA cells, acrylamide treated normal cells, and acrylamide treated OA cells). For cells from healthy donors without arthritis, 2 conditions were measured (untreated and acrylamide-treated). A total of ~2000 individual cells were imaged from the ten donors, representative cells are shown in Figure 1C. Data were imported into a JMP 7.1 database for statistical analysis. After testing for normal distributions, cell aspect ratios of the groups were compared by one-way ANOVA with Tukey-Kramer’s post-hoc correction for multiple comparisons, statistical significance was set at p<0.05.
Finite Element Modeling
The Young’s modulus of the 2% alginate gels was measured experimentally at 11.8kPa The Young’s modulus of the gels were determined from the slope of the equilibrium stress versus strain curves between 5% and 25% strain, as the gels were subjected to unconfined compression under position control at 5% strain increments. At each strain level, the gels were allowed to reach equilibrium (approximately 10 minutes) before recording the compressive force. This stiffness of the alginate was not significantly affected by the presence 4mM acrylamide monomers or the presence of cells at concentrations under 2×106/ml. This stiffness value was then used to create a 3D finite element model of a single alginate-embedded chondrocyte using in MSC.MARC (MSC Software, Santa Ana CA) as shown in Figure 1E–F. The model assumed uniform mechanical properties and an unchanging Poisson’s ratio of 0.15 for the gel and 0.4 for the cells (28–30). Note that the model computes equilibrium modulus and does not account for the viscoelastic behavior of the cell or the gel. The model also does not account for changes in the Poisson’s ratio of the cell upon treatment with acrylamide: vimentin disruption may alter the cellular Poisson’s ratio (21). The cellular stiffness parameter was varied incrementally between 0.2 and 10kPa, and the model calculated the chondrocyte aspect ratio resulting from a net nominal compressive strain of 20% applied to the alginate gel. The cell material property assumption that reproduced the mean experimental aspect ratio was identified as that associated with each cell type and culture condition.
Transduction of GFP-Vimentin
GFP-vimentin and RFP were cloned into a lentiviral expression system (Invitrogen) and used to create stably transfected human chondrocyte strains as described previously (31). The passaged transduced cells were embedded in alginate and treated with acrylamide as described above. Cells were imaged and the deformation of cytosolic RFP compared to that of GFP-vimentin. To quantify the relative deformation, the distance between the edge of the vimentin and the edge of the cytosol was measured in both the X and Y axis as shown in Figure 1D. Means for the control and acrylamide treated groups were compared using Student’s t-test with p<0.05 considered significant.
Results
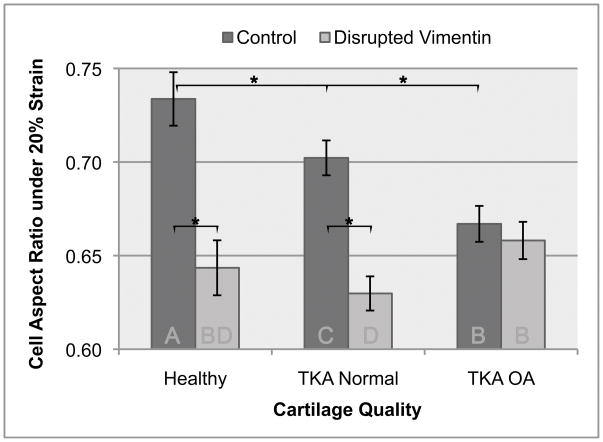
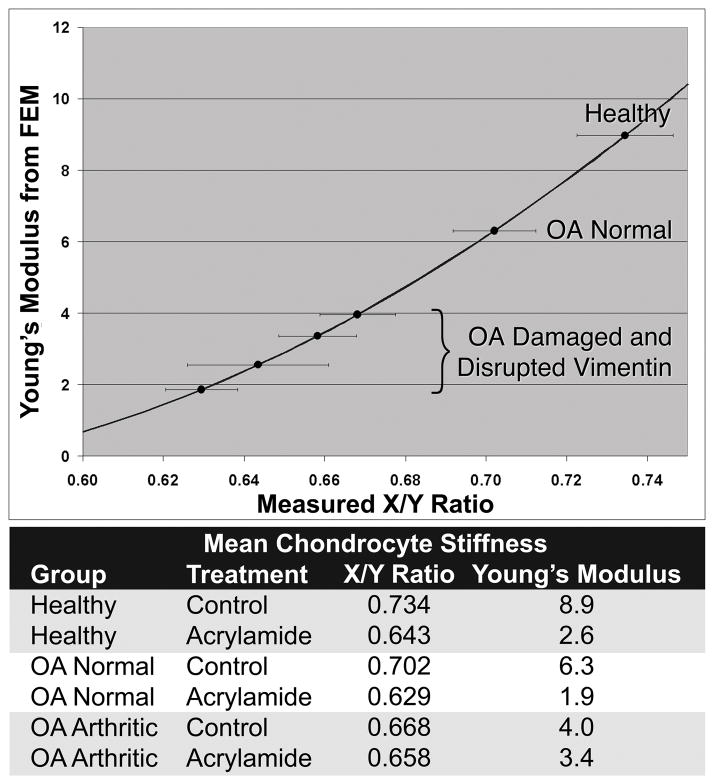
The goal of the study was to compare the contribution of vimentin intermediate filaments to the cytosolic stiffness of chondrocytes from healthy and osteoarthritic cartilage. We found that untreated healthy chondrocytes were the stiffest (the deformation upon strain was the least, the x/y axis ratio was 0.73), and that increasing severity of disease reduced cell stiffness (x/y ratio of arthritic cells was 0.66). Chondrocytes from normal regions of arthritic joints were of intermediate stiffness, and cells from damaged cartilage regions were least stiff (Figure 2). Disruption of the vimentin cytoskeleton decreased the apparent stiffness of chondrocytes. The greatest effect was observed on the stiffest healthy cells, while a reduced effect was observed on normal cells from arthritic joints, and the effect on the stiffness of chondrocytes from damaged arthritic cartilage did not reach statistical significance. Vimentin disruption in healthy chondrocytes reduced their stiffness to that observed in chondrocytes from damaged cartilage (Figure 2). Finite element modeling estimated the cytosolic stiffness at approximately 8.9 kPa, and reduced moduli of 6.3kPa and 4.0kPa were estimated for chondrocytes from OA normal and OA arthritic cartilage, respectively. Upon disruption of vimentin, the Young’s modulus was reduced to approximately 2.8kPa, 1.9kPa, and 3.4kPa for healthy, OA normal, and OA arthritic chondrocytes, respectively (Figure 3).
Figure 2.
Aspect ratio of compressed cells from healthy, TKA-normal, and TKA-OA cartilage, with and without disruption of vimentin with acrylamide treatment. Statistical analysis of ~1800 cell measurements are shown, * indicates p<0.05. Letters on the bottom of the bars indicate groups statistically different from each other by ANOVA with Tukey-Kramer’s post-hoc correction for multiple comparisons; bars with the same letter are not statistically different. Error bars represent 95% confidence interval of the mean.
Figure 3.
Estimated cytosolic Young’s modulus based on cellular deformation in 20% nominal strain. Curve is derived from the finite element model; this assumed a Poisson’s ratios of 0.40 and 0.15 for the cytosol and the alginate, respectively. Horizontal error bars indicate 95% confidence interval of mean cellular aspect ratio. Measured X/Y ratios and estimated Young’s moduli are shown in table form below the graph.
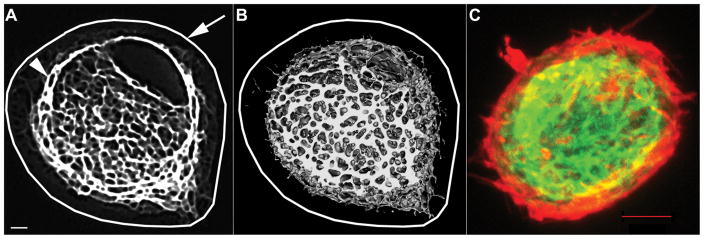
Since relatively little is known about the actual structure of the vimentin cytoskeleton in 3D cultures of chondrocytes, we imaged chondrocytes transduced with lentiviral GFP-vimentin and grown in 3D. Vimentin formed a tight, highly interconnected inner network approximately 1.0 to 1.5um from the outside perimeter (cell membrane) of the chondrocytes (Figure 4). The vimentin network is contained entirely within the cortical actin, whereas the most abundant actin was along the cortical perimeter of the chondrocyte.
Figure 4.
GFP-Vimentin cytoskeleton forms a tight cage within the cell in alginate-embedded chondrocytes A) Single high-resolution confocal slice through the center of a chondrocyte. Arrow indicates superimposed cell outline, arrowhead indicates GFP-Vimentin. B) 3D reconstruction of high-resolution GFP-vimentin network after thresholding in DICOM software, with superimposed cell outline. C) 2D projection confocal image of a GFP-Vimentin-expressing chondrocyte fixed and stained with Phalloidin-Alexa-546 to identify cortical actin filaments (red) and inner GFP-vimentin network. Scale bar is 1um in A and B, 5um in C.
The observation that vimentin forms a tight mesh contained within the central region of the chondrocyte cytosol led us to question whether this inner network would deform to the same extent as the cytosol as a whole. We found that the vimentin network deformed independent of the cytosol, and that the vimentin structure was more resistant to deformation than the cytosol as a whole (Table 1). After disruption of the vimentin network, the vimentin deformation matched that of the cytosol.
Table 1.
Vimentin network and cytosol deform independently. The X and Y dimensions of the vimentin and cytosol were measured in 25 alginate-embedded chondrocytes subjected to 20% nominal strain. The intact vimentin network resisted deformation more than the cytosol, with a consistently higher aspect ratio than the cytosol. After disruption of the vimentin network, the deformation of vimentin was not significantly different from that of the cytosol.
| Mean Aspect Ratio (X/Y dimensions) | Ratio of Vimentin : Cytosol | ||
|---|---|---|---|
| Cytosol | Vimentin | ||
| Control | 0.6389 | 0.7674 | 1.203* |
| Acrylamide | 0.6390 | 0.6268 | 0.984 |
indicates significantly different from acrylamide treated (p<0.01).
To measure of the turnover rate of the vimentin filaments in chondrocytes, FRAP analysis was attempted in 3D. Filament turnover was so slow that we were unable to measure fluorescence recovery within 9 minutes (Figure 5). We observed that the chondrocytes were in constant motion, with rotation and translation movement within their immediate space. At longer time points the bleached areas of vimentin became obscured by the overall rotational and translation motion and distortion of the entire vimentin network. In contrast, actin filament fluorescence recovery was nearly complete within 3 minutes (not shown).
Figure 5.
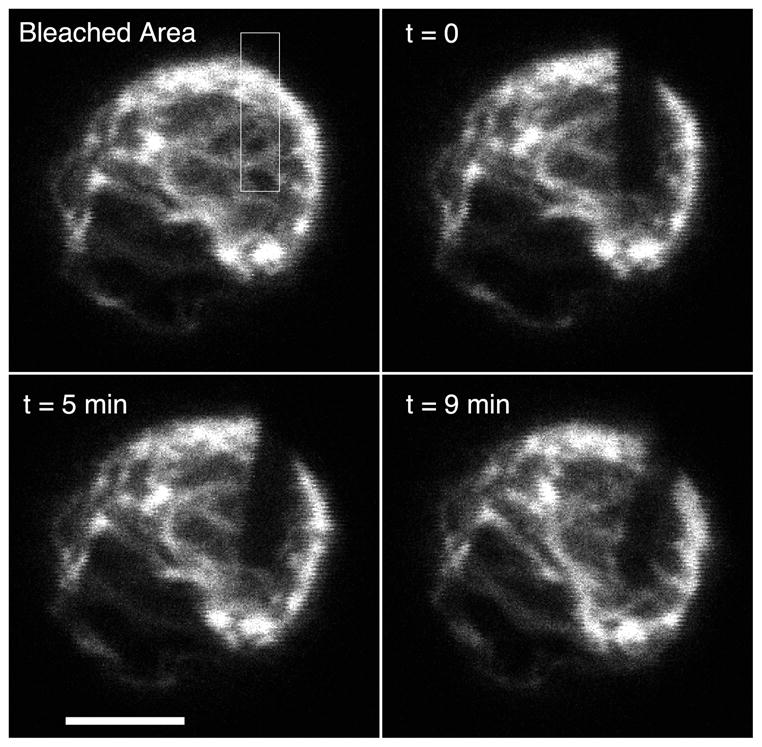
Slow turnover of vimentin cytoskeleton. A single alginate-embedded human chondrocyte expressing GFP-vimentin was photobleached, then observed from time t=0 to 9 minutes by confocal microscopy. There was little recovery of fluorescence in bleached area, suggesting that vimentin filaments form stable structures. Note that translation, rotation and distortion of the vimentin network occur in the living cell even in the absence of filament turnover. Scale 5um
Discussion
Several lines of evidence suggest that alterations in vimentin structure correlate with the progression of osteoarthritis. In adherent cells grown in monolayer, vimentin plays a structural role in maintaining the mechanical integrity of the cells. The functional consequences of the altered vimentin structures in osteoarthritic chondrocytes are largely unknown. We designed this study to determine the role of the vimentin cytoskeleton in providing mechanical properties to primary unpassaged human chondrocytes grown in 3D culture. Notable observations were first, that the overall stiffness of chondrocytes was highest in healthy cartilage, and that a significant portion of this stiffness was lost when vimentin networks were disrupted. Second, cells from more arthritic cartilage were not as stiff, and the stiffness was less affected by vimentin disruption. Third, live-cell imaging of chondrocytes expressing GFP-vimentin revealed that vimentin forms a smaller but very dense network within the cell, which does not extend to the cortical actin shell. Fourth, during compression, this inner vimentin ‘cage’ was much more resistant to compression than the cytosol in general. On vimentin disruption, the vimentin ‘cage’ appeared to lose its stiffness and deformed with the rest of the cytosol. Finally, movement of the vimentin network occurs through rotation, translation, and distortion of the entire network, although turnover of individual vimentin filaments is relatively slow.
A micropipette aspiration study of chondrocytes surprisingly found that vimentin contributed less to cell stiffness of chondrocytes than actin, and found that vimentin disruption by acrylamide only affected stiffness at cytotoxic concentrations(26). However, micropipette aspiration involves testing a small number of cells suspended in culture media, while a dense matrix surrounds in situ chondrocytes. In this study we chose to re-evaluate the contribution of the vimentin network to chondrocyte cell stiffness using chondrocytes embedded in 3D alginate constructs. This technique facilitates stiffness measurements in a larger number of cells, and in a 3D environment more closely resembling that found in cartilage.
A single-cell compression study of middle- and deep-zone bovine chondrocytes in suspension found that vimentin contributed less to the overall compressive stiffness than actin, and that disruption of vimentin with 40mM acrylamide caused the cells to become incompressible, with no loss of volume when compressed (21). Our samples of arthritic human cartilage were not sufficiently large to allow isolation of zonal chondrocytes. This may account for the differences in the studies. Inclusion of surface, middle, and deep chondrocytes in our study may have contributed to the range of cellular deformation we observed within each cell source, since there may be zonal differences in both the vimentin distribution and overall abundance (32).
Fluorescence recovery experiments indicated that the inner vimentin network is very stable in comparison to the cortical actin filaments in 3D cultures of human chondrocytes. Motion of the vimentin network rather than turnover of individual filaments was observed, consistent with a structural role for vimentin.
Elucidating the role of the vimentin cytoskeleton in chondrocyte mechanical properties may have important consequences in the studies of chondrocyte mechanotransduction and of arthritis progression. The extent to which the chondrocyte deforms under physiological strains may play a role in the mechanotransduction pathways that become activated, and may determine whether the cellular response is anabolic or catabolic. In addition to the activation of mechanotransduction pathways, increased cellular deformation might lead to increased susceptibility to mechanical damage at the cellular level, and thus injury to the entire joint.
Supplementary Material
Donor demographics and the number of cells analyzed from each donor.
Acknowledgments
This work was funded by a generous donation from Donald P. and Darlene V. Shiley to the Scripps Health Philanthropic Fund, and NIH grant AG07996.
References
- 1.Zanetti NC, Solursh M. Induction of chondrogenesis in limb mesenchymal cultures by disruption of the actin cytoskeleton. J Cell Biol. 1984;99(1 Pt 1):115–23. doi: 10.1083/jcb.99.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Durrant LA, Archer CW, Benjamin M, Ralphs JR. Organisation of the chondrocyte cytoskeleton and its response to changing mechanical conditions in organ culture. J Anat. 1999;194 ( Pt 3):343–53. doi: 10.1046/j.1469-7580.1999.19430343.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benjamin M, Archer CW, Ralphs JR. Cytoskeleton of cartilage cells. Microscopy research and technique. 1994;28(5):372–7. doi: 10.1002/jemt.1070280503. [DOI] [PubMed] [Google Scholar]
- 4.Langelier E, Suetterlin R, Hoemann CD, Aebi U, Buschmann MD. The chondrocyte cytoskeleton in mature articular cartilage: structure and distribution of actin, tubulin, and vimentin filaments. J Histochem Cytochem. 2000;48(10):1307–20. doi: 10.1177/002215540004801002. [DOI] [PubMed] [Google Scholar]
- 5.Palfrey AJ, Davies DV. The fine structure of chondrocytes. J Anat. 1966;100(Pt 2):213–26. [PMC free article] [PubMed] [Google Scholar]
- 6.Ruiz-Romero C, Lopez-Armada MJ, Blanco FJ. Proteomic characterization of human normal articular chondrocytes: a novel tool for the study of osteoarthritis and other rheumatic diseases. Proteomics. 2005;5(12):3048–59. doi: 10.1002/pmic.200402106. [DOI] [PubMed] [Google Scholar]
- 7.van de Werken R, Gennari M, Tavella S, Bet P, Molina F, Lin S, et al. Modulation of tensin and vimentin expression in chick embryo developing cartilage and cultured differentiating chondrocytes. Eur J Biochem. 1993;217(2):781–90. doi: 10.1111/j.1432-1033.1993.tb18306.x. [DOI] [PubMed] [Google Scholar]
- 8.Finger F, Schorle C, Soder S, Zien A, Goldring MB, Aigner T. Phenotypic characterization of human chondrocyte cell line C-20/A4: a comparison between monolayer and alginate suspension culture. Cells Tissues Organs. 2004;178(2):65–77. doi: 10.1159/000081717. [DOI] [PubMed] [Google Scholar]
- 9.Capin-Gutierrez N, Talamas-Rohana P, Gonzalez-Robles A, Lavalle-Montalvo C, Kouri JB. Cytoskeleton disruption in chondrocytes from a rat osteoarthrosic (OA) -induced model: its potential role in OA pathogenesis. Histol Histopathol. 2004;19(4):1125–32. doi: 10.14670/HH-19.1125. [DOI] [PubMed] [Google Scholar]
- 10.Lambrecht S, Verbruggen G, Verdonk PC, Elewaut D, Deforce D. Differential proteome analysis of normal and osteoarthritic chondrocytes reveals distortion of vimentin network in osteoarthritis. Osteoarthritis Cartilage. 2007 doi: 10.1016/j.joca.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 11.Holloway I, Kayser M, Lee DA, Bader DL, Bentley G, Knight MM. Increased presence of cells with multiple elongated processes in osteoarthritic femoral head cartilage. Osteoarthritis Cartilage. 2004;12(1):17–24. doi: 10.1016/j.joca.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 12.Blain EJ, Gilbert SJ, Hayes AJ, Duance VC. Disassembly of the vimentin cytoskeleton disrupts articular cartilage chondrocyte homeostasis. Matrix Biol. 2006;25(7):398–408. doi: 10.1016/j.matbio.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 13.Henson FM, Vincent TA. Alterations in the vimentin cytoskeleton in response to single impact load in an in vitro model of cartilage damage in the rat. BMC Musculokel Disord. 2008;9:94. doi: 10.1186/1471-2474-9-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stevens AL, Wishnok JS, Chai DH, Grodzinsky AJ, Tannenbaum SR. A SDS-PAGE-liquid chromatography tandem mass spectrometry analysis of bovine cartilage tissue response to mechanical compression injury and the inflammatory cytokines tumor necrosis factor alpha and interleukin-1beta. Arthritis Rheum. 2008;58(2):489–500. doi: 10.1002/art.23120. [DOI] [PubMed] [Google Scholar]
- 15.Janmey PA, Euteneuer U, Traub P, Schliwa M. Viscoelastic properties of vimentin compared with other filamentous biopolymer networks. J Cell Biol. 1991;113(1):155–60. doi: 10.1083/jcb.113.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang N, Stamenovic D. Contribution of intermediate filaments to cell stiffness, stiffening, and growth. Am J Physiol Cell Physiol. 2000;279(1):C188–94. doi: 10.1152/ajpcell.2000.279.1.C188. [DOI] [PubMed] [Google Scholar]
- 17.Henrion D, Terzi F, Matrougui K, Duriez M, Boulanger CM, Colucci-Guyon E, et al. Impaired flow-induced dilation in mesenteric resistance arteries from mice lacking vimentin. J Clin Invest. 1997;100(11):2909–14. doi: 10.1172/JCI119840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eggli PS, Hunziker EB, Schenk RK. Quantitation of structural features characterizing weight- and less-weight-bearing regions in articular cartilage: a stereological analysis of medial femoral condyles in young adult rabbits. Anat Rec. 1988;222(3):217–27. doi: 10.1002/ar.1092220302. [DOI] [PubMed] [Google Scholar]
- 19.Chen J, Yan W, Setton LA. Static compression induces zonal-specific changes in gene expression for extracellular matrix and cytoskeletal proteins in intervertebral disc cells in vitro. Matrix Biol. 2004;22(7):573–83. doi: 10.1016/j.matbio.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 20.Idowu BD, Knight MM, Bader DL, Lee DA. Confocal analysis of cytoskeletal organisation within isolated chondrocyte sub-populations cultured in agarose. Histochem J. 2000;32(3):165–74. doi: 10.1023/a:1004095207330. [DOI] [PubMed] [Google Scholar]
- 21.Ofek G, Wiltz DC, Athanasiou KA. Contribution of the cytoskeleton to the compressive properties and recovery behavior of single cells. Biophys J. 2009;97(7):1873–82. doi: 10.1016/j.bpj.2009.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haudenschild DR, Nguyen B, Chen J, D'Lima DD, Lotz MK. Rho kinase-dependent CCL20 induced by dynamic compression of human chondrocytes. Arthritis Rheum. 2008;58(9):2735–42. doi: 10.1002/art.23797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ragan PM, Chin VI, Hung HH, Masuda K, Thonar EJ, Arner EC, et al. Chondrocyte extracellular matrix synthesis and turnover are influenced by static compression in a new alginate disk culture system. Arch Biochem Biophys. 2000;383(2):256–64. doi: 10.1006/abbi.2000.2060. [DOI] [PubMed] [Google Scholar]
- 24.Eckert BS. Alteration of intermediate filament distribution in PtK1 cells by acrylamide. Eur J Cell Biol. 1985;37:169–74. [PubMed] [Google Scholar]
- 25.Durham HD, Pena SD, Carpenter S. The neurotoxins 2,5-hexanedione and acrylamide promote aggregation of intermediate filaments in cultured fibroblasts. Muscle Nerve. 1983;6(9):631–7. doi: 10.1002/mus.880060903. [DOI] [PubMed] [Google Scholar]
- 26.Trickey WR, Vail TP, Guilak F. The role of the cytoskeleton in the viscoelastic properties of human articular chondrocytes. J Orthop Res. 2004;22(1):131–9. doi: 10.1016/S0736-0266(03)00150-5. [DOI] [PubMed] [Google Scholar]
- 27.Lee DA, Knight MM, Bolton JF, Idowu BD, Kayser MV, Bader DL. Chondrocyte deformation within compressed agarose constructs at the cellular and sub-cellular levels. J Biomech. 2000;33(1):81–95. doi: 10.1016/s0021-9290(99)00160-8. [DOI] [PubMed] [Google Scholar]
- 28.Cloyd JM, Malhotra NR, Weng L, Chen W, Mauck RL, Elliott DM. Material properties in unconfined compression of human nucleus pulposus, injectable hyaluronic acid-based hydrogels and tissue engineering scaffolds. Eur Spine J. 2007;16(11):1892–8. doi: 10.1007/s00586-007-0443-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Freeman PM, Natarajan RN, Kimura JH, Andriacchi TP. Chondrocyte cells respond mechanically to compressive loads. J Orthop Res. 1994;12(3):311–20. doi: 10.1002/jor.1100120303. [DOI] [PubMed] [Google Scholar]
- 30.Trickey WR, Baaijens FP, Laursen TA, Alexopoulos LG, Guilak F. Determination of the Poisson's ratio of the cell: recovery properties of chondrocytes after release from complete micropipette aspiration. J Biomech. 2006;39(1):78–87. doi: 10.1016/j.jbiomech.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 31.Haudenschild DR, Chen J, Steklov N, Lotz MK, D'Lima DD. Characterization of the Chondrocyte Actin Cytoskeleton in Living Three-Dimensional Culture: Response to Anabolic and Catabolic Stimuli. Mol Cell Biomechanics. 2009;6(3):135–44. [PMC free article] [PubMed] [Google Scholar]
- 32.Li S, Duance VC, Blain EJ. Zonal variations in cytoskeletal element organization, mRNA and protein expression in the intervertebral disc. J Anat. 2008;213(6):725–32. doi: 10.1111/j.1469-7580.2008.00998.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Donor demographics and the number of cells analyzed from each donor.