Abstract
Wiskott-Aldrich syndrome protein (WASP) and its homologue neural-WASP (N-WASP) are nucleation promoting factors that integrate receptor signaling with actin cytoskeleton rearrangement. While hematopoietic cells express both WASP and N-WASP, WASP deficiency results in altered cell morphology, loss of podosomes and defective chemotaxis. It was determined that cells from a mouse derived monocyte/macrophage cell line and primary cells of myeloid lineage expressed approximately 15-fold higher levels of WASP relative to N-WASP. To test whether N-WASP can compensate for the loss of WASP and restore actin cytoskeleton integrity, N-WASP was overexpressed in macrophages, in which endogenous WASP expression was reduced by short hairpin RNA (shWASP cells). Many of the defects associated with the loss of WASP, such as podosome-dependent matrix degradation and chemotaxis were corrected when N-WASP was expressed at equimolar level to that of the wild-type WASP. Furthermore, the ability of N-WASP to partially compensate for the loss of WASP may be physiologically relevant since activated murine WASP-deficient peritoneal macrophages, which show enhanced N-WASP expression, also show an increase in matrix degradation. Our study suggests that expression levels of WASP and N-WASP may influence their roles in actin cytoskeleton rearrangement and shed light to the complex intertwining roles WASP and N-WASP play in macrophages.
Keywords: WASP, N-WASP, compensation, podosome, chemotaxis
Introduction
Wiskott-Aldrich Syndrome (WAS) is an X-linked recessive disease characterized by thrombocytopenia, eczema and immunodeficiency. WAS is due to mutations in WAS protein (WASP) that results in either mutant forms of WASP being expressed or in little or no WASP protein being present [1]. WASP, whose expression is restricted to non-erythroid hematopoietic cells [2], along with the ubiquitously expressed neuronal WASP (N-WASP) and WASP family Verproline-homologous protein (WAVE)1, 2 and 3, are members of a family of scaffold proteins that link signals from the cell-surface to the actin cytoskeleton [3]. As actin nucleation promoting factors, WASP family proteins enhance Arp2/3 complex initiated de novo actin polymerization [4]. The essential role of actin cytoskeleton dependent processes in leukocytes, such as determination of cell shape and chemotaxis, is exemplified by the cytoskeletal abnormalities of hematopoietic cells from WAS patients (reviewed in [5, 6]).
Leukocytes require actin nucleation promoting factors to be tightly regulated and yet be responsive to external stimuli to carry out actin rearrangement for vital immune functions. Both WASP and N-WASP exist in an autoinhibitory conformation in resting cells, which is achieved by intramolecular binding of the C-terminal verprolin-homology, cofilin-homology, acidic (VCA) domain to the basic and G protein binding domain (GBD) [7]. This folded conformation effectively conceals the VCA region preventing interaction with actin monomers and the Arp2/3 complex. N-WASP has an additional verproline-homology domain (VVCA), which can interact with one more actin monomer than WASP, resulting in superior actin polymerization activity of N-WASP in vitro [4, 8]. Classically, the interaction of the GBD with Cdc42 was thought to unfold and thus “activate” both WASP and N-WASP, while Rac1 acted through WAVE1-3 proteins. However, a recent systematic in vitro study showed that, while it did not activate WASP, Rac1 was a more potent activator of N-WASP than Cdc42 [9]. Another binding partner of WASP and N-WASP is Phosphatidyl Inositol (4, 5) Phosphate (PtdIns(4,5)P2), which has been reported to synergize with Cdc42 in the activation of WASP [10] and N-WASP [11]. However, Tomasevic et al reported an inhibitory effect of PtdIns(4,5)P2 on WASP but not N-WASP activity [9]. While these studies show the existence of different mechanisms for the regulation of WASP and N-WASP, whether these proteins serve a nonredundant function in the cell is unknown.
The most striking features of WASP deficient macrophages are their chemotaxis defect and the lack of podosomes on their ventral surface [12]. Podosomes mediate adhesion to the extracellular matrix and are capable of matrix degradation [13, 14]. They consist of filamentous (F)-actin - rich core surrounded by loose bundles of F-actin with protein components such as talin and vinculin that are typical of focal contacts. WASP localizes to the F-actin - rich core along with other actin-regulatory proteins, such as cortactin and Arp2/3 [15]. Interestingly, certain aggressive cancer cells and Src-transformed cells possess podosome-like structures called invadopodia that appear to be directly responsible for extra cellular matrix degradation [16]. Invadopodia have similar organization and actin regulatory machinery localization compared to podosomes. However, N-WASP is present in the F-actin core instead of WASP [17]. Subsumed under the term invadosomes, both structures are suspected to play a role in the surveillance of the environment and maintain polarized activities such as chemotaxis and focal degradation of the matrix [16].
N-WASP, originally regarded as the WASP equivalent in non-hematopoietic cells, is also expressed in human peripheral blood monocytes, neutrophils and platelets albeit at potentially lower levels [18]. While the co-expression of closely related proteins suggests potential non-redundant roles for both WASP and N-WASP in hematopoietic cells, several studies suggest that WASP and N-WASP may be able to substitute for one another [19–21]. Platelets from WASP deficient patients and mice have an intact actin assembly system [19]. In addition, WASP or N-WASP deficient mice have similar numbers of T-cells compared to wild type mice, while deficiency of both WASP and N-WASP in the same mouse resulted in severely reduced numbers of T-cell [20]. Furthermore, expression of N-WASP in WASP deficient T-cells partially restored their proliferation [21]. Taken together, these studies suggest an intriguing possibility that enhanced N-WASP expression in WASP deficient macrophages may allow restoration of normal actin dynamics.
Materials and Methods
Cells, transfections, plasmids
All cells were maintained at 37°C in a 5% CO2 incubator. RAW264.7 derived cell lines with reduced WASP expression by short hairpin RNA (shRNA) and control cells expressing non-targeting shRNA were generated as described in [14]. Peritoneal macrophages, RAW 264.7 derived cell lines, and COS-7 cells were all grown in RPMI medium (Mediatech Inc, Manassas, VA) containing 10% new born calf serum (Cambrex, Walkersville, MD), 100 U/ml penicillin and 100 ug/ml streptomycin (Sigma, Saint Louis, MO). Murine bone marrow-derived macrophages (BMM) were isolated and prepared according to a previously published protocol [22], grown in alpha-MEM media (Invitrogen, Carlsbad, CA) with 15% FBS (Sigma, St. Louis, MO), 360 ng/ml recombinant human CSF-1 (Chiron, Emeryville, CA), and 100 U/ml penicillin and 100 ug/ml streptomycin. Lysates of mouse dendritic cells were a generous gift of Dr. Laura Santambrogio.
For stable N-WASP expression, shWASP cells were transfected with either the psrα vector or psrα N -WASP plasmid [23] using Fugene HD (Roche, Indianapolis, IN) then selected with 2mg/mL G418 sulfate until clones were obtained. GFP-N-WASP [24] was expressed in shWASP psrα vector cells using Fugene. Superfect transfection reagent (Qiagen, Valencia, CA) was used to transfect COS-7 cells with either WASP-biosensor-CFP-YFP [25]or N-WASP biosensor CFP-YFP [26] according to manufacturer specification.
Mice and peritoneal macrophages isolation
All procedures involving mice were conducted in accordance with National Institutes of Health regulations concerning the use and care of experimental animals. The study of mice was approved by the Albert Einstein College of Medicine animal use committee. Commercially available 129/svJ control and WASP-deficient mice [27] were purchased from The Jackson Laboratory (Bar Harbor, ME). WASP-deficient mice with mixed background was generated by crossing 129/SvJ WASP-deficient mouse to a control mouse with C57/B6 background and remained in a consistent background throughout breeding. Peritoneal lavage was performed as described previously [28]. Briefly, 1mL of PBS or 4% thioglycollate medium (Sigma, St. Louis, MO) was injected into the peritoneal cavities of mice. Following three to five days of incubation, 10mL of RPMI medium containing 10% new born calf serum, 100 U/ml penicillin and 100 ug/ml streptomycin was used to collect peritoneal macrophages.
Western blotting
Cells were lysed in ice-cold lysis buffer containing either 25 mM Tris, 137 mM NaCl, 1% Triton X-100, and 2 mM EDTA or 150mM NaCl, 50mM Tris, and 1% NP-40; both buffers also included 1 mM orthovanadate, 1 mM benzamidine, 10 µg/ml aprotinin, and 10 µg/ml leupeptin. 5× Laemmli buffer was added and lysates were boiled for 5 minutes. Whole cell lysates were resolved by SDS-PAGE and proteins were transferred onto PVDF membranes (Immobilon-P Millipore, Billerica, MA) that were subsequently blocked with 5% nonfat dry milk in TBS containing 0.1% Tween20 prior to incubation in primary antibodies overnight at 4°C. Primary antibodies included mouse monoclonal anti-GFP (Roche, Indianapolis, IN), mouse monoclonal anti-beta-actin AC-15 (Sigma, St. Louis, MO), rabbit polyclonal anti-N-WASP [23], chicken polyclonal anti-N-WASP/WASP (AE 920), regenerated from sequence indicated in [23], rabbit polyclonal anti-WASP (H-250), and goat polyclonal anti-WAVE2 (both from Santa Cruz Biotechnology, Santa Cruz, CA). Membranes were then washed and incubated with horseradish peroxidase conjugated secondary antibodies, either goat anti-mouse IgG, donkey anti-goat IgG, goat anti-rabbit IgG, or donkey anti -chicken IgY (Jackson Immuno Research, West Grove, PA). Signals were visualized using the SuperSignal West Pico Chemiluminescent Substrate from Pierce (Rockford, IL). Images were acquired using a Kodak Image Station 440 and quantified using Kodak 1D Image Analysis Software. In quantification of Western blots, values were an average generated from several lysates of each condition per experiment run multiple times.
Immunofluorescence microscopy and morphological analysis
Cells plated on 12 mm glass coverslips were serum-starved for at least 3 hours and fixed and stained according to [14]. F-actin was visualized by staining with Alexa 568-phalloidin (Invitrogen, Carlsbad, CA). Vinculin was detected using a specific monoclonal antibody (clone VIN-11-5 or hVIN-1, both from Sigma, St. Louis, MO), followed by incubation with Alexa 488-goat anti-mouse IgG (Invitrogen, Carlsbad, CA).
Morphological parameters were analyzed as follows: a cell displaying migratory morphology was defined by an unambiguous leading and trailing edge [29], podosomes were considered as such when F-actin punctate structures organized in patches were surrounded by a vinculin ring. The elongation index was determined using Image J (http://rsb.info.nih.gov/ij/) by tracing the cells and measuring the ratio of the major axis over the minor axis of the fit ellipse. At least 60 cells per experiment were analyzed for each determination. F-actin core intensity was obtained by identifying the area inside the vinculin ring from F-actin and vinculin merged image and circling the corresponding area on F-actin alone image for intensity measurement. At least 100 podosomes per cell type were analyzed for each experiment. Images were taken using the 60× oil/1.40 phase3 objective of an Olympus IX71 microscope coupled to a Sensicam cooled CCD camera.
Matrix degradation assay
Degradation of fluorescently-labeled fibronectin (FN) by macrophages was determined by a protocol detailed in [14]. Briefly, cells were plated on fluorescently labeled fibronectin for 16 hours and areas of loss of fluorescence were measured as degradation area per cell. At least 50 cells per experiment were analyzed.
Chemotaxis assay
Chemotaxis was measured as previously described [14]. Briefly, 500,000 serum starved cells were added to the upper well of 8-µm pore size inserts (Falcon; BD Biosciences, CA) and incubated at 37°C for 4 hours in the presence or the absence of CSF-1. Cell migration was quantified by counting the number of cells that migrated through the insert in at least 10 randomly selected fields and data was expressed as fold increase of migrated cells in stimulated condition over the unstimulated condition.
Data analysis
Results were considered statistically different when analysis using a Student t-test resulted in differences between two means with a p value of less than 0.05. Error bars signify standard error of the mean.
Results
A murine monocyte/macrophage subline of RAW264.7 transduced with shRNA against WASP was previously shown to recapitulate the chemotactic and podosome defects observed in macrophages isolated from patients with Wiskott Aldrich syndrome (WAS) [14] even though these cells express the WASP homologue N-WASP. Further characterization of these cells showed that endogenous WASP levels were reduced by greater than 90%, while the expression levels of N-WASP remained unaffected, as did levels of WAVE-2, the other dominant WASP/WAVE family member in macrophages [30] (Figure 1A). In addition to the previously described defects on matrix degradation and chemotaxis [14], the reduction of WASP also had clear effects on the morphology of these cells. Consistent with other reports using monocytes from WAS patients [31], shWASP cells appeared to be more round and fewer cells showed a clear head to tail migratory phenotype than control cells (Figure 1B). In fact, when these characteristics were quantified, the percentage of cells exhibiting a migratory morphology decreased in shWASP cells by approximately 60% (Figure 1C). The elongation index, determined by dividing the major axis of each cell by the minor axis, was also decreased by about half in cells with reduced WASP levels (Figure 1D). Additionally, cells producing podosomes, seen as punctate F-actin cores each surrounded by a ring of vinculin, were roughly 60% less prevalent in the population with reduced WASP (Figure 1E). These data further support that shWASP cells recapitulate the hallmarks of WAS macrophages.
Figure 1. WASP regulates macrophage morphology.
(A) WASP was reduced using shRNA in murine monocyte/macrophage cell line (shWASP). Control cells were treated with a non-targeting shRNA sequence and NI denotes non-infected cells. Western blots demonstrated that expression levels of WAVE2 and N-WASP were not affected. The N-WASP antibody recognizes both N-WASP and WASP, a white arrow points towards the band representing N-WASP, a black arrow towards WASP. (B) Images display morphology of control and shWASP cells. ShWASP cells exhibited reduced (C) migratory morphology (n=4), (D) elongation (n=5), and (E) cells with podosomes, (n=4) suggesting that WASP is needed for these activities. Scale bar represents 10µm. * p<0.05
Since both WASP and N-WASP were expressed in monocyte derived cells, the endogenous levels of these proteins were quantified. First, the relative affinity of the antibody recognizing both WASP and N-WASP to be used in this quantification was measured. COS-7 cells were transfected with WASP or N-WASP constructs, both tagged with YFP and CFP, and lysates were probed in Western blots with a chicken polyclonal antibody against N-WASP/WASP. Blots were also probed with a GFP antibody, which binds YFP as well, to normalize for expression level and a beta-actin antibody to control for loading (Figure 2A). The quantification showed that the N-WASP/WASP antibody recognizes N-WASP with an affinity approximately 5.7 fold higher than that for WASP consistent with the fact that the antigen was based on the sequence of N-WASP [23]. Taking this into account, N-WASP and WASP levels were measured using the N-WASP/WASP antibody in the control macrophage cell line and compared to that of other mouse myeloid cells, such as bone marrow derived macrophages (BMM) and immature dendritic cells (DC) [29, 32] (Figure 2B). Using the determined affinity of the antibody, the relative level of WASP to N-WASP in each cell type was calculated. Protein samples were run in serial dilutions in order to confirm that exposure conditions were not saturated. Multiple lysates were taken from each cell type and each lysate run multiple times before averaging together the results. Additionally, similar WASP and N-WASP levels were also obtained using a rabbit polyclonal antibody also against N-WASP/WASP. There were no significant differences between the WASP/N-WASP ratios of the various monocyte derived cell types analyzed. All exhibited considerably higher WASP expression than N-WASP, with control cells showing an average WASP/N-WASP ratio of approximately 15 (Figure 2C). While the ratios of WASP to N-WASP in the primary cells appeared lower than that of control cell line, these differences were not statistically significant. These results indicate that while N-WASP is expressed in monocyte-derived cells, it is at a much lower level than WASP.
Figure 2. Relative expression of N-WASP and WASP in monocyte derived cells.
(A) The relative affinity of an antibody that recognized both WASP and N-WASP was determined by transfecting either epitope tagged N-WASP or WASP into COS-7 cells followed by quantitative Western blot. Values were normalized to β-actin and GFP levels. The white arrow represents endogenous N-WASP in COS-7 cells. Smeared bands could be due to degradation of overexpressed proteins. Anti-N-WASP/WASP demonstrates that the antibody has a 5.7-fold higher affinity for N-WASP than WASP. (B) Representative N-WASP Western blot of a control macrophage cell line (Control), primary immature and mature dendritic cells (DC −/+ LPS respectively), and primary bone marrow-derived macrophages (BMM) is shown. The white arrow indicates bands corresponding to N-WASP and the black arrow indicates WASP bands. (C) The ratio of endogenous WASP to N-WASP was calculated for Control, BMM, and immature DC. Differences in expression between mature and immature dendritic cells were not significant.
It may be possible that the morphological defects associated with the absence of WASP is not due to non-redundant functions between WASP and N-WASP but simply due to the low level of N-WASP expression in macrophages. To address this issue, N-WASP was overexpressed in shWASP cells to determine the extent to which it can compensate for a lack of WASP (Figure 3A). After finding that WASP is expressed roughly 15 times more than N-WASP in control cells, three clones (F6, E7, and D3) were obtained that expressed N-WASP approximately 15 times more than control cells in order to approximate WASP endogenous expression (Figure 3B). When examined by immunofluorescence microscopy, cells overexpressing N-WASP appeared to more closely resemble control cells rather than shWASP cells (Figure 4A). Migratory morphology (Figure 4B) and elongation (Figure 4C) were found to be restored to the levels seen in control cells, while shWASP cells transfected with the psrα vector exhibited a reduction in these parameters similar to that of shWASP cells. In both measurements, differences between the three N-WASP overexpressing clones along with the control were not significant, whereas each were significantly different from the vector control shWASP cells.
Figure 3. Level of N-WASP overexpression in shWASP clones.
(A) Western blotting was performed using cells treated with non-targeting shRNA (control), shWASP (−), vector transfected shWASP cells (vector), or clones from shWASP cells transfected with N-WASP (F6, E7, D3). COS-7 cells were used as a control for endogenous N-WASP. (B) N-WASP levels were determined relative to control. The N-WASP overexpressing shWASP clones expressed approximately 15-fold more N-WASP as compared to endogenous N-WASP.
Figure 4. N-WASP overexpression can rescue morphology defects of WASP deficiency.
Cells treated with a non-targeting shRNA (control), vector transfected shWASP cells (vector), or N-WASP overexpressing clones from shWASP (F6, E7, D3) were plated on coverslips. (A) Representative images show control (top), vector (middle) and N-WASP overexpressing clone E7 (bottom) stained for F-actin (red) and vinculin (green). Scale bar represents 10µm. Insets show a 2µm by 2µm square from the original image, highlighting the vinculin encircled cores of F-actin that distinguish podosomes in both control and N-WASP F6, but not present in vector expressing shWASP cells. (B) The fraction of cells that displayed migratory morphology was determined (n=6). (C) Elongation index was measured using ImageJ (n=5). At least 60 cells per experiment were analyzed for each determination. * p<0.05
The ability of N-WASP to compensate for WASP deficiency in terms of podosome formation was also determined. Quantification of N-WASP overexpressing cells by immunofluorescence microscopy showed that the fraction of cells displaying podosomes was restored to the level seen in control cells following overexpression of N-WASP (Figure 5A). Further analysis of the cells that recovered podosomes showed that the number of podosomes per cell (Figure 5B) and the intensity of F-actin core region of each podosomes (Figure 5C) were comparable to that of the control cells. Interestingly, N-WASP overexpresing clones showed slightly higher number of podosomes per cell compared to control cells, where one clone was statistically significantly different (Figure 5B), which may reflect the superior actin polymerization ability of N-WASP compared to WASP due to the two tandem V motifs of N-WASP [8]. The localization of overexpressed N-WASP was determined using GFP tagged N-WASP since none of the N-WASP antibodies tested could distinguish N-WASP and WASP. Consistent with the recovery of podosomes by N-WASP overexpression, GFP-N-WASP was enriched in podosomes of transfected shWASP cells (Figure 5D). In combination, these data indicate that overexpression of N-WASP can restore the morphological defects and loss of podosomes observed following WASP reduction.
Figure 5. N-WASP overexpression rescues podosome formation.
(A) The fraction of cells containing podosomes treated with non-targeting shRNA (control), vector, or clones from shWASP transfected with N-WASP (F6, E7, D3) was compared (n=3). (B) The number of podosomes per cell and (C) F-actin intensity of the podosomes from the F-actin core region were measured. These assays confirm that the N-WASP overexpressing shWASP cells contain podosomes. Error bars signify SEM. At least 50 cells were analyzed per experiment for (A) and (B). At least 300 podosomes from each cell types were measured for (C). (D) Vector shWASP cells were transfected with GFP-N-WASP, then fixed and stained for F-actin, seen in channels showing Alexa-568 phalloidin (top), GFP (middle) and merged images of GFP and phalloidin fluorescence (bottom). Boxed area is shown at higher magnification in inset. Scale bar represents 10µm. * p<0.05
To further evaluate the ability of N-WASP to compensate for WASP deficiency, whether the restored podosomes of N-WASP overexpressing clones also recovered their function was determined. When plated on fibronectin matrices, the vector control shWASP cells showed significantly less degradation per cell (Figure 6A). N-WASP overexpressing clones degraded matrix at levels comparable to those seen in control cells indicating that the podosomes of N-WASP overexpressing cells were functional. The loss of podosomes is also associated with the loss of CSF-1-mediated chemotaxis in WASP-deficient macrophages. To determine whether N-WASP overexpression could restore the ability of shWASP cells to chemotax towards CSF-1, a transwell migration assay was performed. Intriguingly, N-WASP overexpressing clones migrated at a level comparable to that of control cells while the vector control shWASP cells showed reduced migration in response to CSF-1, (Figure 6C). In conclusion, many of the observed defects in cells with reduced WASP expression can be restored by replacement of WASP with N-WASP.
Figure 6. N-WASP overexpression rescues podosome function and chemotaxis.
(A) Cells were plated on Alexa-568 fibronectin coated-coverslips overnight, then fixed. Degradation area per cell is shown for cells treated with non-targeting shRNA (control), vector shWASP cells (vector), or clones from shWASP transfected with N-WASP (F6, E7, D3) (n=3). (B) Cells were plated on Boyden chamber with or without CSF-1 in the bottom well and allowed to migrate for 4h. At least 10 randomly chosen fields in the bottom well were analyzed to measure the number of migrated cells (n=3). The graph shows fold increase of cell migration over the non-CSF-1 treated cells. Error bars signify SEM. At least 50 cells were analyzed per experiment. * p<0.05
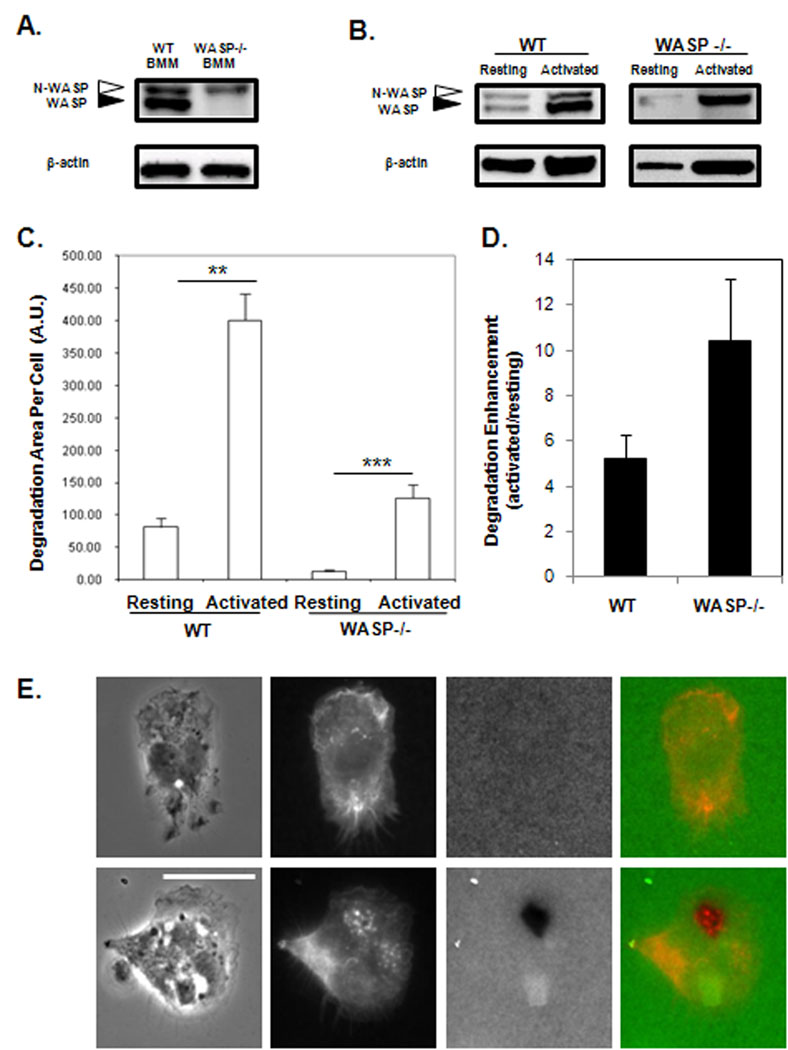
To determine whether N-WASP might compensate for the loss of WASP in vivo, the level of N-WASP expression in primary WASP deficient BMM was examined. BMM from three independent mice per genotype were lysed and multiple lysates were probed on western blots to obtain an average N-WASP expression per genotype. N-WASP expression in WASP deficient BMM was not significantly different compared to wildtype BMM (Figure 7A), consistent with no alteration in N-WASP expression in shWASP cells (Figure 1). However, macrophages are known to increase expression of proteins involved in the immune response following immunological stimulus. Therefore, the level of N-WASP expression in activated compared to resting peritoneal macrophages was examined. Thioglycollate medium was injected into the peritoneum of wildtype and WASP-deficient mice to cause local inflammation. Resident and activated macrophages were then collected by peritoneal lavage from PBS treated or thioglycollate treated mice, respectively. Thioglycollate activated wildtype macrophages showed a significant increase in WASP and N-WASP expression compared to resting macrophages. Similarly, N-WASP expression in activated WASP-deficient peritoneal macrophages increased approximately four fold compared to resting WASP-deficient peritoneal macrophages (Figure 7B). Similar increases expression of WASP and N-WASP were seen in activated macrophages from two different strains of mice, indicating that these are not strain dependent effect (data not shown).
Figure 7. Activated WASP-deficient peritoneal macrophages show enhanced N-WASP expression, podosomes and matrix degradation.
(A) Lysates of bone marrow derived macrophages (BMM) from wildtype (WT) and WASP-deficient mice were probed with an antibody that recognizes both N-WASP (white arrow) and WASP (black arrow) in a Western Blot. (B) A representative Western blot of WASP, N-WASP and β-actin levels in resting and activated WT and WASP-deficient peritoneal macrophages. (C – E) Peritoneal macrophages were plated on Alexa-568 fibronectin coated-coverslips overnight, then fixed and stained for F-actin to identify podosomes. (C) Degradation area per cell is shown for resting and activated WT and WASP-deficient peritoneal macrophages (n=4). Error bars signify SEM. At least 10 randomly chosen fields and 50 cells were analyzed per experiment. ** p<0.01 *** p<0.001 (D) Average degradation enhancement was calculated by dividing the degradation area per cell of activated cells over that of resting cells for each genotype. (E) Representative images of WASP-deficient peritoneal macrophages showing phase (left), Alexa-568 fibronectin (2nd from left), Alexa-647 Phalloidin (3rd from left) and merged images of fibronectin and phalloidin fluorescence (right). Images in the top row display resting cells, the bottom row activated cells. Scale bar represents 10µm.
To determine whether the increase in N-WASP expression in WASP-deficient activated peritoneal macrophages resulted in an increase in their functional capability, the ability of resident and activated peritoneal macrophages to degrade fluorescent matrix was assayed. As expected, non-activated WASP-deficient peritoneal macrophages showed only minimal matrix degradation. However, there was a ten fold increase in the area of matrix degradation by activated WASP-deficient peritoneal macrophages, attaining a similar level of matrix degradation compared to resting wildtype peritoneal macrophages (Supplemental Figure 1 and Figure 7C), while activated wildtype peritoneal macrophages showed only a five-fold increase in the area of matrix degradation compared to resting cells (Figure 7D). Strikingly, investigation of the F-actin arrangements of these cells revealed that activated WASP-deficient macrophages contained podosomes that co-localized with areas of degradation (Figure 7E). These results indicate that an increase in N-WASP expression occurs in vivo in response to an immunological stimulus enhancing the ability of WASP-deficient macrophages to degrade matrix, suggesting that N-WASP compensation for WASP may play a role in the pathogenesis of WAS.
Discussion
The residual chemotactic ability WASP-deficient cells display in in vitro studies [33–35] has been speculated to be an attribute of compensatory contribution by other WASP/WAVE family members [36]. While WASP family proteins promote activation of the Arp2/3 complex through their C-termini, these proteins diverge in binding regions for the interacting adaptor and regulatory molecules, which appear to account for their functional differences [37]. Whereas WASP is exclusively expressed in hematopoietic cells, N-WASP is expressed ubiquitously albeit at low levels [21, 38, 39], consistent with our finding. Calle et al suggested that WASP may play a unique role in osteoclasts due to the profound defects of WASP deficiency in these cells even though N-WASP is expressed [40]. Studies using pathogenic microorganisms that subvert the Arp2/3 dependent actin polymerization pathways suggest WASP-family proteins may not compensate for each other completely. For example, Shigella outer membrane protein VirG only binds to N-WASP and not to any other WASP family members to recruit host F-actin [39]. Shigella motility in N-WASP deficient embryonic stem cell derived fibroblast-like cells was also not rescued by ectopic expression of WASP [38]. However, WASP does compensate for N-WASP in cytoplasmic vaccinia virus motility [38] and Mycobacterium marinum exploits either WASP or N-WASP interchangeably for its intracellular spread [21]. These reports suggest that certain pathogens exploit either WASP or N-WASP for their intracellular spread, yet the functional competency of these proteins to substitute for one another in normal host cell physiology has not been addressed. In this study we demonstrate that while N-WASP is expressed in monocyte-derived cells, it is at significantly lower levels compared to WASP. Furthermore, we provide evidence indicating that many of the defects associated with the loss of WASP can be rectified if N-WASP expression is increased either by forced expression in vitro or following an immune stimulus in vivo.
A hallmark of WAS macrophages is the loss of podosomes [12]. However, podosome-like structures are also present in other non-myeloid cells that do not express WASP such as endothelial cells and smooth muscle cells [16]. In non-myeloid cells, N-WASP mediates invadopodia formation in carcinoma cells and podosome formation in Src-transformed fibroblasts [41–43]. These studies suggest that N-WASP may be able to regulate the formation of podosomes if expressed in sufficient amounts. To test this hypothesis, clones of shWASP cells overexpressing N-WASP at an equivalent molar level to WASP expression seen in control cells were used in this study. In addition to the restoration of macrophage morphology and podosome formation, N-WASP overexpression restored several macrophage functions believed to be podosome dependent, such as matrix degradation and chemotaxis. Given our results that N-WASP overexpressing cells recovered these functions, we examined whether cellular stress, such as inflammation and activation of immune cells, led to a compensatory increase in N-WASP expression in WASP-deficient macrophages in attempt to increase their functionality, however inefficiently. Indeed, activation of WASP-deficient macrophages resulted in an increase in N-WASP expression and matrix degradation capability, proving N-WASP can functionally compensate for WASP. While activated WASP-deficient peritoneal macrophages recovered the level of degradation exhibited by resting wildtype peritoneal macrophages, activated wildtype peritoneal macrophages exhibited superior matrix degradation capability. This difference in degradation capability of activated cells likely result from the fact that thioglycollate induced activation increased not only N-WASP expression but also WASP expression in wildtype cells. In view of the fact that the expression levels of WASP and N-WASP establish the extent of actin cytoskeleton rearragement macrophages can undertake, it is possible that in the absence of N-WASP the cytoskeletal defects of WAS patients may be even more pronounced. The clinical relevance of N-WASP expression status in macrophages and other hematopoietic cells from WAS patients requires further investigation.
While an increase in N-WASP expression rescued many of the cytoskeletal defects of WASP-deficient macrophages, our group has recently showed that the ectopic expression of N-WASP in WASP deficient cells was not able to rescue the defect in Fcγ-R mediated phagocytosis [44]. Correspondingly, N-WASP overexpression did not restore the FcγR-mediated phagocytic defect seen in cells with reduced WASP (data not shown), suggesting that WASP and N-WASP have non-redundant roles in phagocytosis irrespective of their expression level. Park et al suggested that N-WASP may play a role in membrane delivery to the growing phagocytic cup, while WASP may be required for actin polymerization during phagocytosis [44]. Our finding that N-WASP compensated for WASP in certain actin cytoskeletal abnormalities but not all complements predictions made that N-WASP can only partially compensate for WASP due to distinct sets of interactions between the two proteins [38, 40]. In fact, amino acid sequence alignment of WASP and N-WASP reveals that WASP contains a unique insert upstream of GBD, which could confer specific binding partners for WASP but not N-WASP. Furthermore, the proline-rich regions of WASP and N-WASP exhibit 50% sequence divergence, suggesting these two proteins may interact with different sets of SH3 domain containing proteins. For example, Tomasevic et al. reported differential regulation of WASP and N-WASP actin polymerization activities by the SH3 domain containing adaptor proteins Nck1 and Nck2 in vitro [9]. Unfortunately, while many studies report the interaction and/or effect of SH3 domain containing proteins to either WASP or N-WASP (extensively reviewed in [4]), side by side comparison of binding affinities and/or interactions have not been performed. Additional studies are needed to elucidate the specific binding partner(s) of WASP and N-WASP that may contribute to the different roles they assume.
Alternatively, WASP and N-WASP turnover may be regulated differently during podosome formation and phagocytosis. For example, WASP and N-WASP differ in calpain sensitivity [18]. Calpain expression is increased in activated macrophages with enhanced phagocytic ability [33] and its inhibition stabilizes podosomes in DCs [45] while suppressing their proteolytic activity [34]. Since calpain cleaves WASP but not N-WASP, it is plausible that the N-WASP overexpression failed to rescue the phagocytosis defect not because it failed to correct the cytoskeletal abnormality but because N-WASP cannot be cleared by calpain. In conclusion, we have demonstrated N-WASP can compensate for WASP in morphology, podosome formation, matrix degradation, and chemotaxis but not phagocytosis, suggesting WASP and N-WASP have both unique and redundant roles in macrophages. Understanding the precise role N-WASP plays in cytoskeletal rearrangement of macrophages, along with WASP, will further delineate the immunological abnormalities in WAS.
Supplementary Material
Representative images of resting or activated peritoneal macrophages from wildtype (WT) and WASP-deficient (WASP−/−) mice are shown, phase (left) and Alexa-568 fibronectin (right). Scale bar represents 10µm.
Acknowledgments
We thank Dr. Paul Jubinsky and the members of the Condeelis, Stanley and Segall laboratories for helpful comments and discussions. We are grateful to Dr. Laura Santambrogio for her generous gift of cell lysates. This work was supported by grants from the National Institute of Health (GM071828 to D.C., and CA100324 and GM38511 to J.C.).
Abbreviations
- BMM
Bone Marrow-derived Macrophages
- CSF-1
Macrophage Colony Stimulating Factor-1
- DC
Dendritic Cells
- GBD
G Protein Binding Domain
- N-WASP
Neuronal- Wiskott-Aldrich Syndrome protein
- shRNA
Short Hairpin RNA
- WAS
Wiskott-Aldrich Syndrome
- WASP
Wiskott-Aldrich Syndrome Protein
- WAVE
WASP family Verproline-homologous protein
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Beth M. Isaac, Email: bisaac@wesleyan.edu.
Dan Ishihara, Email: dan.ishihara@med.einstein.yu.edu.
Leora M. Nusblat, Email: lnusblat@gmail.com.
Jean-Claude Gevrey, Email: jc_gevrey@yahoo.fr.
Athanassios Dovas, Email: athanassios.dovas@einstein.yu.edu.
John Condeelis, Email: john.condeelis@einstein.yu.edu.
References
- 1.Imai K, Nonoyama S, Ochs HD. WASP (Wiskott-Aldrich syndrome protein) gene mutations and phenotype. Curr Opin Allergy Clin Immunol. 2003;3:427–436. doi: 10.1097/00130832-200312000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Zhu Q, Watanabe C, Liu T, Hollenbaugh D, Blaese RM, Kanner SB, Aruffo A, Ochs HD. Wiskott-Aldrich syndrome/X-linked thrombocytopenia: WASP gene mutations, protein expression, and phenotype. Blood. 1997;90:2680–2689. [PubMed] [Google Scholar]
- 3.Millard TH, Machesky LM. The Wiskott-Aldrich syndrome protein (WASP) family. Trends in biochemical sciences. 2001;26:198–199. doi: 10.1016/s0968-0004(01)01788-1. [DOI] [PubMed] [Google Scholar]
- 4.Takenawa T, Suetsugu S. The WASP-WAVE protein network: connecting the membrane to the cytoskeleton. Nature reviews. 2007;8:37–48. doi: 10.1038/nrm2069. [DOI] [PubMed] [Google Scholar]
- 5.Snapper SB, Rosen FS. The Wiskott-Aldrich syndrome protein (WASP): roles in signaling and cytoskeletal organization. Annu Rev Immunol. 1999;17:905–929. doi: 10.1146/annurev.immunol.17.1.905. [DOI] [PubMed] [Google Scholar]
- 6.Thrasher AJ. WASp in immune-system organization and function. Nat Rev Immunol. 2002;2:635–646. doi: 10.1038/nri884. [DOI] [PubMed] [Google Scholar]
- 7.Kim AS, Kakalis LT, Abdul-Manan N, Liu GA, Rosen MK. Autoinhibition and activation mechanisms of the Wiskott-Aldrich syndrome protein. Nature. 2000;404:151–158. doi: 10.1038/35004513. [DOI] [PubMed] [Google Scholar]
- 8.Yamaguchi H, Miki H, Suetsugu S, Ma L, Kirschner MW, Takenawa T. Two tandem verprolin homology domains are necessary for a strong activation of Arp2/3 complex-induced actin polymerization and induction of microspike formation by N-WASP. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:12631–12636. doi: 10.1073/pnas.190351397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tomasevic N, Jia Z, Russell A, Fujii T, Hartman JJ, Clancy S, Wang M, Beraud C, Wood KW, Sakowicz R. Differential regulation of WASP and N-WASP by Cdc42, Rac1, Nck, and PI(4,5)P2. Biochemistry. 2007;46:3494–3502. doi: 10.1021/bi062152y. [DOI] [PubMed] [Google Scholar]
- 10.Higgs HN, Pollard TD. Activation by Cdc42 and PIP(2) of Wiskott-Aldrich syndrome protein (WASp) stimulates actin nucleation by Arp2/3 complex. J Cell Biol. 2000;150:1311–1320. doi: 10.1083/jcb.150.6.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rohatgi R, Ho HY, Kirschner MW. Mechanism of N-WASP activation by CDC42 and phosphatidylinositol 4, 5-bisphosphate. J Cell Biol. 2000;150:1299–1310. doi: 10.1083/jcb.150.6.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Linder S, Nelson D, Weiss M, Aepfelbacher M. Wiskott-Aldrich syndrome protein regulates podosomes in primary human macrophages. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:9648–9653. doi: 10.1073/pnas.96.17.9648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Linder S. The matrix corroded: podosomes and invadopodia in extracellular matrix degradation. Trends Cell Biol. 2007;17:107–117. doi: 10.1016/j.tcb.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 14.Dovas A, Gevrey JC, Grossi A, Park H, Abou-Kheir W, Cox D. Regulation of podosome dynamics by WASp phosphorylation: implication in matrix degradation and chemotaxis in macrophages. J Cell Sci. 2009;122:3873–3882. doi: 10.1242/jcs.051755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Linder S, Aepfelbacher M. Podosomes: adhesion hot-spots of invasive cells. Trends Cell Biol. 2003;13:376–385. doi: 10.1016/s0962-8924(03)00128-4. [DOI] [PubMed] [Google Scholar]
- 16.Linder S. Invadosomes at a glance. J Cell Sci. 2009;122:3009–3013. doi: 10.1242/jcs.032631. [DOI] [PubMed] [Google Scholar]
- 17.Block MR, Badowski C, Millon-Fremillon A, Bouvard D, Bouin AP, Faurobert E, Gerber-Scokaert D, Planus E, Albiges-Rizo C. Podosome-type adhesions and focal adhesions, so alike yet so different. Eur J Cell Biol. 2008;87:491–506. doi: 10.1016/j.ejcb.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 18.Shcherbina A, Miki H, Kenney DM, Rosen FS, Takenawa T, Remold-O'Donnell E. WASP and N-WASP in human platelets differ in sensitivity to protease calpain. Blood. 2001;98:2988–2991. doi: 10.1182/blood.v98.10.2988. [DOI] [PubMed] [Google Scholar]
- 19.Falet H, Hoffmeister KM, Neujahr R, Hartwig JH. Normal Arp2/3 complex activation in platelets lacking WASp. Blood. 2002;100:2113–2122. [PubMed] [Google Scholar]
- 20.Klein C, Nguyen D, Liu CH, Mizoguchi A, Bhan AK, Miki H, Takenawa T, Rosen FS, Alt FW, Mulligan RC, Snapper SB. Gene therapy for Wiskott-Aldrich syndrome: rescue of T-cell signaling and amelioration of colitis upon transplantation of retrovirally transduced hematopoietic stem cells in mice. Blood. 2003;101:2159–2166. doi: 10.1182/blood-2002-05-1423. [DOI] [PubMed] [Google Scholar]
- 21.Stamm LM, Pak MA, Morisaki JH, Snapper SB, Rottner K, Lommel S, Brown EJ. Role of the WASP family proteins for Mycobacterium marinum actin tail formation. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:14837–14842. doi: 10.1073/pnas.0504663102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stanley ER. Murine bone marrow-derived macrophages. Methods Mol Biol. 1997;75:301–304. doi: 10.1385/0-89603-441-0:301. [DOI] [PubMed] [Google Scholar]
- 23.Miki H, Miura K, Takenawa T. N-WASP, a novel actin-depolymerizing protein, regulates the cortical cytoskeletal rearrangement in a PIP2-dependent manner downstream of tyrosine kinases. EMBO J. 1996;15:5326–5335. [PMC free article] [PubMed] [Google Scholar]
- 24.Yamaguchi H, Lorenz M, Kempiak S, Sarmiento C, Coniglio S, Symons M, Segall J, Eddy R, Miki H, Takenawa T, Condeelis J. Molecular mechanisms of invadopodium formation: the role of the N-WASP-Arp2/3 complex pathway and cofilin. J Cell Biol. 2005;168:441–452. doi: 10.1083/jcb.200407076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cammer M, Gevrey JC, Lorenz M, Dovas A, Condeelis J, Cox D. The mechanism of CSF-1-induced Wiskott-Aldrich syndrome protein activation in vivo: a role for phosphatidylinositol 3-kinase and Cdc42. J Biol Chem. 2009;284:23302–23311. doi: 10.1074/jbc.M109.036384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lorenz M, Yamaguchi H, Wang Y, Singer RH, Condeelis J. Imaging sites of N-wasp activity in lamellipodia and invadopodia of carcinoma cells. Curr Biol. 2004;14:697–703. doi: 10.1016/j.cub.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 27.Snapper SB, Rosen FS, Mizoguchi E, Cohen P, Khan W, Liu CH, Hagemann TL, Kwan SP, Ferrini R, Davidson L, Bhan AK, Alt FW. Wiskott-Aldrich syndrome protein-deficient mice reveal a role for WASP in T but not B cell activation. Immunity. 1998;9:81–91. doi: 10.1016/s1074-7613(00)80590-7. [DOI] [PubMed] [Google Scholar]
- 28.Cox D, Dale BM, Kashiwada M, Helgason CD, Greenberg S. A regulatory role for Src homology 2 domain-containing inositol 5'-phosphatase (SHIP) in phagocytosis mediated by Fc gamma receptors and complement receptor 3 (alpha(M)beta(2); CD11b/CD18) J Exp Med. 2001;193:61–71. doi: 10.1084/jem.193.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wheeler AP, Smith SD, Ridley AJ. CSF-1 and PI 3-kinase regulate podosome distribution and assembly in macrophages. Cell Motil Cytoskeleton. 2006;63:132–140. doi: 10.1002/cm.20111. [DOI] [PubMed] [Google Scholar]
- 30.Kheir WA, Gevrey JC, Yamaguchi H, Isaac B, Cox D. A WAVE2-Abi1 complex mediates CSF-1-induced F-actin-rich membrane protrusions and migration in macrophages. J Cell Sci. 2005;118:5369–5379. doi: 10.1242/jcs.02638. [DOI] [PubMed] [Google Scholar]
- 31.Badolato R, Sozzani S, Malacarne F, Bresciani S, Fiorini M, Borsatti A, Albertini A, Mantovani A, Ugazio AG, Notarangelo LD. Monocytes from Wiskott-Aldrich patients display reduced chemotaxis and lack of cell polarization in response to monocyte chemoattractant protein-1 and formyl-methionyl-leucyl-phenylalanine. J Immunol. 1998;161:1026–1033. [PubMed] [Google Scholar]
- 32.Burns S, Thrasher AJ, Blundell MP, Machesky L, Jones GE. Configuration of human dendritic cell cytoskeleton by Rho GTPases, the WAS protein, and differentiation. Blood. 2001;98:1142–1149. doi: 10.1182/blood.v98.4.1142. [DOI] [PubMed] [Google Scholar]
- 33.Shields DC, Tyor WR, Deibler GE, Banik NL. Increased calpain expression in experimental demyelinating optic neuritis: an immunocytochemical study. Brain Res. 1998;784:299–304. doi: 10.1016/s0006-8993(97)01381-4. [DOI] [PubMed] [Google Scholar]
- 34.Smith ME, van der Maesen K, Somera FP. Macrophage and microglial responses to cytokines in vitro: phagocytic activity, proteolytic enzyme release, and free radical production. J Neurosci Res. 1998;54:68–78. doi: 10.1002/(SICI)1097-4547(19981001)54:1<68::AID-JNR8>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 35.Arkwright PD, Abinun M, Cant AJ. Autoimmunity in human primary immunodeficiency diseases. Blood. 2002;99:2694–2702. doi: 10.1182/blood.v99.8.2694. [DOI] [PubMed] [Google Scholar]
- 36.Calle Y, Chou HC, Thrasher AJ, Jones GE. Wiskott-Aldrich syndrome protein and the cytoskeletal dynamics of dendritic cells. J Pathol. 2004;204:460–469. doi: 10.1002/path.1651. [DOI] [PubMed] [Google Scholar]
- 37.Higgs HN. Actin nucleation: nucleation-promoting factors are not all equal. Curr Biol. 2001;11:R1009–R1012. doi: 10.1016/s0960-9822(01)00612-1. [DOI] [PubMed] [Google Scholar]
- 38.Snapper SB, Takeshima F, Anton I, Liu CH, Thomas SM, Nguyen D, Dudley D, Fraser H, Purich D, Lopez-Ilasaca M, Klein C, Davidson L, Bronson R, Mulligan RC, Southwick F, Geha R, Goldberg MB, Rosen FS, Hartwig JH, Alt FW. N-WASP deficiency reveals distinct pathways for cell surface projections and microbial actin-based motility. Nat Cell Biol. 2001;3:897–904. doi: 10.1038/ncb1001-897. [DOI] [PubMed] [Google Scholar]
- 39.Suzuki T, Mimuro H, Suetsugu S, Miki H, Takenawa T, Sasakawa C. Neural Wiskott-Aldrich syndrome protein (N-WASP) is the specific ligand for Shigella VirG among the WASP family and determines the host cell type allowing actin-based spreading. Cell Microbiol. 2002;4:223–233. doi: 10.1046/j.1462-5822.2002.00185.x. [DOI] [PubMed] [Google Scholar]
- 40.Calle Y, Jones GE, Jagger C, Fuller K, Blundell MP, Chow J, Chambers T, Thrasher AJ. WASp deficiency in mice results in failure to form osteoclast sealing zones and defects in bone resorption. Blood. 2004;103:3552–3561. doi: 10.1182/blood-2003-04-1259. [DOI] [PubMed] [Google Scholar]
- 41.Mizutani K, Miki H, He H, Maruta H, Takenawa T. Essential role of neural Wiskott-Aldrich syndrome protein in podosome formation and degradation of extracellular matrix in src-transformed fibroblasts. Cancer Res. 2002;62:669–674. [PubMed] [Google Scholar]
- 42.Spinardi L, Rietdorf J, Nitsch L, Bono M, Tacchetti C, Way M, Marchisio PC. A dynamic podosome-like structure of epithelial cells. Exp Cell Res. 2004;295:360–374. doi: 10.1016/j.yexcr.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 43.Calle Y, Anton IM, Thrasher AJ, Jones GE. WASP and WIP regulate podosomes in migrating leukocytes. J Microsc. 2008;231:494–505. doi: 10.1111/j.1365-2818.2008.02062.x. [DOI] [PubMed] [Google Scholar]
- 44.Park H, Cox D. Cdc42 Regulates Fc{gamma} Receptor-mediated Phagocytosis through the Activation and Phosphorylation of WASP and N-WASP. Mol Biol Cell. 2009 doi: 10.1091/mbc.E09-03-0230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Calle Y, Carragher NO, Thrasher AJ, Jones GE. Inhibition of calpain stabilises podosomes and impairs dendritic cell motility. J Cell Sci. 2006;119:2375–2385. doi: 10.1242/jcs.02939. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Representative images of resting or activated peritoneal macrophages from wildtype (WT) and WASP-deficient (WASP−/−) mice are shown, phase (left) and Alexa-568 fibronectin (right). Scale bar represents 10µm.