Abstract
Quantitative Sudomotor Axon Reflex Testing is a useful measure of post ganglionic sudomotor function. The test is based on the iontophoresis of an acetylcholine solution which induces a local sweat response. We have previously described a gel-based vehicle that may provide another option for the iontophoresis of acetylcholine. It was our objective to compare the influence of the vehicle (gel versus solution) on sudomotor recordings and perceived discomfort. Results show gel-based vehicles are very similar to solution-based vehicles during Quantitative Sudomotor Axon Reflex Testing.
Keywords: QSART, sudomotor, acetylcholine, gel, solution, vehicle
Introduction
Low et al. (1983) has provided a prior method for assessing and quantifying axon-reflex mediated sudomotor responses by means of the Quantitative Sudomotor Axon Reflex Test (QSART). This method utilizes a Sudorometer, a constant current generator, ACh solution (10% w/v), and a multi-compartmental sweat cell. A dose-response study utilizing ACh solution showed that a 1-molar concentration of acetylcholine was needed to generate a maximal response to 2mA constant current over a 5 minute period (Low et al., 1992).
A new vehicle for delivery of ACh ions has recently been described (Sletten et al., 2009). This vehicle is composed of ACh ions suspended in an agarose gel. A vehicle made of agarose gel optimizes contact with underlying skin and eliminates any potential “hot spots” due to air bubbles that may occur with the standard solution-based vehicle. Theoretically, the gel could reduce the amount of voltage needed to iontophorese ions and potentially reduce patient discomfort during QSART (Sletten et al., 2009).
The objective of the current study was two-fold: 1) to address the influence of the vehicle (gel versus solution) on sudomotor recordings (total sweat volume and time to sweat onset); 2) to address the influence of the vehicle on perceived level of discomfort (as measured by a 11-point visual analog scale).
Method
Participants
Institutional review board approval (Mayo Clinic, Rochester, MN) and participant consent was obtained prior to study. Twenty healthy control participants median age 50 years (18–70 years), median height 169.5 cm (157 to 187 cm), and median weight 70.5 kg (53 to 99 kg) were enrolled. Female to male ratio was 1:1.
Sudomotor Recordings
No food, caffeine, or nicotine was permitted for 8 hours prior to the study. All participants were medication free at the time of the testing; exceptions were made for participants taking oral contraceptives and vitamins. Studies were completed in the Autonomic Disorders Center at the Mayo Clinic in Rochester, MN using a home-built Sudorometer, constant current generator, and multi-compartmental sweat capsules (Low, 1983, 1997). QSART was performed at the medial forearm and proximal foot to assess the integrity of the postganglionic sympathetic sudomotor axon as previously described (Low et al., 1983). Tight control of room temperature and humidity were maintained at 23°C and 25–35%, respectively. Skin preparations were performed according to standard clinical protocol which includes the removal of any excess hair, followed by a four-step cleaning process (acetone, alcohol, water, and dry gauze) (Low and Sletten, 2008). A baseline sweat response was recorded. Once baseline was established, a stimulus of 2 mA constant current was applied for 5 minutes to the stimulus compartment. Sweat responses were recorded for an additional five minutes after discontinuation of the stimulus.
Effect of Formulation on Physiologic Responses and Perceived Discomfort
For the comparison of the gel versus solution formulations, two multi-compartmental sweat capsules were attached to the participant’s medial forearm and proximal foot sites, bilaterally. The stimulus compartments contained identical concentrations of acetylcholine (0.55M) randomized to which side (right or left) would receive the gel and solution formulations. All four sites were studied simultaneously. Measurement of total sweat volume (expressed as µL/10 minutes) and latency (time to onset of sweating), in minutes, were obtained for all participants. Lastly, participants were asked to rate their perceived level of discomfort for each test site immediately following the 5-minute stimulation period using a 11-point visual analog scale (VAS; 0 equals no pain/discomfort and 10 the most severe pain/discomfort felt).
Statistical Analysis
We analyzed the data obtained separately at the forearm and foot sites since volume and latency were expected to differ by regional test sites (Low and Sletten, 2008). To estimate the difference between gel and solution for each subject, we calculated a paired difference defined as the gel value minus the solution value. These paired differences were tested with a two-sided, one-sample Wilcoxon signed rank test. We also report 95% confidence intervals for the median paired difference using the percentile bootstrap method. Due to skewness in the collected data, nonparametric analysis methods were used.
To further assess agreement in volume estimates between the two methods, we calculated the Concordance Correlation Coefficient (CCC) which can be thought of as measure of correlation about the 45 degree line of perfect agreement (Lin, 1989). We also assessed agreement graphically using scatter plots and Bland-Altman style agreement plots (Bland and Altman, 1986).
Results
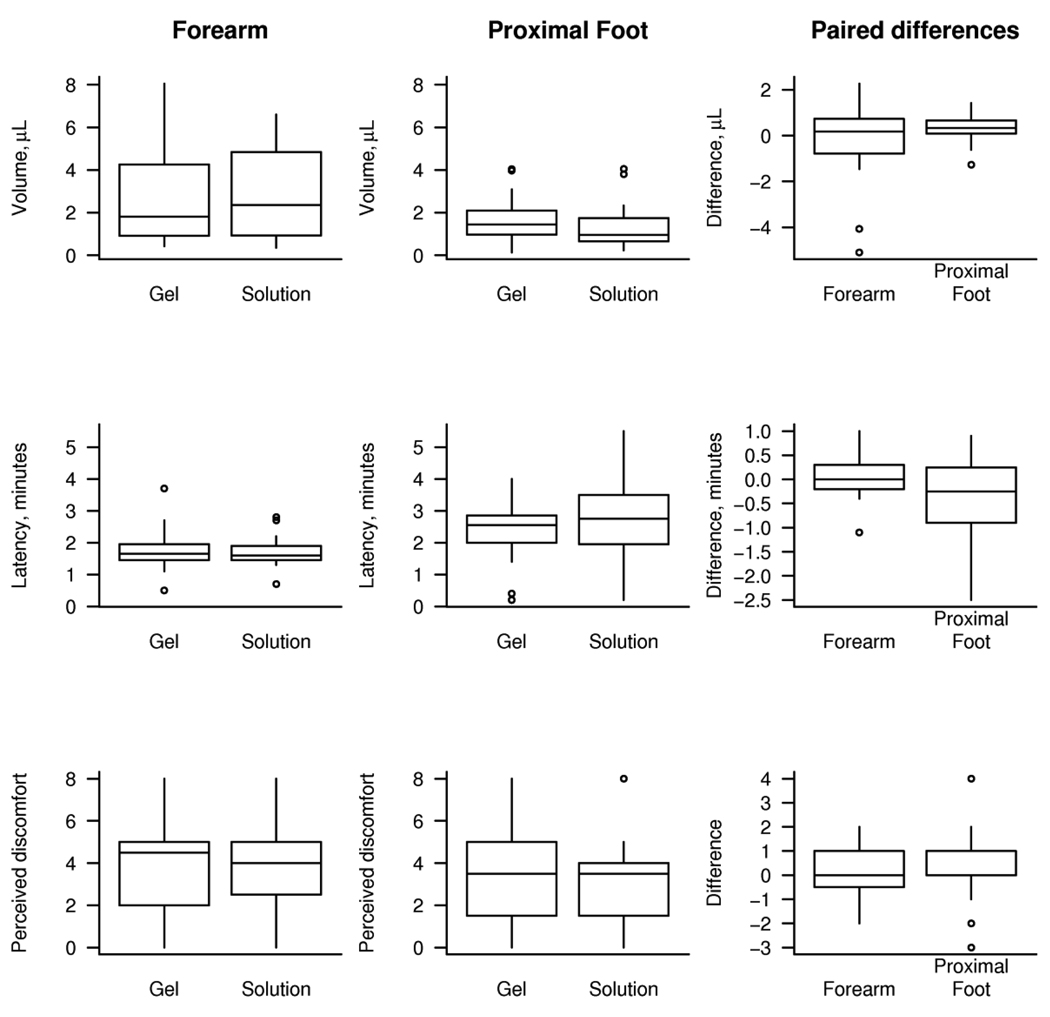
Due to expected differences between the forearm and proximal foot, we report the results for this study separately by site in figure 1 and table 1. At the forearm, we found no significant differences between gel and solution in terms of total sweat volume (p=0.84), latency (p=0.59), or perceived discomfort (p=0.74). At the proximal foot, volume was found to be greater with the gel than the solution by a median value of 0.3 µL (0.16 to 0.52, 95% CI, p=0.009). There was some weak evidence that latency was shorter by a median value of 0.3 minutes, although this was not significant at the 0.05 level (p=0.12). Discomfort was not found to differ significantly at the proximal foot (p=0.40).
Figure 1.
Box plots showing values obtained using gel and solution and the paired difference between gel and solution. Paired differences are calculated from the patient’s value obtained with gel minus their value obtained with solution. The horizontal lines in each box represent the 25th, 50th, and 75th percentiles. The vertical lines extend out to the farthest point within 1.5 times the interquartile range (IQR) with points beyond 1.5 IQRs individually indicated.
Table 1.
Summary statistics
| Volume, µL |
Latency, minutes |
Perceived discomfort, (0 to 10) |
|
|---|---|---|---|
| Forearm | |||
| Median (range): Gel | 1.8 (0.4, 8.1) | 1.6 (1.5, 1.9) | 4.5 (2.0, 5.0) |
| Median (range): Solution | 2.4 (0.3, 6.6) | 1.6 (1.5, 1.9) | 4.0 (2.8, 5.0) |
| Median (range) paired difference* | 0.2 (−5.1, 2.3) | 0.0 (−0.2, 0.3) | 0.0 (−0.2, 1.0) |
| P-value† | 0.84 | 0.59 | 0.74 |
| 95% CI for difference | −0.55 to 0.40 | −0.15 to 0.25 | 0.0 to 1.0 |
| Proximal Foot | |||
| Median (range): Gel | 1.4 (0.1, 4.0) | 2.5 (0.2, 4.0) | 3.5 (0.0, 8.0) |
| Median (range): Solution | 1.0 (0.2, 4.0) | 2.8 (0.2, 5.5) | 3.5 (0.0, 8.0) |
| Median (range) paired difference* | 0.3 (−1.3, 1.4) | −0.3 (−2.5, 0.9) | 0.0 (−3.0, 4.0) |
| P-value† | 0.009 | 0.12 | 0.40 |
| 95% CI for difference | 0.16 to 0.52 | −0.85 to 0.20 | 0.0 to 0.5 |
Calculated as gel value minus solution value
One sample, two-sided signed rank test
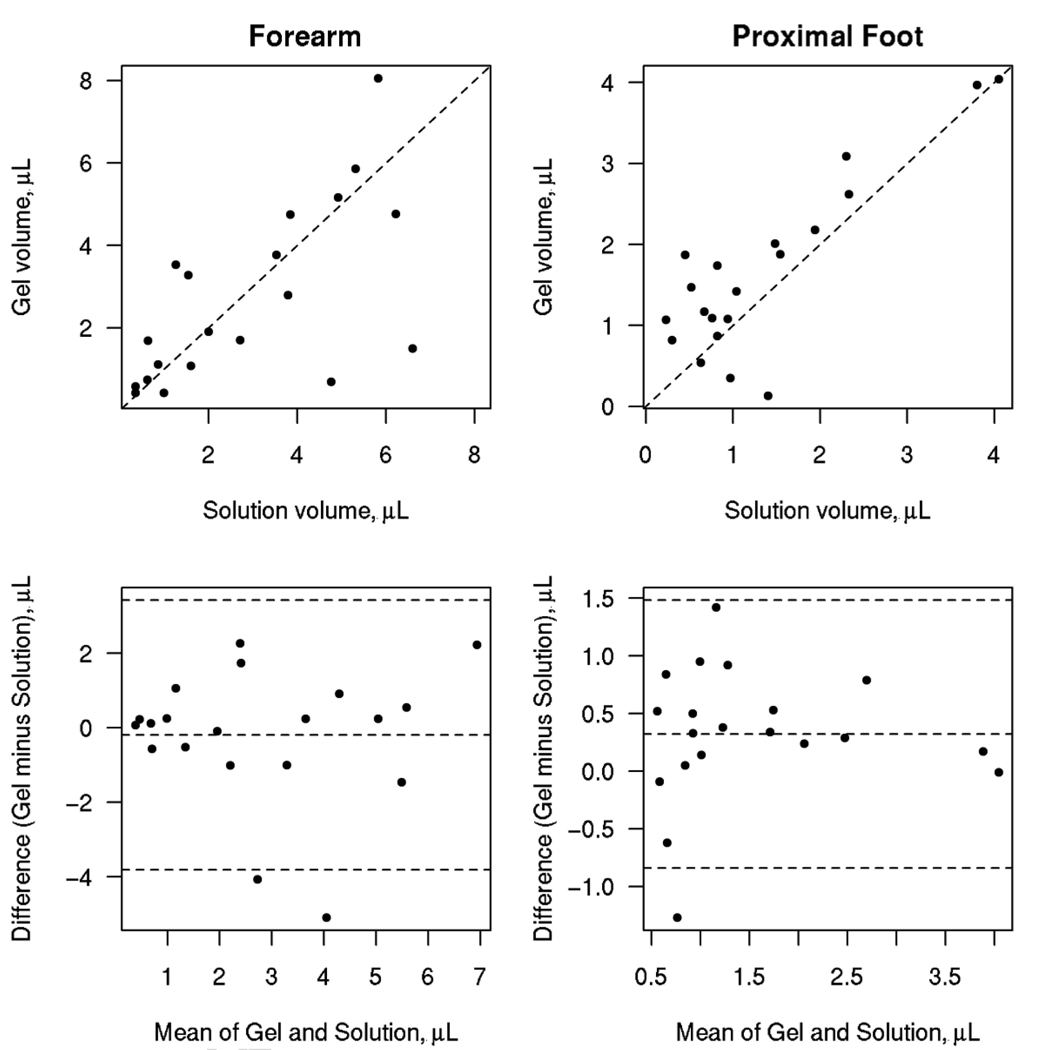
Figure 2 shows scatter plots of the gel versus solution volume and Bland-Altman plots of agreement. For the forearm volume, the CCC (95% CI) was 0.64 (0.29, 0.84) and for the proximal foot the CCC (95% CI) was higher at 0.82 (0.61, 0.92). For the forearm, the limits of agreement were −3.8 µL to 3.4 µL while for the proximal foot the limits were narrower at −0.8 µL to 1.5 µL.
Figure 2.
The top panels show scatter plots showing the relationship between the gel and solution volume estimates. The dashed line represents the 45 degree line of perfect agreement. The bottom panels show Bland-Altman style agreement plots with the dashed lines representing the mean difference and the limits of agreement.
Discussion
The routine clinical evaluation of the autonomic nervous system includes QSART which measures the integrity of the postganglionic sudomotor axon (Low et al., 1983). QSART is useful in the evaluation of several neuropathies, including diabetic neuropathy and in other disorders such as Multiple Systems Atrophy and Pure Autonomic Failure. The physiological basis for the test is the iontophoresis of ACh from sweat capsules placed on the skin. ACh orthodromically stimulates post ganglionic fibers via muscarinic receptors (M3) to result in the sweat response (for review see Low, 2004; Low and Sletten, 2008). However, the test is not without difficulties including patient discomfort, modest skin irritation, and leakage from the stimulation chamber to the sweat collection chamber. We have previously described a gel-based vehicle for the iontophoretic delivery of ACh during QSART in an attempt to increase the efficiency of iontophoresis and improve patient comfort (Sletten et al., 2009).
Issues with skin irritation and patient discomfort are most likely the result of current strength and inefficiency of iontophoresis using standard ionic solutions. Currents of 2 mA (5-minute duration) were required to produce a maximal response to iontophoresis with 1 molar ACh solution (Low et al., 1992). Higher current densities have been hypothesized as the likely cause of patient discomfort and modest skin irritation (Sletten et al., 2009). We have previously used a dose/current response study using ACh at concentrations from 0.0055M to 0.55M at 1–2 mA to assess the effect of the gel vehicle on perceived discomfort as determined by the visual analog scale (VAS). Median VAS values were in the low range (2–3) and only minimally increased at higher currents (2 mA; Sletten et al., 2009). In our comparison between the gel and solution vehicles, there was no significant difference in patient perceived discomfort.
There was no significant difference in regards to sweat volume or latency when gel and solution vehicles were directly compared with the exception favoring the gel vehicle at the foot by a median sweat volume of 0.3 µL, an amount we consider minimal and unlikely to be of clinical significance. Our results indicate that the gel vehicle is at least equivalent to the solution vehicle during QSART in regards to sweat responses and patient comfort. While we found one can expect little to no systematic differences in the volume estimates for one vehicle vs. another, the agreement between the two vehicles was quite good at the foot but less robust at the forearm. The lower CCC at the forearm may be a result of subtle differences in skin characteristics, capsule positioning, or the amount of pressure applied to capsule. Still, we feel that even for subjects relatively far from the line of perfect agreement, the magnitude of the observed difference was of little clinical significance. Therefore in our opinion, our data supports the use of gel-based vehicles as an option for iontophoresis during QSART.
While the most common vehicle used for iontophoresis of ACh is a 10% (wt/v) ionic solution of acetylcholine chloride (Sletten et al., 2005), reagents using a gel-based vehicle are not without precedent. Agarose gel preparations are commonly used to perform diagnostic testing, including the Pilogel® Iontophoretic Discs used in the diagnosis of Cystic Fibrosis (Losty et al., 2006). The greater consistency of gel based products may provide improved surface contact increasing the efficiency of iontophoresis and possibly decreasing patient discomfort during QSART. The added benefit of the increased consistency of gel based formulas are the safety aspects resulting from the prevention of leakage. The leakage of ionic solutions from the sweat capsule may in part play a role in issues with skin irritation.
Conclusions
Gel-based vehicles used for the delivery of ACh ions during the iontophoresis stage of QSART were found to be very similar on average to the solution-based vehicles when considering sudomotor measurements of volume and latency. Furthermore, the use of a gel-based vehicle does not alter the perception of discomfort during the iontophoretic stimulation.
Acknowledgments
We would like to thank Tonette Gehrking and Jade Gehrking for their technical assistance. This work was supported by NIH grants (NS3 2352, NS4 4233, NS4 3364, UL1 RR24150) and Mayo Clinic. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Neurological Disorders and Stroke or the National Institutes of Health.
Abbreviations
- ACh
acetylcholine
- QSART
quantitative sudomotor axon reflex testing
- VAS
Visual Analog Scale
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310. [PubMed] [Google Scholar]
- Lin LI. A concordance correlation coefficient to evaluate reproducibility. Biometrics. 1989;45:255–268. [PubMed] [Google Scholar]
- Losty HC, Wheatley H, Doull I. The evaluation of a novel conductometric device for the diagnosis of cystic fibrosis. Ann. Clin. Biochem. 2006;43:375–381. doi: 10.1258/000456306778520025. [DOI] [PubMed] [Google Scholar]
- Low PA, Caskey PE, Tuck RR, Fealey RD, Dyck PJ. Quantitative sudomotor axon reflex test in normal and neuropathic subjects. Ann. Neurol. 1983;14:573–580. doi: 10.1002/ana.410140513. [DOI] [PubMed] [Google Scholar]
- Low PA, Opfer-Gehrking TL, Kihara M. In vivo studies on receptor pharmacology of the human eccrine sweat gland. Clin. Auton. Res. 1992;2:29–34. doi: 10.1007/BF01824208. [DOI] [PubMed] [Google Scholar]
- Low PA. Evaluation of sudomotor function. Clin. Neurophysiol. 2004;115:1506–1513. doi: 10.1016/j.clinph.2004.01.023. [DOI] [PubMed] [Google Scholar]
- Low PA, Sletten DM. Laboratory evaluation of Autonomic Failure. In: Low PA, Benarroch EE, editors. Clinical Autonomic Disorders. Baltimore: Lippincott, Williams & Wilkins; 2008. pp. 130–163. [Google Scholar]
- Low PA, Denq JC, Opfer-Gehrking TL, Dyck PJ, O'Brien PC, Slezak JM. Effect of age and gender on sudomotor and cardiovagal function and blood pressure response to tilt in normal subjects. Muscle Nerve. 1997;20:1561–1568. doi: 10.1002/(sici)1097-4598(199712)20:12<1561::aid-mus11>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Sletten DM, Nickander KK, Low PA. Stability of acetylcholine chloride solution in autonomic testing. J. Neurol. Sci. 2005;234:1–3. doi: 10.1016/j.jns.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Sletten DM, Kimpinski K, Wiegand SD, Low PA. A novel gel based vehicle for the delivery of acetylcholine in quantitative sudomotor axon reflect testing. Auton Neurosci. 2009 Oct 5;150(1–2):127–130. doi: 10.1016/j.autneu.2009.05.250. Epub 2009 Jun 10. [DOI] [PMC free article] [PubMed] [Google Scholar]