Abstract
In prior studies, we found that activation of cannabinoid-1 receptors in the nucleus tractus solitarii (NTS) prolonged baroreflex-induced sympathoinhibition in rats. In many regions of the central nervous system, activation of cannabinoid-1 receptors presynaptically inhibits γ-aminobutyric acid (GABA) release, disinhibiting postsynaptic neurons. To determine if cannabinoid-1 receptor-mediated presynaptic inhibition of GABA release occurs in the NTS, we recorded miniature inhibitory postsynaptic currents in anatomically-identified second-order baroreceptive NTS neurons in the presence of ionotropic glutamate receptor antagonists and tetrodotoxin. The cannabinoid-1 receptor agonists, WIN 55212-2 (0.3 – 30 μM) and methanandamide (3 μM) decreased the frequency of miniature inhibitory postsynaptic currents in a concentration-dependent manner, an effect that was blocked by the cannabinoid-1 receptor antagonist, N-(piperidin-1-yl)-5-(4-iodophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide (AM 251, 5 μM). Importantly, depolarization of second-order baroreceptive neurons decreased the frequency of miniature inhibitory postsynaptic currents; an effect which was blocked by the cannabinoid-1 receptor antagonist. The data indicate that depolarization of second-order baroreceptive NTS neurons induces endocannabinoid release from the neurons, leading to activation of presynaptic cannabinoid-1 receptors, inhibition of GABA release and subsequent enhanced baroreflex signaling in the NTS. The data suggest that endocannabinoid signaling in the NTS regulates short-term synaptic plasticity and provide a mechanism for endocannabinoid modulation of central baroreflex control.
Introduction
The arterial baroreceptors provide a powerful beat-to-beat regulation of blood pressure via a well-defined central nervous system reflex. The physiological importance of the baroreflex is underscored by the consequences of impaired baroreflex function – volatile blood pressure, orthostatic hypotension, hypertensive crises and, occasionally, malignant vagotonia (Ketch et al., 2002). Previously, we have found functional evidence for modulation of baroreflex function in the nucleus tractus solitarii (NTS) by endocannabinoids (ECBs), including N-arachidonylethanolamine (anandamide) and 2-arachidonoyl glycerol (2-AG) (Brozoski et al., 2005; Seagard et al., 2004, 2005). Microinjection of anandamide into the NTS prolonged baroreflex-induced inhibition of renal sympathetic nerve activity in rats (Seagard et al., 2004). In addition, extending endogenous ECB activity in the NTS by blocking ECB metabolism and uptake also prolonged baroreflex-induced sympathoinhibition (Brozoski et al., 2005). This prolonged baroreflex-induced sympathoinhibition was blocked by prior microinjection of a cannabinoid-1 receptor (CB1R) antagonist, N-piperidinyl-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-pyrazole-3-carboxamide (SR141716). The data suggest a role for CB1R modulation of baroreceptive NTS neuronal function, supported by the finding that ECBs increased the firing of barosensitive NTS neurons (Seagard et al., 2005).
Second-order baroreceptive neurons in the NTS play a pivotal role in control of blood pressure because the signal conditioning at these gateway synapses determines the magnitude, pattern, and duration of the signal transmitted to distal central sites to coordinate baroreflex output (Andresen et al., 1994; Spyer, 1990). Glutamate is the major excitatory neurotransmitter released by baroreceptor afferents and other cardiopulmonary inputs at second-order baroreceptive NTS neurons (Andresen et al., 1994; Bonham et al., 2002; Seagard et al., 2001; Zhang et al., 1997). γ-aminobutyric acid (GABA) is the major inhibitory neurotransmitter released by interneurons in NTS and descending inputs from higher brain regions (Bailey et al., 2008; Gordon et al., 2002). Ultimately, it is the balance of excitatory and inhibitory modulatory influences to second-order baroreceptive NTS neurons that shapes the net NTS output.
Work by others has shown that ECBs are synthesized in response to depolarization (Chevaleyre et al., 2006; Freund et al., 2003). These ECBs serve as retrograde signaling molecules that presynaptically inhibit the release of neurotransmitters, including GABA (Chevaleyre et al., 2006; Freund et al., 2003). Inhibition of GABA release results in depolarization-induced suppression of inhibition (DSI), or disinhibition of postsynaptic neurons (Freund et al., 2003). In terms of modulating baroreflex function, prior microinjection of a GABAA receptor antagonist prevented both the ECB-induced prolongation of sympathoinhibition (Seagard et al., 2004) and activation of barosensitive neurons (Seagard et al., 2005), indicating a role for modulation of GABAergic transmission in the ECB-induced responses. However, the actual mechanism(s) for ECB-induced effects in the NTS were not determined beyond this observation. Interestingly, in NTS slices from young rats (25-30 days old), Accorsi-Mendonca et al. showed that selective CB1/CB2 agonists had no effect on the frequency of spontaneous postsynaptic currents (a mixture of excitatory and inhibitory synaptic currents) in vitro in NTS slices (Accorsi-Mendonca et al., 2008). In light of the in vivo functional studies, the data suggest that expression of ECB receptors may be dependent on age and/or the functional subtypes of neurons in the NTS (e.g. neurons in the baroreflex pathway versus non-baroreceptive neurons).
The present study was carried out to delineate the mechanism(s) by which ECB signaling in the NTS modulates the baroreflex. This study utilized patch clamp electrophysiology of brain slices containing anatomically identified second-order NTS neurons in the baroreflex pathway of adult rats. We examined the effects of endogenously released ECBs, exogenously applied CB1R agonists and CB1R antagonists on GABA signaling to test the hypothesis that ECBs released within the NTS in response to excitation of these neurons modulate GABA transmission presynaptically through a CB1R mediated mechanism. Data obtained support this hypothesis and provide novel information regarding the physiological role of ECBs in regulation of autonomic function in the brainstem.
Materials and Methods
All experimental protocols in this work were reviewed and approved by the University of California Institutional Animal Care and Use Committee in compliance with the Animal Welfare Act and in accordance with Public Health Service Policy on Humane Care and Use of Laboratory Animals.
Sixteen male 11-week-old Sprague-Dawley rats (320 – 370 gm, Charles River Laboratories, Inc. Wilmington, MA, USA) were anesthetized with a combination of ketamine (50 mg/kg, im, Vedco Inc. St. Joseph, MO, USA) and xylazine (8 mg/kg, im, Vedco Inc. St. Joseph, MO, USA). A 4 – 5 mm segment of the aortic depressor nerve between the superior laryngeal nerve and vagus nerve/sympathetic trunk was carefully isolated and placed on a section of parafilm. The fluorescent dye crystals, 1,1′-dilinoleyl-3,3,3′,3′-tetramethylindocarbocyanine, 4-chlorobenzenesulfonate (FAST DiI™ solid; DiI[△]9,12-C18(3), Molecular Probes, Eugene, OR, USA), were placed on the aortic depressor nerve and the area was imbedded with Polyvinylsiloxane gel (Charlisle laboratories Inc. Rockville Centre, NY, USA). To allow for transport of the dye to the terminal boutons, the rats were allowed to recover for two weeks before the experimental protocols as previously reported (Chen et al., 2009; Chen et al., 2005). Pre-emptive analgesia (ketoprofen, 2 mg/kg sc, Fort Dodge Animal Health, Fort Dodge, Iowa, USA) and antibiotics (Baytril 5 mg/kg, im, Bayer HealthCare LLC, Shawnee Mission, Kansas, USA) were given immediately before surgery to prevent post-operative pain and infection. During surgery, the following procedure was used to assess adequacy of anesthesia and supplemental anesthesia (25% of initial dose) was given if 1) the eye blink reflex was present; 2) there was whisker movement; 3) paw withdrawal occurred upon pinch; or 4) irregular or sudden changes in breathing frequency were observed. Fluids (0.9% saline, 2 ml/100 g BW, sc) were administered post-operatively to prevent dehydration. Animals were kept on heating pads during recovery from anesthesia and were checked daily for signs of pain and infection.
Brainstem slice preparation
The rats were anesthetized with a combination of ketamine (50 mg/kg, im) and xylazine (8 mg/kg, im) and decapitated. The brain was rapidly exposed and submerged in ice-cold (< 4°C) high-sucrose artificial cerebrospinal fluid that contained (mM): 3 KCl, 2 MgCl2, 1.25 NaH2PO4, 26 NaHCO3, 10 glucose, 220 sucrose and 2 CaCl2, with a pH of 7.4 when continuously bubbled with 95% O2 / 5% CO2. Brainstem transverse slices (250 μm thick) were cut with Leica VT1000 S vibratome (Leica Microsystems, Inc. Bannockburn, IL, USA). After incubating for 45 min at 37°C in high-sucrose artificial cerebrospinal fluid, the slices were placed in normal artificial cerebrospinal fluid that contained (mM): 125 NaCl, 2.5 KCl, 1 MgCl2, 1.25 NaH2PO4, 25 NaHCO3, 25 glucose and 2 CaCl2, with a pH of 7.4 when continuously bubbled with 95% O2 / 5% CO2. During the experiments a single slice was transferred to the recording chamber, held in place with a silk mesh, and continuously perfused with oxygenated artificial cerebrospinal fluid at a rate of approximately 3 ml/min. The perfusion line consists of an inner tube for artificial cerebrospinal fluid and an outer tube that was connected to a circulating water bath for temperature control. All experiments were performed at 33°-34°C.
Patch-clamp recordings
All whole-cell voltage-clamp recordings were performed on second-order baroreceptive NTS neurons with attached fluorescent aortic depressor nerve boutons. The neurons were visualized with infrared differential interference contrast. The fluorescent boutons were visualized with an optical filter set for DiI (XF108, Omega Optical Inc., Brattleboro, VT, USA) and an image integrating system (InvestiGater, Dage-MTI, Michigan City, IN, USA). Borosilicate glass electrodes were filled with a KCl solution containing (mM): 130 KCl, 5 NaCl, 1 MgCl2, 3 Mg-ATP, 0.2 Na-GTP, 10 EGTA, 10 HEPES. The pH was adjusted to 7.3 with KOH. Once the whole-cell configuration was established, the neuron was voltage-clamped at −60 mV. Miniature inhibitory postsynaptic currents (mIPSCs) were recorded in the presence of the ionotropic glutamate receptor antagonists, 2,3-dihydroxy-6-nitro-7-sulfamoyl-benzo[f]quinoxaline-2,3-dione (NBQX, 10 μM) and (±)-2-amino-5-phosphonovaleric acid (AP-5, 50 μM) and a sodium channel antagonist, tetrodotoxin (1 μM). We and others previously showed that these inhibitory synaptic currents are GABAergic: having a reversal potential for chloride ions and being blocked by GABAA receptor antagonists (Chen et al., 2005, 2009; Glatzer et al., 2005; McDougall et al., 2008; Zhang et al., 2007).
Protocols
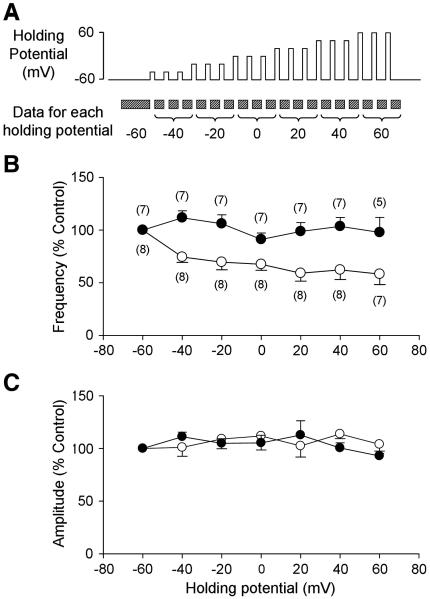
To determine whether activation of CB1Rs in the NTS decreases presynaptic GABA release, mIPSCs were recorded for six min during the control period and five min during perfusion with a cannabinoid receptor agonist, WIN 55212-2 (0.3 – 30 μM). For the WIN concentration-response curve, the doses of WIN were applied in a random order with a recovery time of 7-25 minutes between doses. Since WIN 55212-2 has some nonspecific effects, the response to WIN 55212-2 at concentration at 3 μM, which was found to be an effective functional mid-range dose, was again tested in the presence of the CB1R antagonist, N-(Piperidin-1-yl)-5-(4-iodophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide (AM 251, 5 μM) to verify that the inhibition of GABA release was mediated by activation of CB1Rs. In addition, a more selective CB1R agonist, methanandamide (3 μM), was also tested in the absence and presence of the CB1R antagonist. Finally, to demonstrate that activation of second-order baroreceptive NTS neurons evokes endogenous release of ECBs, leading to presynaptic inhibition of GABA release, the effects of depolarization of the postsynaptic neuron on mIPSC frequency and amplitude were examined in the absence and presence of the CB1R antagonist. The postsynaptic neuron was directly depolarized (−60 mV to +60 mV at 20 mV increments) for 20 s every 60 s with mIPSCs recorded for 40s following the neuronal depolarization (Figure 5A). The responses in mIPSC frequency and amplitude induced by the depolarizing steps were measured at the return of the membrane potential to −60 mV in order to control for the change in driving force for the chloride ions. The decrease in mIPSC frequency was greatest during the first 10 s upon return of the membrane potential to −60mV. The mIPSC frequency then gradually returned to baseline over the next 30 s. Thus the changes in mIPSC frequency reflected the full effect of depolarization. In a separate group of neurons this protocol was performed in the presence of AM 251.
Figure 5.
Effects of postsynaptic membrane depolarization on presynaptic GABA release. A. Depolarization-induced disinhibition protocol. Brief (20 s) depolarizing steps were applied to the postsynaptic neurons every minute. Two minutes of mIPSC recordings before the first depolarizing step was used for the −60 mV data. Each voltage step was applied three times and the mIPSCs after each depolarizing step were analyzed for the data for each step. B. Group data of mIPSC frequency at different postsynaptic membrane potentials in the absence (open circles) and presence (closed circles) of the CB1R antagonist, AM 251 (5 μM). Postsynaptic depolarization significantly decreased mIPSC frequency, but not amplitude, suggesting a decrease in presynaptic GABA release (p<0.05 for postsynaptic membrane potential and interaction, two-way ANOVA.). C. Group data for mIPSC amplitude at different postsynaptic membrane potentials in the absence (open circles) and presence (closed circles) of the CB1R antagonist. Numbers in parentheses indicate number of neurons.
Data Analysis
Data are expressed as means ± SE unless otherwise indicated. Differences were considered significant at p < 0.05. The statistical analyses were performed with SigmaStat software using a student’s t-test, one-way ANOVA, or two-way ANOVA, as described below.
To examine the effect of WIN 55212-2, the frequencies and amplitudes of mIPSCs recorded during the last three min of the control period were averaged as the control value. The same variables for mIPSCs recorded during the last three min of WIN 55212-2 perfusion at each concentration were averaged and expressed as a percent of the control value. The agonist concentration–response curve was analyzed with one-way ANOVA. To determine the effect of the CB1R antagonist on the agonist induced depression, mIPSC frequency during agonist (3 μM) perfusion was expressed as a percent of the control value. The data from neurons used to generate the concentration-response curve for WIN 55212-2 at the 3 μM dose were used to establish the agonist-induced effect in the absence of AM 251. In a separate group of neurons, the effects of the agonist at 3 μM were determined in the presence of AM 251 only. Thus, an unpaired t-test was used. For methanandamine, only one dose (3 μM) was tested and the effects of the agonist were tested both in the absence and presence of AM 251. Thus, the data were compared with a paired t-test.
To determine the effect of depolarization-induced ECB release on presynaptic GABA transmission, mIPSC frequency and amplitude recorded after the 20 s of depolarization were averaged for each depolarizing step. The data were compared with a two-way ANOVA with the postsynaptic membrane potential as one factor and the absence and presence of the CB1R antagonist as the other factor.
Chemicals
WIN 55212-2, methanandamide, and AM 251 were obtained from Tocris (Ballwin, MO, USA). NBQX, AP5, TTX, Mg-ATP, Na-GTP, EGTA, and HEPES were obtained from Sigma (St. Louis, MO, USA). All other chemicals were obtained from Fisher (Fairlawn, NJ, USA).
Results
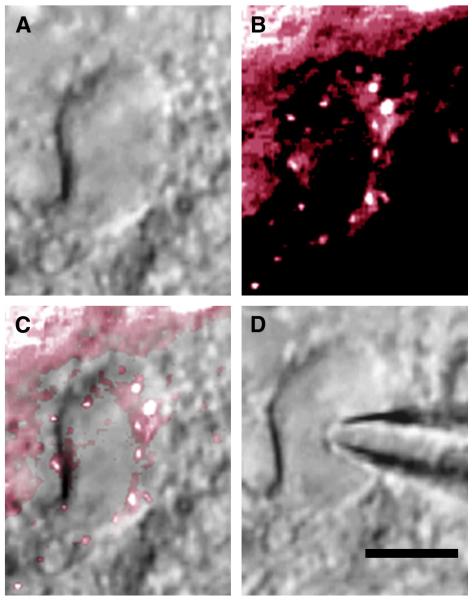
All electrophysiological data were obtained in second-order baroreceptive NTS neurons identified by their possession of fluorescently labeled attached boutons as shown in Figure 1. All neurons studied were located in the dorsal or medial NTS between 250 μm rostral to obex and 250 μm caudal to calamus scriptorius.
Figure 1.
A second-order baroreceptive NTS neuron. A, The neuron viewed with infrared differential interference contrast (IR-DIC). B, The labeled aortic depressor nerve boutons viewed with a fluorescence filter set. C, Overlay of the IR-DIC and fluorescence images. D, Neuron with patch electrode in whole cell configuration. Calibration bar = 10 μm.
Effect of cannabinoid receptor agonists on mIPSCs
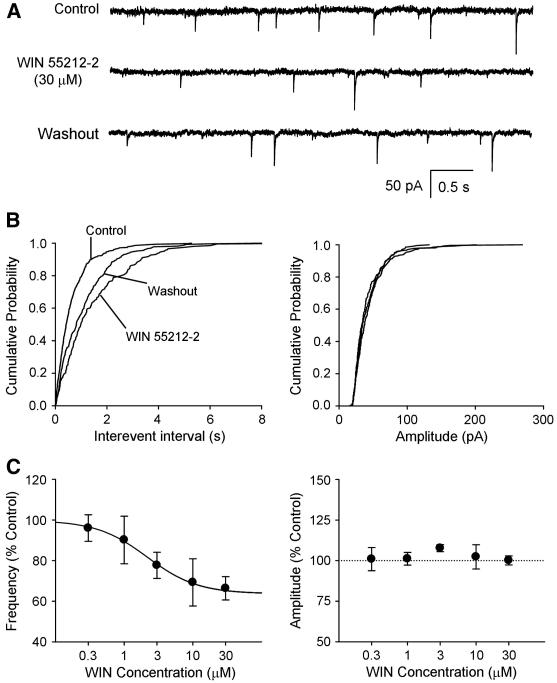
The averaged baseline mIPSC frequency (n = 41) was 1.60 ± 0.24 Hz and the averaged baseline mIPSC amplitude was 63 ± 3 pA. In these cells, the cannabinoid receptor agonist, WIN 55212-2 decreased the frequency but not the amplitude of mIPSCs. Example traces of recorded mIPSCs before, during, and after WIN 55212-2 perfusion are shown in Figure 2A. Figure 2B shows a rightward shift in the cumulative probability of the mIPSC inter-event intervals during WIN 55212-2 perfusion and no change in the cumulative probability of the mIPSC amplitude. The group data (Figure 2C, left), fitted to a sigmoid function, illustrates the concentration-dependent decrease in mIPSC frequency (one-way ANOVA, p < 0.05). The maximum effect of WIN 55212-2 was a 36% decrease in mIPSC frequency. WIN 55212-2 had no consistent effect on the mIPSC amplitude (Figure 2C, right).
Figure 2.
The cannabinoid receptor agonist decreased GABA transmission in a concentration-dependent manner in second-order baroreceptive NTS neurons. A. Example traces of mIPSCs recorded before perfusion (control), during WIN 55212-2 (30 μM) perfusion, and during washout period. B. Cumulative probability of the interevent interval (left) and amplitude (right) before, during, and after WIN 55212-2 perfusion. C, left. Group data showing that WIN 55212-2 decreased mIPSC frequency in a concentration-dependent manner (p<0.05, one-way ANOVA). C, right. Group data showing that WIN 55212-2 had no consistent effect on mIPSC amplitude. Numbers in parentheses indicate number of neurons.
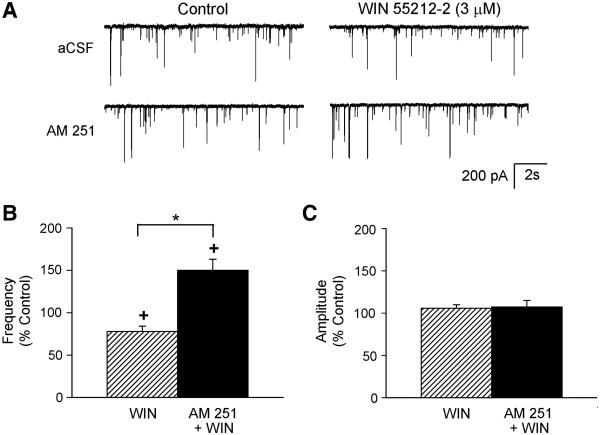
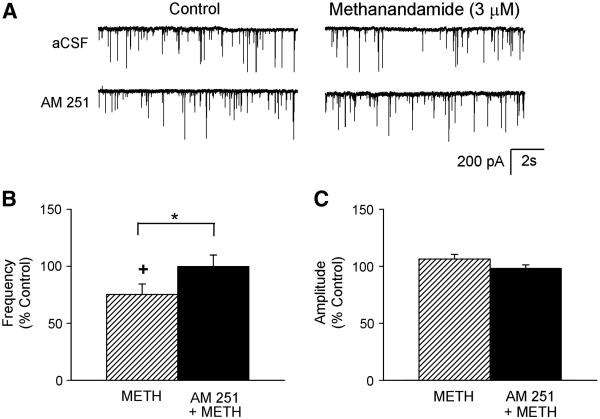
The selective CB1R antagonist, AM 251 (5 μM) prevented WIN 55212-2-induced depression of the mIPSC frequency as shown in Figure 3. Example traces of recorded mIPSCs before and during application of WIN 55212-2 in the absence and presence of the antagonist are shown in Figure 3A. In the absence of antagonist, WIN 55212-2 (3 μM) decreased the mIPSC frequency (78 ± 6 % of control, from 1.1 ± 0.4 Hz to 0.9 ± 0.4 Hz, n=11, Figure 3B) but had no significant effects on the amplitude (106 ± 4 % of control, from 64 ± 9 pA to 69 ± 10 pA, Figure 3C). In the presence of the cannabinoid receptor antagonist, WIN 55212-2 increased mIPSC frequency (150 ± 13 % of control, from 2.0 ± 0.7 Hz to 3.0 ± 1.2 Hz, n=7, Figure 3B) without inducing a significant change in the mIPSC amplitude (107 ± 8 % of control, from 70 ± 9 pA to 78 ± 6 pA, Figure 3C). Since WIN 55212-2 has been reported previously to have non-cannabinoid receptor mediated effects (Pertwee, 2006), a more selective cannabinoid receptor agonist, methanandamide, was also tested in the absence and presence of the antagonist. Example traces of recorded mIPSCs before and during application of methanandimide (3 μM) in the absence and presence of the antagonist AM 251 are shown in Figure 4A. As with WIN 55212-2, methanandamide significantly reduced the mIPSC frequency (75 ± 9 % of control, from 1.5 ± 0.6 Hz to 1.2 ± 0.6 Hz, n=7, Figure 4B), and the selective CB1R antagonist blocked this reduction (100 ± 10 % of control, from 1.5 ± 0.5 Hz to 1.5 ± 0.5 Hz, n=7, Figure 4B). Unlike WIN 55212-2, methanandamide did not increase mIPSC frequency in the presence of the CB1R antagonist, confirming that the increase in mIPSC frequency from WIN 55212-2 was not due to CB1R. Methanandamide had no significant effect on the mIPSC amplitude in the absence (106 ± 4 % of control, from 66 ± 14 pA to 70 ± 15 pA, Figure 4C) and presence of the antagonist (98 ± 3 % of control, from 74 ± 17 pA to 74 ± 19 pA, Figure 4C). These data suggest that activation of presynaptic CB1Rs inhibits GABA release.
Figure 3.
The CB1R antagonist blocked the WIN 55212-2-induced disinhibition. A. Example traces of mIPSCs recorded before (control) and during application of WIN 55212-2 (3 μM) from one cell in the absence (aCSF) and from another cell in the presence of the CB1R antagonist (AM 251, 5 μM). Group data of mIPSC frequency (B) and amplitude (C) in response to exogenous activation of CB1Rs with WIN 55212-2 (3 μM) in the absence (n=11) and presence (n=7) of the CB1R antagonist (AM 251, 5 μM). +p<0.05, control vs. during agonist; *p<0.05, WIN vs AM 251 + WIN.
Figure 4.
The CB1R antagonist blocked the methanandamide-induced disinhibition. A. Example trace of mIPSCs recorded before (control) and during application of methanandamide (3 μM) in the absence and presence of the CB1R antagonist (AM 251, 5 μM) from the same neuron. Group data of mIPSC frequency (B) and amplitude (C) in response to exogenous activation of CB1Rs with methanandamide (METH, 3 μM) in the absence (n = 7) and presence (n = 7) of the CB1R antagonist (AM 251, 5 μM). +p<0.05, control vs. during agonist; *p<0.05, METH vs AM 251 + METH.
Effect of depolarization of second-order baroreceptive neurons on mIPSCs
Depolarization of the identified second-order baroreceptive neuron resulted in a depolarization-dependent decrease in mIPSC frequency (two-way ANOVA; p<0.05 for postsynaptic membrane potential and interaction, Figure 5B), while having no effect on the mIPSC amplitude (Figure 5C). There was a 50% decrease in mIPSC frequency when the postsynaptic neurons were depolarized above 0 mV compared to that at the holding potential of −60 mV (Figure 5B). At the holding potential of −60 mV, the CB1R antagonist (AM 251, 5 μM) had no significant effect on either the mIPSC frequency (110 ± 7 % of control, from 1.8 ± 0.9 Hz to 2.0 ± 0.4 Hz, n=7) or the amplitude (106 ± 5 % of control, from 69 ± 8 pA to 73 ± 8 pA). However, the depolarization-dependent induced decrease in mIPSC frequency was blocked by the CB1R antagonist (Figure 5B). The data suggest that second-order baroreceptive neurons release ECBs upon depolarization that inhibit presynaptic GABA release via activation of the CB1Rs located on the GABAergic terminals. The lack of effect of AM 251 on mIPSC frequency or amplitude at a resting holding potential indicates that excitation of the second-order neuron is required to elicit ECB release, as has been reported for other brain regions (Freund et al., 2003).
Discussion
The present study examined neuronal responses in identified second-order baroreceptive neurons in the NTS. The major findings are: (1) exogenous application of cannabinoid receptor agonists decreased mIPSC frequency without changing mIPSC amplitude; (2) the agonist-induced decrease in mIPSC frequency was blocked by a selective CB1R antagonist, AM 251; (3) direct postsynaptic depolarization of second-order baroreceptive neurons decreased mIPSC frequency in a membrane potential-dependent manner; and (4) the depolarization-induced decrease in mIPSC frequency was blocked with the CB1R antagonist. The decrease in mIPSC frequency due to activation of CB1Rs without an associated increase in mIPSC amplitude is strong evidence that ECBs act presynaptically to reduce GABA release. These data suggest a mechanism whereby activation of second-order baroreceptive neurons elicits release of ECBs, leading to retrograde presynaptic inhibition of GABA release and DSI, or disinhibition. A number of mechanism(s) have been suggested to elicit the CB1R-mediated suppression of neurotransmitter release, including inhibition of voltage-gated calcium channels, activation of a number of potassium channels (KM, KA, and GIRK), and inhibition of release machinery (Chevaleyre et al., 2006; Schlicker et al., 2001). In the present study, we demonstrated ECB-mediated disinhibition in the presence of tetrodotoxin, suggesting that presynaptic CB1Rs may directly interact with the release machinery to reduce GABA release in NTS.
The finding that depolarization-dependent activation of CB1R produces DSI/disinhibition is consistent with our previous report that brief increases in blood pressure increased anandamide content in the NTS (Seagard et al., 2004). Both studies indicate that excitation of second-order baroreceptive neurons in the NTS could result in depolarization-induced release of ECBs. We have focused on CB1R presynaptic inhibition of GABA release in this study because in in vivo studies demonstrate that CB1R activation in the NTS increases discharge of baroreceptive neurons and prolongs baroreflex-induced sympathoinhibition, both of which are consistent with increased excitatory drive (Brozoski et al., 2009; Brozoski et al., 2005; Seagard et al., 2005). This retrograde action of ECBs from the postsynaptic neurons provides a mechanism for activity-dependent modulation of inhibitory synaptic transmission in the NTS.
At a holding membrane potential of −60 mV, AM 251 alone had no significant effect on mIPSC frequency and amplitude. The data suggest that CB1R does not play a significant role in shaping GABAergic transmission at this postsynaptic membrane potential; a more depolarized membrane potential is required for endogenous activation of CB1R. In this regard, the resting membrane potential of the NTS neurons ranges from −40 to −80 mV and averages between −50 and −60 mV ((Barnes et al., 2003; Johnson et al., 1993; Kalia et al., 1993; Miura et al., 1979; Paton et al., 1993; Silva-Carvalho et al., 1995). Together, the data suggest that the functional role of ECB-induced DSI in modulating baroreflex function is minimal under resting conditions. This modulatory role would be enhanced with increases in excitatory input into second-order NTS neurons, such as during acute increase in blood pressure.
ECB/CB1R-mediated disinhibition in the NTS could provide a feed-forward mechanism to enhance baroreflex function by maintaining longer excitation of NTS baroreceptive neurons after initial activation. This type of potentiation of reflex control could be important following acute repeated or exaggerated increases in blood pressure, thereby enhancing the animal’s ability to maintain pressure control in the face of a significant acute challenge. Interestingly, ECB-mediated prolongation of sympathoinhibition is significantly dampened in spontaneously hypertensive rats (SHRs), which have chronically elevated pressure, and thus high amounts of baroreceptor stimulation (Brozoski et al., 2009). Coincident with this result was the finding that NTS CB1R binding sites were significantly reduced in SHRs compared to normotensive rats (Brozoski et al., 2009). Therefore, it is possible that the elevated sympathetic tone and enhanced GABAergic function in the NTS of SHRs (Tsukamoto et al., 1993; Zhang et al., 2007) could be due, in part, to the loss of ECB-mediated inhibition of GABA release. It is not known whether the change in ECB signaling in this model is a cause and/or consequence of the hypertension.
There are two major subtypes of cannabinoid receptors: CB1R and cannabinoid-2 receptor (CB2R). CB1Rs are located predominantly on neurons and nerve terminals and CB2Rs occur mainly on immune cells (Hashimotodani et al., 2007; Pertwee, 2006). This study showed that a selective CB1R antagonist blocked DSI and inhibition of GABA transmission induced by a cannabinoid receptor agonist, indicating that CB2Rs do not play a significant role in modulating GABA transmission in the NTS. Consistent with our findings is a recent study showing a dense punctuate pattern of CB1R expression in the NTS, indicative of presynaptic elements, while CB2R was found in vascular-appearing structures, but not in neuronal elements (Ray et al., 2009).
WIN 55212-2, but not the more selective CB1R agonist, methanandamide, actually increased mIPSC frequency in the presence of CB1R blockage. It has been reported that some of the cannabinoid receptor agonists, including WIN 55212-2, are capable of modulating neurotransmitter release via non-CB1, non-CB2, and non-TRVP1 receptors (Pertwee, 2006). Nevertheless, the CB1R antagonist blocked the selective agonist-induced decreases in mIPSC frequency, suggesting a role for CB1R in modulating inhibitory synaptic transmissions. Related to this finding, Accorsi-Mendonca and colleagues (Accorsi-Mendonca et al., 2008) showed that the CB1R and CB2R antagonists had no effect on the WIN 55212-2-induced decreases in spontaneous synaptic currents (a mixture of excitatory and inhibitory synaptic currents). The reason for the discrepancy between the previous and current studies is not known, but could be related to several factors. The current study examined the effects of WIN 55212-2 on anatomically identified second-order baroreceptive neurons in the NTS, while the previous study examined unidentified NTS neurons. A possible selective effect of CB1R activation of baroreceptive NTS neurons is consistent with our previous study showing that picoejection of a cannabinoid receptor agonist or ECB uptake inhibitor increased discharge in 85% of the identified baroreceptive NTS neurons (Seagard et al., 2005), while an earlier study by Himmi and colleagues (Himmi et al., 1998) showed that only 28% of unidentified NTS neurons increased discharge rate in response to another cannabinoid receptor ligand, Δ9-tetrahydrocannabinol. Together, the data suggest that ECB-mediated DSI is preferentially expressed at NTS neurons within the baroreflex pathway. CB1R antagonists block the GABAergic effects of WIN 55212-2 and methanandamide (present study) and ECB-mediated NTS effects we have found previously have all been blocked by CB1R antagonists (Brozoski et al., 2009; Seagard et al., 2004; Seagard et al., 2005), suggesting that presynaptic inhibition of GABA related to baroreflex activation is mediated primarily by activation of CB1Rs. Finally, we used older rats (13 weeks old) than Accorsi-Mendonca, et al. (22-30 days old), thus results could be affected by developmental changes in cannabinoid receptor expression.
In conclusion, this study demonstrates that activation of CB1Rs in the NTS results in presynaptic inhibition of GABA release, leading to disinhibition of second-order baroreceptive neurons. Although we have not completely characterized the system, our data suggest that ECB-mediated DSI occurs at these neurons. Further, these data are consistent with the hypothesis that ECB-mediated DSI contributes to the prolonged baroreflex sympathoinhibition seen in live animals (Brozoski et al., 2009; Seagard et al., 2004). The findings provide evidence supporting the hypothesis that input into the second-order baroreceptive neuron is shaped by activity and that this mechanism could contribute to exaggerated or prolonged increases in blood pressure.
Acknowledgements
This work is supported by VA Medical Research Funds (J.L.S.), Advancing a Healthier Wisconsin Award from the Medical College of Wisconsin (J.L.S.), American Heart Grant-in-Aid 0750010Z (C.D.), NSF Award IOS 0751613 (C.D.) and NIDA Award DA09155 (C.J.H.). We thank Dr. Betty Guo for editorial assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Accorsi-Mendonca D, Almado CE, Dagostin AL, Machado BH, Leao RM. Inhibition of spontaneous neurotransmission in the nucleus of solitary tract of the rat by the cannabinoid agonist WIN 55212-2 is not via CB1 or CB2 receptors. Brain Res. 2008;1200:1–9. doi: 10.1016/j.brainres.2008.01.011. [DOI] [PubMed] [Google Scholar]
- Andresen MC, Kunze DL. Nucleus tractus solitarius--gateway to neural circulatory control. Annu Rev Physiol. 1994;56:93–116. doi: 10.1146/annurev.ph.56.030194.000521. [DOI] [PubMed] [Google Scholar]
- Bailey TW, Appleyard SM, Jin YH, Andresen MC. Organization and properties of GABAergic neurons in solitary tract nucleus (NTS) J Neurophysiol. 2008;99:1712–1722. doi: 10.1152/jn.00038.2008. [DOI] [PubMed] [Google Scholar]
- Barnes KL, DeWeese DM, Andresen MC. Angiotensin potentiates excitatory sensory synaptic transmission to medial solitary tract nucleus neurons. Am J Physiol Regul Integr Comp Physiol. 2003;284:R1340–1353. doi: 10.1152/ajpregu.00505.2002. [DOI] [PubMed] [Google Scholar]
- Bonham AC, Chen CY. Glutamatergic neural transmission in the nucleus tractus solitarius: N-methyl-D-aspartate receptors. Clin Exp Pharmacol Physiol. 2002;29:497–502. doi: 10.1046/j.1440-1681.2002.03662.x. [DOI] [PubMed] [Google Scholar]
- Brozoski DT, Dean C, Hopp FA, Hillard CJ, Seagard JL. Differential endocannabinoid regulation of baroreflex-evoked sympathoinhibition in normotensive versus hypertensive rats. Auton Neurosci. 2009;150:82–93. doi: 10.1016/j.autneu.2009.05.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brozoski DT, Dean C, Hopp FA, Seagard JL. Uptake blockade of endocannabinoids in the NTS modulates baroreflex-evoked sympathoinhibition. Brain Res. 2005;1059:197–202. doi: 10.1016/j.brainres.2005.08.030. [DOI] [PubMed] [Google Scholar]
- Chen CY, Bechtold AG, Tabor J, Bonham AC. Exercise reduces GABA synaptic input onto nucleus tractus solitarii baroreceptor second-order neurons via NK1 receptor internalization in spontaneously hypertensive rats. J Neurosci. 2009;29:2754–2761. doi: 10.1523/JNEUROSCI.4413-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CY, Bonham AC. Glutamate suppresses GABA release via presynaptic metabotropic glutamate receptors at baroreceptor neurones in rats. J Physiol. 2005;562:535–551. doi: 10.1113/jphysiol.2004.076885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevaleyre V, Takahashi KA, Castillo PE. Endocannabinoid-mediated synaptic plasticity in the CNS. Annu Rev Neurosci. 2006;29:37–76. doi: 10.1146/annurev.neuro.29.051605.112834. [DOI] [PubMed] [Google Scholar]
- Freund TF, Katona I, Piomelli D. Role of endogenous cannabinoids in synaptic signaling. Physiol Rev. 2003;83:1017–1066. doi: 10.1152/physrev.00004.2003. [DOI] [PubMed] [Google Scholar]
- Glatzer NR, Smith BN. Modulation of synaptic transmission in the rat nucleus of the solitary tract by endomorphin-1. J Neurophysiol. 2005;93:2530–2540. doi: 10.1152/jn.00429.2004. [DOI] [PubMed] [Google Scholar]
- Gordon FJ, Sved AF. Neurotransmitters in central cardiovascular regulation: glutamate and GABA. Clin Exp Pharmacol Physiol. 2002;29:522–524. doi: 10.1046/j.1440-1681.2002.03666.x. [DOI] [PubMed] [Google Scholar]
- Hashimotodani Y, Ohno-Shosaku T, Kano M. Endocannabinoids and synaptic function in the CNS. Neuroscientist. 2007;13:127–137. doi: 10.1177/1073858406296716. [DOI] [PubMed] [Google Scholar]
- Himmi T, Perrin J, El Ouazzani T, Orsini JC. Neuronal responses to cannabinoid receptor ligands in the solitary tract nucleus. Eur J Pharmacol. 1998;359:49–54. doi: 10.1016/s0014-2999(98)00630-x. [DOI] [PubMed] [Google Scholar]
- Johnson SM, Felder RB. Effects of aging on the intrinsic membrane properties of medial NTS neurons of Fischer-344 rats. J Neurophysiol. 1993;70:1975–1987. doi: 10.1152/jn.1993.70.5.1975. [DOI] [PubMed] [Google Scholar]
- Kalia M, Schweitzer P, Champagnat J, Denavit-Saubie M. Two distinct phases characterize maturation of neurons in the nucleus of the tractus solitarius during early development: morphological and electrophysiological evidence. J Comp Neurol. 1993;327:37–47. doi: 10.1002/cne.903270104. [DOI] [PubMed] [Google Scholar]
- Ketch T, Biaggioni I, Robertson R, Robertson D. Four faces of baroreflex failure: hypertensive crisis, volatile hypertension, orthostatic tachycardia, and malignant vagotonia. Circulation. 2002;105:2518–2523. doi: 10.1161/01.cir.0000017186.52382.f4. [DOI] [PubMed] [Google Scholar]
- McDougall SJ, Bailey TW, Mendelowitz D, Andresen MC. Propofol enhances both tonic and phasic inhibitory currents in second-order neurons of the solitary tract nucleus (NTS) Neuropharmacology. 2008;54:552–563. doi: 10.1016/j.neuropharm.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura M, Kitamura T. Postsynaptic potentials recorded from medullary neurones following stimulation of carotid sinus nerve. Brain Res. 1979;162:261–272. doi: 10.1016/0006-8993(79)90288-9. [DOI] [PubMed] [Google Scholar]
- Paton JF, Foster WR, Schwaber JS. Characteristic firing behavior of cell types in the cardiorespiratory region of the nucleus tractus solitarii of the rat. Brain Res. 1993;604:112–125. doi: 10.1016/0006-8993(93)90358-t. [DOI] [PubMed] [Google Scholar]
- Pertwee RG. The pharmacology of cannabinoid receptors and their ligands: an overview. Int J Obes (Lond) 2006;30(Suppl 1):S13–18. doi: 10.1038/sj.ijo.0803272. [DOI] [PubMed] [Google Scholar]
- Ray AP, Griggs L, Darmani NA. Delta 9-tetrahydrocannabinol suppresses vomiting behavior and Fos expression in both acute and delayed phases of cisplatin-induced emesis in the least shrew. Behav Brain Res. 2009;196:30–36. doi: 10.1016/j.bbr.2008.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlicker E, Kathmann M. Modulation of transmitter release via presynaptic cannabinoid receptors. Trends Pharmacol Sci. 2001;22:565–572. doi: 10.1016/s0165-6147(00)01805-8. [DOI] [PubMed] [Google Scholar]
- Seagard JL, Dean C, Hopp FA. Properties of NTS neurons receiving input from barosensitive receptors. Ann N Y Acad Sci. 2001;940:142–156. doi: 10.1111/j.1749-6632.2001.tb03673.x. [DOI] [PubMed] [Google Scholar]
- Seagard JL, Dean C, Patel S, Rademacher DJ, Hopp FA, Schmeling WT, Hillard CJ. Anandamide content and interaction of endocannabinoid/GABA modulatory effects in the NTS on baroreflex-evoked sympathoinhibition. Am J Physiol Heart Circ Physiol. 2004;286:H992–1000. doi: 10.1152/ajpheart.00870.2003. [DOI] [PubMed] [Google Scholar]
- Seagard JL, Hopp FA, Hillard CJ, Dean C. Effects of endocannabinoids on discharge of baroreceptive NTS neurons. Neurosci Lett. 2005;381:334–339. doi: 10.1016/j.neulet.2005.02.044. [DOI] [PubMed] [Google Scholar]
- Silva-Carvalho L, Dawid-Milner MS, Goldsmith GE, Spyer KM. Hypothalamic modulation of the arterial chemoreceptor reflex in the anaesthetized cat: role of the nucleus tractus solitarii. J Physiol. 1995;487(Pt 3):751–760. doi: 10.1113/jphysiol.1995.sp020915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spyer KM. The central nervous organization of reflex circulatory control. In: Loewy ADS, editor. Central regulation of autonomic functions. Oxford University Press; New York: 1990. pp. 168–188. K. M. [Google Scholar]
- Tsukamoto K, Sved AF. Enhanced gamma-aminobutyric acid-mediated responses in nucleus tractus solitarius of hypertensive rats. Hypertension. 1993;22:819–825. doi: 10.1161/01.hyp.22.6.819. [DOI] [PubMed] [Google Scholar]
- Zhang J, Mifflin SW. Influences of excitatory amino acid receptor agonists on nucleus of the solitary tract neurons receiving aortic depressor nerve inputs. J Pharmacol Exp Ther. 1997;282:639–647. [PubMed] [Google Scholar]
- Zhang W, Herrera-Rosales M, Mifflin S. Chronic hypertension enhances the postsynaptic effect of baclofen in the nucleus tractus solitarius. Hypertension. 2007;49:659–663. doi: 10.1161/01.HYP.0000253091.82501.c0. [DOI] [PubMed] [Google Scholar]