Abstract
Background
Previous studies describe decreased prostate cancer risk in HIV-infected men. In the U.S., prostate-specific antigen (PSA) screening is common and increases detection of prostate cancer. We evaluated whether the prostate cancer deficit among men with AIDS reflects differential PSA screening.
Methods
Data from the U.S. HIV/AIDS Cancer Match Study were used to calculate standardized incidence ratios (SIRs) for prostate cancer, comparing men with AIDS (N=287,247) to the general population. Further, we estimated PSA testing rates in the Johns Hopkins HIV Clinical Cohort.
Results
Prostate cancer rates increased over time in the general population and beginning in the 1990s were consistently higher than among men with AIDS. Men with AIDS had the same prostate cancer risk as the general population in the pre-PSA era (<1992, SIR=1.00), but significantly reduced risk during the PSA era overall (1992–2007, SIR=0.50) and across age, race, HIV risk group, antiretroviral therapy era, and CD4 counts. Local and regional stage prostate cancer risk was lower among men with AIDS (SIRs 0.49 and 0.14, respectively), but distant stage cancer risk did not differ (SIR=0.85). Among HIV-infected men ≥40 years old, PSA testing was uncommon (18.7% per year), but increased 2.4-fold from 2000 to 2008, after age adjustment.
Conclusion
Prostate cancer risk was decreased by 50% among men with AIDS compared to the general population. This deficit was limited to the PSA era and early stage cancers.
Impact
Our findings suggest that the prostate cancer deficit in HIV-infected men is largely due to differential PSA screening.
Keywords: Prostate cancer, HIV, PSA, AIDS, screening
Introduction
People with human immunodeficiency virus (HIV) infection are at an increased risk of certain cancers, including both acquired immune deficiency syndrome (AIDS)-defining conditions (i.e., Kaposi sarcoma, non-Hodgkin lymphoma and invasive cervical cancer) and non-AIDS-defining cancers (e.g., anal cancer, lung cancer and Hodgkin lymphoma) (1–6). In contrast, several epidemiologic studies have shown a significant inverse association between HIV and prostate cancer risk (2, 3, 5, 7–12). Two meta-analyses reported an overall 30% reduction in prostate cancer risk in HIV-infected men compared to the general population (5, 6).
The reason for the prostate cancer deficit in HIV-infected men is unclear. It has been hypothesized that HIV infection may prevent development of prostate cancer through reductions in androgen levels (3, 6). Alternatively, the deficit may be an artifact driven by low rates of screening with the prostate-specific antigen (PSA) test among HIV-infected men (3, 6, 12).
The U.S. Food and Drug Administration (FDA) approved the use of the PSA test for the monitoring of men with prostate cancer in 1986 and for the diagnosis of prostate cancer in 1992 (13). Subsequent widespread PSA screening in the general U.S. population resulted in a dramatic rise in prostate cancer incidence due to the diagnosis of early stage prostate cancers that were not clinically detectable (14). According to data from the 2000 National Health Interview Survey, 57% of U.S. men age 50 years or older had previously had a PSA test (15). Currently, there are no published estimates of the prevalence of PSA screening among HIV-infected men. However, if the prevalence of PSA screening in HIV-infected men is substantially lower than the U.S. general population, a corresponding deficit in prostate cancer would be anticipated.
In this study, we explored the epidemiology of prostate cancer among HIV-infected men in the U.S. Using data from the HIV/AIDS Cancer Match (HACM) Study, we evaluated prostate cancer risk among men with AIDS, both overall and according to demographic subgroups and cancer stage, to assess whether the patterns observed were consistent with the hypothesis that any deficit in prostate cancers among men with HIV is driven by differential rates of PSA screening. Additionally, we assessed the effect of prostate cancer on survival among men with AIDS. Finally, because nationally representative data on PSA screening among HIV-infected men were not available, we evaluated the frequency of PSA testing in an urban cohort of HIV-infected men in Baltimore, Maryland.
Methods
Our analyses used data from the HACM Study, a linkage of population-based HIV/AIDS and cancer registries in 15 U.S. areas (8). A probabilistic algorithm is used to match records based on name, Social Security number, sex, birth date, death date, and race. Only anonymized data are retained by investigators. The study was approved by institutional review boards at each of the participating registries.
This study included men with AIDS who contributed follow-up from the latter of the start of registry coverage or 4 months after AIDS onset to the earliest of death, the end of complete registry coverage or 60 months after AIDS onset. The 0–3 month period after AIDS diagnosis was excluded from this analysis, because increased medical surveillance at the time of AIDS diagnosis artificially inflates cancer rates during this time period (16). Prostate cancers were identified using cancer registry data, specified according to International Classification of Diseases for Oncology (ICD-O-3) topography code C619 (17). Stage was coded by cancer registries using Surveillance, Epidemiology and End Results (SEER) Program summary stage algorithms.
To estimate the frequency of PSA testing among HIV-infected men, we used data from the Johns Hopkins HIV Clinical Cohort (JHHCC) during 2000–2008. The JHHCC was established in 1989 as a longitudinal study of HIV-infected patients who receive care from the Johns Hopkins AIDS Service (18). JHHCC participants are primarily low income African Americans living in Baltimore, Maryland. Baseline and follow-up demographic characteristics, medical history, and laboratory test results are collected. Information on PSA testing obtained by clinicians was abstracted from electronic medical records. Of note, we were unable to determine if PSA tests were utilized for screening or if they were instead obtained for clinical indication (e.g., urinary obstructive symptoms). Thus, the rate of PSA testing in the JHHCC overestimates PSA screening rates in asymptomatic men.
Statistical analysis
Using HACM Study data, we examined prostate cancer risk in two calendar periods: the era prior to FDA approval of PSA testing for prostate cancer diagnosis (pre-PSA era: 1980–1991), and the PSA era (1992–2007). To compare the risk of prostate cancer in men with AIDS to the general population, we estimated standardized incidence ratios (SIRs) for each attained calendar period. SIRs were calculated as the number of observed cases divided by the number of expected cases, where expected cases were based on prostate cancer incidence rates from cancer registry data and standardized by age, race, calendar year and registry to the AIDS population. Additionally, we present prostate cancer rates directly standardized to the 2000 U.S. general population by age (5-year categories) and race.
We limited additional sub-analyses to person-time contributed during the PSA era (1992–2007). Specifically, prostate cancer incidence rates in the PSA era were calculated across demographic characteristics, antiretroviral therapy calendar period (pre-highly active antiretroviral therapy [HAART] era: 1992–1995, early HAART era: 1996–1999, and late HAART era: 2000–2007), and CD4 count at AIDS diagnosis, and rate ratios (RRs) were estimated with Poisson regression. We calculated SIRs within these strata, using expected cases estimated from rates in the general population, standardized by age, race, calendar year, and registry. Further, to examine whether a deficit in prostate cancers in the PSA era reflected screening, we calculated an additional SIR for the PSA era that used pre-PSA historical cancer rates from the SEER program (1985–1987) for comparison, standardized by age and race. This time period has been used previously to examine prostate cancer rates in the absence of screening, as PSA screening rates in U.S. men began to increase as early as 1988, prior to FDA approval for that purpose (19). For all SIRs, exact two-sided confidence intervals (CIs) were calculated.
Finally, we used two approaches to assess whether men with AIDS were diagnosed later with prostate cancer than men in the general population, as well as the effects of cancer diagnosis on survival. First, for men with AIDS during the PSA era, we calculated SIRs for prostate cancer separately according to stage at cancer diagnosis (local, regional, distant and unknown stage), where a delay in diagnosis would translate into an elevated risk of advanced stage cancer. Second, proportional hazards regression models were used to evaluate the association between prostate cancer and survival among men with AIDS in the PSA era. Men were followed from 4 months after AIDS diagnosis until the earliest of death, the end of available cancer registry data, or 10 years of follow-up. Hazard ratios (HRs) for death from all causes were estimated for any diagnosis of prostate cancer, and for localized/regional, distant, and unknown stage prostate cancers. In these models, we treated the diagnosis of prostate cancer and antiretroviral therapy calendar period as time-dependent covariates. Regression models were additionally adjusted for age at AIDS onset, race and HIV transmission risk group.
JHHCC PSA testing rates were calculated by calendar year and 5-year age group. Poisson regression was used to estimate changes in PSA testing rates over time, adjusting for age. Further, we directly standardized PSA testing rates by age to the 2000 U.S. population (men ≥40 years old) for comparison with general population rates.
Results
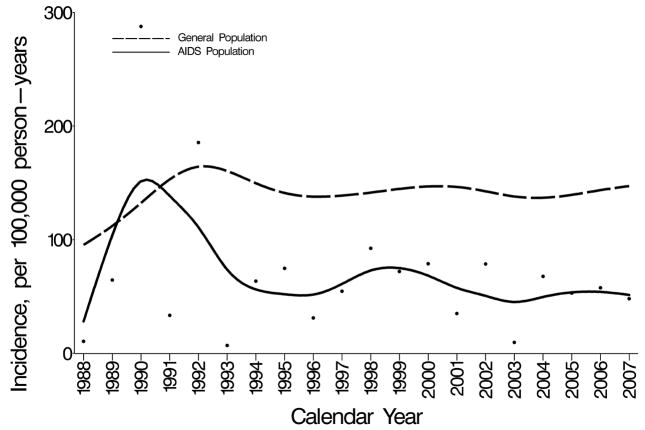
Figure 1 shows incidence rates of prostate cancer in men with AIDS and in the general population, standardized by age and race to the 2000 U.S. general population. From 1988–1992, rates overlapped in the AIDS and general populations; however, rates were unstable and based on few cases in men with AIDS. Prostate cancer incidence increased during this time in the U.S. general population, and after 1992 rates were consistently higher than in men with AIDS.
Figure 1. Prostate cancer incidence among men in the U.S. AIDS and general populations.
Incidence rates in men with AIDS (points), smoothed incidence rates in men with AIDS (solid line), and smoothed incidence rates in men in the general population (dashed line) by calendar year. Incidence rates among men with AIDS and in the general population are age- and race-standardized to the 2000 U.S. population. Data are not presented prior to 1988, because only 3 prostate cancer cases occurred among men with AIDS during 1980–1987.
SIRs confirmed that men with AIDS had the same prostate cancer risk as the general population in the pre-PSA era (SIR=1.00; 95% CI 0.58–1.60, based on n=17 prostate cancer cases among 90,803 men followed during 1980–1991), but they had a significantly reduced risk during the PSA era (SIR=0.50; 95% CI 0.44–0.57, based on n=230 cases among 287,247 men followed during 1992–2007). However, when compared to pre-PSA historical rates (1985–1987), men with AIDS during the PSA era had a modestly increased risk of prostate cancer (SIR=1.17; 95% CI 1.02–1.33).
Among men with AIDS followed during the 1992–2007 PSA era, prostate cancer incidence was 28.3 per 100,000 person-years (Table 1). Prostate cancer risk increased steeply with age, and was highest in black men (incidence RR 2.13; 95% CI 1.59–2.84 compared to white men), and among men in “other/unknown” risk groups (RR: 3.11; 95% CI 1.34–4.13, compared to men who have sex with men). Further, the risk of prostate cancer increased with increasing CD4 cell counts at AIDS (RR=1.54; 95% CI 1.05–2.26, comparing CD4 counts ≥200 cells/mm3 to <50 cells/mm3), and was significantly greater during the early HAART (RR=1.72; 95% CI 1.16–2.56) and late HAART calendar periods (RR=3.12; 95% CI 2.12–4.59), compared to the pre-HAART calendar period.
Table 1.
Prostate cancer risk among men with AIDS during the PSA era (U.S., 1992–2007).
| Observed Cases | Incidence Rate per 100,000 | Rate Ratio | 95% CI | Standardized Incidence Ratio | 95% CI | |
|---|---|---|---|---|---|---|
| Overall | 230 | 28.4 | --- | --- | 0.50 | (0.44–0.57) |
| Age Group years | ||||||
| 15–39 | 3 | 0.8 | 1.0 | 2.06 | (0.43–6.03) | |
| 40–49 | 18 | 6.3 | 0.31 | (0.19–0.50) | ||
| 50–59 | 96 | 104.7 | 34.2 | (21.3–54.9) | 0.50 | (0.41–0.61) |
| 60–69 | 96 | 438.0 | 143.2 | (89.3–229.6) | 0.59 | (0.48–0.72) |
| 70–79 | 16 | 465.7 | 152.2 | (79.4–291.8) | 0.41 | (0.23–0.66) |
| 80+ | 1 | 273.6 | 89.5 | (12.0–665.1) | 0.32 | (0.01–1.81) |
| P-trend<0.001 | ||||||
| Race | ||||||
| White | 71 | 21.1 | 1.0 | 0.50 | (0.39–0.63) | |
| Black | 134 | 44.8 | 2.13 | (1.59–2.84) | 0.53 | (0.44,0.63) |
| Hispanic | 25 | 14.3 | 0.68 | (0.43–1.07) | 0.41 | (0.27–0.61) |
| Risk Group | ||||||
| MSM | 94 | 21.8 | 1.0 | 0.52 | (0.42–0.64) | |
| IDU | 30 | 17.1 | 0.79 | (0.52–1.19) | 0.29 | (0.20–0.42) |
| MSM/IDU | 9 | 15.2 | 0.70 | (0.35–1.38) | 0.55 | (0.25–1.04) |
| Other/unknown | 97 | 67.7 | 3.11 | (2.34–4.13) | 0.62 | (0.50–0.75) |
| Antiretroviral Therapy Era | ||||||
| Pre-HAART (1992–1995) | 34 | 14.8 | 1.0 | 0.42 | (0.29–0.59) | |
| Early HAART (1996–1999) | 87 | 25.4 | 1.72 | (1.16–2.56) | 0.48 | (0.39–0.59) |
| Late HAART (2000–2007) | 109 | 46.1 | 3.12 | (2.12–4.59) | 0.56 | (0.46–0.68) |
| P-trend<0.001 | ||||||
| CD4 count at AIDS | ||||||
| 0–49 cells/mm3 | 74 | 22.7 | 1.0 | 0.43 | (0.34–0.54) | |
| 50–99 cells/mm3 | 27 | 27.6 | 1.27 | (0.78–1.89) | 0.47 | (0.31–0.68) |
| 100–199 cells/mm3 | 88 | 32.8 | 1.45 | (1.06–1.97) | 0.57 | (0.46–0.71) |
| 200+ cells/mm3 | 41 | 35.0 | 1.54 | (1.05–2.26) | 0.56 | (0.41–0.77) |
| P-trend=0.01 | ||||||
When compared to the general population, a significant deficit in prostate cancer risk was observed in 40–79 year-olds (SIRs 0.31–0.59) but not in younger men. Deficits were observed across HIV transmission risk groups, with the greatest deficit observed among injection drug users (SIR=0.29). Further, prostate cancer risk was significantly decreased across racial groups, antiretroviral calendar periods, and regardless of CD4 cell count at AIDS diagnosis, although the deficit appeared to weaken with increasing CD4 count.
When considered by prostate cancer stage, the reduction in risk was limited to local stage (SIR=0.49; 95% CI 0.42–0.57) and regional stage (SIR=0.14; 95% CI 0.06–0.27) disease. Risk of distant stage prostate cancer was similar between men with AIDS and men in the general population (SIR=0.85; 95% CI 0.50–1.34).
Among men with AIDS followed during the PSA era, prostate cancer was associated with a 34% increase in subsequent all-cause mortality (adjusted HR=1.34; 95% CI 1.05–1.71). More specifically (Table 2), local/regional stage prostate cancer was not associated with death (HR =0.93), but distant stage prostate cancer was associated with elevated mortality (HR=4.73). Prostate cancer with an unknown stage at diagnosis was also associated with an increased risk of death (HR=2.16).
Table 2.
Mortality among men with AIDS and prostate cancer (U.S., 1992–2007).
| N | Mortality Rate per 1,000 | Hazard ratio* | 95% CI | |
|---|---|---|---|---|
| No prostate cancer | 100,228 | 64 | 1.0 | |
| Prostate cancer, overall | 224 | 120 | 1.34 | (1.05–1.71) |
| Prostate cancer, by stage | ||||
| Local/regional stage | 13 | 74 | 0.93 | (0.66–1.30) |
| Distant stage | 24 | 645 | 4.73 | (2.80–7.98) |
| Unknown stage | 36 | 269 | 2.16 | (1.34–3.47) |
Hazard ratios were adjusted for age at AIDS onset, race, HIV transmission risk group, and antiretroviral therapy calendar period.
During 2000–2008, 1,216 PSA tests were performed among men in the JHHCC who were age 40 years and older, corresponding to a rate of 18.7% per year (6,488 person-years of follow-up). Over the full time period, the rate of testing was highest in 60–69 year olds (37.9% per year), followed by 50–59 year olds (28.0% per year) and 40–49 year olds (9.5% per year). There was a significant increase in PSA testing rates during 2000–2008 (p-trend<0.0001), with rates more than doubled in 2008 compared to 2000 (rate ratio: 2.4; 95% CI 1.9, 3.2). The age-standardized rate of PSA screening for 2000–2008 was 22.7% per year overall, increasing from 17.1% per year in 2000 to 29.7% per year in 2008.
Discussion
In our study, men with AIDS appeared to have 50% lower risk of prostate cancer than men in the general population, which is consistent with previous studies that have also observed deficits in prostate cancer in HIV-infected men compared to the general population (2, 3, 5, 7–11). In the general U.S. population, PSA screening among men is very common (20) and has contributed to a steep rise in prostate cancer incidence (14). In contrast, we observed lower rates of PSA testing in a cohort of low income HIV-infected men in Baltimore, suggesting that PSA testing may be less common in men with HIV. Although previous studies have proposed biological explanations for the reduced risk of prostate cancer in men with AIDS (3, 6), our results suggest this deficit may be largely driven by differential PSA screening rates in the AIDS and general populations.
PSA screening patterns strongly determine diagnosed rates of prostate cancer. In the U.S., prostate cancer rates began to increase following the identification of PSA as a marker of prostate cancer in 1987 (21) and peaked in 1992 (22). Though rates have been stable since 1995, they remain substantially elevated in comparison to rates in 1986 (22). It has been estimated that approximately 40% of prostate cancers diagnosed among men in the U.S. are detected through screening (19). One study estimated that PSA screening has resulted in the diagnosis of prostate cancer in more than one million additional men in the last 20 years, the majority of whom were age 50 years or older (22). Further, an estimated 67% of all screen-detected prostate cancers are overdiagnoses, meaning that these cancers would never have developed into symptomatic cancers (23).
PSA screening may be more common among men in the U.S. general population than among HIV-infected men. In 2001, 57% of U.S. men age 40 years and older had a PSA test in the previous year (20). This estimate is much higher, for example, than the age-standardized PSA testing rate of 22.7% per year observed in the JHHCC. Further, our estimates provide an upper bound of PSA screening in the JHHCC, because our data captured some PSA tests that might have been obtained for clinical indications rather than for screening in asymptomatic men. We speculate that the low rates of screening reflect clinicians’ perception that the historically poor prognosis of HIV-infected men would limit the test’s utility, coupled with challenges in addressing other medical and social issues in this population. Men in the JHHCC were receiving medical care, and PSA screening rates could be even lower among other HIV-infected populations that lacked access to care, e.g., due to lack of medical insurance. Though annual PSA testing rates in the JHHCC cohort remained lower than the general population, there was a two-fold increase from 2000 to 2008. Given this pattern, it is likely that the rate of PSA testing was less frequent in the years preceding 2000.
Our results imply that prostate cancer rates have been lower among men with AIDS largely because of the dramatic increase in PSA screening in the general population. Specifically, the deficit in prostate cancer that we observed among men with AIDS was limited to the PSA era. Indeed, PSA era incidence rates of prostate cancer in men with AIDS were actually slightly higher than in the general population when we used comparison rates from before the mass uptake of PSA screening. The explanation for this small excess is unclear, but it could reflect limitations in our approach of using historical rates or residual confounding by other factors for which we could not control in the comparison.
Further arguing that screening patterns largely explain the overall reduced risk of prostate cancer, we observed a significant deficit in localized and regional stage prostate cancers among men with AIDS, but no difference for distant stage prostate cancer compared to the general population. If the deficit in prostate cancer risk had a biological explanation, such as lower androgen levels or a direct effect of HIV itself, one would have predicted uniformly reduced risk across prostate cancer stages. Screen-detected cancers are more likely to be early stage and asymptomatic compared to clinically detected cancers (24). Therefore, the disproportional deficit of localized/regional prostate cancer among men with AIDS adds support to the hypothesis that the difference is explained by low rates of screening. Likewise, the deficit in prostate cancer risk was greatest in groups that would have been least likely to have been screened, including injection drug users and men with very low CD4 cell counts. Additionally, we observed that the deficit in prostate cancers was seen across antiretroviral therapy calendar periods. Further arguing against a role of immune suppression, the risk of prostate cancer in immune suppressed transplant recipients is no different from the risk in the general population (5).
Of final note, relative survival following prostate cancer diagnosis in men with AIDS was no worse than in prostate cancer patients in the general population. In men with AIDS, localized/regional prostate cancer was not associated with an increased risk of death (Table 2), similar to what is observed in the general U.S. population (25). Even with lower rates of PSA screening, we did not find an elevated risk of advanced stage prostate cancer in men with AIDS, and the five-fold increase in mortality following distant stage prostate cancer in men with AIDS was similar to that reported for men in the general population (standardized mortality ratio=5.22) (25). Thus, while the public health benefits of PSA screening in the general population are still under debate (26, 27), our results on mortality following a prostate cancer diagnosis do not suggest that men with AIDS are being harmed by the relatively low screening rates.
The main strength of our study was the use of nationally representative data from the HACM Study, which includes men with AIDS in the U.S. over the duration of the AIDS epidemic and before and after introduction of PSA screening. The main limitation of our study was our inability to assess PSA screening prevalence patterns for a larger and more representative HIV population or directly assess prostate cancer incidence in relation to screening. Further, though novel, the PSA testing results presented here from the JHHCC should be interpreted with caution. The JHHCC includes HIV-infected men that are primarily African American and low income, and is not representative of all HIV-infected men in the U.S; thus, PSA testing rates presented here may not be generalizable to all men infected with HIV.
In conclusion, there is a deficit in prostate cancers among American HIV-infected men, which is limited to local and regional stage cancers that are most likely to be detected by PSA screening. A sharp increase in prostate cancer incidence in the general population has largely been driven by widespread use of PSA screening, a practice that appears to be less frequently utilized for HIV-infected men. Combined, these factors suggest that the deficit in prostate cancer observed among HIV-infected men is largely an artifact due to differential PSA screening practices, and is likely not due to a protective effect of HIV against the development of prostate cancer. Further, it appears that the lack of PSA screening in HIV-infected men has not resulted in an increased risk of advanced stage prostate cancer or death.
Acknowledgments
Research support: Intramural research program of the National Cancer Institute. The Johns Hopkins HIV Clinical Cohort is supported by the following National Institutes of Health grants: R01 DA11601, K24 DA00432, R01 AA16893.
This study was funded by the Intramural Research Program of the National Cancer Institute.
The authors thank the HIV/AIDS and cancer registry staff in Colorado; Connecticut; Florida; Georgia; Illinois; Massachusetts; Michigan; New Jersey; Texas; New York, New York; Los Angeles, San Diego, and San Francisco, California; Seattle, Washington; and Washington, D.C. We also thank Tim McNeel for database management.
Footnotes
Data from this manuscript were presented at the American Association for Cancer Research 101st Annual Meeting, Washington, D.C. (April 2010).
References
- 1.Dal Maso L, Polesel J, Serraino D, et al. Pattern of cancer risk in persons with AIDS in Italy in the HAART era. Br J Cancer. 2009;100:840–7. doi: 10.1038/sj.bjc.6604923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Engels EA, Pfeiffer RM, Goedert JJ, et al. Trends in cancer risk among people with AIDS in the United States 1980–2002. AIDS. 2006;20:1645–54. doi: 10.1097/01.aids.0000238411.75324.59. [DOI] [PubMed] [Google Scholar]
- 3.Patel P, Hanson DL, Sullivan PS, et al. Incidence of Types of Cancer among HIV-Infected Persons Compared with the General Population in the United States, 1992–2003. Ann Intern Med. 2008;148:728–36. doi: 10.7326/0003-4819-148-10-200805200-00005. [DOI] [PubMed] [Google Scholar]
- 4.Powles T, Robinson D, Stebbing J, et al. Highly active antiretroviral therapy and the incidence of non-AIDS-defining cancers in people with HIV infection. J Clin Oncol. 2009;27:884–90. doi: 10.1200/JCO.2008.19.6626. [DOI] [PubMed] [Google Scholar]
- 5.Grulich AE, van Leeuwen MT, Falster MO, Vajdic CM. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta-analysis. Lancet. 2007;370:59–67. doi: 10.1016/S0140-6736(07)61050-2. [DOI] [PubMed] [Google Scholar]
- 6.Shiels MS, Cole SR, Kirk GD, Poole C. A meta-analysis of the incidence of non-AIDS cancers in HIV-infected individuals. J Acquir Immune Defic Syndr. 2009;52:611–22. doi: 10.1097/QAI.0b013e3181b327ca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Engels EA, Biggar RJ, Hall HI, et al. Cancer risk in people infected with human immunodeficiency virus in the United States. Int J Cancer. 2008;123:187–94. doi: 10.1002/ijc.23487. [DOI] [PubMed] [Google Scholar]
- 8.Frisch M, Biggar RJ, Engels EA, Goedert JJ. Association of cancer with AIDS-related immunosuppression in adults. JAMA. 2001;285:1736–45. doi: 10.1001/jama.285.13.1736. [DOI] [PubMed] [Google Scholar]
- 9.Gallagher B, Wang Z, Schymura MJ, Kahn A, Fordyce EJ. Cancer incidence in New York State acquired immunodeficiency syndrome patients. Am J Epidemiol. 2001;154:95–104. doi: 10.1093/aje/154.6.544. [DOI] [PubMed] [Google Scholar]
- 10.Herida M, Mary-Krause M, Kaphan R, et al. Incidence of non-AIDS-defining cancers before and during the highly active antiretroviral therapy era in a cohort of human immunodeficiency virus-infected patients. J Clin Oncol. 2003;21:3447–53. doi: 10.1200/JCO.2003.01.096. [DOI] [PubMed] [Google Scholar]
- 11.Silverberg MJ, Chao C, Leyden WA, et al. HIV infection and the risk of cancers with and without a known infectious cause. AIDS. 2009;23:2337–45. doi: 10.1097/QAD.0b013e3283319184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Biggar RJ, Kirby KA, Atkinson J, McNeel TS, Engels E. Cancer risk in elderly persons with HIV/AIDS. J Acquir Immune Defic Syndr. 2004;36:861–8. doi: 10.1097/00126334-200407010-00014. [DOI] [PubMed] [Google Scholar]
- 13.Mariotto AB, Etzioni R, Krapcho M, Feuer EJ. Reconstructing PSA testing patterns between black and white men in the US from Medicare claims and the National Health Interview Survey. Cancer. 2007;109:1877–86. doi: 10.1002/cncr.22607. [DOI] [PubMed] [Google Scholar]
- 14.Potosky AL, Miller BA, Albertsen PC, Kramer BS. The role of increasing detection in the rising incidence of prostate cancer. JAMA. 1995;273:548–52. [PubMed] [Google Scholar]
- 15.Ross LE, Coates RJ, Breen N, et al. Prostate-specific antigen test use reported in the 2000 National Health Interview Survey. Prev Med. 2004;38:732–44. doi: 10.1016/j.ypmed.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 16.Goedert JJ, Cote TR, Virgo P, et al. Spectrum of AIDS-associated malignant disorders. Lancet. 1998;351:1833–9. doi: 10.1016/s0140-6736(97)09028-4. [DOI] [PubMed] [Google Scholar]
- 17.World Health Organization. International Classification of Diseases for Oncology. 3. Geneva: World Health Organization; 2000. [Google Scholar]
- 18.Moore RD. Understanding the clinical and economic outcomes of HIV therapy: the Johns Hopkins HIV clinical practice cohort. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;17 (Suppl 1):S38–S41. doi: 10.1097/00042560-199801001-00011. [DOI] [PubMed] [Google Scholar]
- 19.Draisma G, Etzioni R, Tsodikov A, et al. Lead time and overdiagnosis in prostate-specific antigen screening: importance of methods and context. J Natl Cancer Inst. 2009;101:374–83. doi: 10.1093/jnci/djp001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sirovich BE, Schwartz LM, Woloshin S. Screening men for prostate and colorectal cancer in the United States: does practice reflect the evidence? JAMA. 2003;289:1414–20. doi: 10.1001/jama.289.11.1414. [DOI] [PubMed] [Google Scholar]
- 21.Stamey TA, Yang N, Hay AR, et al. Prostate-specific antigen as a serum marker for adenocarcinoma of the prostate. N Engl J Med. 1987;317:909–16. doi: 10.1056/NEJM198710083171501. [DOI] [PubMed] [Google Scholar]
- 22.Welch HG, Albertsen PC. Prostate cancer diagnosis and treatment after the introduction of prostate-specific antigen screening: 1986–2005. J Natl Cancer Inst. 2009;101:1325–9. doi: 10.1093/jnci/djp278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Welch HG, Black WC. Overdiagnosis in Cancer. J Natl Cancer Inst. 2010;102:1–9. doi: 10.1093/jnci/djq099. [DOI] [PubMed] [Google Scholar]
- 24.Vis AN, Roemeling S, Reedijk AM, Otto SJ, Schroder FH. Overall survival in the intervention arm of a randomized controlled screening trial for prostate cancer compared with a clinically diagnosed cohort. Eur Urol. 2008;53:91–8. doi: 10.1016/j.eururo.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 25.Surveillance Epidemiology and End Results [SEER] program. SEER*Stat Database: Incidence - SEER 9 Regs Limited-Use, Nov 2008 Sub [1973–2006] for SMRs - Link to County Attributes - Total U.S., 1969–2006 Counties. National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch; 2009. released July 2009, based on the November 2008 submission. [Google Scholar]
- 26.Andriole GL, Crawford ED, Grubb RL, III, et al. Mortality results from a randomized prostate-cancer screening trial. N Engl J Med. 2009;360:1310–9. doi: 10.1056/NEJMoa0810696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schroder FH, Hugosson J, Roobol MJ, et al. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med. 2009;360:1320–8. doi: 10.1056/NEJMoa0810084. [DOI] [PubMed] [Google Scholar]