Abstract
Portions of left inferior frontal cortex have been linked to semantic memory both in terms of the content of conceptual representation (e.g., motor aspects in an embodied semantics framework) and the cognitive processes used to access these representations (e.g., response selection). Progressive Nonfluent Aphasia (PNFA) is a neurodegenerative condition characterized by progressive atrophy of left inferior frontal cortex. PNFA can, therefore, provide a lesion model for examining the impact of frontal lobe damage on semantic processing and content. In the current study we examined picture naming in a cohort of PNFA patients across a variety of semantic categories. An embodied approach to semantic memory holds that sensorimotor features such as self-initiated action may assume differential importance for the representation of manufactured artifacts (e.g., naming hand tools). Embodiment theories might therefore predict that patients with frontal damage would be differentially impaired on manufactured artifacts relative to natural kinds, and this prediction was borne out. We also examined patterns of naming errors across a wide range of semantic categories and found that naming error distributions were heterogeneous. Although PNFA patients performed worse overall on naming manufactured artifacts, there was no reliable relationship between anomia and manipulability across semantic categories. These results add to a growing body of research arguing against a purely sensorimotor account of semantic memory, suggesting instead a more nuanced balance of process and content in how the brain represents conceptual knowledge.
Keywords: Semantic Memory, Progressive Nonfluent Aphasia, Category Specificity, Naming, Semantic Categories, Semantic Retrieval, Naming Errors, Embodied Cognition
1. INTRODUCTION
Aurelius Augustine (circa 390 AD) remarked the following about the distributed organization of conceptual knowledge and perceptual features in human memory:
There in memory all things are preserved distinctly and under general heads [categories], each having entered by its own avenue: as light and colors and forms of bodies by the eyes, all sorts of sounds by the ears; all smells by avenues of the nostrils; all tastes by avenue of the mouth.
Confessions of Saint Augustine, Chapter VIII
Well over a millennium later, the durability of Augustine’s distributed feature hypothesis is evident in most contemporary theories of semantic memory (Allport, 1985; Barsalou et al., 2003; Caramazza and Shelton, 1998; Gallese and Lakoff, 2005; Martin, 2007; Martin et al., 1996; Patterson et al., 2007; Rogers and McClelland, 2005; Warrington and McCarthy, 1987). For example, we find a close parallel between Augustine’s words and those of Barsalou (2008), who remarked:
The brain captures states across the modalities and integrates them with a multimodal representation stored in memory (e.g., how a chair looks and feels, the action of sitting, introspections of comfort and relaxation). Later, when knowledge is needed to represent a category (e.g., chair), multimodal representations captured during experiences with its instances are reactivated.
A fully distributed theory of conceptual representation holds that the brain decomposes objects into an array of semantic features. The strongest distributed theories hold that semantic features are grounded across anatomically distinct regions of cortex that lie within or proximal to regions engaged during perception or action (Barsalou, et al., 2003; Gallese and Lakoff, 2005; Martin, 2007; Martin et al., 2000). That is, some features have a clear sensory grounding (e.g., strawberries are RED; sugar tastes SWEET) with representation in modality-specific association cortex, whereas other features have motor grounding (e.g., grasping and striking for hammer) with representation in supplementary and/or premotor cortex.
Many of the claims flowing out of such a distributed semantic feature framework have been substantiated by functional neuroimaging studies investigating the representation of sensory and motor features of words and concepts. For example, generating color associations activates the fusiform gyrus, a region also involved in perception of size and color due to its placement in the ventral visual pathway (Kellenbach et al., 2001; Martin et al., 1995; Simmons, et al., 2007), and probing knowledge about the color of a tomato engages the same anterior projection of the primary visual cortex as viewing a tomato. Similarly, visual motion distinctly activates left ventral premotor cortex and middle temporal gyrus during observation and naming of tools and words denoting mechanical motion (Beauchamp et al., 2002, 2003; Chao et al., 1999; Chao and Martin, 2000; Damasio et al., 2001; Grafton et al., 1997; Kable et al., 2002; Martin et al., 1995; Martin et al., 1996; Perani et al., 1995; Tyler et al., 2003). These regions are also active during motor manipulation (Chao and Martin, 2000; Grabowski et al., 1998; Grafton et al., 1997; Martin et al., 1996). This network of activation is typically assumed to reflect retrieval of action knowledge related to manipulation of objects.
Embodied cognition, a prominent aspect of grounded models of semantic memory, holds that we represent object concepts in semantic memory in terms of our own accrued motor and sensory experiences. That is, our interaction with the environment is the basis for conceptual grounding. As a result, when we are asked to identify some property of an object that is not physically present (e.g., Is a Labrador Retriever larger than a German Shepherd?), we must re-activate a remote sensorimotor experience. This form of mental simulation or imagery has also been referred to as perceptual enactment (Farah and McClelland, 1991; Kosslyn, 2005). An ongoing debate in semantic memory research centers upon the extent to which perceptual enactment mediates conceptual representation.
One view, proposed by Barsalou et al. (1999, 2003, 2008) is that sensorimotor features are necessarily reactivated through attention and memory integration. Others have suggested a similar but more conservative view that the sensorimotor system is the basis for organization and representation of knowledge of actions and objects, but that motor production processes are not required for successful recognition and comprehension of objects and their use (Mahon and Caramazza, 2005; Martin, 2007; Martin and Chao, 2001).
1.1. Category Specificity and the Role of Embodied Cognition
A category specific impairment refers to the selective loss of one domain of knowledge relative to others. These deficits have been reported for broad distinctions such as abstract vs. concrete words (Bonner et al., 2009; Breedin et al., 1994; Reilly et al., 2007; Yi et al., 2007), animate vs. inanimate objects (Caramazza and Shelton, 1998), and actions vs. objects (Bertella et al., 2002; Cappa and Perani, 2003; Damasio and Tranel, 1993; Grossman et al., 2008; Laiacona and Caramazza, 2004). Category specific deficits have also been reported for domains as narrow as fruits and vegetables relative to other natural kinds (Crutch and Warrington, 2003; Samson and Pillon, 2003). By far, the most extensively investigated category impairment occurs between natural kinds (e.g., animals, fruits) and manufactured artifacts (e.g., hammers, cars). The vast majority of neuropsychological case studies have shown worse impairment for naming natural kinds relative to manufactured artifacts (hereafter referred to as artifacts) (Grossman et al., 2002; Humphreys and Forde, 2001; Humphreys and Riddoch, 2003; Keil, 1989).
Although comparatively rare, selective impairments of artifact naming have also been reported (Sacchett and Humphreys, 1992). This is important because it suggests that one of these semantic categories is not inherently more difficult than the other. The two most common sites of lesion associated with impairment in naming manufactured artifacts are the left posterior middle temporal gyrus (Area MT/V5+), a projection of the dorsal visual pathway that is implicated in the processing of mechanical motion, and left ventral premotor cortex (Chao and Martin, 2000; Damasio et al., 2004; Martin et al., 1996).
Two opposing classes of theory, domain-specific and domain-general, have emerged to account for category specific deficits. Domain-specific theories hold that categories of knowledge are localized in the brain (functionally and/or structurally). The most exhaustive possible domain-specific theory holds that subordinate categories (e.g., Labrador Retrievers) and even specific exemplars (e.g., my own Labrador Retriever, MAX) possess unique anatomically-demarcated representations. In general, domain-specific theories take a more parsimonious view and argue for broader categorical distinctions. Perhaps the most influential domain-specific theory of semantic memory is found in the Organized Unitary Content Hypothesis (OUCH) and associated variants (Caramazza and Mahon, 2003, 2006; Hillis et al., 1995; Laiacona et al., 2003; Mahon and Caramazza, 2009). OUCH holds that evolutionary pressures on access to particular semantic domains forced the adaptation of a categorical organization of semantic memory. A second component of OUCH holds that features are organized in distributed clusters. OUCH and related accounts suggest that semantic impairment can emerge from either the loss of an evolutionarily evolved category or the loss of clusters of features that support category knowledge. OUCH offers the possibility for a true category specific loss, a type of pure impairment that is not supported by domain-general theories of semantic memory described below.
Two historically dominant domain-general theories of semantic memory include Sensory-Functional Theory (Warrington and McCarthy, 1987; Warrington and Shallice, 1984) and Sensory-Motor Theory (Martin, 2007; Martin et al., 2000). Sensory-Functional Theory (SFT) is premised upon the idea that sensory detail (e.g., an axe has a long handle) and functional detail (e.g., an axe is used for chopping wood) constitute dissociable semantic systems (Farah and McClelland, 1991; Warrington and Shallice, 1984). Along similar lines, Sensory-Motor Theory (SMT) holds a distinction between sensory and motor properties of objects (e.g., self-initiated action, path and manner of motion) (Chao and Martin, 2000; Martin, 2007; Martin and Chao, 2001; Martin et al., 1996). In this respect, SFT and SMT are consistent with some notions associated with embodied cognition.
Proponents of both SFT and SMT have accounted for category specific naming impairments in terms of the differential weighting of information for natural kinds relative to artifacts. It has been argued that sensory detail assumes differential importance for the representation of natural kinds, whereas motor properties (e.g., you swing it) and/or functional properties (e.g., used for chopping) are more salient attributes of artifacts (Farah and McClelland, 1991; Gonnerman et al., 1997). Because perceptual features are highly inter-correlated in semantic (and neuroanatomical) space, it is possible for brain damage to compromise a set of features such as color knowledge and produce an apparent category-specific impairment for fruits and vegetables since this category of knowledge is thought to depend crucially on color knowledge (for a related account see Tyler et al., 2000).
Although there is lingering debate as to the modular organization of category knowledge, there appears to be somewhat stronger consensus for a fronto-temporal segregation between interpretation of action and motor enactment (frontal) and representation of perceptual features (temporal) (Humphreys and Riddoch, 2003; Lambon Ralph et al., 2007; Martin et al., 2000; Sartori et al., 2007; Shallice, 1988). Anatomical correlates for this distinction include the posterior inferior frontal lobe distribution of motor cortex and the temporal lobe distributions of primary and secondary visual and auditory cortices.
1.2. The role of process in semantic memory
Although many theories of conceptual representation in the brain (including most studies of category-specific deficits) focus on different classes of content, there has also been an increasing realization of the important role of process in semantic memory (Martin and Chao 2001, Koenig and Grossman 2007, Peelle et al., 2009). The common theme in these reports is that information must not only be stored but also actively organized, accessed, and manipulated to support the current contextual demands. This principle was demonstrated elegantly by Thompson-Schill et al. (1997), in which the authors presented participants with a series of tasks that varied in the level of semantic selection required. For example, in a verb generation task, being cued with “wheel” could lead to multiple responses (turn, roll, spin), whereas a word like “kite” has fewer (fly); conditions with more alternatives therefore would require greater selection processes. The authors found that the high-selection conditions led to reliable increases in neural activation in left inferior frontal gyrus. These data indicate the important role that processing plays in accessing and using semantic knowledge, and specifically point toward a contribution of left inferior frontal cortex in selecting semantic responses from among competing alternatives. This is consistent with studies showing that left inferior frontal regions show increased activation when processing sentences containing words with multiple meanings (Rodd et al., 2005); for example, the word “date” could refer to a day of the year or a type of fruit, but its referent is clear in the sentence “There were dates and pears in the fruit bowl”. Again, in this case additional resources are required to assign the appropriate meaning to a word whose concept(s) clearly exist in semantic memory.
1.3. Progressive Nonfluent Aphasia as a Lesion Model
Progressive Nonfluent Aphasia (PNFA) is a variant of primary progressive aphasia that is characterized by damage to multiple regions of the left frontal cortex, including left inferior frontal gyrus, anterior insula, inferior and middle frontal gyri, premotor and supplementary motor cortices, as well as the basal ganglia via the frontal-subcortical loop (Gorno-Tempini et al., 2004; Gorno-Tempini et al., 2004; Grossman et al., 1996; Grossman et al., 2004; Ogar et al., 2007). During much of the disease course, PNFA spares inferolateral temporal lobe regions involved in visual object recognition. The relatively focal distribution of left hemisphere damage incurred in PNFA produces a high degree of anatomical overlap with the site of lesion associated with classical Broca’s aphasia. Thus, one might predict that PNFA and Broca’s Aphasia share many behavioral features, and to a large extent this indeed the case (but see Patterson et al., 2006). Patients with PNFA are commonly reported to have impaired phonological and articulatory aspects of speech production in addition to agrammatism, reduced working memory capacity, and limitations in executive functioning (Ash et al., 2009; Gorno-Tempini et al., 2004; Grossman et al., 1996; Koenig et al., 2006; Thompson et al., 1997). Similar to patients with nonfluent stroke aphasia, PNFA patients also show difficulty comprehending syntactically complex sentences (Peelle et al., 2007; Peelle et al., 2008).
Previous studies have demonstrated that PNFA patients have difficulty naming objects (although less difficulty than other variants of primary progressive aphasia), often using some form of the Boston Naming Test (Gorno-Tempini et al., 2004; Grossman et al., 2004). However, the relatively coarse accuracy measures used previously make it impossible to tell whether these naming declines are due to deficits in semantic content or the processes needed to access this content.
In addition to overall naming difficulty, some PNFA patients show disproportionate impairment for verbs relative to nouns (Hillis et al., 2004). Again, this selective impairment for verbs may have a number of potential causes. Verbs and nouns differ in their grammatical roles; thus, these respective word classes may be vulnerable to syntactic impairment. Another possible explanation for verb deficits in patients with left prefrontal damage is an effect of greater semantic complexity for verbs relative to nouns (i.e., verbs are more difficult than nouns to process) (Breedin et al., 1998). A third potential explanation for a verb deficit is an impairment in the perceptual enactment and gesture of actions (see Arévalo et al., 2007). This modality-specific hypothesis links the motor and language systems through shared motor features of action verbs and manufactured artifacts.
If left inferior prefrontal regions are involved in the category-specific representation of concept knowledge, then PNFA patients should show category-specific impairment for artifacts relative to natural kinds. Furthermore, naming deficits should be amplified for artifacts that have a strong manual manipulation component (such as tools) due to damage to left ventral premotor and supplementary motor areas that are important in the representation of actions such as grasping (for precedent see Arévalo et al., 2007). Conversely, the lack of such category-specific deficits would suggest that it may be a decrease in semantic processing (e.g., response selection) that underlies naming difficulty in PNFA. This result would also argue against a strong view that an embodied account can fully explain all deficits involving semantic memory.
2. METHOD
2.1. Participants
Participants included 9 males and 3 females, who were right-handed, native speakers of English diagnosed with PNFA (n=12) through a consensus review mechanism in accord with published criteria (Grossman, 2010). In a clinical setting, our antemortem diagnostic protocol has proven upon autopsy confirmation to have high sensitivity (100%) and specificity (>90%) for PNFA (Grossman et al., 2007; Hu et al., in press). For PNFA diagnosis, patients must have had an insidious onset of symptoms (no focal stroke or malignancy) and effortful, nonfluent speech with agrammatism and speech-sound errors. Supportive diagnostic speech-language criteria for PNFA included: difficulty with grammatical comprehension, early preservation of word meaning, and late mutism. An assessment of disease severity is supported by significant impairment on measures of visual confrontation naming, category naming fluency, semantic association ability, executive functioning, and global cognitive functioning. For relevant neuropsychological data demographic data, see Table 1.
Table 1.
Demographic and Neuropsychological Data
| Demographic/Measure | PNFA (n=12) | Control (n=24) | |||
|---|---|---|---|---|---|
| Mean | SD | Z-score | Mean | SD | |
| Age (years) | 73.17 | 6.95 | n/a | 69.46 | 7.87 |
| Education (years) | 14.33 | 2.67 | n/a | 15.42 | 8.02 |
| 1 MMSE (of 30) | 22.17 | 6.81 | n/a | 29.22 | .36 |
| 2 Boston Naming Test (15 item) | 8.50 | 5.35 | −4.84 | 14.25 | 1.19 |
| 3 Pyramids and Palm Trees Words | 44.18 | 6.52 | −5.97 | 50.64 | 1.56 |
| 3 Pyramids and Palm Trees Pictures | 42.64 | 7.63 | −5.01 | 50.43 | 1.56 |
| 4 Letter fluency (FAS) | 3.12 | 2.93 | −2.55 | 13.18 | 3.94 |
Mini Mental State Examination (Folstein et al., 1975);
15 item abbreviated version of the original 60-item Boston Naming Test (Kaplan et al., 1976);
The Pyramids and Palm Trees Test (Franklin et al., 1992) examines semantic association ability in two modalities, words and pictures;
Letter fluency naming score represents average words produced when for each cued letter (i.e., F, A, and S) in 60 seconds
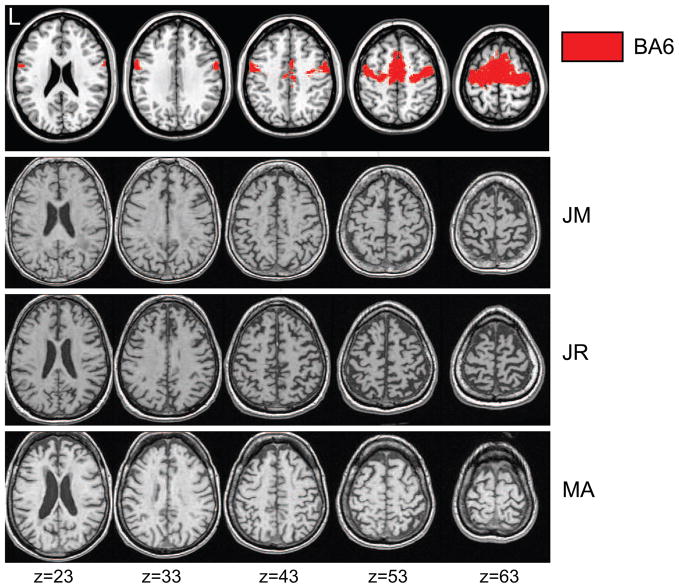
Additional supportive criteria for PNFA include asymmetric atrophy of frontal cortex upon imaging (Neary et al., 1998). Figure 1 represents a series of multi-slice views of left frontal atrophy of varying severity in three of our 12 patients. For comparison, the top row shows in red voxels that have at least an 80% chance of belonging to premotor cortex (BA6) based on cytoarchitectonic probability maps identified using the SPM Anatomy toolbox (Eickhoff et al., 2005; Geyer et al., 1996). Five additional patients had confirmed left frontal lobe atrophy and/or sulcal prominence per clinical radiologic reports. The remaining four patients had contraindications for MR imaging, including ferromagnetic implants (e.g., cardiac pacemaker) and claustrophobia.1
Figure 1. Multi-slice T1 Structural MR Images of Varying Severity PNFA.
Top row: Template brain showing in red regions of premotor cortex (BA6) defined by a probabilistic cytoarchitectonic atlas. Below: Slices from normalized structural MRIs of three individual PNFA patients. MNI coordinates of the axial slice are shown below the figure in mm.
We contrasted patient performance with that of 24 healthy adults who were right-handed, native speakers of English living in the Philadelphia community. The patient and control groups were matched on age, education, and gender (see Table 1). All participants completed an informed consent protocol approved by the University of Pennsylvania institutional review board.
2.2. Materials
Stimuli included 60 black-and-white line drawings from the picture series of Snodgrass and Vanderwart (1980). The drawings represented a range of basic level categories within the superordinate distinctions of natural kinds (n=22 items) and artifacts (n=38 items). The three basic level natural kind categories included: Fruits/Vegetables (n=9), Mammals (n=7), and Non-Mammals (n=6). The six basic level artifacts subcategories included: Clothing/Accessories (n=5), Household Items (n=8), Kitchen Items (n=5), Tools (n=7), Toys (n=7), and Vehicles (n=6).2 Items across the nine basic level categories were matched for familiarity [F(8,48)=.41, p>.05; mean familiarity=525 on a 100–700 scale], visual complexity as assessed by Snodgrass and Vanderwart norms (1980) [F(8,51)=.87, p>05, ns; mean=3.07], and word frequency [F(8,51)=3.35, p>.05, ns; mean written frequency=27.69 per-million words] based on values from the MRC Psycholinguistic Database (Coltheart, 1981).
2.3. Procedure
We presented the 60 line drawings in a fixed random order and scored responses offline. Participants did not receive feedback on accuracy of production. We treated failure to name an item within 60 seconds as an omission/non-response. On trials in which participants made multiple naming attempts, only the final response was accepted.
2.4. Analysis Methodology for Accuracy Data
We examined naming accuracy at two levels of specificity: superordinate and basic. The superordinate category distinction was between natural kinds and artifacts. The basic level distinction spanned the nine categories described previously. The dependent variable in these accuracy analyses was z-score accuracy computed relative to the control distribution for each specific semantic category. We employed z-scores within each of the basic level categories as a means of accounting for baseline variability among normal subjects (for discussion see Laws and Sartori, 2005). For example, we found that healthy older control participants named the Tools category with the lowest overall accuracy. Z-score comparisons allowed us to account for such baseline differences by assessing relative impairment of patients.
2.5. Analysis Methodology for Naming Errors
In addition to the accuracy analysis we conducted two analyses of naming errors. The first of these analyses targeted coarse differences between visual, phonemic, and semantic levels of processing. The second analysis focused on semantic errors. For both error analyses, we examined impaired patients only by setting a threshold of naming impairment (z<−1.96) relative to control participants; of the 12 PNFA patients, 9 met this criterion for having a naming impairment. Thus, only data for these 9 patients were used in the error analyses.
2.5.1. Error Analysis I: Classification of Major Error Types
We first coded major naming errors as the following types:
Visual: Naming a selected part of the target item (e.g., banana → ‘stem’) or substituting a visually similar item from a different semantic category (e.g., asparagus → ‘pencil’)
Phonemic: Distortions or phonemic approximations that share at least one syllable in common with the target (e.g., umbrella → ‘umbellug’)
Unrelated: Real-word responses visually dissimilar and semantically unrelated to the target item (e.g., cat → ‘apple’)
Omission: Non-responses and empty responses (e.g., ’I know… It’s that thing.’)
Semantic: Errors related in meaning to the target item (see below)
2.5.2. Error Analysis II: Classification of Semantic Error Types
We then isolated semantic errors and coded each one as follows:
Coordinate: Responses from the same superordinate semantic category and the same taxonomic level as the target (e.g., zebra → ‘horse’)
Subordinate: Responses that include a specific subordinate exemplar of the target (e.g., dog → ‘poodle’) or a proper name (e.g., volcano → ‘Vesuvius’)
Superordinate: Responses that state the general category to which the target belongs (e.g., dog → ‘animal’)
Functional-Associative: Responses that state a function or action of the target item (e.g., piano → ‘you play music on it’ or ‘you hit the keys’)
Physical Attribute: Responses that describe a feature of the item that is not part of the line drawing (e.g., pumpkin → ‘orange’)
Contextual: responses that identify the context where the target item might be found or used (fish → ‘pond’; or rolling pin → ‘baker’)
3. RESULTS
3.1. Accuracy Analysis Results
Table 2 summarizes overall naming performance. Mean naming accuracy was 71% for patients and 95% for controls. Nine of 12 patients showed the predicted trend toward category impairment for naming artifacts relative to natural kinds [binomial probability, p=.05; paired t(11)=2.10, p=.06]. Finer-grained inspection across the nine basic level categories revealed heterogeneity in naming accuracy, as shown in Figure 2. Consider, for example, naming Tools relative to subsets of Natural Kinds. PNFA patients showed less of an impairment relative to controls in naming tools than naming fruits/vegetables [paired t(11)=3.21, p<.01]. By comparison, PNFA patients showed comparable impairment for naming tools relative to both mammals and non-mammals [paired t(11), p>.05 both].4
Table 2.
Mean Naming Accuracies by Semantic Category
| Group | Measure | Natural (n=22) | Artifacts (n=38) | Natural Kind Subcategories | Manufactured Artifact Subcategories | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Foods (n=9) | Mammals (n=7) | Non-Mammals (n=6) | Clothes (n=5) | Household (n=8) | Kitchen (n=5) | Tools (n=7) | Toys (n=7) | Vehicles (n=6) | ||||
| PNFA | Raw Score | 14.4 | 23.2 | 5.2 | 5.7 | 3.6 | 3.2 | 5.11 | 3.22 | 3.3 | 4.2 | 4.1 |
| % Correct | 65.6 | 61.1 | 58.0 | 81.0 | 59.2 | 64.4 | 63.9 | 64.4 | 47.6 | 60.3 | 68.5 | |
| Z-Score | −6.69 | −9.1 | −8.5 | −2.1 | −4.0 | −5.3 | −2.3 | −5.3 | −2.3 | −6.1 | −2.6 | |
| Control | Raw Score | 21.1 | 36 | 8.8 | 6.7 | 5.6 | 4.9 | 7.9 | 4.9 | 6.0 | 6.8 | 5.5 |
| % Correct | 96 | 95 | 98 | 96 | 93 | 98 | 99 | 98 | 86 | 97 | 92 | |
Note: Means for the PNFA rows above reflect performance of the subset of patients (n=9) we classified as anomic relative to controls
Figure 2. Patient Naming Accuracy Across Basic Semantic Categories.
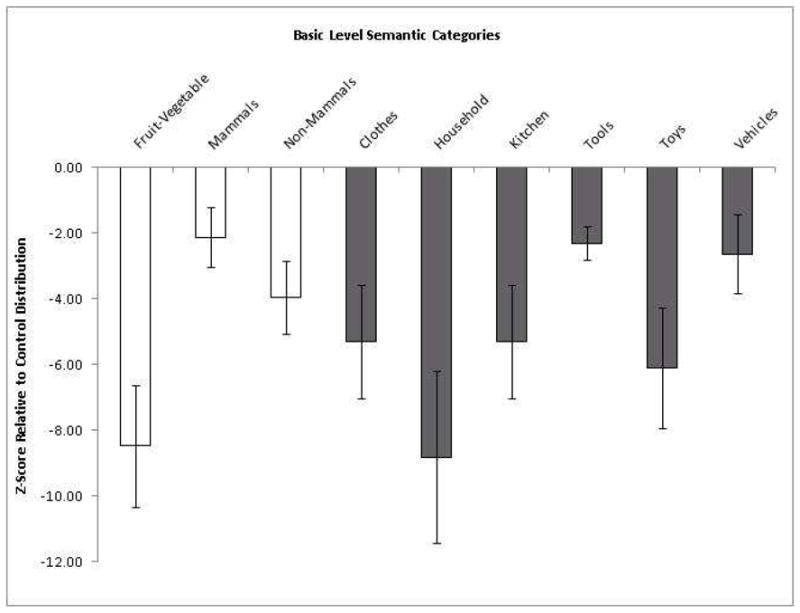
Note: Category naming accuracies for PNFA patients, expressed as z-scores relative to a group of age-matched healthy adults. That is, each error type reflects the Observed Patient Mean minus the Control Mean divided by the Control Standard Deviation. Error bars reflect the Standard Error of the Mean (SEM).
3.1.2. Interim discussion of accuracy data
Patients showed a trend toward naming impairment for artifacts relative to natural kinds. However, there was substantial variability in naming across semantic subcategories. Paradoxically, tools constituted one of the least impaired semantic categories relative to controls. This heterogeneity across basic level exemplars is consistent with previous work suggesting that the general categories such as artifacts and natural kinds may represent overly broad distinctions whose variability is better captured at a finer-grained level of specificity (for reviews see Caramazza and Mahon, 2003, 2006). Dissociations within natural kinds have been observed for knowledge of parts of the human body relative to animals or fruits (Coslett et al., 2002) and for deficits in fruit and vegetable naming in the context of preserved animal naming (Rogers et al., 2004; Samson and Pillon, 2003). Among artifacts, similar effects are apparent such as dissociations for musical instruments relative to other manufactured artifacts (Barbarotto et al., 1995; for discussions and alternate accounts see Dixon et al., 1999; Fung et al., 2001).
Common among the many studies reporting fractionation of artifacts and natural kinds is the idea that latent factor(s) contribute to semantic impairment. For example, the selective loss of color knowledge associated with damage to the ventral visual pathway might logically result in a selective impairment of fruit/vegetable naming because color information plays a central role in object identification for such items (see also De Renzi and Lucchelli, 1994). Alternatively, a latent factor such as impaired ability to grip and manipulate objects appropriately might undermine the lexical-semantic representations of tools (Buxbaum et al., 2000). Controversy persists about the nature of these latent variables. However, embodied cognition has recently emerged as a serious contender for influencing category structure, with aspects such as gesture and manipulability weaving into models of language representation (Arévalo et al., 2007; Hauk et al., 2004). Here, we found an imperfect correspondence between semantic-motor features and naming. That is, theories promoting a reliance on perceptual enactment of grasp or manipulability cannot readily explain the category advantage for tools that we observed. We turn to naming errors as a means of elucidating differences in semantic aspects of naming artifacts and natural kinds in PNFA.
3.2. Error analysis results
Using the z-score criterion described previously, 75% of PNFA patients (n=9) were classified as naming impaired (anomic). Two judges naïve to the study aims then classified naming errors, while a third judge evaluated items that produced disagreement. Inter-rater agreement was 92.1%.
3.2.1. Major Error Results
Figure 1 and Table 3 show the distribution of major error types. We conducted a 2-factor, within-subjects, repeated measures ANOVA nesting major error type (5 levels: Visual, Phonemic, Unrelated, Omission, Semantic) within semantic category (2 levels: Natural Kinds or Artifacts). For each individual error type (e.g., visual errors for artifacts) we calculated the proportion relative to that set size (22 natural kinds, 38 artifacts) for each patient. This ratio conversion permitted direct comparisons across the unequal sample sizes of natural kinds and artifacts. The dependent variable in this analysis was, therefore, proportion of each error type.
Table 3.
Major naming error distribution
| Error Category | Major Error Type |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Visual | Semantic | Phonemic | Unrelated | Empty/Non-response | |||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||
| Total Errors | 1.78 | 1.09 | 10.00 | 4.53 | .44 | .53 | .44 | .73 | 9.78 | 13.30 | |
| Natural Kinds | .11 | .33 | 3.22 | 1.79 | .11 | .33 | 0 | 0 | 3.44 | 5.22 | |
| Artifacts | 1.67 | .87 | 6.78 | 4.78 | .33 | .50 | .44 | .73 | 6.34 | 8.09 | |
Variability in the rates of major error types (i.e., visual, phonemic, semantic, omission) is shown in Figure 2 [main effect of error type F(4,32)=4.75, p<.05]. Patients did not differ significantly in their distributions of major errors for natural kinds and manufactured artifacts. That is, rates of omission, phonemic, and semantic errors were similar when naming tools relative to animals or fruits. This was confirmed by the ANOVA which showed no main effect of semantic category [F(1,32)=.73, p>.05] and the lack of a significant interaction between major error type and semantic category [F(4,32)=1.91, p>.05]. Upon visual inspection of the data (see Figure 1), however, we suspected that a difference in the rates of visual errors may have been masked by the non-significant omnibus ANOVA. A Bonferroni corrected paired samples t-test confirmed that patients made more visual errors for artifacts than natural kinds [t(8)=6.22, p<.001].
3.2.2. Semantic Naming Error Results
In order to examine the distribution of semantic errors, we conducted a 6 (semantic error type: Coordinate, Subordinate, Superordinate, Functional-Associative, Physical Attribute, Contextual) × 2 (superordinate category: natural kinds, artifacts) within subjects ANOVA. The dependent variable in this analysis was the proportion of semantic errors. Again, we standardized the scale of comparison by dividing the raw number of semantic errors by the total number of natural kinds (n=22) or artifacts (n=38) and contrasting the ratios averaged across participants.
The distribution of semantic errors is shown in Figure 3 and listed in Table 4. Patients produced qualitatively different semantic error types as a function of the semantic category of the target item. This was revealed by the significant interaction between superordinate category and semantic error type [F(5,40)=3.41, p<.05] (e.g., participants made more coordinate naming errors for natural kinds relative to artifacts. In addition to this interaction, patients showed variability in their rates of the different types of semantic errors (e.g., there were far more coordinate than physical attribute naming errors) as revealed by a significant main effect of semantic error type [F(5,40)=5.09, p<.01].
Figure 3. Distribution of Major Error Types for Artifacts and Natural Kinds.
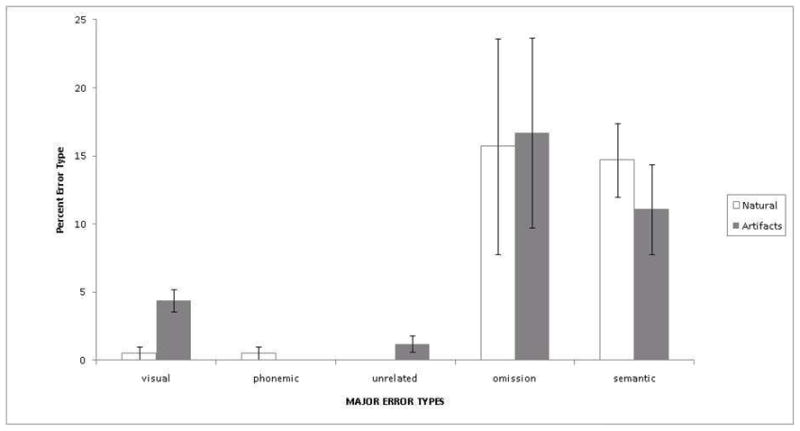
Note: The graph above shows the percentage of errors for natural kinds (white) and artifacts (gray) relative to the set size. For example, we calculated the “semantic error percentage for artifacts” by dividing the mean number of artifact semantic errors (4.22 per patient) by the total number of artifact target names (n=38) and multiplying by 100. Error bars reflect the Standard Error of the Mean (SEM).
Table 4.
Semantic Naming Error Distribution
| Semantic Error Type |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coordinate | Subordinate | Superordinate | Functional-Associative | Physical Attribute | Contextual | |||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Total Errors | 3.7 | 3.1 | .3 | .7 | 1.0 | .9 | 1.2 | 1.6 | .1 | .3 | 1.1 | 2.3 |
| Natural Kinds | 2.0 | 2.0 | .3 | .7 | .6 | .7 | .11 | .3 | .1 | .1 | .1 | .3 |
| Artifacts | 1.7 | 1.8 | 0 | 0 | .4 | .5 | 1.1 | 1.4 | 0 | 0 | 1.0 | 2.0 |
Bonferroni corrected pairwise t-tests showed that patients produce higher proportions of functional-associative errors for artifacts relative to natural kinds [t(8)=2.84, p<.05] and also higher proportions of contextual errors for artifacts relative to natural kinds [t(8)=2.84, p<.05]. Parametric contrasts for pairwise comparisons involving subordinate errors and physical attribute errors were not possible because of zero observations in one category. The two remaining pairwise comparisons, coordinate errors and superordinate errors, were non-significant at a corrected alpha of .05.
3.2.3. Interim discussion of error analyses
The naming error data presented above suggest qualitative differences in perceptual and semantic processing as a function of the target item. Patients made more visual, contextual, and functional-associative errors for artifacts; by contrast, the overwhelmingly dominant error type for natural kinds was coordinate. These distinct error distributions to a large extent honored differences in superordinate category structure (Farah and Feinberg, 2000; Farah and McClelland, 1991; McCrae et al., 2005). Natural kinds tend to have a hierarchical, taxonomic organization and a higher density of shared semantic features than artifacts (see Gonnerman et al., 1997). For example, cats and mice are different species that share great overlap in their features. In contrast, artifacts tend to have more distinctive features and greater dissimilarity across exemplars (e.g., blenders and screwdrivers share few perceptual or functional features and have many distinctive features).
The observed dominance of coordinate naming errors for natural kinds suggests difficulties in distinguishing between exemplars within a semantic category. We envision two possible reasons for this finding. The first is that patients have degraded representations of visual-perceptual feature knowledge associated with object categories that results in semantic concept degradation similar to that of Alzheimer’s disease or semantic dementia, populations who commonly show gross impairment for objects relative to abstract concepts (Bonner et al., 2009; Yi et al., 2007; Breedin et al., 1994; Warrington and Shallice, 1984), and natural kinds may be particularly disadvantaged because of the dependence of this semantic category on visual-perceptual features (see Humphreys and Riddoch, 2003, 2006; Lambon Ralph et al., 2007). The second possibility is that PNFA patients might experience difficulties with controlled retrieval in selecting the appropriate target from amongst many plausible competing alternatives.
Although the degradation of representations of perceptual features has a clear anatomical basis due to disease in visual association cortex in Alzheimer’s disease and semantic dementia (Alladi et al., 2007; Bonner et al., in press; Galton et al., 2001; Grossman et al., 2004; Glosser et al., 2002), much of the cortical visual processing pathway is preserved in PNFA. Therefore, the degradation of visual-perceptual feature knowledge is an unlikely contributor to the preponderance of coordinate naming errors and the overall impairment in accuracy for natural kinds. Instead, we favor a deficit of controlled semantic retrieval — that is, a processing account — to explain anomia for natural kinds in PNFA. This is not to say that deficits in PNFA need be purely due to processing impairments; for example, it is unclear how a pure processing account can account for the observed artifact naming impairment. Thus, we return to latent factors associated with artifacts (i.e., deficits associated with semantic content) to complete the explanation.
PNFA patients, when anomic for artifacts, tended to revert to qualitatively different types of information than they did for natural kinds. Patients made more contextual errors (e.g., knife → ‘you find it in a kitchen’), more functional-associative errors (e.g., knife → ‘you cut with it’), and more visual errors (e.g., ‘asparagus’ → ‘pencil’) for artifacts.3 One explanation for this observed impairment in artifact naming is, in addition to processing deficits, an underlying deficit in the representation of semantic motor features. That is, patients may experience diminished support from motor enactment and simulation processes instantiated in damaged posterior and inferior frontal cortex. Thus, we hypothesize that inferior frontal lobe damage in PNFA results in a dual impairment of controlled semantic retrieval (process) and semantic motor features (content).
There is a clear anatomical basis for this hypothesis in the distribution of posterior frontal lobe regions affected in PNFA. Neighboring and/or overlapping regions of the left posterior inferior frontal cortex have been implicated in both executive aspects of semantic processing and in the enactment, programming, and execution of actions (see also Kan et al., 2006; Postle et al., 2008). From a neuropsychological perspective, therefore, it is not unreasonable to suggest that naming impairment in PNFA has a multifactorial basis, reflecting both compromised executive retrieval processes and degraded motor enactment processes. We view this dual executive-semantic impairment as impacting the representations of natural kinds and artifacts in different ways. The high density of shared semantic features among natural kinds taxes retrieval processes, whereas damage to premotor cortex compromises the representation of motor-action features necessary to simulate artifact use. We elaborate upon this hypothesis in the general discussion to follow.
4. GENERAL DISCUSSION
In the current study on picture naming, progressive aphasia patients with damage to left inferior frontal cortex exhibited more difficulty naming artifacts than natural kinds. However, there are several important caveats to consider before interpreting this overarching trend. The first caveat is that patients showed great heterogeneity in their response accuracy across basic level categories, suggesting a lack of cohesion within the superordinate category distinction. The second caveat is that patients showed no reliable correlation between the classical symptoms of posterior inferior frontal lobe damage and artifact naming. These results are inconsistent with a strict embodiment position that emphasizes a necessary role of motor enactment in the representation of artifacts. Instead, these data support a more conservative view of embodied cognition where the link between language and motor impairment is indirect.
We recently proposed a compromise between embodied and propositional theories of semantic memory (Reilly and Peelle, 2008). Briefly, we favor a theory of dynamic interactivity between modality-neutral and modality-specific sensorimotor processes. In this framework, object concepts are stored as “sparse” abstract representations whose meaning can, and sometimes must, be enriched through perceptual simulation and/or motor enactment processes. We hypothesize that the coordination of these perceptual and motor enrichment processes is to a large extent mediated by frontal-striatal instantiated executive functions. We, therefore, espouse an approach to semantic cognition that emphasizes dual roles of both process (dynamic interactivity) and content (stored semantic features) in how the brain represents object concepts. A multiple component view of semantic memory that emphasizes both active retrieval processes and stored features is now reasonably well-accepted. However, debate persists as to whether there is a true distinction between abstract propositional knowledge and concrete sensorimotor knowledge about objects (Jefferies and Lambon Ralph, 2006; Jefferies et al., 2008; Koenig and Grossman, 2007; Peelle et al., 2009).
A critical component of any neurologically constrained theory of semantic memory is that it must describe what is both necessary and sufficient with respect to distributed representation and perceptual enactment. More specifically, theory should specify the extent to which successful naming of a handtool (e.g., screwdriver) demands activation of somatotopic regions of premotor cortex that support manual grasp. Many studies using a variety of methodologies (e.g., fMRI, transcranial magnetic stimulation, motor evoked potentials) have demonstrated strong associations between action word perception and motor responses (Boulenger, 2006; Glenberg and Kaschak, 2002; Hauk et al., 2004, 2008; but see Postle et al. (2008) for fMRI counterevidence). Moreover, the timecourse of motor-language activation is rapid (almost simultaneous). Action words quickly (i.e., within 200 ms) activate corresponding motor regions and may produce either interference or facilitation when the body part effector (e.g., foot) corresponds to the semantic content of the target action word or phrase (e.g., to kick) (Boulenger et al., 2006).
We recently found such motor-language resonance effects across a number of different behavioral paradigms and stimulus onset asynchronies (Rodriguez and Reilly, unpublished data). For example, healthy young participants showed greater variability in their centers of gravity (i.e., swayed more) during categorical fluency tasks that required generation of motor-related words (e.g., things you do) relative to generation of visually-associated words (e.g., fruits/vegetables). We also found language-motor interference effects in finger-pointing and word production paradigms: Response times to touching a circle on a computer monitor were longer when the circle was preceded by motor-related words (i.e., pencil, write) versus visually-related words (i.e., flower, bloom). Similarly, when word production was preceded by finger-tapping, participants showed increased response latency for motor-related words relative to visually-related words. In summary, there is a wide body of evidence from neuroimaging and behavioral studies to support moderate views of embodiment theory that emphasize interactivity between language and motor processes. One might expect, therefore, to observe converging evidence from neuropsychological case studies. However, this is far from the case: Patient-based evidence is at best equivocal (see Mahon and Caramazza, 2008 for critical review).
Although it is true that some patients with motor impairment exhibit action word impairment (e.g., Motor Neuron Disease: Bak and Hodges, 2004; Grossman et al., 2008; Progressive Supranuclear Palsy: Bak et al., 2006; Parkinson’s Disease on/off dopamine agonists: Boulenger et al., 2008), there are others that suggest motor impairment and naming impairment do not always co-occur. For example, patients with ideomotor apraxia are often unable to execute the motor programming necessary to gesture appropriate use of an object but do not tend to be anomic for the associated items (Rosci et al., 2003; Rothi et al., 1991). Patients with primary temporal lobe pathologies such as Alzheimer’s disease and semantic dementia also tend to show action word deficits despite the relative preservation of posterior frontal lobe structures that support a putative action-object feature segregation (Druks et al., 2006; Reilly et al., 2007; Yi et al., 2007).
Taken together, the current results add to a wider body of neuropsychological evidence demonstrating an imperfect correspondence between embodied cognition and language representation. In this study of naming in PNFA, we find evidence for both degraded representations of objects that depend on motor-action features represented in motor association cortex, and heterogeneity in naming error patterns that is best explained by impaired processing of information represented in semantic memory. At a systems level, a continued challenge remains the development of a synthetic model of semantic memory that can reconcile both the data from patient-based studies and investigations within typical adults.
Figure 4. Distribution of Semantic Naming Errors for Artifacts and Natural Kinds.
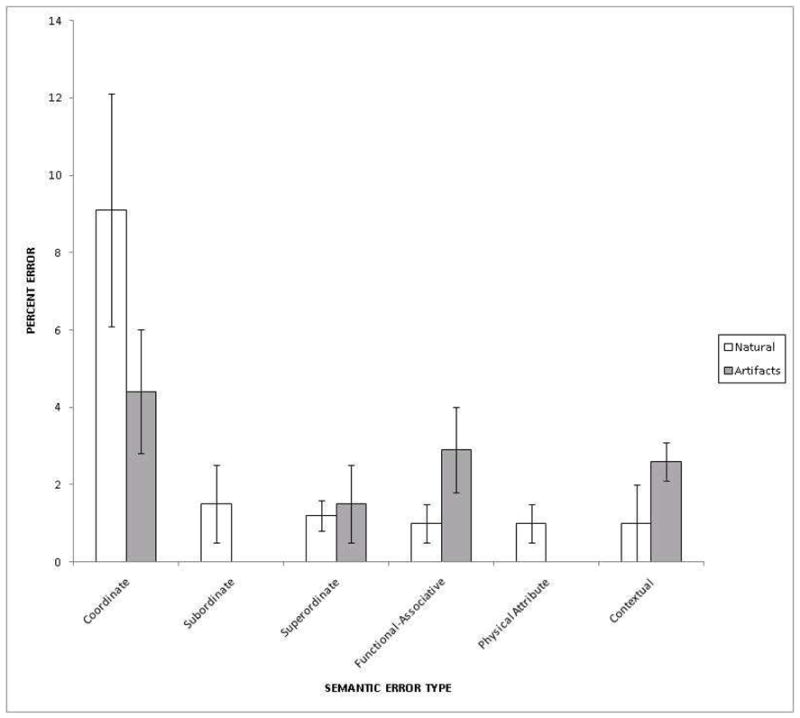
Note: The bars represent percent of each semantic error type incurred within the category of either natural kinds (white) or manufactured artifacts (gray). For example, the proportion of coordinate naming errors for manufactured artifacts pictured above reflects the average number of coordinate naming errors divided by the total number of manufactured artifact exemplars (1.67/38=.044), multiplied by 100. Error bars reflect the Standard Error of the Mean (SEM).
Acknowledgments
This work was supported by US Public Health Service grants K23 DC0101;97 (JR), AG15116 (MG), AG17586 (MG), NS44266 (MG), NS53488 (MG). We are gratefulto Delani Gunawardena and Peachie Moore for their valuable assistance with this manuscript.
Footnotes
Our assumptions rely on a canonical distribution of cortical atrophy in PNFA. Although confirmatory group imaging analyses was precluded, we are able to make reverse inference about the brain based on behavior of a well-characterized clinical population. The validity of such inference is bolstered by the fact that there is near universal consensus across a number of different research groups that the syndrome of PNFA is associated with localized damage to left frontal cortex (Gorno-Tempini et al., 2004; Grossman et al., 2004; Nestor et al., 2003) and that the antemortem diagnostic process employed here has proven to have both high sensitivity and specificity, making misdiagnosis of PNFA exceptionally rare among patients who are assessed repeatedly (Grossman, Libon et al., 2007; Hu et al, in press).
It is important to acknowledge the issue of limited statistical power here both by items and subjects. Our selection of the Snodgrass and Vanderwart (1980) picture series constrained the numbers of items per semantic category. There is, however, great value in the choice of this stimulus set. The Snodgrass series represents a ubiquitous, well-normed, and well-balanced (e.g., visual complexity, frequency, consistency of graphics) set of items that will promote ease of both prospective replication and the possibility of retrospective analyses of the many neuropsychological case studies using this stimulus set. With respect to subjects, although our participant pool seems limited, to our knowledge this sample nevertheless represents the largest single group of patients with PNFA reported to date. An additional strength of the sample is that these patients were not pre-selected for manifesting a category-specific naming impairment, as is sometimes the case in patient studies.
It is possible that visual characteristics (e.g., complexity) are a confounding variable among the items selected for presentation here. However, this possibility is reduced by the fact that items were matched on rated visual complexity (see Method).
There was considerable baseline variability in naming accuracy across the nine basic level categories. Controls named the basic level category “tools” with the lowest overall accuracy (see table 2). We contrasted patient performance between the basic level categories by standardizing the scale of comparison (i.e., z-scores). As a result, although patients named tools with the lowest proportion accuracy, their relative impairment for this category as reflected by z-scores was better than for the other basic level categories. For this reason, although tools were named with the lowest proportion accuracy, tools were paradoxically one of the least impaired categories.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alladi S, Xuereb J, Bak T, Nestor PJ, Knibb J, Patterson K, Hodges JR. Focal cortical presentations of Alzheimer’s disease. Brain. 2007;130(10):2636–2645. doi: 10.1093/brain/awm213. [DOI] [PubMed] [Google Scholar]
- Allport DA. Distributed memory, modular subsystems and dysphasia. In: Newman SK, Epstein R, editors. Current perspectives in dysphasia. Edinburgh: Churchill Livingstone; 1985. pp. 207–244. [Google Scholar]
- Arévalo A, Perani D, Cappa SF, Butler A, Bates E, Dronkers N. Action and object processing in aphasia: From nouns and verbs to the effect of manipulability. Brain and Language. 2007;100(1):79–94. doi: 10.1016/j.bandl.2006.06.012. [DOI] [PubMed] [Google Scholar]
- Ash S, Moore P, Vesely L, Gunawardena D, McMillan C, Anderson C, Avants B, Grossman M. Non-fluent speech in frontotemporal lobar degeneration. Journal of Neurolinguistics. 2009;22:370–383. doi: 10.1016/j.jneuroling.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustine A. In: The Confessions of Saint Augustine. Pusey EB, translator. London: JM Dents and Sons; circa 390 AD. [Google Scholar]
- Bak TH, Hodges JR. The effects of motor neurone disease on language: further evidence. Brain and Language. 2004;89(2):354–361. doi: 10.1016/S0093-934X(03)00357-2. [DOI] [PubMed] [Google Scholar]
- Bak TH, Yancopoulou D, Nestor PJ, Xuereb JH, Spillantini MG, Pulvermuller F, Hodges JR. Clinical, imaging and pathological correlates of a hereditary deficit in verb and action processing. Brain. 2006;129(2):321–332. doi: 10.1093/brain/awh701. [DOI] [PubMed] [Google Scholar]
- Barbarotto R, Capitani E, Spinnler H, Trivelli C. Slowly progressive semantic impairment with category specificity. Neurocase. 1995;1(2):107–119. [Google Scholar]
- Barsalou LW. Perceptual symbols systems. Behavioral and Brain Sciences. 1999;22:577–660. doi: 10.1017/s0140525x99002149. [DOI] [PubMed] [Google Scholar]
- Barsalou LW. Grounded cognition. Annual Review of Psychology. 2008;59:617–645. doi: 10.1146/annurev.psych.59.103006.093639. [DOI] [PubMed] [Google Scholar]
- Barsalou LW, Simmons WK, Barbey AK, Wilson CD. Grounding conceptual knowledge in modality-specific systems. Trends in Cognitive Sciences. 2003;7(2):84–91. doi: 10.1016/s1364-6613(02)00029-3. [DOI] [PubMed] [Google Scholar]
- Beauchamp MS, Lee KE, Haxby JV, Martin A. Parallel visual motion processing streams for manipulable objects and human movements. Neuron. 2002;34(1):149–159. doi: 10.1016/s0896-6273(02)00642-6. [DOI] [PubMed] [Google Scholar]
- Beauchamp MS, Lee KE, Haxby JV, Martin A. FMRI responses to video and point-light displays of moving humans and manipulable objects. Journal of Cognitive Neuroscience. 2003;15(7):991–1001. doi: 10.1162/089892903770007380. [DOI] [PubMed] [Google Scholar]
- Bertella L, Albani G, Greco E, Priano L, Mauro A, Marchi S, Bulla D, Semenza C. Noun-verb dissociation in Parkinson’s disease. Brain and Cognition. 2002;48(2–3):277–280. [PubMed] [Google Scholar]
- Bian H, Grossman M. Frontotemporal lobar degeneration: recent progress in antemortem diagnosis. Acta Neuropathologica. 2007;114(1):23–29. doi: 10.1007/s00401-007-0235-4. [DOI] [PubMed] [Google Scholar]
- Bonner MF, Vesely L, Price C, Anderson C, Richmond L, Farag C, Avants B, Grossman M. Reversal of the concreteness effect in semantic dementia. Cognitive Neuropsychology. 2009;26(6):568–579. doi: 10.1080/02643290903512305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulenger V, Roy AC, Paulignan Y, Deprez V, Jeannerod M, Nazir TA. Cross-talk between language processes and overt motor behavior in the first 200 msec of processing. Journal of Cognitive Neuroscience. 2006;18(10):1607–1615. doi: 10.1162/jocn.2006.18.10.1607. [DOI] [PubMed] [Google Scholar]
- Boulenger V, Mechtouff L, Thobois S, Broussolle E, Jeannerod M, Nazir TA. Word processing in Parkinson’s disease is impaired for action verbs but not for concrete nouns. Neuropsychologia. 2008;46(2):743–756. doi: 10.1016/j.neuropsychologia.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Breedin SD, Saffran EM, Coslett HB. Reversal of the concreteness effect in a patient with semantic dementia. Cognitive Neuropsychology. 1994;11(6):617–660. [Google Scholar]
- Breedin SD, Saffran EM, Schwartz MF. Semantic factors in verb retrieval: An effect of complexity. Brain and Language. 1998;63(1):1–31. doi: 10.1006/brln.1997.1923. [DOI] [PubMed] [Google Scholar]
- Buxbaum LJ, Sirigu A, Schwartz MF, Klatzky R. Cognitive representations of hand posture in ideomotor apraxia. Neuropsychologia. 2003;41(8):1091–1113. doi: 10.1016/s0028-3932(02)00314-7. [DOI] [PubMed] [Google Scholar]
- Cappa SF, Perani D. The neural correlates of noun and verb processing. Journal of Neurolinguistics. 2003;16(2–3):183–189. [Google Scholar]
- Caramazza A, Mahon BZ. The organization of conceptual knowledge: The evidence from category-specific semantic deficits. Trends in Cognitive Sciences. 2003;7(8):354–361. doi: 10.1016/s1364-6613(03)00159-1. [DOI] [PubMed] [Google Scholar]
- Caramazza A, Mahon BZ. The organisation of conceptual knowledge in the brain: The future’s past and some future directions. Cognitive Neuropsychology. 2006;23(1):13–38. doi: 10.1080/02643290542000021. [DOI] [PubMed] [Google Scholar]
- Caramazza A, Shelton JR. Domain-specific knowledge systems in the brain: The animate-inanimate distinction. Journal of Cognitive Neuroscience. 1998;10(1):1–34. doi: 10.1162/089892998563752. [DOI] [PubMed] [Google Scholar]
- Chao LL, Haxby JV, Martin A. Attribute-based neural substrates in temporal cortex for perceiving and knowing about objects. Nature Neuroscience. 1999;2(10):913–919. doi: 10.1038/13217. [DOI] [PubMed] [Google Scholar]
- Chao LL, Martin A. Representation of manipulable man-made objects in the dorsal stream. NeuroImage. 2000;12(4):478–484. doi: 10.1006/nimg.2000.0635. [DOI] [PubMed] [Google Scholar]
- Coltheart M. The MRC Psycholinguistic database. Quarterly Journal of Experimental Psychology. 1981;33(a):497–505. [Google Scholar]
- Coslett HB, Saffran EM, Schwoebel J. Knowledge of the human body: A distinct semantic domain. Neurology. 2002;59(3):357–363. doi: 10.1212/wnl.59.3.357. [DOI] [PubMed] [Google Scholar]
- Crutch SJ, Warrington EK. The selective impairment of fruit and vegetable knowledge: A multiple processing channels account of fine-grain category specificity. Cognitive Neuropsychology. 2003;20(3–6):355–372. doi: 10.1080/02643290244000220. [DOI] [PubMed] [Google Scholar]
- Damasio AR, Tranel D. Nouns and verbs are retrieved with differently distributed neural systems. Proceedings of the National Academy of Sciences USA. 1993;90:4957–4960. doi: 10.1073/pnas.90.11.4957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damasio H, Grabowski TJ, Tranel D, Ponto LL, Hichwa RD, Damasio AR. Neural correlates of naming actions and of naming spatial relations. NeuroImage. 2001;13(6 Pt 1):1053–1064. doi: 10.1006/nimg.2001.0775. [DOI] [PubMed] [Google Scholar]
- Damasio H, Tranel D, Grabowski T, Adolphs R, Damasio A. Neural systems behind word and concept retrieval. Cognition. 2004;92(1–2):179–229. doi: 10.1016/j.cognition.2002.07.001. [DOI] [PubMed] [Google Scholar]
- De Renzi E, Lucchelli F. Are semantic systems separately represented in the brain? The case of living category impairment. Cortex. 1994;30(1):3–25. doi: 10.1016/s0010-9452(13)80322-x. [DOI] [PubMed] [Google Scholar]
- Dixon MJ, Bub DN, Chertkow H, Arguin M. Object identification deficits in dementia of the Alzheimer type: Combined effects of semantic and visual proximity. Journal of the International Neuropsychological Society. 1999;5(4):330–345. doi: 10.1017/s1355617799544044. [DOI] [PubMed] [Google Scholar]
- Druks J, Masterson J, Kopelman M, Clare L, Rose A, Rai G. Is action naming better preserved (than object naming) in Alzheimer’s disease and why should we ask? Brain and Language. 2006;98(3):332–340. doi: 10.1016/j.bandl.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Eickhoff S, Stephan K, Mohlberg H, Grefkes C, Fink G, Amunts K, Zilles K. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. NeuroImage. 2005;25:1325–1335. doi: 10.1016/j.neuroimage.2004.12.034. [DOI] [PubMed] [Google Scholar]
- Farah MJ. The neurological basis of mental imagery: A Computational Analysis. Cognition. 1984;18:245–272. doi: 10.1016/0010-0277(84)90026-x. [DOI] [PubMed] [Google Scholar]
- Farah MJ, McClelland JL. A computational model of semantic memory impairment: Modality specificity and emergent category specificity. Journal of Experimental Psychology: General. 1991;120(4):339–357. [PubMed] [Google Scholar]
- Fung TD, Chertkow H, Murtha S, Whatmough C, Paloquin L, Whitehead V, Templeman FD. The spectrum of category effects in object and action knowledge in dementia of the Alzheimer’s type. Neuropsychology. 2001;15(3):371–379. [PubMed] [Google Scholar]
- Gallese V, Lakoff G. The brain’s concepts: The role of the sensory-motor system in conceptual knowledge. Cognitive Neuropsychology. 2005;22(3):455–479. doi: 10.1080/02643290442000310. [DOI] [PubMed] [Google Scholar]
- Galton CJ, Patterson K, Graham K, Lambon Ralph MA, Williams G, Antoun N, Sahakian BJ, Hodges JR. Differing patterns of temporal atrophy in Alzheimer’s disease and semantic dementia. Neurology. 2001;57(2):216–225. doi: 10.1212/wnl.57.2.216. [DOI] [PubMed] [Google Scholar]
- Geyer S. Series: Advances in Anatomy, Embryology, and Cell Biology. Heidelberg: Springer-Verlag; 2004. The microstructural border between the motor and the cognitive domain in the human cerebral cortex. [DOI] [PubMed] [Google Scholar]
- Glenberg AM, Kaschak MP. Grounding language in action. Psychonomic Bulletin and Review. 2002;9(3):558–565. doi: 10.3758/bf03196313. [DOI] [PubMed] [Google Scholar]
- Glosser G, Baker KM, de Vries JJ, Alavi A, Grossman M, Clark CM. Disturbed visual processing contributes to impaired reading in Alzheimer’s disease. Neuropsychologia. 2002;40(7):902–909. doi: 10.1016/s0028-3932(01)00165-8. [DOI] [PubMed] [Google Scholar]
- Gonnerman LM, Andersen ES, Devlin JT, Kempler D, Seidenberg MS. Double dissociation of semantic categories in Alzheimer’s disease. Brain and Language. 1997;57(2):254–279. doi: 10.1006/brln.1997.1752. [DOI] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Dronkers NF, Rankin KP, Ogar JM, Phengrasamy L, Rosen HJ, Johnson JK, Weiner MW, Miller BL. Cognition and anatomy in three variants of primary progressive aphasia. Annals of Neurology. 2004;55(3):335–346. doi: 10.1002/ana.10825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Murray RC, Rankin KP, Weiner MW, Miller BL. Clinical, cognitive and anatomical evolution from nonfluent progressive aphasia to corticobasal syndrome: a case report. Neurocase. 2004;10(6):426–436. doi: 10.1080/13554790490894011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabowski TJ, Damasio H, Damasio AR. Premotor and prefrontal correlates of category-related lexical retrieval. NeuroImage. 1998;7(3):232–243. doi: 10.1006/nimg.1998.0324. [DOI] [PubMed] [Google Scholar]
- Grafton ST, Fadiga L, Arbib MA, Rizzolatti G. Premotor cortex activation during observation and naming of familiar tools. NeuroImage. 1997;6(4):231–236. doi: 10.1006/nimg.1997.0293. [DOI] [PubMed] [Google Scholar]
- Grossman M. Primary progressive aphasia: clinicopathological correlations. Nature Reviews Neurology. 2010;6(2):88–97. doi: 10.1038/nrneurol.2009.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman M, Anderson C, Khan A, Avants B, Elman L, McCloskey L. Impaired action knowledge in amyotrophic lateral sclerosis. Neurology. 2008;71:1396–1401. doi: 10.1212/01.wnl.0000319701.50168.8c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman M, Koenig P, DeVita C, Glosser G, Alsop D, Detre J, Gee J. The neural basis for category-specific knowledge: An fMRI study. Neuroimage. 2002;15:936–948. doi: 10.1006/nimg.2001.1028. [DOI] [PubMed] [Google Scholar]
- Grossman M, Libon DJ, Forman MS, Massimo L, Wood E, Moore P, et al. Distinct antemortem profiles in patients with pathologically defined frontotemporal dementia. Archives of Neurology. 2007;64(11):1601–1609. doi: 10.1001/archneur.64.11.1601. [DOI] [PubMed] [Google Scholar]
- Grossman M, McMillan C, Moore P, Ding L, Glosser G, Work M, et al. What’s in a name: voxel-based morphometric analyses of MRI and naming difficulty in Alzheimer’s disease, frontotemporal dementia and corticobasal degeneration. Brain. 2004;127(3):628–649. doi: 10.1093/brain/awh075. [DOI] [PubMed] [Google Scholar]
- Grossman M, Mickanin J, Onishi K, Hughes E. Progressive nonfluent aphasia: Language, cognitive, and PET measures contrasted with probable Alzheimer’s disease. Journal of Cognitive Neuroscience. 1996;8(2):135–154. doi: 10.1162/jocn.1996.8.2.135. [DOI] [PubMed] [Google Scholar]
- Hauk O, Davis MH, Kherif F, Pulvermüller F. Imagery or meaning? Evidence for a semantic origin of category-specific brain activity in metabolic imaging. European Journal of Neuroscience. 2008;27:1856–1866. doi: 10.1111/j.1460-9568.2008.06143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauk O, Johnsrude I, Pulvermüller F. Somatotopic representation of action words in human motor and premotor cortex. Neuron. 2004;41:301–207. doi: 10.1016/s0896-6273(03)00838-9. [DOI] [PubMed] [Google Scholar]
- Hillis AE, Rapp BC, Caramazza A. Constraining claims about theories of semantic memory: More on unitary versus multiple semantics. Cognitive Neuropsychology. 1995;12:175–186. [Google Scholar]
- Hillis AE, Sangjin O, Ken L. Deterioration of naming nouns versus verbs in Primary Progressive Aphasia. Annals of Neurology. 2004;55:268–275. doi: 10.1002/ana.10812. [DOI] [PubMed] [Google Scholar]
- Hu FB, McMillan C, Libon D, Leight S, Forman M, Lee V, Trojanowski JQ, Grossman M. Multi-modal predictors for Alzheimer’s Disease in non-fluent Primary Progressive Aphasia. Neurology. doi: 10.1212/WNL.0b013e3181ed9c52. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys GW, Forde EME. Hierarchies, similarity, and interactivity in object recognition: “Category-specific” neuropsychological deficits. Behavioral and Brain Sciences. 2001;24(3):453–509. [PubMed] [Google Scholar]
- Humphreys GW, Riddoch MJ. A case series analysis of “category-specific” deficits of living things: The HIT account. Cognitive Neuropsychology. Special Issue: The organisation of conceptual knowledge in the brain: Neuropsychological and neuroimaging perspectives. 2003;20(3–6):263–306. doi: 10.1080/02643290342000023. [DOI] [PubMed] [Google Scholar]
- Humphreys GW, Riddoch MJ. Features, objects, action: The cognitive neuropsychology of visual object processing, 1984–2004. Cognitive Neuropsychology. 2006;23(1):156–183. doi: 10.1080/02643290542000030. [DOI] [PubMed] [Google Scholar]
- Jefferies E, Lambon Ralph MA. Semantic impairment in stroke aphasia versus semantic dementia: A case-series comparison. Brain. 2006;129(8):2132–2147. doi: 10.1093/brain/awl153. [DOI] [PubMed] [Google Scholar]
- Jefferies E, Patterson K, Lambon Ralph MA. Deficits of knowledge versus executive control in semantic cognition: Insights from cued naming. Neuropsychologia. 2008;46(2):649–658. doi: 10.1016/j.neuropsychologia.2007.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kable JW, Lease-Spellmeyer J, Chatterjee A. Neural substrates of action event knowledge. Journal of Cognitive Neuroscience. 2002;14(5):795–805. doi: 10.1162/08989290260138681. [DOI] [PubMed] [Google Scholar]
- Kan IP, Kable JW, Van Scoyoc A, Chatterjee A, Thompson-Schill SL. Fractionating the left frontal response to tools: Dissociable effects of motor experience and lexical competition. Journal of Cognitive Neuroscience. 2006;18(2):267–277. doi: 10.1162/089892906775783723. [DOI] [PubMed] [Google Scholar]
- Keil FC. Concepts, kinds, and cognitive development. 1. Cambridge: MIT Press; 1989. [Google Scholar]
- Kellenbach ML, Brett M, Patterson K. Large, colorful, or noisy? Attribute- and modality-specific activations during retrieval of perceptual attribute knowledge. Cognitive Affective and Behavioral Neuroscience. 2001;1(3):207–221. doi: 10.3758/cabn.1.3.207. [DOI] [PubMed] [Google Scholar]
- Koenig P, Grossman M. Process and content in semantic memory. In: Hart JJ, Kraut MA, editors. Neural basis of semantic memory. Cambridge: Cambridge University Press; 2007. pp. 247–264. [Google Scholar]
- Koenig P, Smith EE, Grossman M. Semantic categorisation of novel objects in frontotemporal dementia. Cognitive Neuropsychology. 2006;23(4):541–562. doi: 10.1080/02643290542000094. [DOI] [PubMed] [Google Scholar]
- Kosslyn SM. Mental images and the brain. Cognitive Neuropsychology. 2005;22(3–4):333–347. doi: 10.1080/02643290442000130. [DOI] [PubMed] [Google Scholar]
- Laiacona M, Capitani E, Caramazza A. Category-specific semantic deficits do not reflect the sensory/functional organization of the brain: A test of the “sensory quality” hypothesis. Neurocase. 2003;9(3):221–231. doi: 10.1076/neur.9.3.221.15562. [DOI] [PubMed] [Google Scholar]
- Laiacona M, Caramazza A. The noun/verb dissociation in language production: Varieties of causes. Cognitive Neuropsychology. 2004;21(2):103–123. doi: 10.1080/02643290342000311. [DOI] [PubMed] [Google Scholar]
- Lambon Ralph MA, Lowe C, Rogers TT. Neural basis of category-specific semantic deficits for living things: evidence from semantic dementia, HSVE and a neural network model. Brain. 2007;130(Pt 4):1127–1137. doi: 10.1093/brain/awm025. [DOI] [PubMed] [Google Scholar]
- Laws KR, Sartori G. Category Deficits and Paradoxical Dissociations in Alzheimer’s Disease and Herpes Simplex Encephalitis. Journal of Cognitive Neuroscience. 2005;17(9):1453–1459. doi: 10.1162/0898929054985428. [DOI] [PubMed] [Google Scholar]
- Mahon BZ, Caramazza A. The orchestration of the sensory-motor systems: Clues from neuropsychology. Cognitive Neuropsychology. 2005;22(3/4):480–494. doi: 10.1080/02643290442000446. [DOI] [PubMed] [Google Scholar]
- Mahon BZ, Caramazza A. A critical look at the embodied cognition hypothesis and a new proposal for grounding conceptual content. Journal of Physiology: Paris. 2008;102(1–3):59–70. doi: 10.1016/j.jphysparis.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Mahon BZ, Caramazza A. Concepts and categories: A cognitive neuropsychological perspective. Annual Review of Psychology. 2009;60:27–51. doi: 10.1146/annurev.psych.60.110707.163532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A. Neural foundations for conceptual representations: Evidence from functional brain imaging. In: Hart JJ, Kraut MA, editors. Neural Basis of Semantic Memory. Cambridge, UK: Cambridge University Press; 2007. pp. 302–330. [Google Scholar]
- Martin A. The representation of object concepts in the brain. Annual Review of Psychology. 2007;58:25–45. doi: 10.1146/annurev.psych.57.102904.190143. [DOI] [PubMed] [Google Scholar]
- Martin A, Chao LL. Semantic memory and the brain: structure and processes. Current Opinion in Neurobiology. 2001;11:194–201. doi: 10.1016/s0959-4388(00)00196-3. [DOI] [PubMed] [Google Scholar]
- Martin A, Haxby JV, Lalonde FM, Wiggs CL, Ungerleider LG. Discrete cortical regions associated with knowledge of color and knowledge of action. Science. 1995;270(5233):102–105. doi: 10.1126/science.270.5233.102. [DOI] [PubMed] [Google Scholar]
- Martin A, Ungerleider LG, Haxby JV. Category specificity and the brain: The sensory/motor model of semantic representations of objects. In: Gazanniga MS, editor. The New Cognitive Neurosciences. 2. Cambridge, MA: MIT Press; 2000. pp. 1023–1036. [Google Scholar]
- Martin A, Wiggs CL, Ungerleider LG, Haxby JV. Neural correlates of category-specific knowledge. Nature. 1996;379(6566):649–652. doi: 10.1038/379649a0. [DOI] [PubMed] [Google Scholar]
- Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, Black S, Freedman M, Kertesz A, Robert PH, Albert M, Boone K, Miller BL, Cummings J, Benson DF. Frontotemporal lobar degeneration: A consensus on clinical diagnostic criteria. Neurology. 1998;51(6):1546–1554. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- Nestor PJ, Graham NL, Fryer TD, Williams GB, Patterson K, Hodges JR. Progressive non-fluent aphasia is associated with hypometabolism centred on the left anterior insula. Brain. 2003;126:2406–2418. doi: 10.1093/brain/awg240. [DOI] [PubMed] [Google Scholar]
- Ogar JM, Dronkers NF, Brambati SM, Miller BL, Gorno-Tempini ML. Progressive nonfluent aphasia and its characteristic motor speech deficits. Alzheimer Disease and Associated Disorders. 2007;21(4):S23–30. doi: 10.1097/WAD.0b013e31815d19fe. [DOI] [PubMed] [Google Scholar]
- Patterson K, Graham NL, Lambon Ralph MA, Hodges JR. Progressive non-fluent aphasia is not a progressive form of non-fluent (post-stroke) aphasia. Aphasiology. 2006;20(9–11):1018–1034. [Google Scholar]
- Patterson K, Nestor PJ, Rogers TT. Where do you know what you know? The representation of semantic knowledge in the human brain. Nature Reviews Neuroscience. 2007;8(12):976–987. doi: 10.1038/nrn2277. [DOI] [PubMed] [Google Scholar]
- Peelle JE, Cooke A, Moore P, Vesely L, Grossman M. Syntactic and thematic components of sentence processing in progressive nonfluent aphasia and nonaphasic frontotemporal dementia. Journal of Neurolinguistics. 2007;20(6):482–494. doi: 10.1016/j.jneuroling.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peelle JE, Troiani V, Gee J, Moore P, McMillan C, Vesely L, Grossman M. Sentence comprehension and voxel-based morphometry in progressive nonfluent aphasia, semantic dementia, and nonaphasic frontotemporal dementia. Journal of Neurolinguistics. 2008;21(5):418–432. doi: 10.1016/j.jneuroling.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peelle JE, Troiani V, Grossman M. Interaction between process and content in semantic memory: An fMRI study of noun feature knowledge. Neuropsychologia. 2009;47:995–1003. doi: 10.1016/j.neuropsychologia.2008.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perani D, Cappa SF, Bettinardi V, Bressi S, Gorno-Tempini M, Matarrese M. Different neural systems for the recognition of animals and man-made tools. Neuroreport. 1995;6(12):1637–1641. doi: 10.1097/00001756-199508000-00012. [DOI] [PubMed] [Google Scholar]
- Postle N, McMahon KL, Ashton R, Meredith M, de Zubicaray GI. Action word meaning representations in cytoarchitectonically defined primary and premotor cortices. Neuroimage. 2008;43(3):634–644. doi: 10.1016/j.neuroimage.2008.08.006. [DOI] [PubMed] [Google Scholar]
- Reilly J, Cross K, Troiani V, Grossman M. Single word semantic judgments in Semantic Dementia: Do phonology and grammatical class count? Aphasiology. 2007;21(6/7/8):558–569. [Google Scholar]
- Reilly J, Peelle JE. Effects of semantic impairment on language processing in Semantic Dementia. Seminars in Speech and Language. 2008;29:32–43. doi: 10.1055/s-2008-1061623. [DOI] [PubMed] [Google Scholar]
- Rodd JM, Davis MH, Johnsrude IS. The neural mechanisms of speech comprehension: fMRI studies of semantic ambiguity. Cerebral Cortex. 2005;15:1261–1269. doi: 10.1093/cercor/bhi009. [DOI] [PubMed] [Google Scholar]
- Rogers TT, Lambon Ralph MA, Garrard P, Bozeat S, McClelland JL, Hodges JR, Patterson K. Structure and Deterioration of Semantic Memory: A Neuropsychological and Computational Investigation. Psychological Review. 2004;111(1):205–235. doi: 10.1037/0033-295X.111.1.205. [DOI] [PubMed] [Google Scholar]
- Rosci C, Chiesa V, Laiacona M, Capitani E. Apraxia is not associated to a disproportionate naming impairment for manipulable objects. Brain and Cognition. 2003;53(2):412–415. doi: 10.1016/s0278-2626(03)00156-8. [DOI] [PubMed] [Google Scholar]
- Rothi LJ, Ochipa C, Heilman KM. A cognitive neuropsychological model of limb praxis. Cognitive Neuropsychology. 1991;8(6):443–458. [Google Scholar]
- Rogers TT, McClelland JL. A Parallel Distributed Processing Approach to Semantic Cognition: Applications to Conceptual Development. Gershkoff Stowe, Lisa. 2005 [Google Scholar]
- Sacchett C, Humphreys GW. Calling a squirrel a squirrel but a canoe a wigwam: A category-specific deficit for artefactual objects and body parts. Cognitive Neuropsychology. 1992;9(1):73–86. [Google Scholar]
- Samson D, Pillon A. A case of impaired knowledge for fruit and vegetables. Cognitive Neuropsychology. Special Issue: The organisation of conceptual knowledge in the brain: Neuropsychological and neuroimaging perspectives. 2003;20(3–6):373–400. doi: 10.1080/02643290244000329. [DOI] [PubMed] [Google Scholar]
- Sartori G, Gnoato F, Mariani I, Prioni S, Lombardi L. Semantic relevance, domain specificity and the sensory/functional theory of category-specificity. Neuropsychologia. 2007;45(5):966–976. doi: 10.1016/j.neuropsychologia.2006.08.028. [DOI] [PubMed] [Google Scholar]
- Shallice T. Specialisation within the semantic system. Cognitive Neuropsychology. 1988;5(1):133–142. [Google Scholar]
- Simmons WK, Ramjee V, Beauchamp MS, McRae K, Martin A, Barsalou LW. A common neural substrate for perceiving and knowing about color. Neuropsychologia. 2007;45(12):2802–2810. doi: 10.1016/j.neuropsychologia.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snodgrass JG, Vanderwart M. A standardized set of 260 pictures: Norms for name agreement, image agreement, familiarity, and visual complexity. Journal of Experimental Psychology: Human Learning and Memory. 1980;6(2):174–215. doi: 10.1037//0278-7393.6.2.174. [DOI] [PubMed] [Google Scholar]
- Thompson CK, Ballard KJ, Tait ME, Weintraub S, Mesulam MM. Patterns of language decline in non-fluent primary progressive aphasia. Aphasiology. 1997;11(4–5):297–331. [Google Scholar]
- Tyler LK, Moss HE, Durrant-Peatfield MR, Levy JP. Conceptual structure and the structure of concepts: A distributed account of category-specific deficits. Brain and Language. 2000;75(2):195–231. doi: 10.1006/brln.2000.2353. [DOI] [PubMed] [Google Scholar]
- Tyler LK, Stamatakis EA, Dick E, Bright P, Fletcher P, Moss H. Objects and their actions: Evidence for a neurally distributed semantic system. NeuroImage. 2003;18:542–557. doi: 10.1016/s1053-8119(02)00047-2. [DOI] [PubMed] [Google Scholar]
- van Schie HT, Toni I, Bekkering H. Comparable mechanisms for action and language: neural systems behind intentions, goals, and means. Cortex. 2006;42(4):495–498. doi: 10.1016/s0010-9452(08)70385-x. [DOI] [PubMed] [Google Scholar]
- Warrington EK, McCarthy RA. Categories of knowledge. Further fractionations and an attempted integration. Brain. 1987;110 (Pt 5):1273–1296. doi: 10.1093/brain/110.5.1273. [DOI] [PubMed] [Google Scholar]
- Warrington EK, Shallice T. Category specific semantic impairments. Brain. 1984;107:829–854. doi: 10.1093/brain/107.3.829. [DOI] [PubMed] [Google Scholar]
- Yi H, Moore P, Grossman M. Reversal of the concreteness effect for verbs in semantic dementia. Cognitive Neuropsychology. 2007;21(1):1–19. doi: 10.1037/0894-4105.21.1.9. [DOI] [PubMed] [Google Scholar]