Abstract
The scaffold protein kinase suppressor of Ras 1 (KSR1) is critical for efficient activation of ERK in a number of cell types. Consistent with this, we observed a defect in ERK activation in thymocytes that lack KSR1. Interestingly, we found that the defect was much greater after PMA stimulation than by CD3 activation. Since ERK activation is thought to be important for thymocyte development, we analyzed thymocyte selection in KSR1-deficient (KSR1−/−) mice. We found that positive selection in two different TCR transgenic models, HY and AND, was normal. On the other hand, negative selection in the HY model was slightly impaired in KSR1−/− mice. However, a defect in negative selection was not apparent in the AND TCR model system or in an endogenous superantigen-mediated model of negative selection. These results suggest that, despite a requirement for KSR1 for full ERK activation in thymocytes, full and efficient ERK activation is not essential for the majority of thymocyte selection events.
Keywords: Kinase Suppressor of Ras, ERK, thymocyte development
Introduction
T cell development is a complex, multi-step process that begins with seeding of the thymus by progenitor cells arising from the bone marrow. Progenitor cells progress through three distinct stages before exiting the thymus as mature T cells into the periphery. These developmental stages can be characterized by the expression of the cell surface markers CD25, CD44, the co-receptors CD4 and CD8, as well as the TCR itself. Early in development, thymocytes that lack expression of either co-receptor (DN) begin to rearrange and test their TCR α-chains. Once successful generation of the TCR α-chain has been accomplished, thymocytes begin the processes of positive and negative selection. At this stage, both CD4 and CD8 co-receptors are expressed (DP) and interactions with self-peptide and MHC molecules are critical in determining thymocyte fate. Thymocytes must receive the appropriate signal through their TCR to undergo positive selection in order to escape death by neglect and develop into CD4 or CD8 lineage cells [1, 2]. Further, the signal delivered through the TCR via MHC/self-peptide must not be too strong or the programmed cell death of thymocytes will be induced, a process termed negative selection [3, 4].
The critical role of the ERK-MAPK signaling cascade in T cell development has been well studied but the results have been inconsistent [5–12]. Many of these studies used transgenic mice expressing dominant-negative or constitutively active forms of MAPK pathway components. These studies generated conclusions that ERK was implicated in either positive but not negative selection, or in both positive and negative selection [3, 6, 8, 9, 12]. A more definitive study used conditional deletion to remove both ERK isoforms at various stages of thymocyte development [7]. These studies demonstrated that thymocytes lacking both isoforms of ERK have a partial developmental block at the DN3 stage. If ERK was deleted following the DN3 stage, however, a complete block in positive selection but not negative selection was observed [7, 13]. Interestingly, when double ERK knockout mice were analyzed on a TCR transgenic background, some positive selection did occur. This study also suggested that ERK2 plays a more important role in CD4 T cell development compared to CD8 T cell development [7]. More recent work has suggested that the degree and duration of ERK activation may distinguish positive and negative selection and possibly CD4 versus CD8 lineage decisions [14]. All of this work suggests that modulators of ERK activation may play an important role in thymocyte differentiation.
KSR1 was originally identified as a positive regulator of Ras signaling in C. elegans and Drosophila and homologues were subsequently discovered in mammals [15–17]. Further studies demonstrated that KSR1 is a scaffold molecule that binds critical components of the MAPK cascade and is essential for ERK activation in a variety of cell types [18]. In the immune system, KSR1 is critical for the production of pro-inflammatory cytokines by innate immune cells in response to stress signals and required for efficient activation of peripheral T cells [18, 19]. Little is known, however, about the role of KSR1 in the development of T cells, although a cursory examination revealed no gross abnormalities [18].
In this study, we examined the role of KSR1 in thymocyte development. As expected, KSR1 deletion resulted in impairment of ERK activation in thymocytes following TCR stimulation. Interestingly, this diminished ERK activation had only minimal effects on T cell development. Positive selection was normal in both KSR1−/− AND (CD4+) and HY (CD8+) TCR transgenic mice. Negative selection also appeared normal in KSR1−/− AND mice, but was slightly impaired in male HY KSR1−/− mice. Negative selection in a third model of negative selection, endogenous superantigen deletion, also appeared normal. These data indicate that a minimal amount of ERK activation may be sufficient to sustain thymocyte maturation and that strong activation of ERK may only be required for negative selection of certain TCR expressing thymocytes.
Results
KSR1 is required for efficient ERK activation in thymocytes
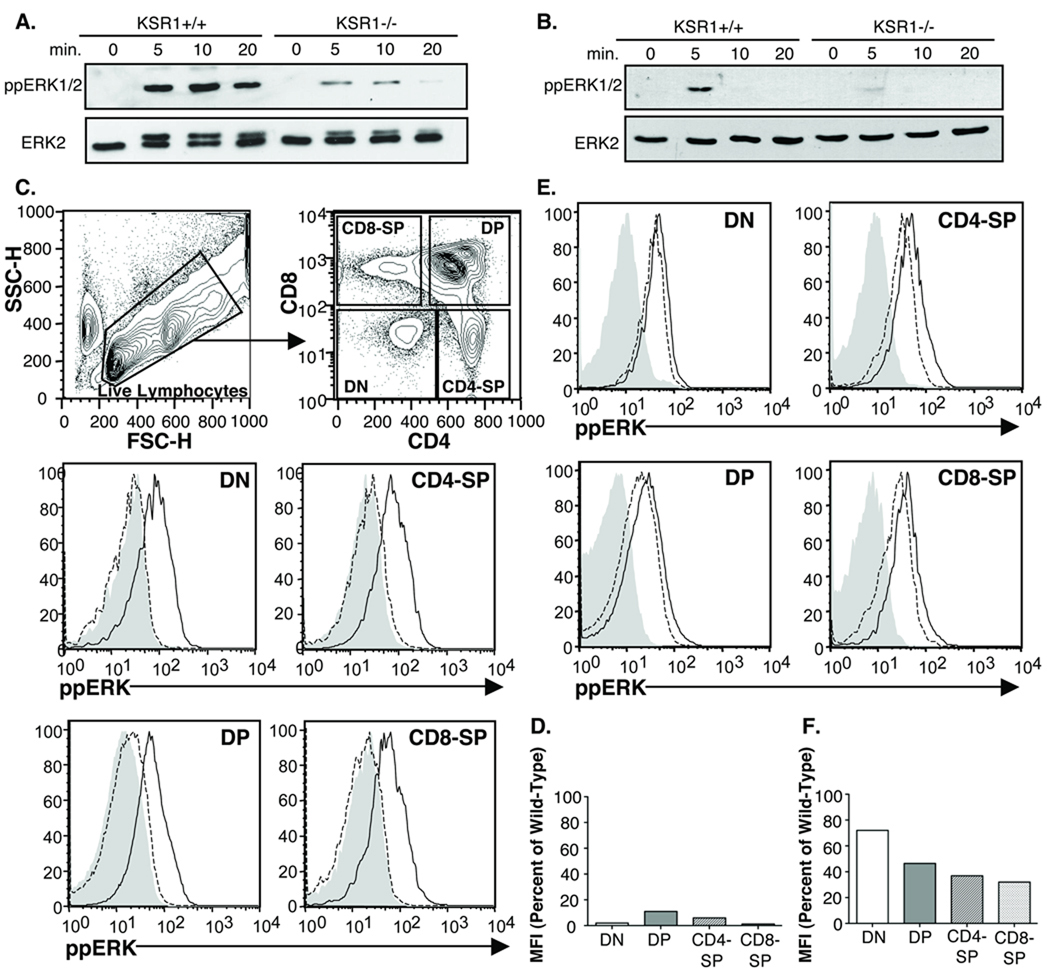
KSR1 has been shown to be required for the efficient activation of ERK in a number of cell types [18–22]. We previously reported a defect in ERK activation in peripheral T cells in response to PMA or CD3-crosslinking [18]. To determine the extent to which ERK activation in thymocytes also requires KSR1, we stimulated KSR1 wild-type or knockout thymocytes with PMA (Fig 1A) or anti-CD3 (Fig 1B) for various time-points, lysed the cells and measured the level of activated ERK using an ERK phospho-specific antibody. As expected, there was a significant defect in ERK activation in KSR1−/− thymocytes downstream of both stimuli. Interestingly, we noted that the defect after PMA stimulation was reproducibly always more significant than after CD3 stimulation.
Figure 1. ERK activation is impaired in KSR1−/− thymocytes.
Thymi from wild-type or KSR1−/− mice were processed into a single-cell suspension. 1 × 107 cells were stimulated for the indicated time-points with either (A) 40 nM PMA or (B) 5ug/mL anti-CD3 (2C11). Cells were lysed in NP-40 buffer, run on SDS-PAGE gel, transferred and blotted for total or phospho-ERK. ECL western blotting substrate was used for detection. Blots are representative of 3 experiments from 3 separate mice each. (C, E) FACS analysis of ERK activation in thymocyte subsets. Filled histogram represents wild-type unstimulated, solid black line is wild-type following 3 minutes of anti-CD3 stimulation and dashed black line is KSR1−/− after 3 minutes anti-CD3. FACS plots are representative of 2 experiments each. (D,F). Quantification of FACS data was achieved by dividing the MFI of KSR1−/− thymocyte subsets following stimulation by the MFI of the corresponding wild-type population after subtraction of baseline MFI. Bar graph is the average of 2 experiments.
We quantified the ERK activation defect using flow cytometric analysis using the phospho-ERK antibody (Figure 1 C–F). This also allowed us to measure the ERK activation defect in individual thymocyte subsets. The analysis confirmed that there is a significant ERK activation defect after PMA activation and that it is more significant that the defect after CD3 activation (Fig 1C–F). The ERK activation defect in KSR1−/− thymocytes appeared to be greatest in CD4 and CD8 SP with a smaller but consistent defect in the DN and DP subsets. These data indicate that KSR1 is required for the full activation of ERK in thymocytes but suggests that the role of KSR1 may be more important for PMA stimulation as compared to CD3 activation.
Positive selection is normal in the HY TCR transgenic KSR1-deficient mice
The defect in ERK activation in KSR1−/− thymocytes and previous data suggesting that ERK activation is critical for thymocyte development [5–12, 23] led us to analyze thymocyte development in KSR1−/− mice. Since we previously reported grossly normal thymocyte development in KSR1-deficient mice with a polyclonal TCR repertoire [18], we crossed KSR1−/− mice to two different TCR transgenic mice, the MHC-II restricted TCR transgenic AND [24] and the MHC-I restricted HY TCR [25], to examine thymocyte selection in the context of a clonal TCR repertoire.
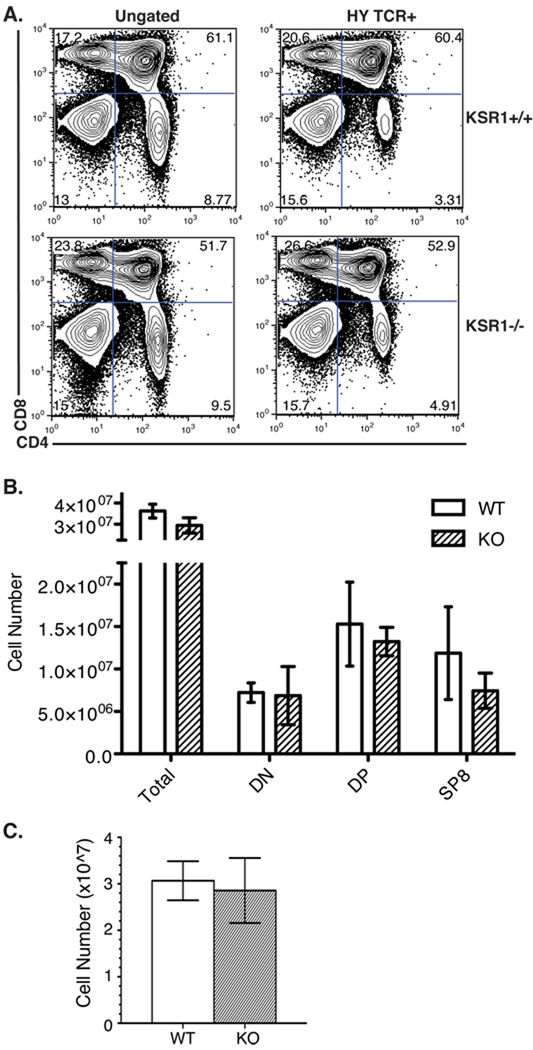
Since ERK has mainly been implicated in positive selection [7–9, 12], we first analyzed female HY TCR transgenic mice to determine the effect of KSR1 on positive selection of CD8 HY TCR thymocytes. In female mice, because of the absence of the peptide from the male antigen, the HY+ T cells are not deleted but are instead positively selected by interaction with an unknown endogenous peptide [25]. Flow cytometric analysis of these mice demonstrated that the percentages and cell numbers of DN, DP, and SP were comparable between KSR1−/− and wild-type HY thymi when either total or HY TCR+ thymocytes were analyzed (Figure 2A, B). There were similar numbers of peripheral HY TCR+ CD8+ T cells in female wild-type and knockout mice (Figure 2C). These data suggest that KSR1 is not required for the positive selection of these cells.
Figure 2. Positive selection is normal in KSR1−/− HY female mice.
Single cell suspensions of (A, B) thymocytes or (C) splenocytes from HY female wild-type or KSR1−/− mice were counted and stained with antibodies to CD4, CD8, CD3 and HY TCR. Cells were collected on a BD FACSCalibur and analyzed using Flowjo software. Representative FACS plots are shown either ungated (left) or gated on HY TCR+ cells (right) in (A). Cell numbers are representative of 4 mice per group and are shown as mean ± SD (B, C). Differences between groups are not significant (P > 0.05; unpaired Student’s t-test).
Negative selection is impaired in HY TCR transgenic KSR1−/− mice
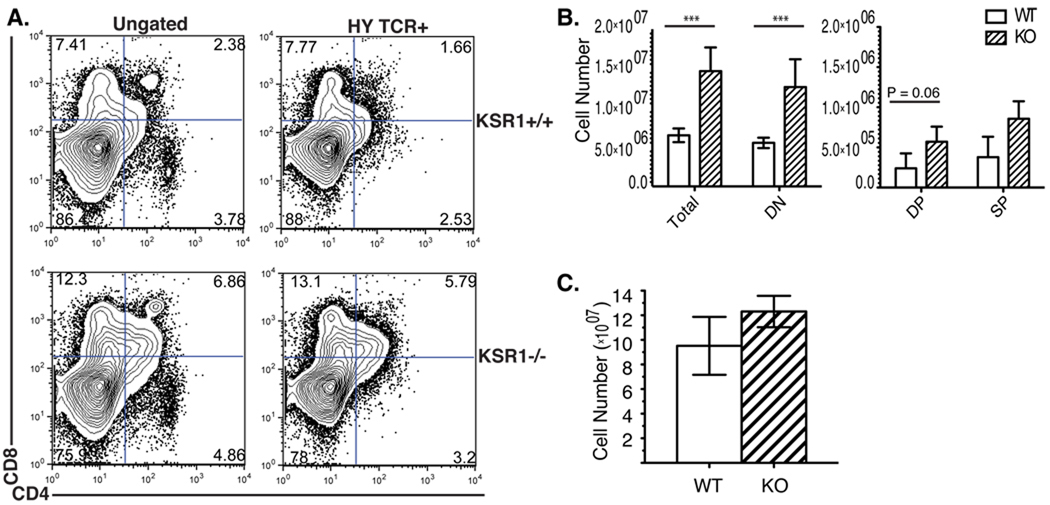
We next examined whether there was negative selection defect in HY male mice. The HY TCR recognizes a male antigen in the context of H-2b MHC class I leading to negative selection of thymocytes in male mice [25]. Due to negative selection, wild-type male HY TCR transgenic mice have small thymi that contain mostly DN thymocytes and a limited number of DP and CD8-SP thymocytes [25]. KSR1 deficient mice, however, had increased thymocyte numbers compared to wild-type mice (Fig 3A, B). The increased cell number was characterized by a significant increase in the DN population, and a trend increase in the DP population. Because negative selection occurs before the DP stage in HY male mice, the accumulation of DN thymocytes indicates there is a defect in negative selection in KSR1−/− HY male [25–27]. Since the mice used in our study were not on a RAG-deficient background, we analyzed HY TCR+ thymocytes using a clonotypic antibody (T3.70) [28]. These results gave similar results with a significant increase in the DN and a trend increase in the DP thymocyte subset (Fig 3A, B). Analysis of HY TCR+ CD8 T cells in in the periphery, however, did not show any significant differences between KSR1−/− mice compared to wild-type (Fig 3C). These data are consistent with a mild defect in negative selection in HY TCR transgenic T cells in KSR1−/− mice.
Figure 3. Negative selection in KSR1−/− male HY mice is slightly impaired.
Single cell suspensions of (A, B) thymi and (C) spleen from HY male wild-type or KSR1−/− mice were counted using trypan blue exclusion and stained with antibodies to CD4, CD8, CD3 and HY TCR. Cells were collected on a BD FACSCalibur and analyzed using Flowjo software. Representative FACS plots are shown either ungated (left) or gated on HY TCR+ cells (right) in (A). Cell numbers are representative of 4 mice per group and are shown as mean ± SD (B, C). Unless otherwise specified, differences between groups are not significant (P > 0.05). ***P<0.001, unpaired Student’s t-test.
Positive selection is normal in AND TCR transgenic KSR1−/− mice
We next assessed positive and negative selection using a second TCR transgenic model. We chose the AND TCR transgenic mouse since it is an MHCII-restricted TCR and would allow analysis of CD4 thymocyte selection and also would allow us to examine both positive and negative selection. AND TCR transgenic mice bear a Vα11Vβ3 TCR that recognizes pigeon cytochrome c peptide bound to MHC II H-2k and H-2b molecules [24]. However, thymocytes that develop on the H-2k haplotype have small thymi with a reduction of DP thymocytes most likely due to partial clonal deletion and have therefore been utilized as a model of negative selection [29].
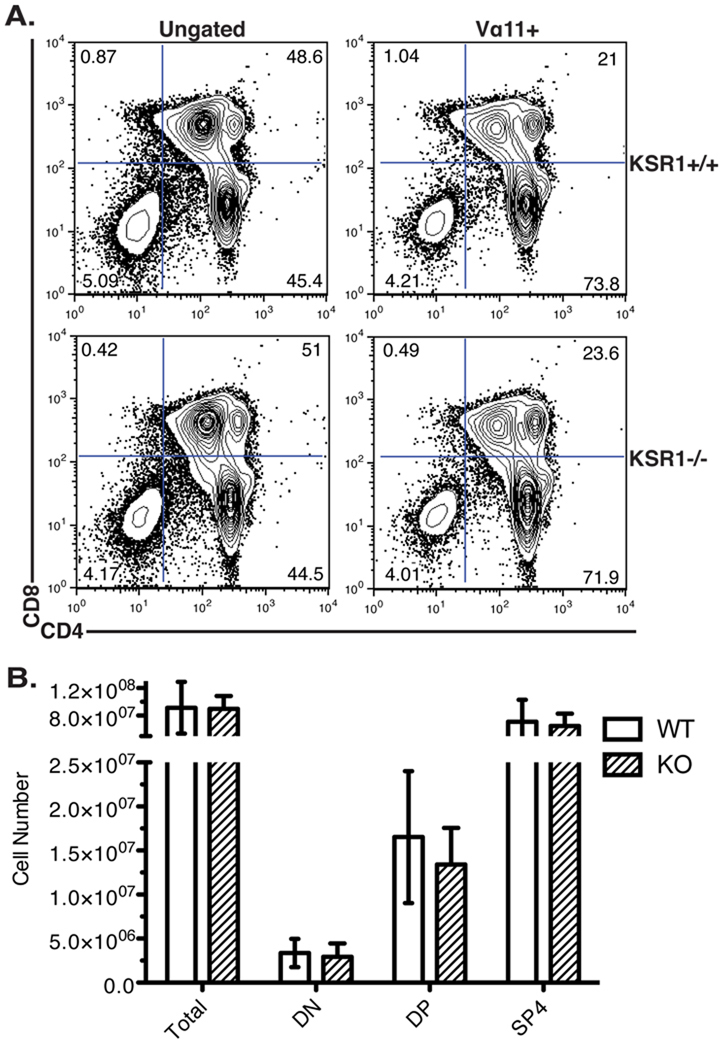
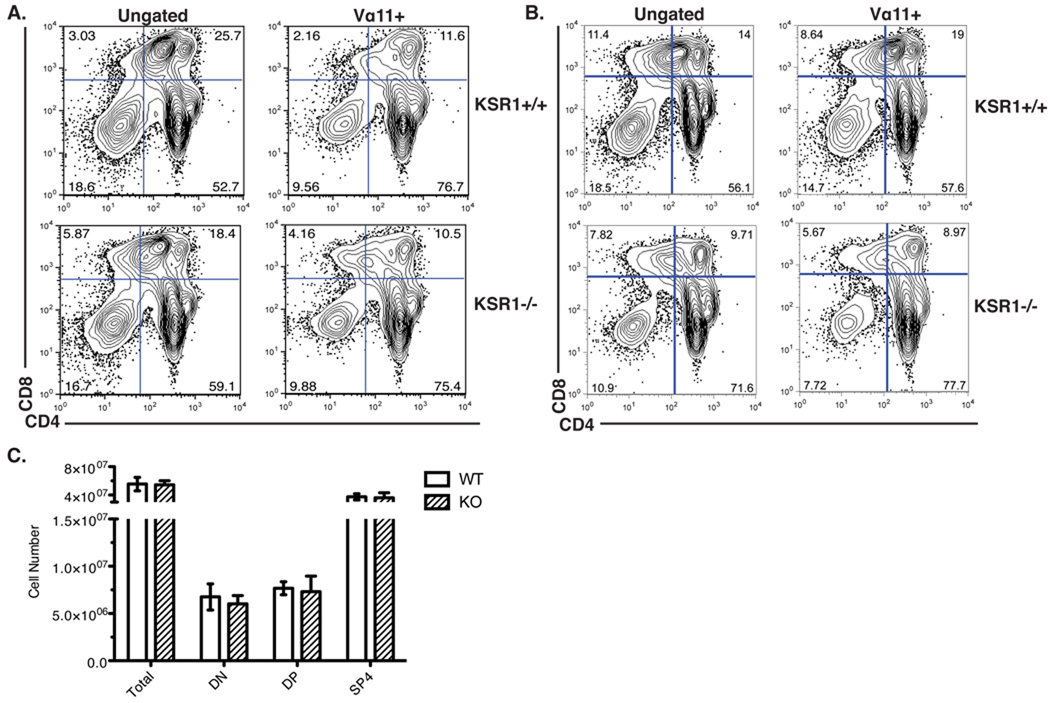
We first compared the thymocyte profiles of wild-type and KSR1−/− AND mice on the positively selecting C57BL/6 background (H-2b) [24] (Fig 4). There was a similar percentage and absolute number of DN, DP or SP thymocytes between wild-type or KSR1−/− mice. This was also true when we restricted our analysis to thymocytes expressing the transgenic AND receptor (TCR Vα11+) (Figure 4). These data indicate that, similar to our results in HY TCR mice, KSR1 is dispensable for efficient positive selection of CD4+ AND T cells.
Figure 4. Positive selection is normal in KSR1−/− AND TCR transgenic mice.
Thymi from AND TCR wild-type or KSR1−/− mice on an H-2b haplotype were made into single-cell suspension, counted and stained with antibodies to CD4, CD8, CD3 and Vα11. Data was collected using a BD FACSCalibur and analyzed using Flowjo software. Representative FACS plots are shown either ungated (left) or gated on the AND TCR+ cells (right) in (A). Cell numbers are representative of 6 mice per group and are shown as mean +/− SD (B). Differences between groups are not significant (P > 0.05; unpaired Student’s t-test).
Negative selection appears normal in AND TCR transgenic KSR1−/− mice
To determine whether negative selection is affected by the absence of KSR1 in the AND TCR mouse model, we analyzed the thymic selection of AND TCR transgenic thymocytes on the weakly negative-selecting H-2k haplotype [29, 30] (Fig 5A, C).
Figure 5. Negative selection in KSR1−/− AND mice is normal.
Thymi from AND TCR wild-type or KSR1−/− mice on an (A) H-2k or (B) H-2bxk haplotype were counted and made into single-cell suspension and stained with antibodies to CD4, CD8, CD3 and Vα11. Data were collected using a BD FACSCalibur and analyzed using Flowjo software. Representative FACS plots are shown either ungated (left) or gated on the AND TCR+ cells (right) in (A). Cell numbers shown are for analyses on the H-2k haplotype and are representative of 4 mice per group and are shown as mean ± SD (C). Differences between groups are not significant (P > 0.05; unpaired Student’s t-test).
We observed similar percentages and numbers of DN, DP and SP thymocytes between wild-type and KSR1−/− AND mice indicating that negative selection in this model is unaffected by the loss of KSR1 (Fig 5A, C). We also analyzed the selection of AND T cells in mice with the heterozygous H-2bxk haplotype, a background that should have a lower negative selection stimulus [29]. Again, the percentages and total numbers of the thymocyte populations were comparable between wild-type and KSR1−/−mice on this background (Fig 5B). These data indicate that, unlike in HY male mice, negative selection in the AND TCR transgenic mouse model does not require KSR1-dependent ERK activation.
Endogenous superantigen-mediated negative selection is normal in KSR1−/− mice
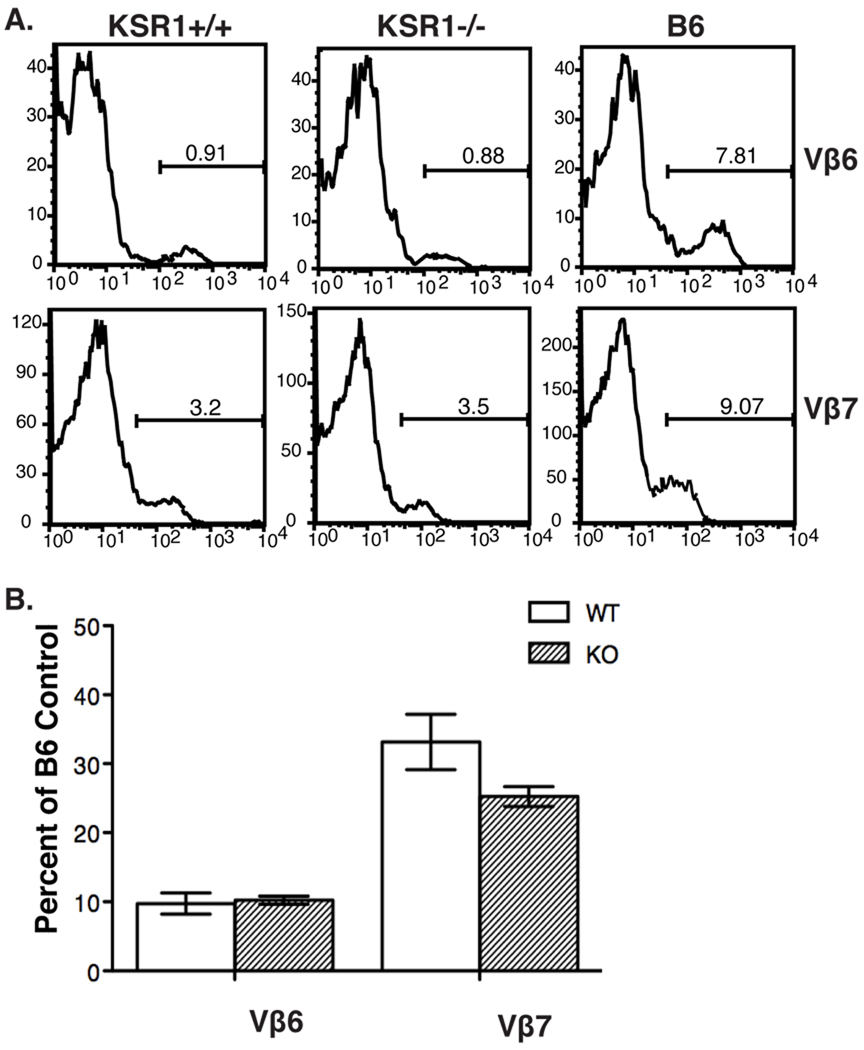
Because we observed different results regarding negative selection in the absence of KSR1 in two different mouse models, we next analyzed negative selection of T cells in response to an endogenous superantigen. We used KSR1 deficient mice on the DBA1/LacJ background because they express the endogenous retroviral superantigen MMTV-7. MMTV-7 expression in wild-type mice results in deletion of T cells expressing the TCR Vβ-6, 7, 8.1 and 9 chains by negative selection [31]. To determine if KSR1 is important for negative selection in this model, we compared the representation of these Vβ chains in splenocytes from wild-type or KSR1−/− on the DBA1/LacJ background (Fig 6). These analyses showed that the representation of TCRVβ-6 and 7 in splenic T cells was not significantly different between wild-type and KSR1−/− mice. These data show that the negative selection mediated by endogenous superantigen on the DBA/LacJ background is not effected by the absence of KSR1.
Figure 6. Negative selection mediated by endogenous superantigen is normal in KSR1−/− DBA1 mice.
KSR1 DBA1/LacJ wild-type or knockout splenocytes were counted and stained with antibodies to CD3, CD4, CD8 and the indicated Vβ TCR antibodies. Data was collected using a BD FACSCalibur and analyzed with Flowjo software. Representative histograms indicating the percentage of Vβ positive cells in T cells from C57BL/6, KSR1 wild-type or knockout splenocytes are shown in A. Cell numbers are shown as percentage of B6 control mouse and are representative of 5 mice per group (B).
Discussion
KSR1 is a scaffold that plays a role in facilitating ERK activation. It is thought to function by binding all of the components of the Ras-MAP kinase pathway, Raf, MEK and ERK. Preassembly of these components is thought to facilitate that the rapid and efficient activation of ERK. Consistent with this idea, all studies to date show that the absence of KSR1 leads to an attenuation of ERK activity in a wide variety of different cells [18–22]. Because the intensity and duration of ERK activation has been implicated in the development of thymocytes [8, 9, 32, 33], we were interested to test whether the absence of KSR1 would have an effect on the positive and negative selection of thymocytes. Surprisingly, our analysis, using several different models, showed that KSR1 was basically dispensable for both positive and negative thymocyte selection.
Our findings are in contrast to a previous study on the role of KSR1 in thymocyte development that suggested it was important for positive selection [34]. In that study, overexpression of KSR1 was delivered to thymocytes by retroviruses [34] and resulted in a partial block in positive selection. While our study used a variety of in vivo models of positive and negative selection, the previous study relied on in vitro reaggregate cultures [34]. Differences between the studies could be due to the different approaches used. Also, overexpression of scaffold proteins is problematic as it can act to titer down concentrations of binding partners, possibly resulting in off-target effects on pathways in addition to the ERK-MAPK pathway [35]. No data was presented regarding the effect of KSR1 overexpression on negative selection [34].
Numerous studies have directly implicated ERK in thymocyte development [7–11]. While initial studies in the ERK1−/− mouse indicated that there was a slight defect in thymocyte maturation [10], subsequent studies failed to find any defect [7]. Mice lacking both isoforms of ERK, ERK1 and ERK2, have a partial block in thymocyte development at the DN3 stage [7] and a complete block in positive selection. Surprisingly, when the ERK1/2 double KO was bred to two different TCR transgenic mice, OT-1 and AND, a small percentage of thymocytes could still be positively selected suggesting that ERK is important but not absolutely required for positive selection [7]. This defect in positive selection is consistent with studies using transgenic mice expressing dominant-negative (DN) forms of Ras and MEK under the control of the Lck promoter[5, 36, 37]. Our studies showing decreased, but clearly detectable, ERK activity in KSR1 deficient thymocytes is consistent with the idea that only a threshold amount of ERK activity is required to mediate positive selection.
The role of ERK in negative selection is more controversial. Experiments performed using transgenic mice expressing a dominant negative form of Ras or MEK reported normal negative selection using a superantigen mediated deletion model or the HY TCR transgenic model [5, 36, 37]. Inhibitor studies added confusion to the field with some studies showing a role for ERK in negative selection and others showing no role [6, 8, 12, 32]. For example, inhibition of ERK by the MEK inhibitor, PD98059, in fetal thymic organ cultures showed no defects in either anti-CD3 mediated or HY TCR male antigen mediated negative selection [12]. In contrast, another group using the P14 TCR transgenic fetal thymic organ cultures showed defects in negative selection with the same inhibitor [8] and was confirmed in at least two other transgenic TCR models [6]. More recently, Hedrick’s group showed that there was no negative selection defect in ERK1/2 double knockout OT-1 CD8 transgenic TCR thymocytes both in vitro and in vivo [13]. Our results with KSR1 deficient mice showing a mild negative selection defect in HY-thymocytes is consistent with a role for ERK in negative selection but could be due to some idiosyncrasy with the HY TCR transgenic system. It is also possible that the role of ERK in negative selection is dependent on differences in the affinity of the pMHC:TCR complex.
While all previous studies show that the absence of KSR1 leads to the general attenuation of ERK activation, we were surprised to find that the role of KSR1 was more important for PMA than for CD3 stimulation. To our knowledge, these are the first data that implicate a scaffold in one but not another similar pathway. One possible explanation is that PMA stimulates a second pathway that enhances KSR1 recruitment to the membrane. Since PMA stimulates Ras exclusively via RasGRP and CD3 stimulates Ras through both RasGRP and SOS [38], another possibility is that KSR1 might function specifically in the RasGRP but not the SOS pathway. We are currently exploring between these possibilities and others using a variety of biological and computational approaches.
We also noted that the magnitude of the ERK defect varied by thymocyte subset. After CD3 stimulation, the ERK defect was greatest in the SP subsets and less in the DP and DN subsets. The relatively small defect in ERK activation after CD3 stimulation in the DP subset, could explain the absence of a developmental defect in KSR1 deficient thymocytes. What explains the differences of KSR1 function in thymocyte subsets is unclear, but it is interesting to speculate that this is due to differences in the signaling potential between SP versus DN and DP cells. DN and DP cells exhibit low-level expression of both the TCR and RasGRP that would lead one to speculate they have a low signaling potential [39]. Lower overall levels of Ras activation might result in changes between the ratio of activated Ras and the number of KSR1 molecules, which are known to influence the efficiency of KSR1 mediated ERK activation [35, 40]. It is also possible that the decreased signaling potential influences the feedback loops between RasGRP and SOS leading to unexpected changes in levels of ERK activation [41].
In conclusion, our data demonstrate that KSR1 influences the efficient ERK activation in developing thymocytes and that disruption of this pathway has few, if any, effects on thymocyte development. Progression of immature thymocytes through the DN and DP stages was uninhibited in KSR1−/− thymi, indicating that suboptimal ERK activation is enough for thymocytes to proceed through developmental checkpoints that require TCR signaling. Consistent with previous studies, we found a more complex role for ERK in negative selection. Of the three model systems examined in this study, attenuated ERK activation diminished the efficiency of negative selection only for the HY TCR. Determining the exact nature of the role of ERK activity in negative selection will help shed light on the signaling mechanisms responsible for distinguishing positive and negative selection.
Materials and Methods
Mice
KSR1−/− mice were previously generated on a DBA1/LacJ background [18]. For TCR transgenic experiments, these mice were backcrossed more than 10 times to C57BL/6 (Jackson Laboratory). KSR1−/− TCR transgenic mice were generated by breeding KSR1−/− C57BL/6 mice with AND [24] (Jackson Laboratory) or HY [25] TCR (Taconic) transgenic mice. AND mice were crossed with AKR.B6 mice (Jackson Laboratory) to generate AND TCR transgenic mice with the H-2k haplotype. Superantigen deletion experiments were performed in the original DBA1/LacJ KSR−/− mice. All mice were housed under specific pathogen-free condition in the Washington University animal facilities in accordance with institutional guidelines.
Western Blot
Single cell suspensions were generated from thymi excised from 6–8 week old mice. Total thymocytes were stimulated with 40 ng/mL PMA or 5 µM anti-CD3 for various time-points, lysed in NP-40 buffer and resolved on a 10% SDS-PAGE gel. Total ERK and ppERK were detected using polyclonal rabbit antibodies from Santa Cruz (anti-ERK2) and Cell Signaling Technology [anti-pERK1/2, (Thr202/Tyr204)], respectively. HRP-conjugated anti-Rabbit secondary antibody (Jackson ImmunoResearch) followed by ECL western blotting substrate (Pierce) was used for detection.
Detection of Phosho-ERK by FACS
Single cell suspensions were generated from thymi of 4–6 week old mice. Cells were stimulated with 1µg biotinylated anti-CD3 (BD Biosciences) followed by 1µg/mL unconjugated SA (Jackson Immunoresearch) for the 3 minutes followed by fixation with 4% PFA and permeablization with 95% methanol. Cells were first stained with anti-pERK1/2, (Thr202/Tyr204) from Cell Signaling overnight and then stained with CD4 APC and CD8 PE-Cy5 antibodies from BD Biosciences and an anti-rabbit PE conjugated secondary (Jackson ImmunoResearch).
Thymocyte and Splenocyte FACS Analysis
FACS analysis was performed on single-cell suspensions of thymus and spleen. Following passage through a cell strainer (Fisher), cell suspensions were pelleted and resuspended in PBS + 2% FBS and counted using trypan blue exclusion. Cells were then stained with various combinations of the following antibodies from BD Biosciences: CD4 FITC, Vβ9 FITC, CD4 PE, Vβ6 PE, Vβ7 PE, Vβ8.1 PE, HY TCR PE, Vα11 PE or eBiosciences: CD8 PECy7 and CD3 APC. Samples were run on a BD FACSCalibur instrument and analyzed using FlowJo software.
Supplementary Material
Acknowledgements
The authors would like to thank Rob Lewis for providing KSR1−/− DBA1/LacJ mice. This work was supported by the NIH (R37-AI57966-AS and T32-AI07163-EF) and the Howard Hughes Medical Institute.
References
- 1.Sha WC, Nelson CA, Newberry RD, Kranz DM, Russell JH, Loh DY. Positive and negative selection of an antigen receptor on T cells in transgenic mice. Nature. 1988;336:73–76. doi: 10.1038/336073a0. [DOI] [PubMed] [Google Scholar]
- 2.Jameson SC, Hogquist KA, Bevan MJ. Positive selection of thymocytes. Annu Rev Immunol. 1995;13:93–126. doi: 10.1146/annurev.iy.13.040195.000521. [DOI] [PubMed] [Google Scholar]
- 3.Mariathasan S, Jones RG, Ohashi PS. Signals involved in thymocyte positive and negative selection. Semin Immunol. 1999;11:263–272. doi: 10.1006/smim.1999.0182. [DOI] [PubMed] [Google Scholar]
- 4.Siggs OM, Makaroff LE, Liston A. The why and how of thymocyte negative selection. Curr Opin Immunol. 2006;18:175–183. doi: 10.1016/j.coi.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 5.Alberola-Ila J, Forbush KA, Seger R, Krebs EG, Perlmutter RM. Selective requirement for MAP kinase activation in thymocyte differentiation. Nature. 1995;373:620–623. doi: 10.1038/373620a0. [DOI] [PubMed] [Google Scholar]
- 6.Bommhardt U, Scheuring Y, Bickel C, Zamoyska R, Hunig T. MEK activity regulates negative selection of immature CD4+CD8+ thymocytes. J Immunol. 2000;164:2326–2337. doi: 10.4049/jimmunol.164.5.2326. [DOI] [PubMed] [Google Scholar]
- 7.Fischer AM, Katayama CD, Pages G, Pouyssegur J, Hedrick SM. The role of erk1 and erk2 in multiple stages of T cell development. Immunity. 2005;23:431–443. doi: 10.1016/j.immuni.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 8.Mariathasan S, Ho SS, Zakarian A, Ohashi PS. Degree of ERK activation influences both positive and negative thymocyte selection. Eur J Immunol. 2000;30:1060–1068. doi: 10.1002/(SICI)1521-4141(200004)30:4<1060::AID-IMMU1060>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 9.McNeil LK, Starr TK, Hogquist KA. A requirement for sustained ERK signaling during thymocyte positive selection in vivo. Proc Natl Acad Sci U S A. 2005;102:13574–13579. doi: 10.1073/pnas.0505110102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pages G, Guerin S, Grall D, Bonino F, Smith A, Anjuere F, Auberger P, Pouyssegur J. Defective thymocyte maturation in p44 MAP kinase (Erk 1) knockout mice. Science. 1999;286:1374–1377. doi: 10.1126/science.286.5443.1374. [DOI] [PubMed] [Google Scholar]
- 11.Sharp LL, Schwarz DA, Bott CM, Marshall CJ, Hedrick SM. The influence of the MAPK pathway on T cell lineage commitment. Immunity. 1997;7:609–618. doi: 10.1016/s1074-7613(00)80382-9. [DOI] [PubMed] [Google Scholar]
- 12.Sugawara T, Moriguchi T, Nishida E, Takahama Y. Differential roles of ERK and p38 MAP kinase pathways in positive and negative selection of T lymphocytes. Immunity. 1998;9:565–574. doi: 10.1016/s1074-7613(00)80639-1. [DOI] [PubMed] [Google Scholar]
- 13.McGargill MA, Ch'en IL, Katayama CD, Pages G, Pouyssegur J, Hedrick SM. Cutting edge: Extracellular signal-related kinase is not required for negative selection of developing T cells. J Immunol. 2009;183:4838–4842. doi: 10.4049/jimmunol.0902208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bommhardt U, Basson MA, Krummrei U, Zamoyska R. Activation of the extracellular signal-related kinase/mitogen-activated protein kinase pathway discriminates CD4 versus CD8 lineage commitment in the thymus. J Immunol. 1999;163:715–722. [PubMed] [Google Scholar]
- 15.Therrien M, Chang HC, Solomon NM, Karim FD, Wassarman DA, Rubin GM. KSR, a novel protein kinase required for RAS signal transduction. Cell. 1995;83:879–888. doi: 10.1016/0092-8674(95)90204-x. [DOI] [PubMed] [Google Scholar]
- 16.Kornfeld K, Hom DB, Horvitz HR. The ksr-1 gene encodes a novel protein kinase involved in Ras-mediated signaling in C. elegans. Cell. 1995;83:903–913. doi: 10.1016/0092-8674(95)90206-6. [DOI] [PubMed] [Google Scholar]
- 17.Sundaram M, Han M. The C. elegans ksr-1 gene encodes a novel Raf-related kinase involved in Ras-mediated signal transduction. Cell. 1995;83:889–901. doi: 10.1016/0092-8674(95)90205-8. [DOI] [PubMed] [Google Scholar]
- 18.Nguyen A, Burack WR, Stock JL, Kortum R, Chaika OV, Afkarian M, Muller WJ, Murphy KM, Morrison DK, Lewis RE, McNeish J, Shaw AS. Kinase suppressor of Ras (KSR) is a scaffold which facilitates mitogen-activated protein kinase activation in vivo. Mol Cell Biol. 2002;22:3035–3045. doi: 10.1128/MCB.22.9.3035-3045.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fusello AM, Mandik-Nayak L, Shih F, Lewis RE, Allen PM, Shaw AS. The MAPK scaffold kinase suppressor of Ras is involved in ERK activation by stress and proinflammatory cytokines and induction of arthritis. J Immunol. 2006;177:6152–6158. doi: 10.4049/jimmunol.177.9.6152. [DOI] [PubMed] [Google Scholar]
- 20.Yan F, Polk DB. Kinase suppressor of ras is necessary for tumor necrosis factor alpha activation of extracellular signal-regulated kinase/mitogen-activated protein kinase in intestinal epithelial cells. Cancer Res. 2001;61:963–969. [PubMed] [Google Scholar]
- 21.Ohmachi M, Rocheleau CE, Church D, Lambie E, Schedl T, Sundaram MV. C. elegans ksr-1 and ksr-2 have both unique and redundant functions and are required for MPK-1 ERK phosphorylation. Curr Biol. 2002;12:427–433. doi: 10.1016/s0960-9822(02)00690-5. [DOI] [PubMed] [Google Scholar]
- 22.Kortum RL, Costanzo DL, Haferbier J, Schreiner SJ, Razidlo GL, Wu MH, Volle DJ, Mori T, Sakaue H, Chaika NV, Chaika OV, Lewis RE. The molecular scaffold kinase suppressor of Ras 1 (KSR1) regulates adipogenesis. Mol Cell Biol. 2005;25:7592–7604. doi: 10.1128/MCB.25.17.7592-7604.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O'Shea CC, Crompton T, Rosewell IR, Hayday AC, Owen MJ. Raf regulates positive selection. Eur J Immunol. 1996;26:2350–2355. doi: 10.1002/eji.1830261012. [DOI] [PubMed] [Google Scholar]
- 24.Kaye J, Hsu ML, Sauron ME, Jameson SC, Gascoigne NR, Hedrick SM. Selective development of CD4+ T cells in transgenic mice expressing a class II MHC-restricted antigen receptor. Nature. 1989;341:746–749. doi: 10.1038/341746a0. [DOI] [PubMed] [Google Scholar]
- 25.Kisielow P, Bluthmann H, Staerz UD, Steinmetz M, von Boehmer H. Tolerance in T-cell-receptor transgenic mice involves deletion of nonmature CD4+8+ thymocytes. Nature. 1988;333:742–746. doi: 10.1038/333742a0. [DOI] [PubMed] [Google Scholar]
- 26.Bouillet P, Purton JF, Godfrey DI, Zhang LC, Coultas L, Puthalakath H, Pellegrini M, Cory S, Adams JM, Strasser A. BH3-only Bcl-2 family member Bim is required for apoptosis of autoreactive thymocytes. Nature. 2002;415:922–926. doi: 10.1038/415922a. [DOI] [PubMed] [Google Scholar]
- 27.von Boehmer H, Kisielow P. Negative selection of the T-cell repertoire: where and when does it occur? Immunol Rev. 2006;209:284–289. doi: 10.1111/j.0105-2896.2006.00346.x. [DOI] [PubMed] [Google Scholar]
- 28.Teh HS, Kishi H, Scott B, Von Boehmer H. Deletion of autospecific T cells in T cell receptor (TCR) transgenic mice spares cells with normal TCR levels and low levels of CD8 molecules. J Exp Med. 1989;169:795–806. doi: 10.1084/jem.169.3.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matechak EO, Killeen N, Hedrick SM, Fowlkes BJ. MHC class II-specific T cells can develop in the CD8 lineage when CD4 is absent. Immunity. 1996;4:337–347. doi: 10.1016/s1074-7613(00)80247-2. [DOI] [PubMed] [Google Scholar]
- 30.Vasquez NJ, Kaye J, Hedrick SM. In vivo and in vitro clonal deletion of double-positive thymocytes. J Exp Med. 1992;175:1307–1316. doi: 10.1084/jem.175.5.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marrack P, Winslow GM, Choi Y, Scherer M, Pullen A, White J, Kappler JW. The bacterial and mouse mammary tumor virus superantigens; two different families of proteins with the same functions. Immunol Rev. 1993;131:79–92. doi: 10.1111/j.1600-065x.1993.tb01531.x. [DOI] [PubMed] [Google Scholar]
- 32.Mariathasan S, Zakarian A, Bouchard D, Michie AM, Zuniga-Pflucker JC, Ohashi PS. Duration and strength of extracellular signal-regulated kinase signals are altered during positive versus negative thymocyte selection. J Immunol. 2001;167:4966–4973. doi: 10.4049/jimmunol.167.9.4966. [DOI] [PubMed] [Google Scholar]
- 33.Adachi S, Iwata M. Duration of calcineurin and Erk signals regulates CD4/CD8 lineage commitment of thymocytes. Cell Immunol. 2002;215:45–53. doi: 10.1016/s0008-8749(02)00012-6. [DOI] [PubMed] [Google Scholar]
- 34.Laurent MN, Ramirez DM, Alberola-Ila J. Kinase suppressor of Ras couples Ras to the ERK cascade during T cell development. J Immunol. 2004;173:986–992. doi: 10.4049/jimmunol.173.2.986. [DOI] [PubMed] [Google Scholar]
- 35.Burack WR, Shaw AS. Signal transduction: hanging on a scaffold. Curr Opin Cell Biol. 2000;12:211–216. doi: 10.1016/s0955-0674(99)00078-2. [DOI] [PubMed] [Google Scholar]
- 36.Swan KA, Alberola-Ila J, Gross JA, Appleby MW, Forbush KA, Thomas JF, Perlmutter RM. Involvement of p21ras distinguishes positive and negative selection in thymocytes. EMBO J. 1995;14:276–285. doi: 10.1002/j.1460-2075.1995.tb07001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alberola-Ila J, Hogquist KA, Swan KA, Bevan MJ, Perlmutter RM. Positive and negative selection invoke distinct signaling pathways. J Exp Med. 1996;184:9–18. doi: 10.1084/jem.184.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roose JP, Mollenauer M, Gupta VA, Stone J, Weiss A. A diacylglycerol-protein kinase C-RasGRP1 pathway directs Ras activation upon antigen receptor stimulation of T cells. Mol Cell Biol. 2005;25:4426–4441. doi: 10.1128/MCB.25.11.4426-4441.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Priatel JJ, Teh SJ, Dower NA, Stone JC, Teh HS. RasGRP1 transduces low-grade TCR signals which are critical for T cell development, homeostasis, and differentiation. Immunity. 2002;17:617–627. doi: 10.1016/s1074-7613(02)00451-x. [DOI] [PubMed] [Google Scholar]
- 40.Locasale JW, Chakraborty AK. Regulation of signal duration and the statistical dynamics of kinase activation by scaffold proteins. PLoS Comput Biol. 2008;4:e1000099. doi: 10.1371/journal.pcbi.1000099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roose JP, Mollenauer M, Ho M, Kurosaki T, Weiss A. Unusual interplay of two types of Ras activators, RasGRP and SOS, establishes sensitive and robust Ras activation in lymphocytes. Mol Cell Biol. 2007;27:2732–2745. doi: 10.1128/MCB.01882-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.