Abstract
Background
Estimates of human papillomavirus (HPV) concordance among sexual partners are important for various public health activities, from counseling individual patients to predicting the impact of HPV vaccination.
Methods
We systematically searched PubMed and EMBASE for studies of HPV concordance among heterosexual couples published through 2008 in English. Two coders independently abstracted data using standardized forms. We integrated concordance data using random-effects meta-analysis.
Results
Thirty studies (33 study populations) that met inclusion criteria reported concordance data for 2,972 couples. Most studies were cross-sectional, cohort studies conducted in Europe or Asia that used DNA hybridization to test for HPV, sometimes in conjunction with polymerase chain reaction (PCR). Overall, 25.5% (95% CI: 17.2%-36.1%) of couples were infected with 1 or more of the same HPV types. Among couples with both members HPV-positive, 63.2% (95% CI: 49.1%-75.3%) were infected with 1 or more of the same viral types. Positive concordance was higher for female partners of men with HPV infections than for male partners of women with HPV infections. Positive concordance was also higher for studies using PCR and for the few studies that recruited men with HPV-related disease.
Conclusions
Sexual partners of HPV-infected individuals had high rates of HPV infection, suggesting a need for increased attention to this group.
Impact
Our refined estimates of HPV concordance can inform clinical encounters and public health planning. Future HPV concordance studies should use more rigorous research designs, characterize their participants in greater detail, and study more meaningful populations.
Keywords: concordance, HPV, human papillomavirus, neoplasia, cancer
Introduction
Anogenital human papillomavirus (HPV) infection is a common sexually transmitted infection (STI) that preferentially infects squamous epithelial cells (1, 2). At least twenty million people in the U.S. are currently infected with HPV, and 5.5 million people become infected annually (3). Although most HPV infections clear spontaneously (4–6), some nononcogenic HPV types (mainly types 6 and 11) cause anogenital warts (7, 8). Persistent infection with oncogenic HPV types, if left untreated, can lead to cancers of the cervix, vagina, vulva, penis, anus, oropharynx, and possibly skin and lung (9–12). Most cervical cancer deaths occur in the developing world (13), primarily because of lack of access to screening and treatment. While the U.S. has much lower rates of cervical cancer, it spends about $4 billion annually on treating and managing cervical disease and anogenital warts (14) and an additional $418 million annually on other HPV-related diseases (15).
A key contributor to the incidence of HPV-related disease is the high transmissibility of HPV, estimated to be 40% (median value with range of 5%-100%) per unprotected sexual act (16). Persistent HPV infection also increases the risk for disease and sexual partners acquiring the virus. Among women, the median duration of infection is usually less than a year, but oncogenic HPV infections last longer than nononcogenic HPV infections (4, 6, 17, 18). Although fewer data on infection duration have been reported for men, findings suggest that HPV infections clear more quickly for men than for women, and men have similar duration of infection for oncogenic and nononcogenic HPV types (5). While the extent to which sexual partners both harbor anogenital HPV infection has important implications for public health, including helping to understand the risks that partners of infected individuals face and increasing the precision of HPV vaccine cost-effectiveness analyses, previous studies of HPV concordance have yielded mixed findings. To address this question, we conducted a systematic review and meta-analysis of the existing literature on HPV DNA concordance among heterosexual couples.
Materials and Methods
Data Sources and Searches
Two investigators (PR and WP) independently searched PubMed and EMBASE databases for studies published through December 2008 whose title, abstract, or keywords referred to HPV infection or concordance among sexual partners. Search terms were ((HPV OR human papillomavirus) OR (papillomavirus infection)) AND (transmission OR concordance). We also manually searched reference sections of identified papers to locate additional studies for inclusion.
Search Strategy and Study Selection
Two investigators (PR and WP) independently screened titles, abstracts, and articles for eligibility using predefined criteria described below. We required that studies reported findings in English on anogenital HPV infection. We excluded studies that reported data only on non-anogenital HPV infections (e.g., oral infections only). We required that studies determined the HPV status of participants using molecular methods (e.g., DNA hybridization, polymerase chain reaction [PCR]), as opposed to relying solely on clinical examination or histopathology, since some individuals with no clinical evidence of HPV infection are positive for HPV DNA (19) while others with acetowhite anogenital lesions are HPV DNA-negative (20). We required that studies reported data on heterosexual couples, as HPV transmission dynamics in same-sex couples may differ.
Data Extraction and Quality Assessment
Two investigators (PR and WP) independently completed standardized data extraction forms to code study and participant characteristics that could affect HPV concordance. Study characteristics included study design, specimen collection method, HPV types examined, and HPV detection methods. Participant characteristics included demographics (age and marital status), health (history of HPV-related disease or other STIs and whether males were circumcised), sexual history (age at first intercourse and number of lifetime sexual partners), and relationship characteristics (length of time couples had been together, condom use, and whether couples were monogamous). A third investigator (NB) resolved the few coding disagreements. For case-control studies, we treated cases and controls as separate study samples, as “cases” (e.g., women with cervical cancer) may have higher chances of infecting their partners with HPV than would “controls” (e.g., women with normal Pap smear test results).
Reviewers extracted data on the status of each partner for infection with any HPV type and HPV types 6, 11, 16, and 18. We examined these 4 HPV types as they are the primary causes of HPV-related diseases (e.g., genital warts, cervical cancer, and anal cancer (7–9)) and recently licensed HPV vaccines protect against 2 or all 4 of these viral types (21–23). Reviewers created measures of positive concordance, defined as both partners having the HPV outcome of interest. Thus, both partners being negative did not increase concordance. We usually refer to positive concordance simply as concordance for the remainder of this report. We examined any-type concordance (both partners had HPV), same-type concordance (both partners had 1 or more HPV types in common), and type-specific concordance for HPV types 6, 11, 16, and 18.
We required that studies reported HPV infection data for both members of couples in a manner that allowed us to assess at least 1 of these HPV concordance measures. We included type-specific concordance only for studies that reported data that did not combine multiple HPV types (e.g., the HPV DNA probe indicated the presence of either HPV type 16 or 18). During data extraction, we excluded articles that inconsistently reported concordance data that queries to the authors did not resolve.
Data Synthesis and Analysis
We pooled data across studies using random-effects meta-analysis to examine any-type concordance, same-type concordance, and type-specific HPV concordance among couples. Using methods similar to those from previous studies (24, 25), we determined if concordance levels for HPV types 6, 11, 16, an 18 were higher than chance would predict by calculating expected concordance levels and using random-effects meta-analysis to calculate odds ratios (ORs) and 95% confidence intervals (CIs). Analyses also examined sex-dependent same-type concordance, defined as the proportion of men who had the same HPV types (1 or more types) as their HPV-positive female partners and the proportion of women who had the same HPV types (1 or more types) as their HPV-positive male partners. We examined potential correlates of sex-dependent concordance using random-effects meta-regression, using two-tailed tests and 0.05 as the critical alpha. Analyses excluded a study that reported data on only 1 couple, because the absence of study-level variance precluded its inclusion in random-effects meta-analyses. For random-effects meta-analyses, we report I2 values as an indication of heterogeneity among studies. We conducted all analyses using Comprehensive Meta-Analysis Version 2 software (Englewood, NJ).
Results
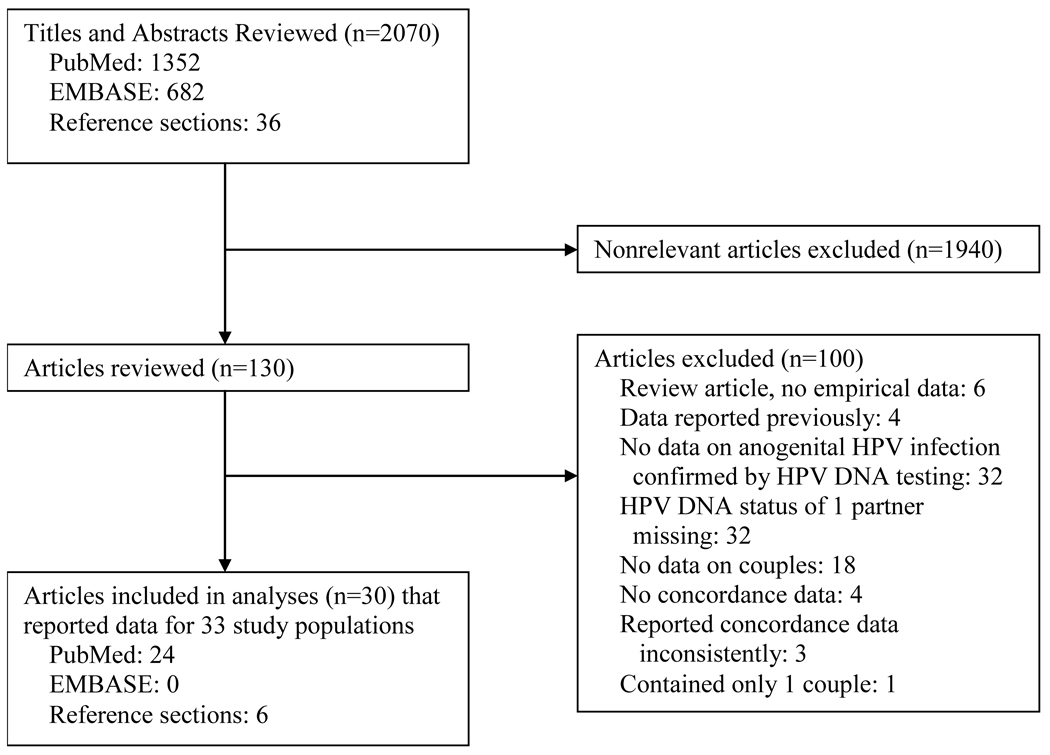
Of the 2,070 titles and abstracts and 130 articles that we reviewed, 30 articles met inclusion criteria (Figure 1) (24–53). These articles reported data from 33 study populations that included HPV concordance data for 2,972 couples (median=45, range=4–499) (Table 1). Studies provided too few data to report meaningful summary statistics on participant characteristics. Year of study publication ranged from 1985 to 2008 (median=1994). Study locations included Europe (52%), Asia (36%), Latin America (15%), and the United States (9%). Most studies used cohort (76%) and cross-sectional (88%) study designs. Researchers collected specimens from a wide range of anatomic sites using various methods. To detect HPV, most studies used DNA hybridization alone (42%) or in combination with PCR (42%). While most studies (61%) tested for 5 or more HPV types, the most common were HPV types 16 (100%), 18 (94%), 6 (70%), and 11 (70%).
Figure 1.
Study flow diagram
Table 1.
Characteristics of studies (n=30) and study populations (n=33) included in analyses
| Author, Year |
Location | Study Design |
Study Sampling |
Study Population | Female Specimen Collection |
Male Specimen Collection |
HPV Detection |
HPV Types | No. of Couples |
|---|---|---|---|---|---|---|---|---|---|
|
Overall (33 study populations) 1994 or earlier: 52% 1995 or later: 48% |
Europe: 52% Asia: 36% Latin America: 15% United States: 9% |
Co: 76% Ca: 24% X: 88% L: 12% |
Cn: 88% P: 12% |
-- | -- | -- |
H: 85% PCR: 58% H+PCR: 42% |
16: 100% 18: 94% 6: 70% 11: 70% 5+ types total: 61% 4 types total or less:39% |
Median: 45 Range: 4–499 |
| Baken et al., 1995 (26) | United States | Co, X | Cn | Heterosexual couples attending a sexually transmitted diseases clinic | Vulvovaginal, cervical, perianal swabs | Penile swabs | H, PCR | 6,11,16,18,31,33, 35,42,43,44,45, 51,52,56 |
45 |
| Bar-Am and Niv, 2007 (27) | Israel* | Co, X | Cn | Females with CIN3 undergoing cone biopsy at 1 hospital and their male partners | Cervical specimens | Penile shaft, scrotum, perianal area swabs | H | 16,18,31,33,35, 45,52,56 |
74 |
| Benevolo et al., 2008 (28) | Italy | Co, X | Cn | Women with current or past CIN (1–3) and/or a positive HPV DNA test and their stable male partners | Cervicovaginal brushings | Penile brushings | H, PCR | 6,11,16,18,31,33, 35,39,40,43,44, 45,51,52,53,56, 58,59,66,68,73,82 |
55 |
| Bleeker et al., 2005 (24) | Netherlands | Co, X | Cn | Heterosexual couples recruited from coloposcopy clinic; women had mild dyskaryosis or worse noted by cytological examination of the cervix and/or CIN noted by colposcopy or histological examination. | Cervical scrapings | Penile scrapings | H, PCR | 6,11,16,18,26,31, 33,34,35,39,40, 42,43,44,45,51, 52,53,54,55,56, 57,58,59,61,66, 68,70,71,72,73, 81,82/MM4, 82/IS39,83,84, cand89 |
181 |
| Campion et al., 1985 (29) | United Kingdom | Ca, X | Cn | Female partners of men with penile condylomata acuminata | Urethral, high-vaginal, and endocervical swabs and smears | Penile and perianal biopsies | H | 6,11,16 | 9 |
| Franceschi et al., 2002a (30) | Spain, Columbia, Thailand | Ca, X | P | Reports data from 7 case-control studies; case women were newly-diagnosed with ICC or CIS and men were stable partners of these women | Cervical scrapings | Penile swabs (distal urethra, glans, coronal sulcus) | H, PCR | 6,11,16,18,31,33, "other types" does not specify |
499 |
| Franceschi et al., 2002b (30) | Spain, Columbia, Thailand | Ca, X | P | Reports data from 7 case-control studies; control women were population-based (2 studies) or hospital-based (5 studies) and men were stable partners of these women | Cervical scrapings | Penile swabs (distal urethra, glans, coronal sulcus) | H, PCR | 6,11,16,18,31,33, "other types" does not specify |
465 |
| Gal et al., 1989 (31) | Israel | Co, L | Cn | Women referred to dysplasia clinic with diagnosis of an HPV-related lesion and their male partners | Colposcopy/biopsy from HPV-related lesions and CIN | Colposcopy/biopsy from HPV-related lesions | H | 6,11,16,18 | 76 |
| Giovannelli et al., 2007 (25) | Italy | Co, X | Cn | Consecutive HPV-positive women with abnormal Pap smears and their husbands or current stable male partners | Cervical brushings | Penile brushings (shaft, foreskin, coronal sulcus, frenulum, glans), urethral brushings, semen | H, PCR | 6,11,16,18,31,33, 35,39,40,42,43, 44,45,51,52,54, 70,74,53,56,58, 59,66,68; also used a 43 type amplifier to detect HPV DNA |
45 |
| Giraldo et al., 2008 (32) | Brazil* | Co, X | Cn | Men who were asymptomatic partners of women with a histopathological diagnosis of LGSIL | Not reported | Penile brushings (base, body, balanopreputial folds, preputium, distal urethra) | H | 16,18,31,33,35,39, 45,51,52,56,58, 59,68 |
54 |
| Golijow et al., 2005 (33) | Argentina | Co, X | Cn | Men attending a urological department with an HPV-positive female partner | Not reported | First void urine specimens | PCR | 6,11,16,18,31,33, 34,57 |
112 |
| Gomousa-Michael et al., 1997 (34) | Greece | Co, X | Cn | Females with HPV or SIL and their male partners | Cervical specimens using cytobrush, colposcopy, punch biopsy | Urethral samples using cytobrush | H | 6,11,16,18,31,33, 35 |
20 |
| Gross et al., 1986 (35) | Germany* | Co, X | Cn | Male patients with Bowenoid papulosis or genital warts and females with CIN 1–3 or VIN 1–3 plus the partners of these people | Cervical and vulvar lesion specimens | Penile lesions | H | 6,11,16,18 | 50 |
| Gupta et al., 2006a (36) | India* | Ca, X | Cn | Cases were women with histologically confirmed ICC and their husbands | Cervical biopsy and urine | Penile swabs (intrameatal and distal urethra, external surface of the glans and coronal sulcus), urine | PCR | 16,18 | 30 |
| Gupta et al., 2006b (36) | India* | Ca, X | Cn | Controls were women with normal or inflammatory or negative cervical cytology and their husbands | Cervical scrapings (ectocervix, surface of the cervical portio) and urine | Penile swabs (intrameatal and distal urethra, external surface of the glans and coronal sulcus), urine | PCR | 16,18 | 30 |
| Hernandez et al., 2008 (37) | United States | Co, L | Cn | Couples attending a university health clinic | Cervical smear, swabs, cytobrush from ectocervix and endocervix, anal swabs, oral cytobrushings, dominant hand swabs, first-catch urine samples | Swabs from penis (glans/corona, shaft), scrotum, inner foreskin (for uncircumcised men), and anus; oral cytobrushings; dominant hand swabs, first-catch urine samples, semen | PCR | 6,11,16,18,26,31, 33,35,39,40,42, 45,51,52,53,54,55, 56,58,59,61,62, 64,66,67,68,69, 70,71,72,73,81, 82,83,84,IS39, 6P6108 |
25 |
| Hillman et al., 1993 (38) | United Kingdom | Co, L | Cn | Couples in which 1 partner had clinically apparent anogenital warts | Urethral loop, cervical scrapes and cytological brushings from endocervix, cytobrushings from anal canal, rectal swab, buccal scrapes, vaginal washes, biopsies, blood | Urethral loop, rectal swabs, penile samples (glans, shaft, scrotum), cytobrushings from anal canal, buccal scrapes, biopsies, blood | H, PCR | 6,11,16,18,31,33 | 4 |
| Hippelainen et al., 1994 (39) | Finland | Co, X | Cn | Consecutive women referred for examination for a newly detected abnormal Pap smear and their male partners | Punch biopsies | Biopsies | H, PCR | 6,11,16,18,31,33, 42 |
270 |
| Ho et al., 1993 (40) | Singapore | Co, X | Cn | Consecutive women undergoing colopscopic evaluation of abnormal Pap smears and their husbands | Cervical smear or biopsy | Penile (shaft, foreskin, glans) smears or punch biopsy | PCR | 16 | 17 |
| Inoue et al., 1992 (41) | Japan | Co, X | Cn | Women attending colposcopy clinic and their male partners | Swabs from uterine ectocervix | Semen | H, PCR | 16,18 | 23 |
| Konno et al., 1990 (42) | Japan | Co, X | Cn | Couples presenting with genital warts (female vulvar and cervical condylomata and penile condylomata from male partners) to a sexually transmitted diseases clinic | Cervical or vulvar biopsies | Penile biopsies | H | 6,11,16,18 | 12 |
| Kyo et al., 1994a (43) | Japan | Ca, X | P | Cases were women with ICC or CIN and their male partners | Cervical scrapes from uterine ectocervix | Semen | H, PCR | 16,18 | 12 |
| Kyo et al., 1994b (43) | Japan | Ca, X | P | Controls were women presenting for evaluation of infertility with normal Pap smears and their male partners | Cervical scrapes from uterine ectocervix | Semen | H, PCR | 16,18 | 41 |
| Monsonego et al., 1993 (44) | France | Co, X | Cn | Women with anogenital tract HPV infection (clinical warts, cytological abnormality, or colposcopic abnormality) and their male partners | Samples from exocervix and vagina using an Ayre spatula, cytobrushings from endocervix, and biopsies from lesions on cervix, vagina, vulva, perineum, and anus | Penile biopsies and cellular material from urethral meatus | H | 6,11,16,18, undetermined type (not type 6,11,16, or 18) |
350 |
| Nakazawa et al., 1991 (45) | Japan | Co, X | Cn | Women who underwent hysterectomy because of cervical neoplasms and their male partners | Swabs from ectocervix | Urine | H, PCR | 16,18 | 23 |
| Nicolau et al., 2005 (46) | Brazil | Co, X | Cn | HPV-positive women and their stable male partners | Not reported | Brushings from glans, prepuce internal surface (including sulcus and corona), distal urethra, prepuce external surface (in conjunction with cutis penis), scrotum, and anus; biopsies from subclinical or clinically apparent lesions | H | 6,11,16,18,31,33, 35,39,42,43,44,45, 51,52,56,58,59, 68 |
50 |
| Nieminen et al., 1991 (47) | Finland | Ca, X | Cn | Cases were regular male partners of women positive for HPV DNA from the cervix | Cervical samples | Semen | H | 6,11,16,18,31,33, 35 |
17 |
| Rintala et al., 2005 (48) | Finland | Co, L | Cn | Cohort of young pregnant women, fathers-to-be, and infants to assess familial transmission; study included consecutive families | Cervical smear; brushings from vagina, exocervix, endocervix; scrapings from cervical mucosa and oral mucosae | Semen, scrapings from the urethral mucosa and oral muscosae | H, PCR | 16,18,31,33,35, 39,45,51,52,54, 56,58 |
76 |
| Rosemberg et al., 1988 (49) | United States | Co, X | Cn | Males who have had contact with known HPV-infected females | Not reported | Urethral cytobrushings | H | 6,11,16,18 | 75 |
| Rotola et al., 1994 (50) | Italy | Co, X | Cn | Male partners of females who were diagnosed as having HPV genital infection by colposcopy, cytology, or histology | Cervical specimens | Biopsies from penile, scrotum, pubis, and perianal regions | H | 6,11,16,18,33 | 15 |
| Schneider et al., 1988 (51) | Germany* | Co, X | Cn | Male partners of women with documented HPV-associated lesions of the cervix | Cervical specimens | Penile swabs (preputial sac, glans penis, fossa navicularis, penile shaft), | H | 6,11,16,18,31 | 156 |
| Strand et al., 1995 (52) | Sweden* | Co, X | Cn | Women referred to colposcopy clinic due to recently detected HPV infection or an abnormal Pap smear and their male partners | Specimens from cervical or vaginal lesions | Cytobrushings from glans penis, sulcus, preputium, and penile shaft; sample from distal part of urethra taken with a plastic probe; biopsies from any clinical abnormality | H, PCR | 6,11,16,18,31,33, 35,42,43,44,51, 52,56,58 |
25 |
| Wickenden et al., 1988 (53) | United Kingdom | Co, X | Cn | Men and women presenting at a clinic for genital warts who brought their partners into the clinic within 28 days | Cervical scrapings; biopsies from exophytic warts (could be cervical, vulval, perianal, or pharyngeal) | Biopsies from exophytic warts (could be penile, perianal, or pharyngeal) | H | 6,11,16,18 | 36 |
Note. Co = cohort study, Ca = case-control study, X = cross-sectional data, L = longitudinal data, Cn = convenience sample, P = population-based sample, H = DNA hybridization methods, PCR = polymerase chain reaction, HPV = human papillomavirus, CIN = cervical intraepithelial neoplasia, CIS = carcinoma in situ, SIL = squamous intraepithelial lesion, LGSIL = low-grade squamous intraepithelial lesion, ICC = invasive cervical cancer, VIN = vulvar intraepithelial neoplasia.
Study location not stated explicitly but inferred from authors’ location
Both partners in 37.7% of couples were infected with any type of HPV (Table 2). In 25.5% of couples, both partners were infected with 1 or more of the same HPV types. More couples had both members infected with HPV type 16 (9.0%) or type 6 (8.6%) than with type 11 (4.6%) or type 18 (2.2%). Concordance was higher than chance would predict for HPV types 11, 16, and 18 (all p<0.05), while concordance for HPV type 6 reached borderline statistical significance (p=0.06) (Table 3). In couples where both members were HPV-positive, 63.2% were infected with 1 or more of the same HPV types (Table 2).
Table 2.
HPV concordance
| No. Studies |
Total No. Couples |
No. Concordant Couples |
Concordance (95% CI) |
I2 | |
|---|---|---|---|---|---|
| Same HPV type* | 19 | 1249 | 263 | 0.255 (0.172–0.361) | 88 |
| Any HPV type | 33 | 2972 | 975 | 0.377 (0.293–0.468) | 93 |
| Of couples with both partners infected, percent who have the same HPV type* |
18 | 538 | 263 | 0.632 (0.491–0.753) | 79 |
| HPV type 6 | 9 | 632 | 29 | 0.086 (0.031–0.231) | 83 |
| HPV type 11 | 9 | 632 | 18 | 0.046 (0.018–0.113) | 69 |
| HPV type 16 | 21 | 1949 | 132 | 0.090 (0.051–0.154) | 87 |
| HPV type 18 | 17 | 1819 | 16 | 0.022 (0.010–0.046) | 56 |
Note. Concordance estimates are from random effects meta-analysis. HPV = human papillomavirus, CI = confidence interval.
Both members of the couple infected with 1 or more of the same HPV types
Table 3.
Expected and observed type-specific HPV concordance
| HPV Prevalence, n (%) |
HPV Concordance, n (%) |
|||||||
|---|---|---|---|---|---|---|---|---|
| No. Studies | No. Couples | Females | Males | Expected | Observed | OR (95% CI) | I2 | |
| HPV 6 | 5 | 340 | 49 (14.4) | 41 (12.1) | 5.9 (1.7) | 18 (5.3) | 6.60 (0.92–47.50)† | 65 |
| HPV 11 | 5 | 512 | 32 (6.3) | 21 (4.1) | 1.3 (0.3) | 7 (1.4) | 7.56 (2.63–21.74)** | 0 |
| HPV 16 | 12 | 1615 | 479 (29.7) | 169 (10.5) | 50.1 (3.1) | 107 (6.6) | 3.42 (2.25–5.20)** | 0 |
| HPV 18 | 6 | 1464 | 134 (9.2) | 28 (1.9) | 2.6 (0.2) | 10 (0.7) | 7.91 (2.43–25.73)* | 26 |
Note. Table reports unweighted frequencies and percentages along with odds ratios and 95% confidence intervals from random-effects meta-analysis. Table includes only studies that reported data required for these calculations. HPV = human papillomavirus, OR = odds ratio, CI = confidence interval.
p=0.06,
p<0.05,
p<0.001
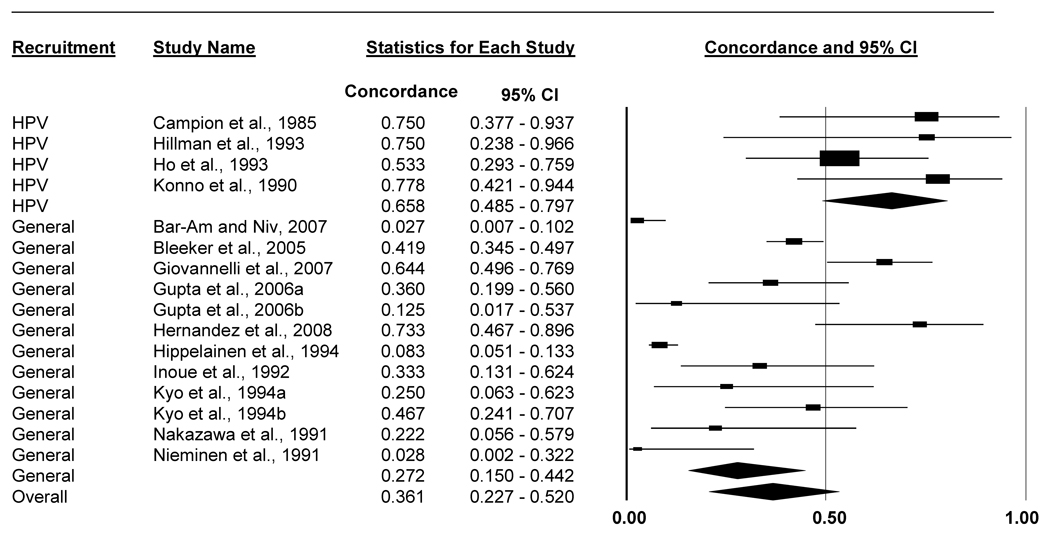
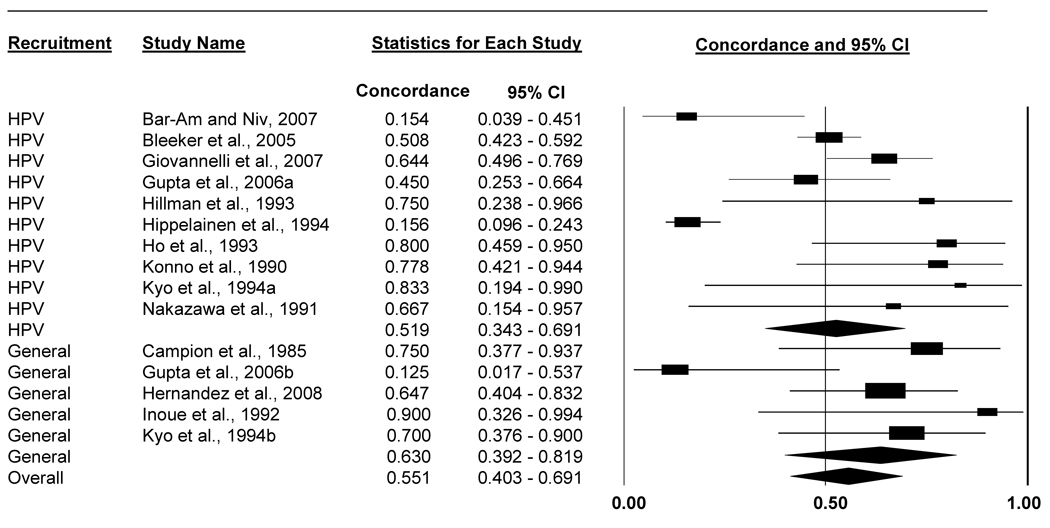
Same-type concordance for people with HPV-infected partners was lower for men than women (Table 4, Figures 2 and 3). Among male partners of HPV-positive women, 36.1% (95% CI: 22.7%-52.0%) were infected with 1 or more of the same HPV types. Conversely, among female partners of HPV-positive men, 55.1% (95% CI: 40.3%-69.1%) were infected with 1 or more of the same HPV types.
Table 4.
Sex-dependent HPV concordance
| No. Studies |
Total No. Couples with HPV-positive Females |
No. Couples with Males with Same HPV types* |
Concordance (95% CI) |
I2 | |
|---|---|---|---|---|---|
| All studies | 16 | 605 | 173 | 0.361 (0.227–0.520) | 86 |
| Studies that recruited couples with male partners who had HPV-related disease |
4 | 36 | 24 | 0.658 (0.485–0.797) | 0 |
| Studies without HPV-disease-based inclusion criteria for males |
12 | 569 | 149 | 0.272 (0.150–0.442) | 88 |
| Total No. Couples with HPV-positive Males |
No. Couples with Females with Same HPV types* |
||||
| All studies | 15 | 381 | 173 | 0.551 (0.403–0.691) | 75 |
| Studies that recruited couples with female partners who had HPV-related disease |
10 | 334 | 144 | 0.519 (0.343–0.691) | 82 |
| Studies without HPV-disease-based inclusion criteria for females |
5 | 47 | 29 | 0.630 (0.392–0.819) | 45 |
Note. Concordance estimates are from random effects meta-analysis. Analyses included all partners, regardless of HPV status, of HPV-positive individuals for whom studies reported appropriate concordance data. HPV = human papillomavirus, CI = confidence interval.
Both members of the couple infected with 1 or more of the same HPV types
Figure 2.
Forest plot of percent of men with the same HPV types (1 or more types) as their HPV-positive female partners. Analyses included all men, regardless of HPV status, whose female partners were HPV-positive and for whom studies reported appropriate concordance data. Results stratified by whether the study recruited couples that included men with HPV-related disease.
Figure 3.
Forest plot of percent of women with the same HPV types (1 or more types) as their HPV-positive male partners. Analyses included all women, regardless of HPV status, whose male partners were HPV-positive and for whom studies reported appropriate concordance data. Results stratified by whether the study recruited couples that included women with HPV-related disease.
We further refined these sex-dependent concordance estimates by stratifying on whether studies recruited individuals with HPV or HPV-related disease (e.g., invasive cervical cancer) and their partners. Men with HPV-positive female partners had 1 or more of the same HPV types more often in studies that recruited men with HPV-related disease (65.8%, 95% CI: 48.5%-79.7%) compared to studies without this inclusion criterion for men (27.2%, 95% CI: 15.0%-44.2%) (p=0.002; Table 4). We did not find this difference for women. Same-type concordance was equally high in studies that recruited couples with women having HPV-related disease (51.9%, 95% CI: 34.3%-69.1%) compared to studies that did not recruit women with HPV-related disease (63.0%, 95% CI: 39.2%-81.9%) (p=0.463).
We further examined these sex-dependent concordance estimates for potential correlates. For men, we examined only studies that did not recruit men with HPV-related disease (k=12 studies), since this was shown to strongly affect concordance levels. Because we did not find this difference for women, we included all studies (k=15 studies). Among men, studies that used PCR to detect HPV had higher same-type concordance (k=10 studies, 35.0%, 95% CI: 20.5%-52.9%) compared to studies that did not use PCR (k=2 studies, 2.7%, 95% CI: 0.8%-8.9%) (p<0.001), where concordance was defined as the proportion of men positive for 1 or more of the same HPV types as their HPV-infected female partners. None of the other variables examined (specimen collection site for men, number of HPV types tested for, year of article publication, study location) were associated with sex-dependent concordance. Among women, number of HPV types tested for, year of article publication, whether the study used PCR, and study location were not associated with sex-dependent concordance.
Discussion
Our comprehensive review of data from several thousand heterosexual couples from 4 continents found moderate to high positive concordance, defined as both partners having the HPV outcome of interest. Concordance was greater than one would expect by chance for HPV types 11, 16 and 18, and results were suggestive for HPV type 6. While 2 small primary studies have reported similar results (24, 25), our meta-analysis confirms these findings in a substantially larger and more diverse sample. Of couples in which both members were HPV-positive, about two thirds (63.2%) were infected with 1 or more of the same HPV types. This level of concordance is consistent with the high transmissibility of HPV (16).
Our finding that female coital partners of HPV-positive men were more likely to be infected with the same types of HPV (1 or more types) compared to male partners of HPV-positive women makes sense, given that women may be more susceptible to HPV infection and take longer to clear HPV infections than men (4–6, 17, 18, 54). Exposure to HPV in men often involves the keratinized epithelium of the penis that may be less susceptible to HPV infection than the mucosal epithelium of the cervix (54). HPV infections may also persist longer in women compared to men. Estimates of the median duration of HPV infections among women have ranged from 4.3–11.1 months for nononcogenic HPV types and from 6.5–14.8 months for oncogenic HPV types (4, 6, 17, 18). Among men, the median duration of HPV infections is about 6 months for both oncogenic and nononcogenic HPV types (5), with circumcision possibly reducing HPV acquisition and speeding clearance (55–57). The gender difference did not appear in studies where recruitment involved individuals with HPV-related disease. However, in studies where recruitment did not involve individuals with HPV-related disease, the difference was apparent and may be partly due to sampling from suboptimal anatomic sites and difficulty in obtaining adequate specimens, problems that occur more often when testing men for HPV (58, 59).
HPV concordance in heterosexual couples has important clinical and public health implications. HPV infection and subsequent HPV-related disease pose a substantial burden worldwide (10). Female partners of men with HPV-related disease should be encouraged to get screened for HPV-related disease given that they have a high likelihood of concomitant infection and that most HPV infections in couples are of the same viral types. Screening may also benefit male partners of HPV-infected women, though an HPV DNA test has not yet been approved for clinical use in men. Partners of HPV-infected individuals may receive additional benefits from educational counseling and screening for other STIs if encouraged to see a healthcare provider (60). However, only 62% of healthcare providers encourage women with either abnormal Pap smears or positive HPV tests to tell their sexual partners to see a clinician (61). We are not aware of data regarding referral of female partners of men with HPV-related disease, but high concordance levels suggest clinicians may be missing many opportunities to encourage HPV-infected patients to notify their partners and encourage them to seek care. It is likely that many of these partners are unknowingly infected with HPV and may be at risk for HPV-related disease.
Uninfected sexual partners may be an important target population for HPV vaccination, provided they are in the recommended age range for HPV vaccine. The Advisory Committee on Immunization Practices (ACIP) currently recommends routine vaccination of females aged 11–12 years with catch-up vaccination for females through age 26 (21, 22), while providing a permissive recommendation for HPV vaccination of males aged 9–26 years (23). The benefits of vaccination to individuals seronegative to HPV types included in the vaccine are clear, and emerging evidence suggests that HPV vaccine may also help people who previously had and cleared an infection (62, 63), though additional research among such individuals is needed. It is unlikely that people have been infected with all 4 HPV types in the quadrivalent HPV vaccine (64), making the vaccine a potentially beneficial prophylaxis for people already exposed to some HPV types. Women’s higher rates of infection when their partners are HPV-positive lend further support to the recommendations of the ACIP, which made a stronger recommendation for HPV vaccination among age-appropriate females than males (21–23). Our findings may provide HPV vaccine cost-effectiveness analyses with increased precision when estimating the potential consequences of having a sexual partner who is HPV-positive and how it may differ for males and females.
Strengths of our meta-analysis include a comprehensive search strategy, careful data extraction methods, and use of random-effects modeling techniques. The few additional studies identified after we searched PubMed suggests that we identified most relevant published studies. We identified some important sources of variability in study concordance estimates, but because of poor reporting by primary studies, we were unable to identify additional sources of variation that we believe are likely to exist but are presently unknown. For this reason, we believe that these concordance levels should be viewed as tentative estimates that may differ for some populations.
As the field matures, new concordance studies should use more rigorous research designs. All but a few studies were cross-sectional, meaning many of the studies were not able to establish the temporality or direction of HPV transmission between sexual partners. Longitudinal designs of HPV discordant couples would more effectively address the dynamics of HPV transmission, clearance, and persistent infection (37). More rigorous research designs will have the added benefit of allowing researchers to move beyond description to more robust hypothesis testing. More complex study designs would also allow researchers to better understand first transmission, re-infection and back-and-forth passage within couples, concordance in couples in which 1 partner has received HPV vaccine, and concordance after treatment for HPV-related lesions.
Concordance studies should also characterize their participants in greater detail and study more meaningful populations. Fewer studies recruited men with HPV-related disease than recruited women with such disease. Many studies did not report basic information on participant demographics, health, sexual history, and relationship characteristics. While studies often used state of the art methods for determining HPV DNA prevalence, some studies also did not report basic information on study design and methods. Table 5 describes basic participant, couple, and study characteristics that we recommend future HPV concordance and transmission studies consider collecting and reporting. The studies in our meta-analysis sampled geographically diverse populations, including many in developing countries where the majority of cervical cancer deaths occur (13). However, no studies were conducted in Africa, parts of which have the highest cervical cancer mortality rates in the world (13).
Table 5.
Characteristics that future HPV concordance and transmission studies should collect and report.
| Study Characteristics |
| Years of data collection |
| Study location |
| Study design (e.g., longitudinal) |
| Sampling and recruitment methods |
| Response rate |
| Specimen collection sites and methods |
| HPV detection methods |
| All HPV types tested for |
| Participant Characteristics |
| Age |
| Race / ethnicity |
| Socioeconomic status (e.g., education) |
| Number of lifetime sexual partners |
| Age at first sexual intercourse |
| Sexual behavior with same-sex partners |
| History of sexually transmitted infections |
| Circumcision status for males |
| Couple Characteristics |
| Duration of relationship |
| Marital status |
| Condom use |
| Whether couple is monogamous |
Our findings can help inform cost-effectiveness analyses, which have helped guide regulatory and funding policies regarding HPV vaccine. High quality longitudinal studies are needed to better understand HPV concordance and transmission among heterosexual couples. In future efforts, researchers also should be more diligent in reporting characteristics of participants, couples, and their studies. Reporting such detailed information about participants and couples may help identify high-risk subsets of individuals that warrant focused interventions to prevent HPV-related disease. Our findings suggest the need for greater attention to sexual partners of HPV-infected individuals.
Acknowledgement
Financial Support: Supported in part by research grants from the American Cancer Society (MSRG-06-259-01-CPPB) and the Cancer Control Education Program at Lineberger Comprehensive Cancer Center (R25 CA57726).
We thank Evan Myers for his feedback on an earlier draft of this paper.
Footnotes
Conflicts of interest: Authors have received research grants from Merck & Co., Inc. (NB, PR) and GlaxoSmithKline (NB), but neither has received honoraria or consulting fees from these companies. These funds were not used to support this research study. WP reports no conflicts.
References
- 1.Partridge JM, Koutsky LA. Genital human papillomavirus infection in men. Lancet Infect Dis. 2006;6:21–31. doi: 10.1016/S1473-3099(05)70323-6. [DOI] [PubMed] [Google Scholar]
- 2.Gillison ML, Shah KV. Chapter 9: Role of mucosal human papillomavirus in nongenital cancers. J Natl Cancer Inst Monogr. 2003;31:57–65. doi: 10.1093/oxfordjournals.jncimonographs.a003484. [DOI] [PubMed] [Google Scholar]
- 3.Cates W., Jr Estimates of the incidence and prevalence of sexually transmitted diseases in the United States. American Social Health Association Panel. Sex Transm Dis. 1999;26:S2–S7. doi: 10.1097/00007435-199904001-00002. [DOI] [PubMed] [Google Scholar]
- 4.Giuliano AR, Harris R, Sedjo RL, et al. Incidence, prevalence, and clearance of type-specific human papillomavirus infections: The Young Women's Health Study. J Infect Dis. 2002;186:462–469. doi: 10.1086/341782. [DOI] [PubMed] [Google Scholar]
- 5.Giuliano AR, Lu B, Nielson CM, et al. Age-specific prevalence, incidence, and duration of human papillomavirus infections in a cohort of 290 US men. J Infect Dis. 2008;198:827–835. doi: 10.1086/591095. [DOI] [PubMed] [Google Scholar]
- 6.Trottier H, Mahmud S, Prado JC, et al. Type-specific duration of human papillomavirus infection: Implications for human papillomavirus screening and vaccination. J Infect Dis. 2008;197:1436–1447. doi: 10.1086/587698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown DR, Schroeder JM, Bryan JT, Stoler MH, Fife KH. Detection of multiple human papillomavirus types in condylomata acuminata lesions from otherwise healthy and immunosuppressed patients. J Clin Microbiol. 1999;37:3316–3322. doi: 10.1128/jcm.37.10.3316-3322.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lacey CJ, Lowndes CM, Shah KV. Chapter 4: Burden and management of non-cancerous HPV-related conditions: HPV-6/11 disease. Vaccine. 2006;24 doi: 10.1016/j.vaccine.2006.06.015. S3/35–41. [DOI] [PubMed] [Google Scholar]
- 9.Gillison ML, Chaturvedi AK, Lowy DR. HPV prophylactic vaccines and the potential prevention of noncervical cancers in both men and women. Cancer. 2008;113:3036–3046. doi: 10.1002/cncr.23764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parkin DM, Bray F. Chapter 2: The burden of HPV-related cancers. Vaccine. 2006;24 doi: 10.1016/j.vaccine.2006.05.111. S3/11–25. [DOI] [PubMed] [Google Scholar]
- 11.Pfister H. Chapter 8: Human papillomavirus and skin cancer. J Natl Cancer Inst Monogr. 2003;31:52–56. doi: 10.1093/oxfordjournals.jncimonographs.a003483. [DOI] [PubMed] [Google Scholar]
- 12.Klein F, Amin Kotb WF, Petersen I. Incidence of human papilloma virus in lung cancer. Lung Cancer. 2009;65:13–18. doi: 10.1016/j.lungcan.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 13.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 14.Insinga RP, Dasbach EJ, Elbasha EH. Assessing the annual economic burden of preventing and treating anogenital human papillomavirus-related disease in the US: Analytic framework and review of the literature. Pharmacoeconomics. 2005;23:1107–1122. doi: 10.2165/00019053-200523110-00004. [DOI] [PubMed] [Google Scholar]
- 15.Hu D, Goldie S. The economic burden of noncervical human papillomavirus disease in the United States. Am J Obstet Gynecol. 2008;198 doi: 10.1016/j.ajog.2008.03.064. 500.e1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burchell AN, Richardson H, Mahmud SM, et al. Modeling the sexual transmissibility of human papillomavirus infection using stochastic computer simulation and empirical data from a cohort study of young women in Montreal, Canada. Am J Epidemiol. 2006;163:534–543. doi: 10.1093/aje/kwj077. [DOI] [PubMed] [Google Scholar]
- 17.Goodman MT, Shvetsov YB, McDuffie K, et al. Prevalence, acquisition, and clearance of cervical human papillomavirus infection among women with normal cytology: Hawaii Human Papillomavirus Cohort Study. Cancer Res. 2008;68:8813–8824. doi: 10.1158/0008-5472.CAN-08-1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Munoz N, Mendez F, Posso H, et al. Incidence, duration, and determinants of cervical human papillomavirus infection in a cohort of Colombian women with normal cytological results. J Infect Dis. 2004;190:2077–2087. doi: 10.1086/425907. [DOI] [PubMed] [Google Scholar]
- 19.Syrjanen S, Saastamoinen J, Chang FJ, Ji HX, Syrjanen K. Colposcopy, punch biopsy, in situ DNA hybridization, and the polymerase chain reaction in searching for genital human papillomavirus (HPV) infections in women with normal PAP smears. J Med Virol. 1990;31:259–266. doi: 10.1002/jmv.1890310404. [DOI] [PubMed] [Google Scholar]
- 20.Nuovo GJ, Blanco JS, Leipzig S, Smith D. Human papillomavirus detection in cervical lesions nondiagnostic for cervical intraepithelial neoplasia: Correlation with papanicolaou smear, colposcopy, and occurrence of cervical intraepithelial neoplasia. Obstet Gynecol. 1990;75:1006–1011. [PubMed] [Google Scholar]
- 21.Markowitz LE, Dunne EF, Saraiya M, et al. Quadrivalent human papillomavirus vaccine: Recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2007;56:1–24. [PubMed] [Google Scholar]
- 22.Centers for Disease Control and Prevention. FDA licensure of bivalent human papillomavirus vaccine (HPV2, Cervarix) for use in females and updated HPV vaccination recommendations from the Advisory Committee on Immunization Practices (ACIP) MMWR Morb Mortal Wkly Rep. 2010;59:626–629. [PubMed] [Google Scholar]
- 23.Centers for Disease Control and Prevention. FDA licensure of quadrivalent human papillomavirus vaccine (HPV4, Gardasil) for use in males and guidance from the Advisory Committee on Immunization Practices (ACIP) MMWR Morb Mortal Wkly Rep. 2010;59:630–632. [PubMed] [Google Scholar]
- 24.Bleeker MC, Hogewoning CJ, Berkhof J, et al. Concordance of specific human papillomavirus types in sex partners is more prevalent than would be expected by chance and is associated with increased viral loads. Clin Infect Dis. 2005;41:612–620. doi: 10.1086/431978. [DOI] [PubMed] [Google Scholar]
- 25.Giovannelli L, Bellavia C, Capra G, et al. HPV group- and type-specific concordance in HPV infected sexual couples. J Med Virol. 2007;79:1882–1888. doi: 10.1002/jmv.21015. [DOI] [PubMed] [Google Scholar]
- 26.Baken LA, Koutsky LA, Kuypers J, et al. Genital human papillomavirus infection among male and female sex partners: Prevalence and type-specific concordance. J Infect Dis. 1995;171:429–432. doi: 10.1093/infdis/171.2.429. [DOI] [PubMed] [Google Scholar]
- 27.Bar-Am A, Niv J. The role of HPV DNA in the evaluation and follow-up of asymptomatic male sexual partners of females with CIN3. Eur J Gynaecol Oncol. 2007;28:207–210. [PubMed] [Google Scholar]
- 28.Benevolo M, Mottolese M, Marandino F, et al. HPV prevalence among healthy Italian male sexual partners of women with cervical HPV infection. J Med Virol. 2008;80:1275–1281. doi: 10.1002/jmv.21189. [DOI] [PubMed] [Google Scholar]
- 29.Campion MJ, Singer A, Clarkson PK, McCance DJ. Increased risk of cervical neoplasia in consorts of men with penile condylomata acuminata. Lancet. 1985;1:943–946. doi: 10.1016/s0140-6736(85)91724-6. [DOI] [PubMed] [Google Scholar]
- 30.Franceschi S, Castellsague X, Dal Maso L, et al. Prevalence and determinants of human papillomavirus genital infection in men. Br J Cancer. 2002;86:705–711. doi: 10.1038/sj.bjc.6600194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gal D, Friedman M, Mitrani-Rosenbaum S. Transmissibility and treatment failures of different types of human papillomavirus. Obstet Gynecol. 1989;73:308–311. [PubMed] [Google Scholar]
- 32.Giraldo PC, Eleuterio J, Jr, Cavalcante DI, Goncalves AK, Romao JA, Eleuterio RM. The role of high-risk HPV-DNA testing in the male sexual partners of women with HPV-induced lesions. Eur J Obstet Gynecol Reprod Biol. 2008;137:88–91. doi: 10.1016/j.ejogrb.2006.12.026. [DOI] [PubMed] [Google Scholar]
- 33.Golijow CD, Perez LO, Smith JS, Abba MC. Human papillomavirus DNA detection and typing in male urine samples from a high-risk population from Argentina. J Virol Methods. 2005;124:217–220. doi: 10.1016/j.jviromet.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 34.Gomousa-Michael M, Deligeorgi-Politi H, Condi-Paphiti A, Rammou-Kinia R, Ghionis J, Belca-Hari K. Human papillomavirus identification and typing of both sexual partners. Acta Cytol. 1997;41:244–250. doi: 10.1159/000332450. [DOI] [PubMed] [Google Scholar]
- 35.Gross G, Ikenberg H, de Villiers E, Schneider A, Wagner D, Gissmann L. Bowenoid papulosis: A venereally transmissible disease as reservoir for HPV 16. In: Peto R, zur Hausen H, editors. Viral etiology of cervical cancer. Cold Spring Harbor, N.Y.: Cold Spring Harbor Laboratory; 1986. pp. 149–165. [Google Scholar]
- 36.Gupta A, Arora R, Gupta S, et al. Human papillomavirus DNA in urine samples of women with or without cervical cancer and their male partners compared with simultaneously collected cervical/penile smear or biopsy specimens. J Clin Virol. 2006;37:190–194. doi: 10.1016/j.jcv.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 37.Hernandez BY, Wilkens LR, Zhu X, et al. Transmission of human papillomavirus in heterosexual couples. Emerg Infect Dis. 2008;14:888–894. doi: 10.3201/eid1406.070616.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hillman RJ, Ryait BK, Botcherby M, Taylor-Robinson D. Changes in HPV infection in patients with anogenital warts and their partners. Genitourin Med. 1993;69:450–456. doi: 10.1136/sti.69.6.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hippelainen MI, Yliskoski M, Syrjanen S, et al. Low concordance of genital human papillomavirus (HPV) lesions and viral types in HPV-infected women and their male sexual partners. Sex Transm Dis. 1994;21:76–82. doi: 10.1097/00007435-199403000-00004. [DOI] [PubMed] [Google Scholar]
- 40.Ho L, Tay SK, Chan SY, Bernard HU. Sequence variants of human papillomavirus type 16 from couples suggest sexual transmission with low infectivity and polyclonality in genital neoplasia. J Infect Dis. 1993;168:803–809. doi: 10.1093/infdis/168.4.803. [DOI] [PubMed] [Google Scholar]
- 41.Inoue M, Nakazawa A, Fujita M, Tanizawa O. Human papillomavirus (HPV) type 16 in semen of partners of women with HPV infection. Lancet. 1992;339:1114–1115. doi: 10.1016/0140-6736(92)90708-b. [DOI] [PubMed] [Google Scholar]
- 42.Konno R, Shikano K, Horiguchi M, et al. Detection of human papillomavirus DNA in genital condylomata in women and their male partners by using in situ hybridization with digoxygenin labeled probes. Tohoku J Exp Med. 1990;160:383–390. doi: 10.1620/tjem.160.383. [DOI] [PubMed] [Google Scholar]
- 43.Kyo S, Inoue M, Koyama M, Fujita M, Tanizawa O, Hakura A. Detection of high-risk human papillomavirus in the cervix and semen of sex partners. J Infect Dis. 1994;170:682–685. doi: 10.1093/infdis/170.3.682. [DOI] [PubMed] [Google Scholar]
- 44.Monsonego J, Zerat L, Catalan F, Coscas Y. Genital human papillomavirus infections: Correlation of cytological, colposcopic and histological features with viral types in women and their male partners. Int J STD AIDS. 1993;4:13–20. doi: 10.1177/095646249300400104. [DOI] [PubMed] [Google Scholar]
- 45.Nakazawa A, Inoue M, Fujita M, Tanizawa O, Hakura A. Detection of human papillomavirus type 16 in sexual partners of patients having cervical cancer by polymerase chain reaction. Jpn J Cancer Res. 1991;82:1187–1190. doi: 10.1111/j.1349-7006.1991.tb01777.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nicolau SM, Camargo CG, Stavale JN, et al. Human papillomavirus DNA detection in male sexual partners of women with genital human papillomavirus infection. Urology. 2005;65:251–255. doi: 10.1016/j.urology.2004.09.031. [DOI] [PubMed] [Google Scholar]
- 47.Nieminen P, Koskimies AI, Paavonen J. Human papillomavirus DNA is not transmitted by semen. Int J STD AIDS. 1991;2:207–208. doi: 10.1177/095646249100200313. [DOI] [PubMed] [Google Scholar]
- 48.Rintala MA, Grenman SE, Puranen MH, et al. Transmission of high-risk human papillomavirus (HPV) between parents and infant: A prospective study of HPV in families in Finland. J Clin Microbiol. 2005;43:376–381. doi: 10.1128/JCM.43.1.376-381.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rosemberg SK, Reid R, Greenberg M, Lorincz AT. Sexually transmitted papillomaviral infection in the male: II. The urethral reservoir. Urology. 1988;32:47–49. doi: 10.1016/0090-4295(88)90452-9. [DOI] [PubMed] [Google Scholar]
- 50.Rotola A, Costa S, Monini P, et al. Impact of sexual habits on the clinical evaluation of male HPV infection. Eur J Epidemiol. 1994;10:373–380. doi: 10.1007/BF01719659. [DOI] [PubMed] [Google Scholar]
- 51.Schneider A, Kirchmayr R, De Villiers EM, Gissmann L. Subclinical human papillomavirus infections in male sexual partners of female carriers. J Urol. 1988;140:1431–1434. doi: 10.1016/s0022-5347(17)42065-9. [DOI] [PubMed] [Google Scholar]
- 52.Strand A, Rylander E, Wilander E, Zehbe I. HPV infection in male partners of women with squamous intraepithelial neoplasia and/or high-risk HPV. Acta Derm Venereol. 1995;75:312–316. doi: 10.2340/0001555575312316. [DOI] [PubMed] [Google Scholar]
- 53.Wickenden C, Hanna N, Taylor-Robinson D, et al. Sexual transmission of human papillomaviruses in heterosexual and male homosexual couples, studied by DNA hybridisation. Genitourin Med. 1988;64:34–38. doi: 10.1136/sti.64.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thompson DL, Douglas JM, Jr, Foster M, et al. Seroepidemiology of infection with human papillomavirus 16, in men and women attending sexually transmitted disease clinics in the United States. J Infect Dis. 2004;190:1563–1574. doi: 10.1086/423817. [DOI] [PubMed] [Google Scholar]
- 55.Lu B, Wu Y, Nielson CM, et al. Factors associated with acquisition and clearance of human papillomavirus infection in a cohort of US men: A prospective study. J Infect Dis. 2009;199:362–371. doi: 10.1086/596050. [DOI] [PubMed] [Google Scholar]
- 56.Tobian AA, Serwadda D, Quinn TC, et al. Male circumcision for the prevention of HSV-2 and HPV infections and syphilis. N Engl J Med. 2009;360:1298–1309. doi: 10.1056/NEJMoa0802556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Giuliano AR, Lazcano E, Villa LL, et al. Circumcision and sexual behavior: Factors independently associated with human papillomavirus detection among men in the HIM study. Int J Cancer. 2009;124:1251–1257. doi: 10.1002/ijc.24097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Giuliano AR, Nielson CM, Flores R, et al. The optimal anatomic sites for sampling heterosexual men for human papillomavirus (HPV) detection: The HPV detection in men study. J Infect Dis. 2007;196:1146–1152. doi: 10.1086/521629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Flores R, Abalos AT, Nielson CM, Abrahamsen M, Harris RB, Giuliano AR. Reliability of sample collection and laboratory testing for HPV detection in men. J Virol Methods. 2008;149:136–143. doi: 10.1016/j.jviromet.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 60.Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2006. MMWR. 2006;55:1–94. [PubMed] [Google Scholar]
- 61.Hoover K, Friedman A, Montano D, Kasprzyk D, Greek A, Hogben M. What about the partners of women with abnormal pap or positive HPV tests? Sex Transm Dis. doi: 10.1097/OLQ.0b013e31818eb765. (In press). [DOI] [PubMed] [Google Scholar]
- 62.Munoz N, Manalastas R, Jr, Pitisuttithum P, et al. Safety, immunogenicity, and efficacy of quadrivalent human papillomavirus (types 6, 11, 16, 18) recombinant vaccine in women aged 24–45 years: A randomised, double-blind trial. Lancet. 2009;373:1949–1957. doi: 10.1016/S0140-6736(09)60691-7. [DOI] [PubMed] [Google Scholar]
- 63.Olsson SE, Kjaer SK, Sigurdsson K, et al. Evaluation of quadrivalent HPV 6/11/16/18 vaccine efficacy against cervical and anogenital disease in subjects with serological evidence of prior vaccine type HPV infection. Hum Vaccin. 2009;5(10) doi: 10.4161/hv.5.10.9515. [DOI] [PubMed] [Google Scholar]
- 64.Markowitz LE, Sternberg M, Dunne EF, McQuillan G, Unger ER. Seroprevalence of human papillomavirus types 6, 11, 16, and 18 in the United States: National Health and Nutrition Examination Survey 2003–2004. J Infect Dis. 2009;200:1059–1067. doi: 10.1086/604729. [DOI] [PubMed] [Google Scholar]