Abstract
Background
Melanoma incidence has been correlated strongly and positively with both socioeconomic status (SES) and lower latitude and other measures of ambient ultraviolet radiation (UVR). However, because high SES populations may be co-located in areas of high UVR, we assessed their joint influences on melanoma occurrence, so as to better target subpopulations for melanoma education and screening.
Methods
We obtained from the California Cancer Registry information regarding 23,564 incident cases of invasive cutaneous melanoma among non-Hispanic white residents between January 1, 1998 and December 31, 2002. We geocoded each case based on residence at diagnosis and linked previously tested neighborhood measures of SES and average annual UVR to calculate age-adjusted incidence rates, rate ratios, and corresponding 95% confidence intervals (CI). Poisson regression was used to calculate multivariately adjusted rate ratios.
Results
UVR was significantly and positively associated with melanoma incidence only among persons living in the top 40% of California neighborhoods ranked by SES. People in neighborhoods of the highest SES and UVR categories had 60% higher rates of melanoma than those from neighborhoods in the lowest categories (rate ratio 1.60; 95% CI 1.02–2.51).
Conclusion
Our findings indicate that UVR and SES interact to influence melanoma occurrence, and suggest that socioeconomic gradients in melanoma incidence are not explained entirely by UVR.
Impact
Cancer prevention and early detection educational efforts should be targeted to high SES groups in areas of high UVR exposure. Contextual measures of both SES and UVR should be considered important determinants of melanoma occurrence in future studies.
Keywords: Melanoma, SES, UV, geocode, risk, model
BACKGROUND
Melanoma represents a substantial and growing component of the cancer burden in light-skinned populations worldwide(1). Among the non-Hispanic white population of the United States (US) over the past two decades, melanoma incidence has increased by over 3% per year(2). At both the individual- and population-levels, an important predictor of melanoma risk is socioeconomic status (SES) (3–6). Among US whites, melanoma incidence rates are at least twice as high among persons with college educations or high household incomes compared with those in the lowest categories of education or income (7, 8).
Several factors may mediate the positive association of SES with melanoma occurrence. At the individual level, higher SES correlates with behaviors related to exposure to ultraviolet radiation (UVR), specifically lifetime number of sunburns, increased tanning bed use and intermittent exposure to high levels of UVR during vacations to sunny areas(3). In a large cohort of Norwegian women, associations of melanoma risk with educational status were explained completely by lifetime number of sunburns as well as latitude of residence(9). However, at the neighborhood level, it is unclear whether UVR explains the association with SES, especially because higher SES neighborhoods are often co-located in areas with higher average annual UVR, including waterfront or coastal areas. To our knowledge, no prior studies have assessed the separate contributions to melanoma incidence of neighborhood measures of SES and UVR, perhaps because they lacked the large size and adequate heterogeneity with respect to both SES and latitude.
Disentangling the influences of SES and UVR will help to better target geographically-defined subpopulations for melanoma screening or sun safety intervention. To quantify the joint influences of these factors on melanoma incidence rates, we took advantage of a large and unselected series of non-Hispanic white melanoma patients in California. This population is remarkable for its size, heterogeneity with respect to SES and UVR, and for having among the highest melanoma incidence rates reported worldwide(10).
MATERIALS AND METHODS
We obtained from the California Cancer Registry (CCR), the population-based cancer registry for the state of California, data regarding all 23,564 incident cases of malignant cutaneous melanoma (International Classification of Disease for Oncology, 3rd Edition [ICD-O-3], topography codes C44.0 through C44.9; histology codes 8720–8790) diagnosed between January 1, 1998 and December 31, 2002 among non-Hispanic white males and females residing in California at time of diagnosis. We limited analyses to the peri-censal diagnostic period 1998–2002 because the denominators needed to calculate census-tract level cancer rates were available only for decennial census years. The vast majority of these cases were reported by a hospital or physician with only 46 patients (0.2%) reported by death certificates only. Demographic and tumor information for each newly diagnosed melanoma case was abstracted directly from medical records(11). Population denominator estimates were based on 2000 US Census data for census tracts of residence as described below.
Neighborhood-level socioeconomic status
Individual-level patient SES characteristics (e.g., education, income, occupation) are not collected routinely by most US cancer registries, including the CCR. However, since residence is collected and routinely geocoded, SES characteristics of the geographic region in which the patient resided at the time of diagnosis can be obtained from the US Census Bureau. For the 2000 Census, the smallest geographic unit with both SES information and the detailed, race-specific population counts needed to calculate cancer incidence rates was the census tract, an area containing on average 4000 residents. Instead of relying on a single measure of SES, we used a multifactorial socioeconomic index developed by Yost et al. used previously to demonstrate substantial socioeconomic gradients in breast cancer incidence(12) and Hodgkin lymphoma(13). This method employed principal components analysis to develop a single index from seven census indicator variables of SES (education index, median household income, percent living 200% below poverty level, percent blue-collar workers, percent older than 16 in workforce without job, median rent, and median house value) and thus incorporates information about cost of living, which varies within California. Using this index, we assigned a standardized score to each census tract in California in 2000. Individuals for whom tract group of residence at diagnosis was unknown or the residence could not be adequately assigned to a census block group (n=1,307, 5.5%) were randomly allocated to a tract within the same county. These cases did not differ significantly from patients with known tract group by UVR, age, or sex. Standardized scores assigned to each census tract were ranked and categorized into quintiles (1= lowest, 5= highest).
Ambient ultraviolet radiation exposure
We calculated average annual ambient UVR exposure according to the census tract of residence at diagnosis of melanoma, using previously described methods (14). Briefly, we derived 1 kilometer-square ground surface level UVR values using spatial smoothing techniques for data from 215 UVR measurement stations throughout the United States, adjusted for local climate and terrain features. The resulting surface of ground level UV for California was intersected with the census tract centroids for all census tracts in California to provide a measure of potential residential average annual UVR exposure (measured in Watt-hours per meter squared, Wh/m2). UVR could not be calculated for the 2.2% of cases (n=509) who resided where the boundary of the continuous UVR surface and the discrete census tract boundary did not overlap (e.g., state border). This objective measurement approach for UVR exposure has been previously shown to correlate with melanoma incidence rates(14). Census tracts were ranked by UVR and categorized so as to represent relatively equal quartiles of the total California population.
SES quintile and UVR quartile data were also mapped to visualize geographic distributions.
Statistical analysis
We used SEER*Stat version 6.5.1 (National Cancer Institute, Bethesda, MD) to calculate age-adjusted (2000 population standard) melanoma incidence rates and corresponding 95% confidence intervals by SES quintile and UVR quartile.
To separate the influences on melanoma incidence rates of neighborhood SES and UVR, we stratified age-adjusted incidence rates jointly by these characteristics. To formally test associations of SES and UVR with incidence rates in each group of interest, we used Poisson regression, performed in SAS PROC GENMOD, to calculate incidence rate ratios (IRR) across SES and UVR categories and to evaluate goodness of fit.
The Poisson distribution was used to model the distribution of cell counts collected from the CCR in a multi-way contingency table. In the regression model, the outcome is number of patients with a new diagnosis of invasive melanoma during 1998–2002. Using the 2000 census population for the state, an offset was included in the model based on the natural log of the population, serving to normalize the fitted cell means to a per-person-at-risk basis. To correct for moderate overdispersion, the model included a dispersion parameter estimated by the ratio of the square root of model deviance to its associated degrees of freedom, replacing the model’s log-likelihood function with a quasi-likelihood function. The data met all model assumptions. Confidence intervals and p-values for IRR estimates were calculated using a two-sided test. Comparisons were considered statistically significant if the corresponding Wald chi-square p-value was below the alpha level of 0.05.
RESULTS
Table 1 shows the characteristics of the 23,564 melanoma patients ultimately included in analyses. Over 60% of melanoma patients were male, and males were slightly older at diagnosis (mean age at diagnosis: 61 years) than females (mean age at diagnosis: 56 years).
TABLE 1.
Demographic characteristics of 23,564 non-Hispanic white persons diagnosed with invasive melanoma, California 1998–2002.
| Male | Female | Total | |
|---|---|---|---|
| N (%) |
N (%) |
N (%) |
|
| Age at diagnosis | |||
| 0–34 | 792 (5.7) | 1,189 (12.3) | 1,981 (8.4) |
| 35–49 | 2,772 (19.9) | 2,617 (27.2) | 5,389 (22.9) |
| 50–64 | 4,124 (29.6) | 2,501 (26.0) | 6,625 (28.1) |
| 65–79 | 4,397 (31.6) | 2,228 (23.1) | 6,625 (28.1) |
| 80+ | 1,842 (13.2) | 1,102 (11.4) | 2,944 (12.5) |
| Total | 13,927 | 9,637 | 23,564 |
| Socioeconomic Status* | |||
| 1 (lowest) | 823 (5.9) | 551 (5.7) | 1,374 (5.8) |
| 2 | 1,759 (12.6) | 1,275 (13.2) | 3,034 (12.9) |
| 3 | 2,739 (19.7) | 1,939 (20.1) | 4,678 (19.9) |
| 4 | 3,673 (26.4) | 2,702 (28.0) | 6,375 (27.1) |
| 5 (highest) | 4,933 (35.4) | 3,170 (32.9) | 8,103 (34.4) |
| Ultraviolet Radiation1 | |||
| 3900–4914 | 3519 (25.3) | 2473 (25.7) | 5992 (25.4) |
| 4915–5025 | 3556 (25.5) | 2569 (26.7) | 6125 (26.0) |
| 5026–5099 | 3350 (24.1) | 2262 (23.5) | 5612 (23.8) |
| 5100+ | 3199 (23.0) | 2127 (22.1) | 5326 (22.6) |
| Missing | 303 (2.2) | 206 (2.1) | 506 (2.2) |
UVR and SES measured at census tract of residence at time of cancer diagnosis
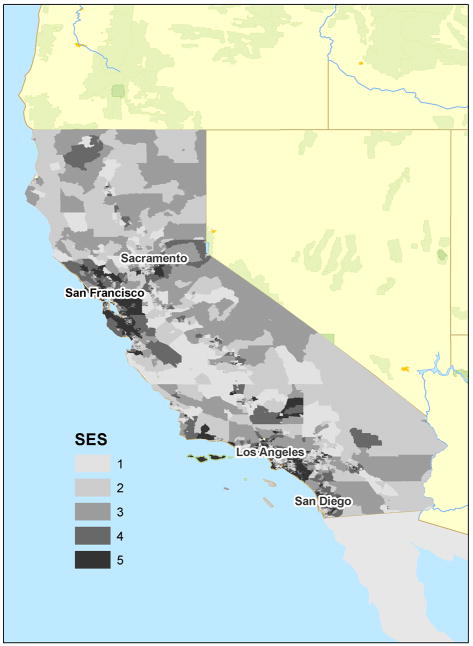
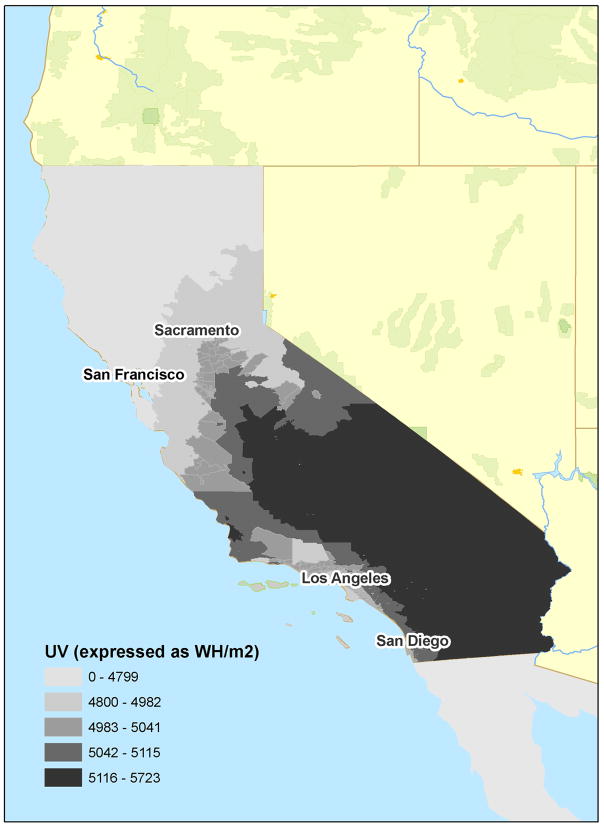
Age-adjusted incidence rates of melanoma were calculated jointly by neighborhood SES and average annual UVR (Tables 2 and 3). The highest rates of melanoma observed were among males living in the highest 20% of neighborhoods ranked by SES (over 45 cases per 100,000), 2.5 times higher than for the lowest rates observed among women living in lowest 40% of neighborhoods. Notably, incidence rates were higher in the top quintile of neighborhoods ranked by SES than the top quartile ranked by UVR. Incidence rates did not vary according to UVR among males or females living in the lowest 60% of neighborhoods ranked by SES. Figure 1a shows the distribution of SES in California, showing higher concentrations of higher SES quintiles in the coastal Bay Area and Orange County regions. Figure 1b shows the wide distribution of UVR in California, showing the generally inverse association of latitude with UVR.
TABLE 2.
Incidence rates of invasive melanoma by sex, and jointly by neighborhood measures of socioeconomic status (SES), and ambient ultraviolet radiation (UVR). California, 1998–2002.
| Males | SES*1 (low) | SES*2 | SES*3 | SES*4 | SES*5 (high) |
|---|---|---|---|---|---|
| UVR* |
Rate† (95% CI) |
Rate† (95% CI) |
Rate† (95% CI) |
Rate† (95% CI) |
Rate† (95% CI) |
| 3900–4914 | 29.3 (24.3, 34.9) | 21.7 (19.5, 24.0) | 29.3 (26.9, 31.8) | 26.9 (25.5, 28.8) | 34.6 (33.0, 36.4) |
| 4915–5025 | 24.5 (20.5, 29.1) | 29.3 (26.3, 32.6) | 27.2 (25.1, 29.4) | 34.2 (32.2, 36.4) | 46.3 (44.0, 48.7) |
| 5026–5099 | 28.4 (24.1, 33.3) | 23.4 (21.0, 26.0) | 31.2 (28.9, 33.7) | 39.0 (36.5, 41.6) | 49.5 (46.9, 52.3) |
| 5100+ | 28.7 (25.9, 31.8) | 26.4 (24.4, 28.4) | 30.0 (28.0, 32.1) | 42.1 (39.4, 45.0) | 45.4 (41.1, 50.1) |
| Females | |||||
| 3900–4914 | 18.1 (14.3, 22.5) | 14.9 (13.2, 16.9) | 17.7 (16.0, 19.6) | 18.6 (17.2, 20.1) | 20.4 (19.2, 21.7) |
| 4915–5025 | 12.9 (10.1, 16.3) | 17.9 (15.7, 20.4) | 20.3 (18.5, 22.1) | 23.2 (21.6, 25.0) | 29.4 (27.6, 31.4) |
| 5026–5099 | 18.6 (15.2, 22.5) | 16.9 (14.8, 19.1) | 19.2 (17.4, 21.1) | 25.3 (23.4, 27.4) | 26.7 (24.8, 28.7) |
| 5100+ | 16.6 (14.5, 18.8) | 16.4 (14.9, 18.0) | 17.7 (16.2, 19.3) | 26.0 (23.9, 28.2) | 29.7 (26.2, 33.6) |
UVR and SES measured at census tract of residence at time of cancer diagnosis
Incidence rate per 100,000 person-years, age-adjusted (2000 standard population)
TABLE 3.
Adjusted melanoma incidence rate ratio (IRR) according to ultraviolet radiation exposure (UVR) at residence of diagnosis among non-Hispanic white persons, 1998–2002, California.
| UVR* | Adjusted for sex and age | Adjusted for sex, age, and SES* | ||
|---|---|---|---|---|
| IRR† (95% CI) |
P-value |
IRR† (95% CI) |
P-value |
|
| 3900–4914 | 1.00 | Reference | 1.00 | Reference |
| 4915–5025 | 1.20 (1.03, 1.36) | 0.004 | 1.24 (1.12, 1.37) | <0.0001 |
| 5026–5099 | 1.22 (1.07, 1.38) | 0.002 | 1.28 (1.15, 1.42) | <0.0001 |
| 5100+ | 1.08 (0.95, 1.23) | 0.25 | 1.26 (1.13, 1.41) | <0.0001 |
UVR and SES measured at census tract of residence at time of cancer diagnosis
Incidence rate ratio obtained through a quasi-likelihood function (a log-likelihood function divided by a dispersion parameter).
FIGURE 1.
Distribution of an index of socioeconomic status (a) and ambient ultraviolet radiation (b), by census tract of residence, California, 2000.
To rule out a possibly confounding influence of age, we used multivariate Poisson regression to assess differences in IRR among groups. We observed increasing incidence rates with increasing quintile of neighborhood SES (Table 2). UVR remained significantly and positively associated with incidence among higher SES populations, suggesting that socioeconomic gradients in melanoma incidence are not explained entirely by collocation of higher SES neighborhoods in regions of high UVR (Tables 3 and 4).
TABLE 4.
Adjusted melanoma incidence rate ratio (IRR) according to neighborhood-level socioeconomic status (SES) at residence of diagnosis among non-Hispanic white persons, 1998–2002, California.
| SES* | Adjusted for sex and age | Adjusted for sex, age, and UVR* | ||
|---|---|---|---|---|
| IRR† (95% CI) |
P-value |
IRR† (95% CI) |
P-value |
|
| 1(low) | 1.00 | Reference | 1.00 | Reference |
| 2 | 0.93 (0.75, 1.15) | 0.51 | 0.95 (0.79, 1.15) | 0.62 |
| 3 | 1.07 (0.87, 1.30) | 0.52 | 1.09 (0.91, 1.30) | 0.36 |
| 4 | 1.28 (1.05, 1.55) | 0.013 | 1.32 (1.11, 1.58) | 0.002 |
| 5 (high) | 1.48 (1.23, 1.79) | <0.0001 | 1.58 (1.32, 1.88) | <0.0001 |
UVR and SES measured at census tract of residence at time of cancer diagnosis
Incidence rate ratio obtained through a quasi-likelihood function (a log-likelihood function divided by a dispersion parameter).
Table 5 shows that adding an interaction term for UVR and SES to the Poisson regression model confirmed a statistically significant interaction. Melanoma incidence rates were significantly higher in the higher UVR categories for the high SES group only compared to the 1st UVR quartile and low SES group. For the highest SES quintile, IRRs across increasing quartiles of UVR were essentially the same: 1.61 (CI: 1.06–2.43), 1.59 (CI: 1.05–2.41), and 1.60 (CI: 1.02–2.51), respectively.
Table 5.
Adjusted melanoma incidence rate ratio (IRR) according to neighborhood-level socioeconomic status (SES) and ultraviolet radiation (UVR) at residence of diagnosis among non-Hispanic white persons, 1998–2002, California.
| UVR* | SES*1 (low) | SES*2 | SES*3 | SES*4 | SES*5 (high) |
|---|---|---|---|---|---|
| IRR† (95% CI) | IRR† (95% CI) | IRR† (95% CI) | IRR† (95% CI) | IRR† (95% CI) | |
| 3900–4914 | 1.00 | 0.79 (0.50, 1.25) | 1.03 (0.50, 1.25) | 0.97 (0.64, 1.49) | 1.18 (0.78, 1.78) |
| 4915–5025 | 0.82 (0.47, 1.45) | 1.02 (0.65, 1.62) | 1.01 (0.66, 1.56) | 1.23 (0.81, 1.86) | 1.61 (1.06, 2.43)‡ |
| 5026–5099 | 1.02 (0.60, 1.74) | 0.87 (0.55, 1.38) | 1.07 (0.70, 1.65) | 1.38 (0.91, 2.10) | 1.59 (1.05, 2.41)‡ |
| 5100+ | 0.97 (0.67, 1.53) | 0.94 (0.60, 1.41) | 1.00 (0.66, 1.54) | 1.43 (0.94, 2.18) | 1.60 (1.02, 2.51)‡ |
UVR and SES measured at census tract of residence at time of cancer diagnosis
Incidence rate ratio obtained through a quasi-likelihood function (a log-likelihood function divided by a dispersion parameter).
p-value= <0.05
DISCUSSION
Our current understanding of melanoma etiology implicates two major risk factors for melanoma: genetic predisposition and history of exposure to UVR (15–18). Behaviors related to UVR exposure, including age-specific history of sun exposure, sunburns and use of tanning salons have been repeatedly associated with melanoma risk, as have markers of environmental UVR including lower latitude and higher elevation. Many of these UVR exposure behaviors have been associated with educational status and other measures of SES. Our study was able to use a large, representative series of melanoma cases enhanced with neighborhood-level information regarding SES and UVR to measure the combined influence of SES and UVR on melanoma occurrence patterns. Our results showed that persons living in the highest SES quintile had a nearly 50% higher rate of invasive melanoma compared to the lowest quintile (after adjustment for sex and age) a finding similar to those calculated using individual-level measure of SES (7, 8, 19) which suggested at least two-fold increases in risk. We observed that melanoma incidence rates were more strongly associated with neighborhood SES than UVR, but that UVR remained significantly and positively associated with incidence among higher SES neighborhoods, suggesting that socioeconomic gradients in melanoma incidence are not explained entirely by UVR. These observations underscore the true interactive effect of SES and UVR on melanoma incidence.
Higher SES neighborhoods may include more persons with access to recreational (i.e., sun vacation) UVR exposure typically involving short-term, high-intensity exposure which has been strongly associated with melanoma development. Persons living in high SES neighborhoods may have more time to participate in outdoor leisure activities such as gardening or sports (20); may travel more frequently to high altitude or low latitude vacation destinations where UVR exposure is greater (21) or may have cultural preferences for tanned skin (22–24). This pattern of intermittent sun exposure may be different than those for persons living in low and middle SES neighborhoods who may have more consistent, cumulative UVR exposure without the vacation-related intense bursts of exposure. Regardless of the etiologic pathways explaining our observations, our findings squarely target geographic areas of high SES and annual UVR as a high-risk population for melanoma who should be targeted for cancer prevention outreach and education.
We consider it unlikely that geographic differences in melanoma reporting or screening practices explain these patterns. Our prior studies of melanoma incidence trends over time in California confirmed doublings of rates in all neighborhood SES groups between 1988–92 and 1998–2002, implying change independent of access to care (2). Evidence of increased underreporting (25–28) or delayed reporting (29) of melanoma to cancer registries over time is unlikely to explain our findings, because there is no indication that that physician reporting of melanoma differs according to SES (25), and it is unlikely to be related to ambient UVR levels .
We used area-level SES and UVR measures because individual-level information regarding education, , income, skin sensitivity, skin type or specific sun exposure history are generally not collected by population-based cancer registries in the US. Our area-level average annual UVR measure does not capture specific sun exposure history, including history of sunburn or intermittent exposure to intense UV, both of which have been associated previously with melanoma risk as well as with SES. Our use of area-level measures may have introduced the ecological fallacy whereby group-level effects are not necessarily comparable to those calculated from individual-level information. If individual-level information were available, we could have examined separately the individual- and area-level effects of SES and UVR to better understand contextual effects of neighborhood characteristics above and beyond those conferred by individual-level characteristics. For example, there may be cultural preferences for tanned skin or for participating in outdoor or indoor tanning activities in neighborhoods with high SES and UVR. Regardless, to minimize heterogeneity within area-level categories, we utilized the smallest geographic designation for which cancer incidence rates could be calculated (the census tract). Our observed area-level SES effects are generally consistent with studies that were able to calculate incidence rates using individual-level information regarding education and SES and are also consistent with the suggestion that area-level effects may underestimate individual-level effects (30–33).
A related limitation involves our use of address at residence at the time of diagnosis for assignment of area-level SES and UVR. In general, cancer registries do not collect information regarding duration of residence at this address, nor any residential history information; thus average annual UVR at place of diagnosis may not necessarily correlate with exposure in earlier life which may be most relevant to melanoma development (34–36). We also were unable to analyze time trends in melanoma incidence by UVR exposure, since National Oceanic and Atmospheric Association (NOAA)-derived measures were only reliable for more recent time periods. As our analysis was limited to non-Hispanic whites in California, our findings may not be generalizable to other races or ethnicities, especially those with different skin types.
The rapid increase in melanoma occurrence over the past 50 years underscores the importance of characterizing high risk populations for meaningful public health intervention. Our results underscore the importance of targeting cancer prevention and early detection educational efforts to high SES groups who additionally live in areas of high ambient UVR exposure. Our analysis also reflected the inadequacy of models which included only the individual effects of SES or UVR on melanoma incidence. These results highlight the importance of incorporating both individual and contextual measures of both SES and UVR in future melanoma studies.
Acknowledgments
This study was supported by a developmental cancer research award from the Stanford Cancer Center. The collection of cancer incidence data used in this study was supported by the California Department of Public Health as part of the statewide cancer reporting program mandated by California Health and Safety Code Section 103885; the National Cancer Institute's Surveillance, Epidemiology and End Results Program under contract N01-PC-35136 awarded to the Cancer Prevention Institute of California (formerly the Northern California Cancer Center), contract N01-PC-35139 awarded to the University of Southern California, and contract N01-PC-54404 awarded to the Public Health Institute; the Centers for Disease Control and Prevention's National Program of Cancer Registries, under agreement 1U58DP00807-01 awarded to the Public Health Institute; and R01-ES015552 from the NIEHS and R01-CA121052 from NCI. The authors have no financial conflicts to declare. The ideas and opinions expressed herein are those of the authors and endorsement by the State of California, Department of Public Health the National Cancer Institute, and the Centers for Disease Control and Prevention or their Contractors and Subcontractors is not intended nor should be inferred. We thank Zaria Tatalovich and Jennifer Boldrick for their contributions to this study.
References
- 1.Geller AC, Miller DR, Annas GD, et al. Melanoma incidence and mortality among US whites, 1969–1999. Jama. 2002;288(14):1719–20. doi: 10.1001/jama.288.14.1719. [DOI] [PubMed] [Google Scholar]
- 2.Linos E, Swetter SM, Cockburn MG, Colditz GA, Clarke CA. Increasing burden of melanoma in the United States. J Invest Dermatol. 2009;129(7):1666–74. doi: 10.1038/jid.2008.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.MacKie RM, Hauschild A, Eggermont AM. Epidemiology of invasive cutaneous melanoma. Ann Oncol. 2009;20(Suppl 6):vi1–7. doi: 10.1093/annonc/mdp252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perez-Gomez B, Aragones N, Gustavsson P, et al. Do sex and site matter? Different age distribution in melanoma of the trunk among Swedish men and women. Br J Dermatol. 2008;158(4):766–72. doi: 10.1111/j.1365-2133.2007.08429.x. [DOI] [PubMed] [Google Scholar]
- 5.Rimpela AH, Pukkala EI. Cancers of affluence: positive social class gradient and rising incidence trend in some cancer forms. Soc Sci Med. 1987;24(7):601–6. doi: 10.1016/0277-9536(87)90064-5. [DOI] [PubMed] [Google Scholar]
- 6.Harrison RA, Haque AU, Roseman JM, Soong SJ. Socioeconomic characteristics and melanoma incidence. Ann Epidemiol. 1998;8(5):327–33. doi: 10.1016/s1047-2797(97)00231-7. [DOI] [PubMed] [Google Scholar]
- 7.Clegg LX, Reichman ME, Miller BA, et al. Impact of socioeconomic status on cancer incidence and stage at diagnosis: selected findings from the surveillance, epidemiology, and end results: National Longitudinal Mortality Study. Cancer Causes Control. 2009;20(4):417–35. doi: 10.1007/s10552-008-9256-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mouw T, Koster A, Wright ME, et al. Education and risk of cancer in a large cohort of men and women in the United States. PLoS One. 2008;3(11):e3639. doi: 10.1371/journal.pone.0003639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Braaten T, Weiderpass E, Kumle M, Lund E. Explaining the socioeconomic variation in cancer risk in the Norwegian Women and Cancer Study. Cancer Epidemiology, Biomarkers & Prevention. 2005;14(11 Pt 1):2591–7. doi: 10.1158/1055-9965.EPI-05-0345. [DOI] [PubMed] [Google Scholar]
- 10.Parkin DM, Whelan SL, Ferlay J, Teppo L, Thomas DB, editors. Cancer Incidence in Five Continents. Lyon: IARC; 2002. [Google Scholar]
- 11.Hankey BF, Ries LA, Edwards BK. The surveillance, epidemiology, and end results program: a national resource. Cancer Epidemiol Biomarkers Prev. 1999;8(12):1117–21. [PubMed] [Google Scholar]
- 12.Yost K, Perkins C, Cohen R, Morris C, Wright W. Socioeconomic status and breast cancer incidence in California for different race/ethnic groups. Cancer Causes & Control. 2001;12(8):703–11. doi: 10.1023/a:1011240019516. [DOI] [PubMed] [Google Scholar]
- 13.Clarke CA, Glaser SL, Keegan TH, Stroup A. Neighborhood socioeconomic status and Hodgkin's lymphoma incidence in California. Cancer Epidemiology, Biomarkers & Prevention. 2005;14(6):1441–7. doi: 10.1158/1055-9965.EPI-04-0567. [DOI] [PubMed] [Google Scholar]
- 14.Tatalovich Z, Wilson JP, Mack T, Yan Y, Cockburn M. The objective assessment of lifetime cumulative ultraviolet exposure for determining melanoma risk. J Photochem Photobiol B. 2006;85(3):198–204. doi: 10.1016/j.jphotobiol.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 15.Cockburn M, Black W, McKelvey W, Mack T. Determinants of melanoma in a case-control study of twins. Cancer Causes & Control. 2001;12(7):615–25. doi: 10.1023/a:1011271117496. [DOI] [PubMed] [Google Scholar]
- 16.Shahbazi M, Pravica V, Nasreen N, et al. Association between functional polymorphism in EGF gene and malignant melanoma. Lancet. 2002;359(9304):397–401. doi: 10.1016/S0140-6736(02)07600-6. [DOI] [PubMed] [Google Scholar]
- 17.Wachsmuth RC, Gaut RM, Barrett JH, et al. Heritability and gene-environment interactions for melanocytic nevus density examined in a U.K. adolescent twin study. Journal of Investigative Dermatology. 2001;117(2):348–52. doi: 10.1046/j.0022-202x.2001.01415.x. [DOI] [PubMed] [Google Scholar]
- 18.Whiteman DC, Watt P, Purdie DM, et al. Melanocytic nevi, solar keratoses, and divergent pathways to cutaneous melanoma. Journal of the National Cancer Institute. 2003;95(11):806–12. doi: 10.1093/jnci/95.11.806. [DOI] [PubMed] [Google Scholar]
- 19.Brown TT, Quain RD, Troxel AB, Gelfand JM. The epidemiology of sunburn in the US population in 2003. J Am Acad Dermatol. 2006;55(4):577–83. doi: 10.1016/j.jaad.2006.05.029. [DOI] [PubMed] [Google Scholar]
- 20.Lindstrom M, Hanson BS, Ostergren PO. Socioeconomic differences in leisure-time physical activity: the role of social participation and social capital in shaping health related behaviour. Soc Sci Med. 2001;52(3):441–51. doi: 10.1016/s0277-9536(00)00153-2. [DOI] [PubMed] [Google Scholar]
- 21.Ternowetsky G. Holiday Taking and Socio-Economic Status in Australia. Leisure Studies. 1983;(2):31–44. [Google Scholar]
- 22.Autier P, Dore JF, Lejeune F, et al. Cutaneous malignant melanoma and exposure to sunlamps or sunbeds: an EORTC multicenter case-control study in Belgium, France and Germany. EORTC Melanoma Cooperative Group. Int J Cancer. 1994;58(6):809–13. doi: 10.1002/ijc.2910580610. [DOI] [PubMed] [Google Scholar]
- 23.Dellavalle RP, Schilling LM, Chen AK, Hester EJ. Teenagers in the UV tanning booth? Tax the tan. Arch Pediatr Adolesc Med. 2003;157(9):845–6. doi: 10.1001/archpedi.157.9.845. [DOI] [PubMed] [Google Scholar]
- 24.Koh HK, Bak SM, Geller AC, et al. Sunbathing habits and sunscreen use among white adults: results of a national survey. Am J Public Health. 1997;87(7):1214–7. doi: 10.2105/ajph.87.7.1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cockburn M, Swetter SM, Peng D, et al. Melanoma underreporting: why does it happen, how big is the problem, and how do we fix it? J Am Acad Dermatol. 2008;59(6):1081–5. doi: 10.1016/j.jaad.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koh HK, Geller A, Miller DR, Clapp RW, Lew RA. Underreporting of cutaneous melanoma in cancer registries nationwide. J Am Acad Dermatol. 1992;27(6 Pt 1):1035–6. doi: 10.1016/s0190-9622(08)80285-x. [DOI] [PubMed] [Google Scholar]
- 27.Merlino LA, Sullivan KJ, Whitaker DC, Lynch CF. The independent pathology laboratory as a reporting source for cutaneous melanoma incidence in Iowa, 1977–1994. J Am Acad Dermatol. 1997;37(4):578–85. doi: 10.1016/s0190-9622(97)70175-0. [DOI] [PubMed] [Google Scholar]
- 28.Zippin C, Lum D, Hankey BF. Completeness of hospital cancer case reporting from the SEER Program of the National Cancer Institute. Cancer. 1995;76(11):2343–50. doi: 10.1002/1097-0142(19951201)76:11<2343::aid-cncr2820761124>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 29.Clegg LX, Feuer EJ, Midthune DN, Fay MP, Hankey BF. Impact of reporting delay and reporting error on cancer incidence rates and trends. J Natl Cancer Inst. 2002;94(20):1537–45. doi: 10.1093/jnci/94.20.1537. [DOI] [PubMed] [Google Scholar]
- 30.Krieger N, Chen JT, Waterman PD, et al. Geocoding and monitoring of US socioeconomic inequalities in mortality and cancer incidence: does the choice of area-based measure and geographic level matter?: the Public Health Disparities Geocoding Project. Am J Epidemiol. 2002;156(5):471–82. doi: 10.1093/aje/kwf068. [DOI] [PubMed] [Google Scholar]
- 31.Krieger N, Williams DR, Moss NE. Measuring social class in US public health research: concepts, methodologies, and guidelines. Annu Rev Public Health. 1997;18:341–78. doi: 10.1146/annurev.publhealth.18.1.341. [DOI] [PubMed] [Google Scholar]
- 32.Singh GK, Miller BA, Hankey BF. Changing area socioeconomic patterns in U.S. cancer mortality, 1950–1998: Part II--Lung and colorectal cancers. J Natl Cancer Inst. 2002;94(12):916–25. doi: 10.1093/jnci/94.12.916. [DOI] [PubMed] [Google Scholar]
- 33.Singh GK, Miller BA, Hankey BF, Feuer EJ, Pickle LW. Changing area socioeconomic patterns in U.S. cancer mortality, 1950–1998: Part I--All cancers among men. J Natl Cancer Inst. 2002;94(12):904–15. doi: 10.1093/jnci/94.12.904. [DOI] [PubMed] [Google Scholar]
- 34.Nikolaou VA, Sypsa V, Stefanaki I, et al. Risk associations of melanoma in a Southern European population: results of a case/control study. Cancer Causes Control. 2008;19(7):671–9. doi: 10.1007/s10552-008-9130-0. [DOI] [PubMed] [Google Scholar]
- 35.Solomon CC, White E, Kristal AR, Vaughan T. Melanoma and lifetime UV radiation. Cancer Causes Control. 2004;15(9):893–902. doi: 10.1007/s10552-004-1142-9. [DOI] [PubMed] [Google Scholar]
- 36.Whiteman DC, Whiteman CA, Green AC. Childhood sun exposure as a risk factor for melanoma: a systematic review of epidemiologic studies. Cancer Causes Control. 2001;12(1):69–82. doi: 10.1023/a:1008980919928. [DOI] [PubMed] [Google Scholar]