Abstract
Arf GAPs (ADP-ribosylation factor GTPase activating proteins) are essential components of Arf (ADP-ribosylation factor) signaling pathways. Arf GAPs stimulate the hydrolysis of GTP to GDP to transition Arf from the active, GTP bound, state to the inactive, GDP bound, state. Based on this activity, Arf GAPs were initially proposed to function primarily or exclusively as terminators of Arf signaling. Further studies of Arf GAPs have revealed that they also function as effectors of Arf signaling in at least a few steps or processes in which Arfs are not directly involved. In this review we discuss the non-canonical functions of Arf GAPs and address several key questions in the field, including: whether (1) Arf GAPs are terminators or effectors of Arf signaling, (2) Arf GAPs positively or negatively regulate COPI assembly, (3) Arf GAPs are involved in vesicle fission, and (4) Arf GAPs regulate vesicle uncoating.
Keywords: Arf, ArfGAP1, COPI, GAP
Introduction
ADP-ribosylation factors (Arfs) are a family within the Ras superfamily of ∼20 kDa regulatory GTP binding proteins that includes 29 members in humans [1]. Arfs are ubiquitously expressed in eukaryotic cells and function as key regulators of membrane traffic. Arfs dynamically associate with membranes, where they facilitate the initial steps in vesicle biogenesis by regulating the recruitment of adaptor proteins for coat assembly and lipid modifying enzymes for transmembrane protein cargo accumulation.[2]. While it is likely that Arfs participate in parallel pathways directed toward the early steps of carrier biogenesis (e.g., modifications to lipid microenvironment) it is unclear whether Arfs have additional roles in the subsequent steps of vesicle biogenesis (e.g. fission and uncoating) or even whether Arfs persist on mature vesicles (discussed below). Like all regulatory GTP binding proteins, Arfs cycle between GTP and GDP bound states and function in cells as molecular switches, controlled by the nucleotide bound (e.g., see Fig. 1). Exchange of bound GDP for GTP results in enhanced association with membranes and causes a conformational change that increases the affinity of Arf for effector proteins responsible for Arf signaling. When GTP is hydrolyzed to GDP Arf dissociates from the membrane and affinity for effector proteins is lost, thereby terminating Arf signaling. The intrinsic exchange rate of GDP for GTP by Arfs in cells is thought to be very slow and Arfs have no detectable intrinsic GTPase activity in vitro [3]. Thus, the GTPase cycle is mediated by guanine nucleotide exchange factors (GEFs) that facilitate the exchange of bound GDP for GTP and GTPase activating proteins (GAPs) that promote the hydrolysis of GTP to GDP. Despite these general properties of GEFs and GAPs it is too limiting to view these modulators of Arf activity simply as activators or terminators of Arf, respectively, for reasons to be explained below. In this review we will focus on the Arf GAPs, particularly those at the Golgi, and will review the diverse roles and evolving models of Arf GAPs in membrane traffic pathways as well as the controversies in the literature.
Figure 1.
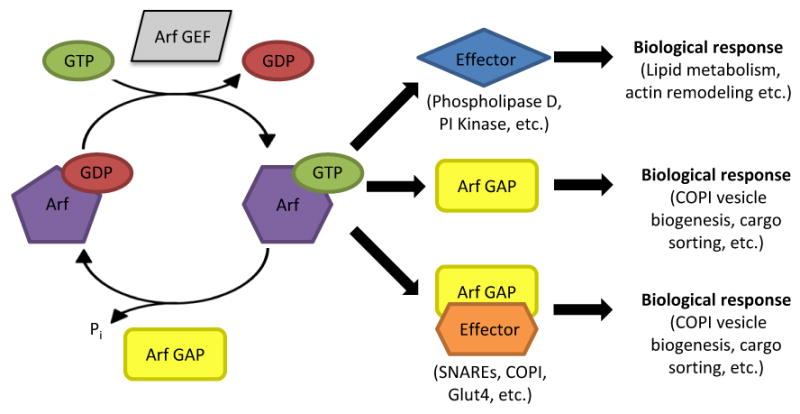
Model of Arf GAP functions in Arf-dependent signaling and termination. The GTP cycle of Arf depends upon Arf GEFs to facilitate the exchange of bound GDP for GTP and Arf GAPs to stimulate hydrolysis of GTP to GDP. GTP binding by Arf induces a conformational change that increases the affinity of Arf for effector proteins ultimately leading to a biological response, mediated by effectors. As discussed in the text, association with Arf GAPs or with Arf GAP/effector complexes also results in biological responses, suggesting that Arf GAPs can serve as Arf effectors. Thus, Arf GAPs have at least dual roles in regulating Arf signaling as terminators and effectors. This conclusion also points to the regulation of Arf GAP activity as a critical, yet understudied, aspect of Arf and Arf GAP signaling.
The Arf GAP family is encoded by at least 31 genes in humans. These proteins are defined by the presence of the Arf GAP domain which consists of a zinc-finger motif of four cysteines with specific spacing terminating with a catalytic arginine residue (CX2CX16CX2CX4R) [4-5]. Early alignment and simple phylogenetic analyses of proteins containing the Arf GAP domain identified 10 sub-families in eukaryotes [5]. This conclusion was confirmed and evolutionary relationships better defined in a much more exhaustive study (J. Dacks and R.A. Kahn, manuscript in preparation). Six of these were already present in the earliest common ancestor, with the other 4 arising at later stages in animal evolution (Mowbrey, Kahn, and Dacks; manuscript in preparation). Thus, this is an ancient family of cell regulators that share at least one biochemical activity and are likely to have developed a number of distinct and overlapping cellular functions.
The presence of the Arf GAP domain has been a very reliable indicator of Arf GAP activity, though by no means has every predicted Arf GAP protein been shown to possess it, but it is not revealing of the specificity for GTPase. Rather, the specificity of any Arf GAP for Arf family members is very poorly understood. This is further confounded by some apparent differences in specificities between in vitro and cell-based Arf GAP assays [6]. The Arf family includes the “true” Arfs, that typically share >60% sequence identity, the Arf-like (Arl) proteins, that are ∼40-60% identical to any Arf, and the Sar GTPases that are 20-40% identical to an Arf [1]. The Arf GAPs studied to date are highly specific to the Arfs and have displayed little or no activity against the few Arl or Sar proteins tested. There is also very little difference in substrate specificity for most Arf GAPs among the Arfs seen in in vitro assays (Arf1-6). It is likely that the localization of the Arf GAP is a key determinant of its biological substrates and functions. Finally, the entirety of proteins with Arf GAP activity is unlikely to be limited to those containing an Arf GAP domain. A recent report describes the first purification of a GAP, ELMOD2, with activity against an Arl, Arl2 [7]. Despite the lack of an Arf GAP domain, ELMOD2 was found to have GAP activity against Arfs. Thus, we clearly still have much to learn about the potential for cross-signaling between Arf and Arl pathways as a result of overlapping specificities of their regulators and effectors.
The study of Arf GAPs has resulted in several conflicting models of their cellular function(s) [8-9]. Of the 31 Arf GAPs in humans, members of the ArfGAP1 and ArfGAP2/3 sub-groups have been studied most extensively and those functions are the basis for many models of the function of Arf GAPs in general. Thus, the majority of this review is based upon studies of ArfGAP1 and ArfGAP2/3. Extrapolation of these models to other Arf GAPs is tempting but risky, particularly for the larger Arf GAPs that contain many additional domains.
This review will address key questions in the field including whether or not (1) Arf GAPs are terminators or effectors of Arf signaling, (2) Arf GAPs positively or negatively regulate COPI assembly, (3) Arf GAPs are involved in vesicle fission, and (4) Arf GAPs regulate vesicle uncoating.
Are Arf GAPs terminators or effectors of Arf signaling?
Arfs lack detectable intrinsic GTPase activity and thus to complete the canonical GTPase cycle absolutely require a GTPase activating protein (GAP) for hydrolysis of bound GTP [3]. Arf GAP activity was first described and shown to be sensitive to phospholipids by Randazzo and Kahn [10]. Shortly thereafter the first purification of an Arf GAP, ArfGAP1, was achieved by members of the Cassel lab [11]. Because the only in vitro assay for Arf GAPs is GTP hydrolysis, and it is assumed that Arf-GTP is the only active conformer, Arf GAPs were originally assigned the role of terminators of Arf signaling pathways. This model was supported by the finding that over-expression of ArfGAP1 disrupted the Golgi apparatus [12]. This observation was interpreted as analogous to the treatment of cells with Brefeldin A, which lowers cellular levels of Arf-GTP by preventing Arf activation [12-13], but detailed comparisons were not performed. Consistent with a role for Arf GAPs in terminating Arf signaling, knockdown of ArfGAP1-3 resulted in higher cellular levels of activated Arf and Golgi morphology and cell viability defects [14]. Thus, the initial model for the function of Arf GAPs was the down-regulation of Arf signaling by stimulating GTP hydrolysis.
Additional testing of the actions of Arf GAPs as down regulators of Arf signaling commonly took advantage of two types of designed mutations; those homologous to Arf1-Q71L [15], resulting in an Arf that is resistant to the actions of GAPs, and those homologous to ArfGAP1-R50K that result in an ArfGAP with catalytic capacity that is reduced by several orders of magnitude [16]. For instance, AGAP2 associates with AP-1 positive recycling endosomes and over-expression of AGAP2 accelerates the exit of transferrin from this compartment. Over-expression of the catalytically dead mutant, AGAP2-R618K, or co-expression of AGAP2 with Arf1-Q71L failed to enhance transferrin recycling thereby linking this phenotype to the catalytic activity of AGAP2 [17]. In another example, over-expression of Git2-short results in the re-distribution β-COP from the cis-Golgi and the loss of actin stress fibers and paxillin containing focal adhesions throughout the cell [18]. Co-expression of Arf1-Q71L suppressed these phenotypes while the expression of a catalytically inactive Git2-short mutant had no effect on the localization of β-COP or on the abundance of actin stress fibers and focal adhesions. Similarly, increased expression of yeast Arf GAPs was shown to reduce the toxicity resulting from excess Arf activity [20], as would be expected for a down-regulator of Arf activity. Thus, Arf GAP activity of at least these Arf GAPs plays an essential role in their cellular functions.
But is the activation of GTP hydrolysis the only function of members of this large protein family or do they have additional functions? Simply the existence of a large number of domains involved in several aspects of cell signaling in most sub-families of Arf GAPs (excepting the ArfGAP1-3 group) may be taken as prima facie evidence for multiple roles in cell signaling that may act in concert with or independent of GAP activity. The model that Arf GAPs act solely as terminators of Arf signaling was first challenged experimentally by work in the yeast S. cerevisiae, which suggested that Arf GAPs can function as effectors of Arf. Four of the six proteins that contain Arf GAP domains were identified in a screen for suppressors of the loss of Arf function [19]. Increasing expression of the Arf GAPs Gcs1, Glo3, Age11, or Age2 was able to rescue the lethality resulting from Arf insufficiency. If Arf GAPs functioned solely as terminators of Arf signaling, as suggested by their biochemical Arf GAP activity, over-expression of Arf GAPs should only exacerbate the effects of the loss of Arf, not rescue them. In the same study, deletion of the Arf GAP Gcs1 resulted in supersensitivity to fluoride in a manner similar to the loss of Arf1. Thus, loss of Arf GAP produced the same phenotype as the loss of Arf1, suggesting again that they function in the same pathway but not antagonistically, as would be predicted from a pure negative regulator. Thus, Arf GAPs may act either to attenuate or mediate Arf signaling. If Arf GAPs function as both effectors and terminators of Arf, then Arf GAP activity must be strictly regulated to allow a non-catalytic interaction with Arf to facilitate effector function and a catalytic interaction with Arf to terminate Arf signaling. Studies of Arf GAP kinetics in vitro have relied heavily on the use of isolated GAP domains and are almost certain to have overlooked critical aspects of the regulation of Arf GAP activity by domains within the Arf GAP or by other binding partners. For instance, both the PH and BAR domains of ASAP1 have roles in regulating its Arf GAP activity. The PH domain is functionally integrated with the Arf GAP domain and is required for Arf GAP activity[21]. The BAR domain of ASAP1 inhibits Arf GAP activity and has been shown to be essential for the function of ASAP1 in cells [22-23]. Thus, domains outside of the canonical Arf GAP domain are essential for ASAP1 Arf GAP activity and may be key regulators of the function of ASAP1 in cells.
A third line of investigation provides even stronger, and more direct, evidence that at least one Arf GAP is a required component in the generation of Arf-dependent carriers of membrane traffic. Both yeast and human orthologs of the ArfGAP2/3 sub-family bind directly to both coat components and cargo molecules [24-28]. Yeast Glo3 facilitates the formation of a tripartite priming complex with Arf1 and any of three ER (endoplasmic reticulum)-Golgi v-SNARES for the recruitment of coatomer in vitro [29]. Interaction between COPI and SNARE was not observed in the absence of Glo3 and addition of Glo3 to the reaction mixture prior to the addition of coatomer was required. Thus, Glo3 interaction with cargo was proposed to precede coatomer binding. Similarly, human ArfGAP2 and ArfGAP3 were shown to be recruited to membranes through interactions with COPI and act downstream of Arf, or in concert with other Arf effectors [30]. Thus, in these studies orthologs of the ArfGAP2/3 family function as effectors rather than as simple down-regulators of Arf in the context of cargo and coat recruitment. Of course, there is no reason they cannot act in both capacities and more likely this is the case.
Human ArfGAP1 was also found to couple cargo to the COPI coat but did so in a manner that was independent of Arf. Like ArfGAP2/3 orthologs, mammalian ArfGAP1 binds directly to both coatomer and several SNARE proteins and facilitates the interaction between the cargo and the coat [24, 31-32]. But in contrast to the Glo3/ArfGAP2/3 studies, the ArfGAP1 complex had no requirement for Arf. GAP activity was not required to facilitate the interaction between coat and cargo as shown by use of an ArfGAP1 mutant that lacks catalytic activity, ArfGAP1-R50K [31]. These data suggest that ArfGAP1 has a role in cargo sorting that is independent of both Arf and GAP activity in a manner similar to but different from Glo3. In this model ArfGAP1 forms a priming complex with cargo for the recruitment of coatomer. In either instance, interaction with coatomer and cargo were mediated by regions of Arf GAP outside of the catalytic domain and these effects could be recapitulated with deletion mutants lacking the catalytic domain, further suggesting that these functions can act independently of GAP activity [31, 33]. Thus, ArfGAP1 clearly has the potential to function as an effector of Arf and in processes or pathways that do not involve direct binding to Arf. A more general conclusion that will likely be repeated in other systems and with other Arf GAPs is that the presence of an Arf GAP in a pathway does not in itself establish a role for an Arf in that same pathway.
The duality of ArfGAPs as both terminators and essential contributing components of Arf signaling extends to at least one of the larger, multi-domain ArfGAPs. ACAP1 is involved in the traffic of cargo from recycling endosomes to the plasma membrane, interacts simultaneously with Arf6, clathrin, and the recycling cargo Glut4, and co-localized with each on recycling endosomes [34-35]. Knockdown of either Arf6 or ACAP1 resulted in the loss of clathrin staining on recycling endosomes and inhibited Glut4 recycling to the plasma membrane. Knockdown of Arf6 also resulted in the loss of ACAP1 from recycling endosomes and prevented binding of Glut4 to ACAP1 and clathrin. Thus, ACAP1 functioned as an Arf6 effector in facilitating the interaction between coat and cargo and in regulating Glut4 recycling. However, expression of the catalytically dead ACAP1 mutant, ACAP1-R448Q, did not rescue the ACAP1 knockdown phenotype suggesting that GAP activity was also required for the regulation of Glut4 recycling. Thus, ACAP1 functions as both an Arf6 effector and as an Arf GAP in the same pathway supporting both the model for Arf GAPs as terminators of Arf signaling and as effectors of Arf.
Models for GAPs acting as positive components in signaling are also not novel to the Arf family. It is well documented that regulators of G-protein signaling (RGS) possess both the canonical function as GAPs, terminating G-protein coupled receptor signaling, and non-canonical roles as Gα and Gβγ effectors and as regulators of pathways independent of GPCR signaling (for a recent review see [36]). Regulation of GPCR signaling pathways by RGSs is also quite complex as RGSs coordinate the timing and amplitude of these pathways [37]. RGSs facilitate the rapid termination of GPCR signaling when stimulus is removed but must also allow for sufficiently high levels of activation upon stimulation. To allow for signal transduction, RGSs must either potentiate G-protein activation or GAP activity must be inhibited and there is evidence to support both mechanisms. Thus, RGSs regulate both the activation and termination of G-protein signaling in response to stimulus. A parallel may be drawn for the Arf GAPs in Arf signaling pathways. If Arf GAP activity remained high at all times, the result would be unproductive cycling of Arf on and off the membrane. Thus, timing of Arf GAP activity must be coordinated in order to allow for and terminate Arf signaling, though this is currently an understudied aspect of Arf GAP biology.
Together, there is a compelling and growing amount of data revealing that the prevailing model for the function of Arf GAPs in cells solely as terminators of Arf signaling is too limited. The literature supports a much more complex model for Arf GAPs that includes both the termination of Arf signaling, Arf effector function, and roles in other pathways that are independent of Arf. A model for the roles of Arf GAPs in Arf-dependent functions is presented in Figure 1.
Does ArfGAP1 positively or negatively regulate COPI assembly?
On the surface this would appear to be a straightforward and readily addressed issue. However, it has proven to be probably the most controversial question in models of Arf GAP function because mainly two different laboratories have conducted parallel studies and obtained opposing results and conclusions. The ArfGAP1 and ArfGAP2/3 subfamilies play critical roles in retrograde traffic from the Golgi to the ER, although no single member is essential for this process, based upon the lack of phenotype upon depletion of any one of the ArfGAP1-3 proteins in mammalian cells or Gcs1 or Glo3 in yeast [14, 20, 38]. The yeast ortholog of ArfGAP1 is Gcs1 and a single representative exists of the ArfGAP2/3 family in yeast, Glo3. Loss of all three, ArfGAP1-3, in mammalian cells, or of Gcs1 and Glo3 in yeast, result in severe defects in membrane traffic [14, 20, 38]. This double deletion in yeast causes accumulation of ER membrane and blocks secretion at an early stage. In vitro transport assays also revealed a defect in retrograde transport from the Golgi [38]. Depletion of ArfGAP1-3 in mammalian cells resulted in the loss of Golgi morphology and a redistribution of Golgi retrograde cargos to the ER-Golgi intermediate compartment (ERGIC). Cells depleted of ArfGAP1-3 were also defective for retrograde transport from the Golgi using an in vivo transport assay [14]. Thus, the ArfGAP subfamily is required for COPI-mediated retrograde transport from the Golgi to the ER and may also display secondary phenotypes. The bulk of the literature on the mammalian ArfGAP subfamilies is comprised of studies of ArfGAP1 but the overlapping and compensatory functions of ArfGAP2-3 for ArfGAP1 suggest that these findings may also extend to ArfGAP2-3. While there appears to be widespread agreement on the importance of ArfGAP1-3 in retrograde traffic from the Golgi, the mechanisms involved remain in dispute.
The use of reconstitution assays for aspects of membrane traffic has proven a very powerful means of demonstrating the roles of many components in the assembly or disassembly of carriers. However, such studies are often plagued by the potential for artifacts resulting from non-physiological concentrations of one or more reactants. These processes are clearly the result of many protein-protein and protein-lipid interactions of relatively low affinity that can be made to proceed in vitro despite the lack of components that may be required in vivo. The purity of membrane or protein preparations is also a confounding factor, particularly when testing the requirement for a protein that may act catalytically (e.g., an Arf GAP) and present in relatively low levels, compared to coat proteins that are predicted to serve structural roles.
A study by members of the Hsu laboratory offered a potential mechanism for the function of ArfGAP1 in regulating retrograde traffic from the Golgi by serving as a component of the COPI coat [32]. In this study, a two stage COPI vesicle reconstitution assay was used to show that ArfGAP1 persisted on COPI coated vesicles following their release from isolated Golgi donor membrane. COPI coated vesicles generated in this manner were similar in morphology and size to COPI vesicles reconstituted from isolated Golgi in previous studies and migrated in a similar manner on sucrose gradients. Immunogold EM confirmed the presence of the COPI coat and ArfGAP1 on these vesicles. ArfGAP1 levels on COPI coated vesicles were stoichiometric with the COPI component β-COP as determined by quantitative immunoblot analysis. These data were interpreted as evidence that ArfGAP1 is a component of the COPI coat. These findings were corroborated in a similar study in yeast in which endogenous Glo3 was found to co-purify with COPI coated vesicles reconstituted from isolated Golgi and another study in which ArfGAP2 and ArfGAP3 were associated with COPI coated vesicles reconstituted from isolated Golgi and cytosol, suggesting that ArfGAP2/3 members are components or are stably associated with these vesicles [25-26]. Further analysis of COPI coated vesicles from Hsu's study revealed that Arf was not associated with these vesicles by immunoblot or immunogold EM [32].
Work from the Wieland laboratory appears to refute the finding that ArfGAP1 is a component of the COPI coat and is consistent with the majority of the results from COPI vesicle reconstitution assays [39]. Using a variation of a COPI vesicle reconstitution assays, ArfGAP1 was not required for the formation of the COPI coated vesicles. Instead, ArfGAP1antagonized the accumulation of COPI coated vesicles in a dose-dependent manner, though it is not clear whether ArfGAP1 prevented the generation of vesicles or whether it triggered the loss of the COPI coat. Vesicles generated in this manner were homogeneous, had morphology consistent with previous COPI coated vesicle preparations, and migrated to the same place in sucrose gradients. Quantitative immunoblot analysis of purified vesicles revealed that Arf levels were stoichiometric with β-COP. These findings were consistent with the results of previous vesicle reconstitution assays from isolated Golgi which identified the seven subunits of COPI and Arf as the minimal components of COPI coated vesicles [40-41]. Yet another COPI vesicle reconstitution using liposomes and chemically defined components yielded the same requirement of only COPI and Arf for vesicle reconstitution and also showed that addition of the catalytic domain of ArfGAP1 antagonized COPI vesicle biogenesis [42-43]. Thus, two prevailing models for the minimal components of COPI vesicles exist. In Hsu's model, COPI vesicles consist of COPI and ArfGAP1 but not Arf, whereas Wieland's model includes COPI and Arf but not ArfGAP1.
The study from the Wieland laboratory also suggested that the assay used to identify ArfGAP1 as a coat component could not reliably discern COPI coated vesicles from donor membrane fragments. In Hsu's assay, the level of β-COP in immunoblots of the supernatant after incubating purified Golgi with Arf, COPI, and ArfGAP1 was used as an assay of COPI vesicle reconstitution [32]. The same assay was used to show that ArfGAP1 was released from the Golgi pellet in a manner similar to COPI. Wieland's group used the same assay and showed that ArfGAP1 was released from the Golgi pellet independent of the release of COPI by preventing vesicle fission with GTPγS and Arf1-Q71L [39]. Thus, release of ArfGAP1 was not necessarily coupled to COPI vesicle reconstitution. Centrifugation of these vesicle fractions at 200,000×g, which is predicted to pellet all membranes, resulted in an ArfGAP1 containing pellet that did not contain detectable amounts of COPI. Thus, ArfGAP1 associated non-specifically with membrane fragments released from the donor membrane that did not result from COPI vesicle budding, suggesting ArfGAP1 was a contaminant of COPI vesicle preparations rather than a component of the COPI coat. These findings are consistent with the observations that ArfGAP1 is capable of binding membranes directly, through its ALPS domain, in a process that is thought to be enhanced with increasing membrane curvature [44]. COPI may stabilize this association through a direct interaction with ArfGAP1. Thus, it is unclear whether ArfGAP1 simply bound non-specifically with the highly curved COPI coated vesicles or whether it was a functional component of the COPI coat.
It appears clear from the literature that ArfGAP1 is not an obligate, stoichiometric component of the COPI coat. COPI and Arf appear to be the minimal components of the COPI coat and vesicles can be reconstituted from both isolated Golgi and liposomes using only those components [40, 42]. Although Hsu's group presented compelling evidence that ArfGAP1 is a component of the COPI coat it is still unclear whether this association is the result of non-specific binding by ArfGAP1 to the membrane or a specific and essential interaction with COPI. Perhaps the most intriguing finding was that COPI vesicles containing ArfGAP1 did not contain Arf. These data are consistent with both in vivo data suggesting that COPI persists on membrane after the dissociation of Arf and proteomic analysis of COPI coated vesicles in which Arfs and ArfGAP1 were not detected [45-50]. There is also evidence suggesting that the accumulation of cargo in nascent buds or mature carriers is dependent upon GTP hydrolysis, or at least is compromised by the use of GTPγS or Arf1-Q71L in vitro and in cells and that ArfGAP1 plays a direct role in this type of cargo sorting [51-54]. Thus, addition of ArfGAP1 to vesicle reconstitution assays may have resulted in the generation of more physiologically relevant COPI coated vesicles that retain the COPI coat but not Arf.
Is ArfGAP1 required for fission of COPI coated vesicles?
Fission of coated vesicles from donor membranes has also been argued to require ArfGAP1 though this is another point of controversy in the field [32, 55]. A common feature of nearly all COPI reconstitution assays is the necessity of some manipulation for the release of vesicles from the donor membrane, with the possible exception of liposome based assays. When isolated Golgi membranes are incubated with a source of GTP, COPI, and Arf, coated buds are clearly visible by EM but are arrested at a stage preceding vesicle fission [56]. Several manipulations have been reported to overcome this block in vesicle fission/maturation including mechanical disruption via pipette shearing, high salt washes, or the addition of ArfGAP1 or palmitoyl-coenzyme A (palm-CoA) [32, 39, 41, 57]. Thus, two prevailing models exist for the fission of COPI coated vesicles. In one model, ArfGAP1 plays a critical role in a manner that also involves palm-CoA. In the other model, tethering interactions link COPI vesicles to the donor membrane and are disrupted experimentally by high salt treatment. In the two-stage COPI reconstitution assay, addition of ArfGAP1 to Golgi membrane pre-bound with Arf and COPI was sufficient to release COPI vesicles [32]. ArfGAP1-mediated fission was later found to also require residual Brefeldin-A ADP-ribosylated Substrate (BARS) bound to the donor membrane [55]. COPI vesicle reconstitution could be blocked by more stringent washing of the donor membrane that removed BARS or by the addition of an inhibitory antibody against BARS. ArfGAP1 and BARS interact directly and both were required for the efficient release of COPI coated vesicles. Thus, ArfGAP1 and BARS function together in stimulating vesicle fission. BARS also binds palm-CoA and this binding enhanced both vesicle reconstitution and the binding of BARS to ArfGAP1. Addition of palm-CoA to stringently washed Golgi did not allow for the release of COPI vesicles. Thus, BARS is at least one factor that mediates COPI fission stimulated by palm-CoA. These findings suggest that ArfGAP1 and BARS represent key machinery for COPI vesicle fission in cells.
At least two groups have reported that a salt wash is sufficient to release COPI coated vesicles [39, 41]. When compared to the release of COPI by ArfGAP1, a 250mM KCl wash was considerably more efficient at releasing COPI coated vesicles and could overcome blocks by GTPγS and Arf1-Q71L [39]. Thus, COPI vesicle fission was mediated independently of Arf-mediated GTP hydrolysis and ArfGAP1. A likely mechanism for the fission of vesicles in this manner is the disruption of protein-protein interactions that link vesicle buds to the donor membrane.
Whether fission of COPI coated vesicles is mediated by ArfGAP1 and BARS or the disruption of tethering factors in cells remains to be determined. Release of COPI coated vesicles in reconstitution assays precludes the absolute requirement of ArfGAP1 in the fission process but says little about the precise mechanism. Wieland's group offered an intriguing model in which either or both mechanisms may facilitate the release of COPI coated vesicles [39]. The tethering protein GMAP-210 links curved or vesicular membrane to flat membrane in an Arf1- and GTP- dependent manner [58]. Thus, disruption of tethering mediated by GMAP-210 may be a convergent mechanism of both high salt treatment and addition of ArfGAP1 in stimulating the release of COPI coated vesicles. Hydrolysis of GTP stimulated by ArfGAP1 would terminate the interaction between GMAP-210 and Arf to release the vesicle whereas high salt treatment may disrupt the protein-protein interaction directly. Thus, ArfGAP1, BARS, and tethering factors such as GMAP-210 are prime candidates for further study of COPI fission machinery.
Do Arf GAPs regulate uncoating?
A widely proposed role for Arf GAPs in membrane traffic is to stimulate vesicle uncoating at destination or acceptor membranes. This model was first proposed based solely upon the suggested presence of a high molar ratio of Arf-GTP to COPI and the obvious need to remove the COPI coat prior to fusion [40]. This model was supported by the finding that GTP hydrolysis by Arf was required for the dissociation of COPI from the vesicle linking Arf GAP activity to vesicle uncoating [59]. When GTPγS or Arf1-Q71L was used in in vitro transport assays, coated vesicles formed but transport was inhibited. Examination of the vesicles by EM revealed an accumulation of coated vesicles with few or no uncoated vesicles detected. Thus, uncoating was proposed to be linked to the ability of Arf to hydrolyze GTP. Because Arfs lack detectable intrinsic GTPase activity, Arf GAPs were implicated in triggering vesicle uncoating by stimulating GTP hydrolysis. Addition of any full length ArfGAP1-3 or the isolated catalytic domain of ArfGAP1 to COPI coated vesicles derived from liposomes resulted in vesicle uncoating supporting a potential role for Arf GAP activity in this process [27, 30, 43].
The control of vesicle uncoating by Arf GAPs was further elucidated by the finding that COPI enhanced ArfGAP1 catalytic activity. Addition of COPI to the reaction mixture of Δ17-Arf1 and the catalytic domain of ArfGAP1 enhanced GAP activity by several orders of magnitude in a dose-dependent manner [60]. These findings provided the first mechanism by which an Arf GAP activity may be regulated to ensure a productive context for this activity and are consistent with either a role for Arf GAPs in vesicle uncoating or biogenesis, as these in vitro observations do not differentiate between a possible hydrolysis and release of Arf during vesicle biogenesis from a regulated hydrolysis that is triggered by another factor at the site of vesicle fusion. These findings were later challenged using full-length myristoylated Arf1 as a substrate where addition of COPI to the reaction mixture had no effect on GTP hydrolysis by ArfGAP1 [16]. Full-length ArfGAP1 was also found to exhibit considerably greater Arf GAP activity in the absence of COPI than the catalytic domain used in Goldberg's original work. The two conflicting datasets were later reconciled by a more detailed kinetic study of ArfGAP1 [61]. In this study, COPI enhanced ArfGAP1 activity against both myristoylated Arf1 and Δ17-Arf1 by increasing the affinity of Arf1 for ArfGAP1. As COPI affected the Km and not the kcat, the experimental conditions in the previous study may have prevented the detection of the effects of COPI on ArfGAP1 activity. Thus, COPI was confirmed to have the potential for allosteric regulation of ArfGAP1 activity and may be a key regulator of this activity during COPI vesicle biogenesis or uncoating by coordinating GTP hydrolysis.
Kinetic analysis of Arf1 and COPI membrane dynamics appears to refute the model for the regulation of vesicle uncoating by Arf GAPs at the destination or acceptor membrane by suggesting that dissociation of COPI from membrane was not linked to the dissociation of Arf [45]. Using live-cell imaging with fluorescently tagged constructs, COPI-GFP (GFP fused to ε-COP) was found to persist on the membrane longer than Arf1-GFP; as the half-life of COPI-GFP was 35 sec and that of Arf1-GFP only 15 sec. Treatment of cells with BFA which would prevent rebinding of Arf to the membrane resulted in a half-life of 13 sec for Arf1-GFP and 30 sec for COPI-GFP, further suggesting that the dissociation of COPI-GFP was not coupled to the dissociation of Arf1-GFP. When Arf1-Q71L-GFP was used, both Arf1-Q71L-GFP and COPI-GFP were irreversibly associated with the membrane, suggesting that GTP hydrolysis is required for dissociation of Arf1-GTP and subsequently COPI-GFP. These findings suggest that GTP hydrolysis (and dissociation of Arf1) is essential and upstream of COPI dissociation or uncoating but is not necessarily the uncoating trigger.
Another argument against the model that Arf GAP-stimulated hydrolysis of GTP on Arfs acts as a trigger for vesicle uncoating at a destination membrane is that this model requires that Arf be present in the mature carriers, and presumably at levels approaching those of adaptors, e.g., COPI. However, proteomic analyses of three different purified Arf-dependent vesicle preparations (COPI [48], AP-1 [46-47, 49] and AP-3 [50] coated vesicles) each reported finding no Arfs among the large lists of protein components. Though it is dangerous to make hard conclusions from negative data of this kind, these data raise serious doubts about the presence of Arfs on mature carriers. Thus, GTP hydrolysis by Arf and, therefore, Arf GAP activity is ultimately a pre-requisite for vesicle uncoating though it is unlikely to be the trigger. Rather, we propose that GTP hydrolysis by Arfs occurs during the process of carrier biogenesis and leads to release of Arfs prior to release of the mature carrier from the donor membrane.
Conclusions
Arf GAPs are absolutely required for Arf-mediated GTP hydrolysis but their function in cells is not limited to this activity. Studies in yeast and mammalian cells have shown at least some Arf GAPs to be effectors of Arfs and to have the potential to mthat do not involve Arf. The small, single domain ArfGAP1 subfamily is the most thoroughly studied and has been proposed to facilitate multiple steps of COPI vesicle biogenesis, including uncoating of COPI coated vesicles. Which of these activities may be attributed to coordinating Arf-mediated GTP hydrolysis remains unclear but it is unlikely that this event mediates all of the proposed functions of ArfGAP1 in the COPI pathway. It will be important in the near future to extend molecular testing of Arf GAP actions to other types of carriers, using different Arf-dependent adaptors (e.g., AP-1, GGAs, and Mints) both to test the generalities of observations made with COPI carriers and in hopes of providing new insights. But further study of ArfGAP1 is also required both to reconcile the controversial findings summarized in this review and to elucidate the precise mechanisms of ArfGAP1 in these pathways. The study of ArfGAP1 has been a very profitable means of determining a model for the function of Arf GAPs in membrane traffic but it is dangerous to assume these models apply to other Arf GAPs. ArfGAP1 is among the smallest member of the Arf GAPs which are generally large proteins with diverse domain architecture linked only by the presence of the relatively small Arf GAP domain. Thus, studies of ArfGAP1 provide a starting point for the study of other Arf GAPs but each likely facilitates diverse functions and should be considered independently.
Acknowledgments
The authors thank Dr. Paul A. Randazzo for his critical reading of the manuscript and support from the National Institutes of Health (RAK, GM067226 and GM061268) and American Heart Association (09PRE2140029).
Abbreviations used
- Arf
ADP-ribosylation factor
- GAP
GTPase-activating protein
- GEF
guanine nucleotide exchange factor
- ER
endoplasmic reticulum
- ERGIC
endoplasmic reticulum-Golgi intermediate compartment
- SNARE
soluble N-ethylmaleimide-sensitive factor attachment protein receptors
- GDP
guanosine 5-diphosphate
- GTP
guanosine 5-triphosphate
- SDS-PAGE
sodium dodecylsulfate polyacrylamide gel electrophoresis
Footnotes
Note that AGE1 and AGE2 (Arf GAP with Effector function) were originally named SAT1 and SAT2 (Suppressors of arf1-ts) but were later changed to avoid a conflict with another gene in the Saccharomyces Genome Database.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Works Cited
- 1.Kahn RA, Cherfils J, Elias M, Lovering RC, Munro S, Schurmann A. Nomenclature for the human Arf family of GTP-binding proteins: ARF, ARL, and SAR proteins. J Cell Biol. 2006;172:645–50. doi: 10.1083/jcb.200512057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kahn RA. Toward a model for Arf GTPases as regulators of traffic at the Golgi. FEBS Lett. 2009;583:3872–9. doi: 10.1016/j.febslet.2009.10.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kahn RA, Gilman AG. The protein cofactor necessary for ADP-ribosylation of Gs by cholera toxin is itself a GTP binding protein. J Biol Chem. 1986;261:7906–11. [PubMed] [Google Scholar]
- 4.Cukierman E, Huber I, Rotman M, Cassel D. The ARF1 GTPase-activating protein: zinc finger motif and Golgi complex localization. Science. 1995;270:1999–2002. doi: 10.1126/science.270.5244.1999. [DOI] [PubMed] [Google Scholar]
- 5.Kahn RA, Bruford E, Inoue H, Logsdon JM, Jr, Nie Z, Premont RT, et al. Consensus nomenclature for the human ArfGAP domain-containing proteins. J Cell Biol. 2008;182:1039–44. doi: 10.1083/jcb.200806041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cuthbert EJ, Davis KK, Casanova JE. Substrate specificities and activities of AZAP family Arf GAPs in vivo. Am J Physiol Cell Physiol. 2008;294:C263–70. doi: 10.1152/ajpcell.00292.2007. [DOI] [PubMed] [Google Scholar]
- 7.Bowzard JB, Cheng D, Peng J, Kahn RA. ELMOD2 is an Arl2 GTPase-activating protein that also acts on Arfs. J Biol Chem. 2007;282:17568–80. doi: 10.1074/jbc.M701347200. [DOI] [PubMed] [Google Scholar]
- 8.Nie Z, Randazzo PA. Arf GAPs and membrane traffic. J Cell Sci. 2006;119:1203–11. doi: 10.1242/jcs.02924. [DOI] [PubMed] [Google Scholar]
- 9.Spang A, Shiba Y, Randazzo PA. Arf GAPs: Gatekeepers of vesicle generation. FEBS Lett. 2010;584:2646–51. doi: 10.1016/j.febslet.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Randazzo PA, Kahn RA. GTP hydrolysis by ADP-ribosylation factor is dependent on both an ADP-ribosylation factor GTPase-activating protein and acid phospholipids. J Biol Chem. 1994;269:10758–63. [PubMed] [Google Scholar]
- 11.Makler V, Cukierman E, Rotman M, Admon A, Cassel D. ADP-ribosylation factor-directed GTPase-activating protein. Purification and partial characterization. J Biol Chem. 1995;270:5232–7. doi: 10.1074/jbc.270.10.5232. [DOI] [PubMed] [Google Scholar]
- 12.Aoe T, Cukierman E, Lee A, Cassel D, Peters PJ, Hsu VW. The KDEL receptor, ERD2, regulates intracellular traffic by recruiting a GTPase-activating protein for ARF1. EMBO J. 1997;16:7305–16. doi: 10.1093/emboj/16.24.7305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peyroche A, Antonny B, Robineau S, Acker J, Cherfils J, Jackson CL. Brefeldin A acts to stabilize an abortive ARF-GDP-Sec7 domain protein complex: involvement of specific residues of the Sec7 domain. Mol Cell. 1999;3:275–85. doi: 10.1016/s1097-2765(00)80455-4. [DOI] [PubMed] [Google Scholar]
- 14.Saitoh A, Shin HW, Yamada A, Waguri S, Nakayama K. Three homologous ArfGAPs participate in coat protein I-mediated transport. J Biol Chem. 2009;284:13948–57. doi: 10.1074/jbc.M900749200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang CJ, Rosenwald AG, Willingham MC, Skuntz S, Clark J, Kahn RA. Expression of a dominant allele of human ARF1 inhibits membrane traffic in vivo. J Cell Biol. 1994;124:289–300. doi: 10.1083/jcb.124.3.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Szafer E, Pick E, Rotman M, Zuck S, Huber I, Cassel D. Role of coatomer and phospholipids in GTPase-activating protein-dependent hydrolysis of GTP by ADP-ribosylation factor-1. J Biol Chem. 2000;275:23615–9. doi: 10.1074/jbc.M003171200. [DOI] [PubMed] [Google Scholar]
- 17.Nie Z, Fei J, Premont RT, Randazzo PA. The Arf GAPs AGAP1 and AGAP2 distinguish between the adaptor protein complexes AP-1 and AP-3. J Cell Sci. 2005;118:3555–66. doi: 10.1242/jcs.02486. [DOI] [PubMed] [Google Scholar]
- 18.Mazaki Y, Hashimoto S, Okawa K, Tsubouchi A, Nakamura K, Yagi R, et al. An ADP-ribosylation factor GTPase-activating protein Git2-short/KIAA0148 is involved in subcellular localization of paxillin and actin cytoskeletal organization. Mol Biol Cell. 2001;12:645–62. doi: 10.1091/mbc.12.3.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang CJ, Cavenagh MM, Kahn RA. A family of Arf effectors defined as suppressors of the loss of Arf function in the yeast Saccharomyces cerevisiae. J Biol Chem. 1998;273:19792–6. doi: 10.1074/jbc.273.31.19792. [DOI] [PubMed] [Google Scholar]
- 20.Zhang CJ, Bowzard JB, Anido A, Kahn RA. Four ARF GAPs in Saccharomyces cerevisiae have both overlapping and distinct functions. Yeast. 2003;20:315–30. doi: 10.1002/yea.966. [DOI] [PubMed] [Google Scholar]
- 21.Kam JL, Miura K, Jackson TR, Gruschus J, Roller P, Stauffer S, et al. Phosphoinositide-dependent activation of the ADP-ribosylation factor GTPase-activating protein ASAP1. Evidence for the pleckstrin homology domain functioning as an allosteric site. J Biol Chem. 2000;275:9653–63. doi: 10.1074/jbc.275.13.9653. [DOI] [PubMed] [Google Scholar]
- 22.Jian X, Brown P, Schuck P, Gruschus JM, Balbo A, Hinshaw JE, et al. Autoinhibition of Arf GTPase-activating protein activity by the BAR domain in ASAP1. J Biol Chem. 2009;284:1652–63. doi: 10.1074/jbc.M804218200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nie Z, Hirsch DS, Luo R, Jian X, Stauffer S, Cremesti A, et al. A BAR domain in the N terminus of the Arf GAP ASAP1 affects membrane structure and trafficking of epidermal growth factor receptor. Curr Biol. 2006;16:130–9. doi: 10.1016/j.cub.2005.11.069. [DOI] [PubMed] [Google Scholar]
- 24.Eugster A, Frigerio G, Dale M, Duden R. COP I domains required for coatomer integrity, and novel interactions with ARF and ARF-GAP. EMBO J. 2000;19:3905–17. doi: 10.1093/emboj/19.15.3905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lewis SM, Poon PP, Singer RA, Johnston GC, Spang A. The ArfGAP Glo3 is required for the generation of COPI vesicles. Mol Biol Cell. 2004;15:4064–72. doi: 10.1091/mbc.E04-04-0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frigerio G, Grimsey N, Dale M, Majoul I, Duden R. Two human ARFGAPs associated with COP-I-coated vesicles. Traffic. 2007;8:1644–55. doi: 10.1111/j.1600-0854.2007.00631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kliouchnikov L, Bigay J, Mesmin B, Parnis A, Rawet M, Goldfeder N, et al. Discrete determinants in ArfGAP2/3 conferring Golgi localization and regulation by the COPI coat. Mol Biol Cell. 2009;20:859–69. doi: 10.1091/mbc.E08-10-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luo R, Ha VL, Hayashi R, Randazzo PA. Arf GAP2 is positively regulated by coatomer and cargo. Cell Signal. 2009;21:1169–79. doi: 10.1016/j.cellsig.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rein U, Andag U, Duden R, Schmitt HD, Spang A. ARF-GAP-mediated interaction between the ER-Golgi v-SNAREs and the COPI coat. J Cell Biol. 2002;157:395–404. doi: 10.1083/jcb.200112092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weimer C, Beck R, Eckert P, Reckmann I, Moelleken J, Brugger B, et al. Differential roles of ArfGAP1, ArfGAP2, and ArfGAP3 in COPI trafficking. J Cell Biol. 2008;183:725–35. doi: 10.1083/jcb.200806140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee SY, Yang JS, Hong W, Premont RT, Hsu VW. ARFGAP1 plays a central role in coupling COPI cargo sorting with vesicle formation. J Cell Biol. 2005;168:281–90. doi: 10.1083/jcb.200404008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang JS, Lee SY, Gao M, Bourgoin S, Randazzo PA, Premont RT, et al. ARFGAP1 promotes the formation of COPI vesicles, suggesting function as a component of the coat. J Cell Biol. 2002;159:69–78. doi: 10.1083/jcb.200206015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schindler C, Rodriguez F, Poon PP, Singer RA, Johnston GC, Spang A. The GAP domain and the SNARE, coatomer and cargo interaction region of the ArfGAP2/3 Glo3 are sufficient for Glo3 function. Traffic. 2009;10:1362–75. doi: 10.1111/j.1600-0854.2009.00952.x. [DOI] [PubMed] [Google Scholar]
- 34.Dai J, Li J, Bos E, Porcionatto M, Premont RT, Bourgoin S, et al. ACAP1 promotes endocytic recycling by recognizing recycling sorting signals. Dev Cell. 2004;7:771–6. doi: 10.1016/j.devcel.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 35.Li J, Peters PJ, Bai M, Dai J, Bos E, Kirchhausen T, et al. An ACAP1-containing clathrin coat complex for endocytic recycling. J Cell Biol. 2007;178:453–64. doi: 10.1083/jcb.200608033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sethakorn N, Yau DM, Dulin NO. Non-canonical functions of RGS proteins. Cell Signal. 2010 doi: 10.1016/j.cellsig.2010.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ross EM. Coordinating speed and amplitude in G-protein signaling. Curr Biol. 2008;18:R777–R83. doi: 10.1016/j.cub.2008.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Poon PP, Cassel D, Spang A, Rotman M, Pick E, Singer RA, et al. Retrograde transport from the yeast Golgi is mediated by two ARF GAP proteins with overlapping function. EMBO J. 1999;18:555–64. doi: 10.1093/emboj/18.3.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beck R, Adolf F, Weimer C, Bruegger B, Wieland FT. ArfGAP1 activity and COPI vesicle biogenesis. Traffic. 2009;10:307–15. doi: 10.1111/j.1600-0854.2008.00865.x. [DOI] [PubMed] [Google Scholar]
- 40.Serafini T, Orci L, Amherdt M, Brunner M, Kahn RA, Rothman JE. ADP-ribosylation factor is a subunit of the coat of Golgi-derived COP-coated vesicles: a novel role for a GTP-binding protein. Cell. 1991;67:239–53. doi: 10.1016/0092-8674(91)90176-y. [DOI] [PubMed] [Google Scholar]
- 41.Ostermann J, Orci L, Tani K, Amherdt M, Ravazzola M, Elazar Z, et al. Stepwise assembly of functionally active transport vesicles. Cell. 1993;75:1015–25. doi: 10.1016/0092-8674(93)90545-2. [DOI] [PubMed] [Google Scholar]
- 42.Bremser M, Nickel W, Schweikert M, Ravazzola M, Amherdt M, Hughes CA, et al. Coupling of coat assembly and vesicle budding to packaging of putative cargo receptors. Cell. 1999;96:495–506. doi: 10.1016/s0092-8674(00)80654-6. [DOI] [PubMed] [Google Scholar]
- 43.Reinhard C, Schweikert M, Wieland FT, Nickel W. Functional reconstitution of COPI coat assembly and disassembly using chemically defined components. Proc Natl Acad Sci U S A. 2003;100:8253–7. doi: 10.1073/pnas.1432391100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bigay J, Casella JF, Drin G, Mesmin B, Antonny B. ArfGAP1 responds to membrane curvature through the folding of a lipid packing sensor motif. EMBO J. 2005;24:2244–53. doi: 10.1038/sj.emboj.7600714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Presley JF, Ward TH, Pfeifer AC, Siggia ED, Phair RD, Lippincott-Schwartz J. Dissection of COPI and Arf1 dynamics in vivo and role in Golgi membrane transport. Nature. 2002;417:187–93. doi: 10.1038/417187a. [DOI] [PubMed] [Google Scholar]
- 46.Blondeau F, Ritter B, Allaire PD, Wasiak S, Girard M, Hussain NK, et al. Tandem MS analysis of brain clathrin-coated vesicles reveals their critical involvement in synaptic vesicle recycling. Proc Natl Acad Sci U S A. 2004;101:3833–8. doi: 10.1073/pnas.0308186101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Borner GH, Harbour M, Hester S, Lilley KS, Robinson MS. Comparative proteomics of clathrin-coated vesicles. J Cell Biol. 2006;175:571–8. doi: 10.1083/jcb.200607164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gilchrist A, Au CE, Hiding J, Bell AW, Fernandez-Rodriguez J, Lesimple S, et al. Quantitative proteomics analysis of the secretory pathway. Cell. 2006;127:1265–81. doi: 10.1016/j.cell.2006.10.036. [DOI] [PubMed] [Google Scholar]
- 49.Girard M, Allaire PD, McPherson PS, Blondeau F. Non-stoichiometric relationship between clathrin heavy and light chains revealed by quantitative comparative proteomics of clathrin-coated vesicles from brain and liver. Mol Cell Proteomics. 2005;4:1145–54. doi: 10.1074/mcp.M500043-MCP200. [DOI] [PubMed] [Google Scholar]
- 50.Salazar G, Craige B, Wainer BH, Guo J, De Camilli P, Faundez V. Phosphatidylinositol-4-kinase type II alpha is a component of adaptor protein-3-derived vesicles. Mol Biol Cell. 2005;16:3692–704. doi: 10.1091/mbc.E05-01-0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lanoix J, Ouwendijk J, Stark A, Szafer E, Cassel D, Dejgaard K, et al. Sorting of Golgi resident proteins into different subpopulations of COPI vesicles: a role for ArfGAP1. J Cell Biol. 2001;155:1199–212. doi: 10.1083/jcb.200108017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nickel W, Malsam J, Gorgas K, Ravazzola M, Jenne N, Helms JB, et al. Uptake by COPI-coated vesicles of both anterograde and retrograde cargo is inhibited by GTPgammaS in vitro. J Cell Sci. 1998;111(Pt 20):3081–90. doi: 10.1242/jcs.111.20.3081. [DOI] [PubMed] [Google Scholar]
- 53.Lanoix J, Ouwendijk J, Lin CC, Stark A, Love HD, Ostermann J, et al. GTP hydrolysis by arf-1 mediates sorting and concentration of Golgi resident enzymes into functional COP I vesicles. EMBO J. 1999;18:4935–48. doi: 10.1093/emboj/18.18.4935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pepperkok R, Whitney JA, Gomez M, Kreis TE. COPI vesicles accumulating in the presence of a GTP restricted arf1 mutant are depleted of anterograde and retrograde cargo. J Cell Sci. 2000;113(Pt 1):135–44. doi: 10.1242/jcs.113.1.135. [DOI] [PubMed] [Google Scholar]
- 55.Yang JS, Lee SY, Spano S, Gad H, Zhang L, Nie Z, et al. A role for BARS at the fission step of COPI vesicle formation from Golgi membrane. EMBO J. 2005;24:4133–43. doi: 10.1038/sj.emboj.7600873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Weidman P, Roth R, Heuser J. Golgi membrane dynamics imaged by freeze-etch electron microscopy: views of different membrane coatings involved in tubulation versus vesiculation. Cell. 1993;75:123–33. [PubMed] [Google Scholar]
- 57.Serafini T, Rothman JE. Purification of Golgi cisternae-derived non-clathrin-coated vesicles. Methods Enzymol. 1992;219:286–99. doi: 10.1016/0076-6879(92)19029-6. [DOI] [PubMed] [Google Scholar]
- 58.Drin G, Morello V, Casella JF, Gounon P, Antonny B. Asymmetric tethering of flat and curved lipid membranes by a golgin. Science. 2008;320:670–3. doi: 10.1126/science.1155821. [DOI] [PubMed] [Google Scholar]
- 59.Tanigawa G, Orci L, Amherdt M, Ravazzola M, Helms JB, Rothman JE. Hydrolysis of bound GTP by ARF protein triggers uncoating of Golgi-derived COP-coated vesicles. J Cell Biol. 1993;123:1365–71. doi: 10.1083/jcb.123.6.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Goldberg J. Structural basis for activation of ARF GTPase: mechanisms of guanine nucleotide exchange and GTP-myristoyl switching. Cell. 1998;95:237–48. doi: 10.1016/s0092-8674(00)81754-7. [DOI] [PubMed] [Google Scholar]
- 61.Luo R, Randazzo PA. Kinetic analysis of Arf GAP1 indicates a regulatory role for coatomer. J Biol Chem. 2008;283:21965–77. doi: 10.1074/jbc.M802268200. [DOI] [PMC free article] [PubMed] [Google Scholar]


