1. INTRODUCTION
Ischemic preconditioning (IPC) by brief ischemia induced robust tolerance to ischemia in rat retina (Roth et al. 1998). Events underlying IPC neuroprotection are under intense study (Ettaiche et al. 2001; Nonaka et al. 2001; Sakamoto et al. 2001; Toprak et al. 2002; Kamphuis et al. 2007a; Thiersch et al. 2008; Zhu et al. 2008). Mechanisms uncovered to date include altered expression of a variety of genes (Kamphuis et al. 2007b), attenuation of apoptosis (Zhang et al. 2002), and involvement of adenosine (Li and Roth 1999), HIF-1α (Zhu et al. 2007), erythropoietin (Dreixler et al. 2009b), and protein kinases including Akt, PKC, and p38 (Dreixler et al. 2009a; Dreixler et al. 2009b; Dreixler et al. 2009c). Other stimuli induce a state of endogenous neuroprotection, or “cross-tolerance” (Franco et al. 2008). Cross-tolerance is significant because it affords the opportunity to “mimic” IPC’s neuroprotection, and therefore, has potential direct clinical relevance. Multiple mechanisms leading to ischemic damage render it difficult to design effective neuroprotection (McCulloch and Dewar 2001). Exogenously administered neuroprotectants may be toxic or non-specific. An exciting alternative with profound physiological and therapeutic implications is to use IPC to harness the endogenous cellular protective capacity (Roth et al. 1998). Principal advantages are inhibiting multiple pathways of ischemia-reperfusion injury, and a lower likelihood of non-specificity. Studying IPC provides a unique opportunity to understand endogenous protective mechanisms counteracting ischemia. Clinical studies suggest the existence of IPC in the brain in humans who experience transient ischemic attacks (Wegener et al. 2004).
The recently uncovered phenomenon of post-conditioning (Post-C) approaches endogenous neuroprotection from the opposite end of ischemia from IPC. Post-C is a brief period of ischemia during the reperfusion phase after the prolonged ischemia has ended. There has been an enormous recent basic and clinical research interest in Post-C, with over 155 papers appearing on Medline in 2006–09, mostly involving myocardium and brain. Species, aging, and organ differences are emerging, as well as dependence upon the length and severity of ischemia, where conflicting results have appeared (Darling et al. 2005; Tang et al. 2006; Manintveld et al. 2007). The mechanisms of Post-C are incompletely explained, and may be distinct from those of IPC. Part of the distinction is that IPC exerts its effects by changing the molecular environment of cells before, during, or after ischemia. In contrast, Post-C, by definition, can only be acting during the reperfusion phase. However, cellular changes during ischemia and reperfusion differ, and thus it is likely that mechanisms of neuroprotection are, at least in part, distinct. Post-C is highly attractive not only as another means of examining endogenous mechanisms of neuroprotection, but also as a potentially applicable and clinically translatable strategy to prevent retinal cell death. Therefore an understanding of its methods has high translational relevance.
A recent study (Fernandez et al. 2009) observed neuroprotective effects of Post-C in the rat retina. After 40 min of ischemia, Post-C enhanced recovery of the electroretinogram (ERG) b-wave from approximately 33% to 90% of baseline, and, after 60 min of ischemia, from 10% to 35%. Post-C reduced but did not prevent retinal ganglion cell loss after ischemia, and was effective up to 60 min after the onset of reperfusion following 40 min of ischemia. The authors showed that blockade of protein synthesis 1 min prior to Post-C, but not 6 h after, prevented the neuroprotection. This finding suggests that Post-C activates an early change in protein synthesis. However, the mechanisms of Post-C neuroprotection in the retina are not known. One possible explanation of the effectiveness of Post-C is that it augments intrinsic neuroprotective mechanisms initiated during ischemia. Increasing duration of the damaging ischemic insult may therefore impact the effectiveness of Post-C. Additionally, we hypothesized that IPC and Post-C, by operating on opposite sides of damaging ischemia, would provide additive neuroprotection to each one applied alone, particularly in more severe ischemia, where Post-C or IPC did not completely ameliorate ischemic injury (Dreixler et al. 2009a; Dreixler et al. 2009b; Dreixler et al. 2009c; Fernandez et al. 2009). To test this theory, we examined the effect of combining IPC and Post-C. To begin to examine the mechanisms of Post-C, we examined its impact upon cell death produced by apoptosis, a major mechanism of retinal cell death following ischemia which, in previous studies, was found to be attenuated by IPC (Singh et al. 2001; Dreixler et al. 2009a; Dreixler et al. 2009b; Dreixler et al. 2009c). Finally, inflammatory cell infiltration after ischemia (Jo et al. 2003) was examined to determine if Post-C altered this manifestation of the inflammatory response.
2. METHODS
2.1. Retinal Preconditioning and Post-conditioning and Ischemia
Procedures (Roth et al. 2006; Dreixler et al. 2008) conformed to the Association for Research in Vision and Ophthalmology Resolution on the Use of Animals in Research and were approved by our Animal Care Committee. Wistar rats (200–250 gm) purchased from Harlan (Indianapolis, IN) were maintained on a 12 h on/12 h off light cycle.
IPC was produced using two different methods, both of which were previously used in our laboratory (Roth et al. 2006; Dreixler et al. 2008). For increased IOP preconditioning (“IOP PC”), the intraocular pressure (IOP) was increased for 8 min to 160 – 165 mm Hg using a pressurized bag of sterile ocular irrigating solution (BSS: Alcon, Fort Worth, TX) connected to a 30-g needle placed under direct vision in the center of the anterior chamber. For ligation IPC, a 2.0 silk ligature was placed around the optic nerve and blood vessels behind the globe of one eye and pulled through a small length of polyethylene tubing (PE-200, Intramedic; Becton-Dickinson, Parsippany, NJ). By pushing the tubing toward the eyes while clamping the suture to maximal tightness, we produced complete ocular ischemia (Roth et al. 1998; Rosenbaum et al. 2001). Our previous studies have shown that the IPC paradigms produce no morphological or functional retinal changes, but protect the retina from ischemic morphological and functional changes (Roth et al. 2006; Dreixler et al. 2008; Dreixler et al. 2009b).
For retinal ischemia, rats were anesthetized with chloral hydrate, 275 mg/kg i.p., and IOP increased to 130 – 135 mm Hg for 45 or 55 min. To prevent an influence of hypothermia (Faberowski et al. 1989), temperature was maintained at 36–37 C using a servo–controlled heating blanket (Harvard Apparatus, Natick, MA). To preclude a preconditioning influence of hypoxia (Zhu et al. 2007), oxygen saturation was measured with a pulse oximeter (Ohmeda; Louisville, CO) on the rat’s tail. Supplemental oxygen, when necessary to maintain O2 saturation > 94%, was administered using a cannula placed in front of the nares and mouth. Blood pressure was monitored during the ischemia experiments using a non-invasive tail blood pressure device (IITC Life Science; Woodland Hills, CA).
Post-conditioning was accomplished by allowing reperfusion for 5 min after ischemia, confirmed by direct observation under the operating microscope, and then increasing the IOP to 160 –165 mm Hg for 8 minutes, after which the anterior chamber needle was removed. Groups of rats were studied in which IPC was performed 24 h prior to ischemia, followed by Post-C. The two different methods of IPC were studied because it was not known if the method of IPC might influence the results of combining IPC and Post-C, either due to technical reasons or due to a specific mechanism associated with a specific means of producing IPC.
2.2. Electroretinography Before and after Retinal Ischemia
Procedures we used have been described in detail previously (Roth et al. 2003; Roth et al. 2006, Dreixler, 2008). Animals were dark-adapted for at least 2 h before ERG recordings. For baseline and post–ischemic (i.e., after 7 days) follow–up ERG, and during pre-conditioning, rats were injected i.p. with ketamine (35 mg/kg), and xylazine (5 mg/kg). Corneal analgesia was with 1–2 drops of 0.5% proparacaine (Alcon, Ft. Worth, TX). Pupils were dilated with 0.5% tropicamide (Alcon), and cyclomydril (0.2% cyclopentolate HCl and 1% phenylephrine HCl (Alcon).
The ERG was recorded at baseline (prior to the experiments) and at 7 days after ischemia by placing platinum needle electroencephalogram electrodes (Grass, Providence, RI) in contact with the corneal surfaces of both eyes, and a reference electrode on the tongue. Needles were bent to provide maximum longitudinal contact between the metal and cornea, completely avoiding contact between the sharp tip of the needle and the cornea. Electrode wires were embedded in putty, shaped specially for each rat, thus enabling recording of responses from the same contact point in the cornea throughout the experiment. The cornea was intermittently irrigated with a balanced salt solution (Alcon) to maintain adequate electrical contact and to prevent exposure keratopathy.
Stimulus-intensity ERG analysis was achieved on a UTAS-E 4000 ERG system with a full-field Ganzfeld stimulator (LKC Technologies), and the rat’s head centered 7 in from the stimulator. The low pass filter was 0.05 Hz and the high pass 500 Hz. Flash intensity varied electronically from 0.37 log cd.s/m2 to 1.40 log cd.s/m2, with settings confirmed by photometry (EG & G Model 550 photometer, Electro-Optics, Boulder, CO). Responses were averaged for 3 to 5 flashes delivered 18 to 30 s apart depending upon flash intensity, with number of flashes decreasing and time between them increasing with flash intensity. Flashes were progressively delivered from the lowest intensity to the highest to prevent possible effect upon dark adaptation, and at least 1 min elapsed between the series of flashes for the intensity settings. This timing was effective in preventing attenuation of dark adaptation. Oscillatory potentials (OPs) were measured by extracting OP wavelet components from the ERG waveforms using Fast Fourier transformation (Matlab, The MathWorks, Inc., Natick, MA). Sum of the root mean squares (Sum RMS) of the amplitudes of the OP wavelets was calculated (Bui et al. 2005).
2.3. Histology
Eyes enucleated on the seventh day after ischemia were immediately fixed in Davidson’s fixative (11% glacial acetic acid, 2% neutral buffered formalin and 32% ethanol in H2O) for 24 h, then transferred to 70% ethanol for 24 h and stored in PBS at 4°C. Eyes were embedded in paraffin, sectioned to 4 μm and stained with hematoxylin and eosin (H&E). Sections were examined by light microscopy and retinal ganglion cell (RGC) counts quantified as described earlier (Roth et al. 1998; Junk et al. 2002; Dreixler et al. 2009b). The determination of the cells in the inner nuclear layer (INL) or outer nuclear layer was accomplished by capturing images of the retinal sections using Micron (Westover Scientific, Mill Creek, WA). Several cell regions of interest (ROI) were selected (around 1500 μm from the optic nerve bundle) and the numbers of cells were manually counted and corrected for the calculated area of the ROI.
2.4. Fluorescent TUNEL
Fluorescent TUNEL was performed using a Fluorescein FragEL DNA Fragmentation Detection Kit (Calbiochem, La Jolla, CA) as described previously (Singh et al. 2001; Zhang et al. 2002). Briefly, 7 μm thick frozen retinal sections were fixed and hydrated in 4% formaldehyde followed by TBS immersion, permeabilized with proteinase K in 10 mM Tris pH=8 (1:100), and then labeled by TdT enzymatic reaction.
2.5. Immunohistochemistry
Enucleated eyes were fixed at room temperature in 4% paraformaldehyde for 3 h. After removal of the anterior segment, the posterior portion of the eye was postfixed in the same fixative overnight at 4°C before being placed in 25% sucrose for a second overnight period at 4°C for cryoprotection. Eyecups were embedded in OCT compound (Sakura Finetec, Torrance, CA) and cut into 10-μm-thick cryosections.
Primary antibodies that we used (all at 1:50 concentration) included: rabbit polyclonal anti-MCP-1 (Santa Cruz Biotechnology, Santa Cruz, CA) to recognize infiltrating monocytes, rabbit polyclonal anti-MIP-1α (Santa Cruz Biotechnology) for macrophages, FITC-conjugated mouse monoclonal anti-CD45 (BD Biosciences, Pharmingen, San Diego, CA) for leukocytes, and biotin-conjugated mouse monoclonal anti-Thy-1 (BD Biosciences) to recognize retinal ganglion cells. Sections were then exposed to the appropriate secondary antibodies: rhodamine red X-conjugated avidin (Invitrogen, Eugene, OR) or goat anti-rabbit IgG fluorescein-conjugate (1:500, Invitrogen). Antifade mounting media containing DAPI to identify nuclei (Oncogene Research, San Diego, CA) was applied and sections were cover-slipped.
2.6. Imaging and Image analysis
For imaging frozen retinal sections (immunohistochemistry and TUNEL), we utilized a fluorescence microscope (Olympus IX81 inverted microscope), a Fast firewire Retiga EXi chilled CCD camera, and a 40X oil lens. Excitation/dichroic/emission settings were 480/40 nm-505LP-535/30 nm for rhodamine and 530–550 nm – 570DM-590LP for greens (FITC and fluorescein). TUNEL positive cells were identified as previously reported (Singh et al. 2001; Zhang et al. 2002). Quantification of the fluorescent intensities was performed using NIH Image J v.1.33, adapted from our previous methods (Roth et al. 2003). Mean intensity was determined from a whole retinal region extending from the retinal ganglion cell layer into the photoreceptor layer. The mean intensities were normalized to the paired normal eye.
2.7. Data handling and statistical analysis
To account for day-to-day variation in the normal eye, and for simultaneous reference to the baseline in the ischemic eye, as previously described (Roth et al. 2006; Dreixler et al. 2008; Dreixler et al. 2009b; Dreixler et al. 2009c) we used the three highest intensity values of 0.37, 0.87, and 1.4 log cd.s/m2 for comparisons of electroretinography data between groups. For simplicity, data for 0.87 log cd.s/m2 are shown in the bar graph figures; the other values for these sets of comparisons appear in the Tables. A- and b-wave data were normally distributed as confirmed using normality plots and skewness/kurtosis in Stata version 10.0 (College Station, TX). All a- and b-wave data were statistically analyzed using the Scheffe multiple-comparison post hoc test (Stata 10.0). Sum RMS of the OPs was not normally distributed, hence we used the Kruskal –Wallis test for multiple comparisons and Mann-Whitney rank sum comparison between the experimental groups (Stata 10.0).
For histopathological data analysis, we could not assume a normal distribution and therefore used the Kruskal –Wallis test for multiple comparisons and Mann-Whitney rank sum comparison between the experimental groups. Similarly, for fluorescent TUNEL, Kruskal-Wallis test assessed change in percentage of TUNEL positive RGC cells. The Mann-Whitney test compared results between two of the groups.
3. RESULTS
3.1. Post-C and 45 minutes of ischemia
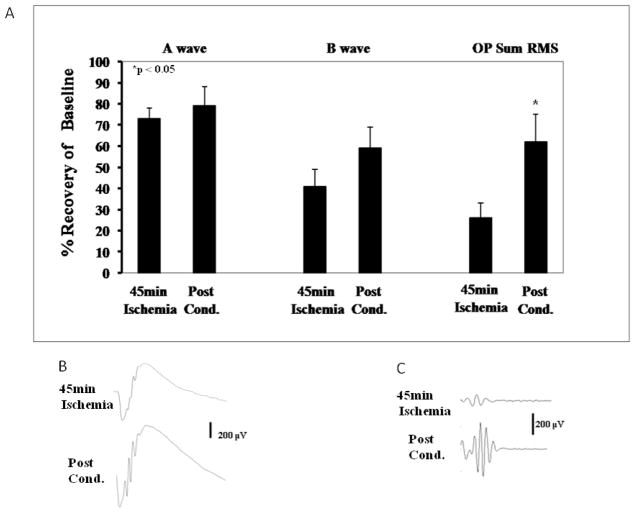
The results for the normalized and corrected waveforms at a representative flash intensity of 0.87 log cd.s/m2 are shown in Fig. 1. Recovery of the a- and b-waves was relatively unchanged with Post-C as compared to the ischemia only group (Fig. 1A) when ischemia lasted 45 min. Recovery of SUM RMS OPs (oscillatory potentials), normalized and corrected at 0.87 log.cd/m2, was significantly increased with ischemia + Post-C about 2-fold to 62 ± 14% (p = 0.04), compared to the 45 min ischemia alone (26 ± 7%; Fig. 1A).
Figure 1.
A. Recovery of the a- and b-waves, but not the oscillatory potentials (OPs), was relatively unchanged with Post-C (n = 9) at 7 days after ischemia as compared to the 45 min ischemia-only group (n = 8), shown at a representative flash intensity of 0.87 log cd.s/m.2 Post-C was 8 min of ischemia applied at 5 min of reperfusion after 45 min of ischemia ended; B. Representative ERG traces. RMS = root mean square amplitude of oscillatory potentials. C. Representative OP transformations.
Histopathological examination found that the number of retinal ganglion cells (RGCs), the numbers of cells per unit area in the retinal inner nuclear layer (INL) and outer nuclear layer (ONL) were not significantly changed (data not shown).
3.2. IPC and 55 minutes of ischemia
The normalized and corrected a-wave recovery at intensity 0.87 log cd.s/m2 was unchanged after ischemia of 55 min duration with either method of IPC (ANOVA compared to ischemia, p = 0.24; Fig. 2A, Table 1). The b-wave recovery was significantly increased approximately 3–4 fold with ligation IPC to 41 ± 1% (p = 0.05) and significantly increased with IOP-IPC to 47 ± 4% (p = 0.01) as compared to the ischemia only (12 ± 2%; ANOVA between the three groups p = 0.01; Fig. 2A; Table 1). Recovery of the RMS of the OP SUM RMS was significantly increased approximately 6–7 fold with ligation IPC to 24 ± 2% (p = 0.01) and increased with IOP-IPC to 28 ± 4% (p = 0.01) as compared to the 55 min ischemia alone (4 ± 1%; Kruskal-Wallis multiple comparison between the three groups p = 0.01; Fig. 2D; Table 1). Analysis of the normalized % change values for the three highest flash intensities is summarized in Table 1.
Figure 2.
A. Recovery of the b-wave, and the oscillatory potentials (OPs) 7 days after 55 min of ischemia was significantly enhanced by two methods of ischemic preconditioning (IPC), as compared to the 55 min ischemia group (n = 6), shown at a representative flash intensity of 0.87 log cd.s/m.2 IPC was 8 min of ischemia by elevated intraocular pressure (IOP-IPC; n = 6), or 5 min ligation of the central retinal artery (Ligation-IPC; n = 6), at 24 h prior to ischemia. B. Representative ERG traces. C. Representative OP transformations. RMS = root mean square amplitude of oscillatory potentials.
Table 1.
ERG analysis comparing amplitude of recovery at 7 days after 55 min ischemia with or without one of the two different IPC paradigms at the three highest flash intensities. These results (mean ± s.e.m.) were corrected for day-to-day variation in the normal eye, and were referenced to the baseline in the ischemic eye. IPC 24 h prior to ischemia resulted in significant improvement in recovery of b-wave and OP amplitudes, but not the a-wave.
| a-wave | Normalized % change (0.37 log cd.s/m2) | Normalized % change (0.87 log cd.s/m2) | Normalized % change (1.4 log cd.s/m2) |
|---|---|---|---|
| Ischemia (55 min) (n = 6) | 55 ± 6 | 54 ± 5 | 70 ± 3 |
| Ligation IPC (n = 6) | 71 ± 3 | 73 ± 2 | 70 ± 3 |
| IOP IPC (n = 6) | 85 ± 4 | 82 ± 4 | 81 ± 5 |
| Significant p-values | n/a | n/a | n/a |
| b-wave | Normalized % change (0.37 log cd.s/m2) | Normalized % change (0.87 log cd.s/m2) | Normalized % change (1.4 log cd.s/m2) |
| Ischemia (55 min) (n = 6) | 10 ± 1 | 12 ± 2 | 16 ± 2 |
| Ligation IPC (n = 6) | 39 ± 11 | 41 ± 13 | 45 ± 1 |
| IOP IPC (n = 6) | 43 ± 52 | 47 ± 44 | 52 ± 45 |
| Significant p-values |
10.02 v. ischemia 20.01 v. ischemia |
30.05 v. ischemia 40.01 v. ischemia |
50.01 v. ischemia |
| OP (Summed root mean square amplitude) | Normalized % change (0.37 log cd.s/m2) | Normalized % change (0.87 log cd.s/m2) | Normalized % change (1.4 log cd.s/m2) |
| Ischemia (55 min) (n = 6) | 5 ± 1 | 4 ± 1 | 3 ± 1 |
| Ligation IPC (n = 6) | 25 ± 21 | 24 ± 23 | 26 ± 25 |
| IOP IPC (n = 6) | 30 ± 42 | 28 ± 44 | 27 ± 46 |
| Significant p-values |
10.01 v. ischemia 20.03 v. ischemia |
30.01 v. ischemia 40.01 v. ischemia |
50.01 v. ischemia 60.01 v. ischemia |
Ischemic retinae showed disorganization, cell loss and infiltration of inflammatory cells in the inner retina (Fig. 3), changes completely blunted by prior IPC. Histological examination of the retinas 7 d after ischemia showed that the number of RGCs significantly differed between the 3 groups (Table 2A; Kruskal–Wallis test p= 0.003). The number of RGCs in the ischemic retinae significantly decreased from 11.0 ± 0.5 for IOP IPC + ischemia (p=0.004; n= 6) and 11.1 ± 0.07 for ligation IPC + ischemia (p=0.004; n= 6) to 5.5 ± 0.7 (n=6) in the ischemia only group. The number of cells per unit area (μm2) × 100 for both the INL (Table 2B; Kruskal–Wallis test p= 0.01) and ONL (Table 2C; Kruskal–Wallis test p= 0.003) were significantly decreased with 55 min ischemia as compared to the two IPC paradigms. The number of cells/area in the INL for the ischemic retinae significantly decreased from 2.6 ± 0.1 (p=0.01; n=6) for both IOP IPC + ischemia and for ligation IPC + ischemia to 2.0 ± 0.1 (n=6) in the ischemia only group. The number of cells/area in the ONL for the ischemic retinae significantly decreased from 6.2 ± 0.3 (p=0.004; n=6) for IOP IPC + ischemia and 6.1 ± 0.2 (p=0.004; n=6) for ligation IPC + ischemia to 4.5 ± 0.1 (n=6) in the ischemia only group.
Figure 3.
Representative histopathological images of hematoxylin and eosin-stained retinae in 5 μm thick sections for each of the experimental groups that had 55 min of ischemia. These sections were prepared from retinae removed from the rats at 7 days following ischemia. Arrows indicate layers demonstrating cell loss. Asterisks denote regions of inflammatory cell infiltration (also see Figure 5). Retinal cell layers are labeled in a normal retina in the bottom image. 55 min of ischemia resulted in retinal cell loss and disorganization of the retina, accompanied by inflammatory reaction. IPC or Post-C effectively prevented these changes, but combining IPC and Post-C produced a result resembling ischemia without IPC or Post-C.
Table 2.
| A: Number of retinal ganglion cells in the ischemic retinae 7 days after 55 min ischemia alone compared to the number of retinal ganglion cells in the ischemic retinae for both forms of IPC. Ischemia produced a significant decrease in RGCs. | |||
|---|---|---|---|
| Number of RGCs | p-value vs. Ischemia | p-value vs. IOP IPC | |
| 55 min Ischemia | 5.5 ± 0.7 | --- | |
| 55 min Ischemia + IOP IPC | 11.0 ± 0.5 | 0.004 | --- |
| 55 min Ischemia + Ligation IPC | 11.1 ± 0.7 | 0.004 | 1.00 |
| B: Number of INL cells/area (μm2; × 100) in the ischemic retinae 7 days after 55 min ischemia alone compared to the number of cells/area in the ischemic retinae for both forms of IPC. Ischemia produced a significant decrease in number of cells in the INL/area. | |||
| Number of INL cells/area | p-value vs. Ischemia | p-value vs. IOP IPC | |
| 55 min Ischemia | 2.0 ± 0.1 | --- | |
| 55 min Ischemia + IOP IPC | 2.6 ± 0.1 | 0.01 | --- |
| 55 min Ischemia + Ligation IPC | 2.6 ± 0.1 | 0.01 | 0.87 |
| C: Number of ONL cells/area (μm2; × 100) in the ischemic retinae 7 days after 55 min ischemia alone compared to the number of cells/area in the ischemic retinae for both forms of IPC. Ischemia produced a significant decrease in number of cells in the ONL/area. | |||
| Number of ONL cells/area | p-value vs. Ischemia | p-value vs. IOP IPC | |
| 55 min Ischemia | 4.5 ± 0.1 | --- | |
| 55 min Ischemia + IOP IPC | 6.2 ± 0.3 | 0.004 | --- |
| 55 min Ischemia + Ligation IPC | 6.1 ± 0.2 | 0.004 | 0.57 |
3.3. Post-C and 55 minutes of ischemia
The a-wave recovery was not significantly increased compared to the ischemia only group (Fig. 4A; Table 3). The b-wave recovery was significantly increased about 5-fold to 60 ± 7% (p = 0.008) as compared to the 55 min ischemia group (12 ± 2%; Fig. 4A; Table 3). The OP SUM RMS recovery was significantly increased with ischemia + Post-C about 10-fold to 51 ± 7% as compared to the 55 min ischemia (4 ± 1%; p= 0.03; Fig. 4A; Table 3). The analysis of the normalized % change values for the three highest flash intensities is summarized in Table 3.
Figure 4.
A. Recovery of the b-wave as well as the oscillatory potentials (OPs) 7 d after ischemia was significantly enhanced with Post-C (n = 5) as compared to the 55 min ischemia group (n = 6) at a representative flash intensity of 0.87 log cd.s/m.2 Post-C was 8 min of ischemia applied 5 min after reperfusion following 55 min of ischemia. B. Representative ERG traces. C. Representative OP transformations. RMS = root mean square amplitude of oscillatory potentials.
Table 3.
ERG analysis comparing amplitude of recovery at 7 days after 55 min ischemia and ischemia + post conditioning, at the three highest flash intensities. These results (mean ± s.e.m.) were corrected for day-to-day variation in the normal eye, and were referenced to the baseline in the ischemic eye. Post-conditioning significantly enhanced recovery after ischemia of the amplitudes of the b-wave and OPs, but not the a-wave.
| a-wave | Normalized % change (0.37 log cd.s/m2) | Normalized % change (0.87 log cd.s/m2) | Normalized % change (1.4 log cd.s/m2) |
|---|---|---|---|
| Ischemia (55 min) (n = 6) | 55 ± 6 | 54 ± 5 | 70 ± 3 |
| Post-C (n = 5) | 109 ± 12 | 72 ± 10 | 84 ± 8 |
| Significant p-values | n/a | n/a | n/a |
| b-wave | Normalized % change (0.37 log cd.s/m2) | Normalized % change (0.87 log cd.s/m2) | Normalized % change (1.4 log cd.s/m2) |
| Ischemia (55 min) (n = 6) | 10 ± 1 | 12 ± 2 | 16 ± 2 |
| Post-C (n = 5) | 74 ± 11 | 60 ± 7 | 57 ± 6 |
| Significant p-values | 0.007 | 0.008 | 0.010 |
| OP (Summed root mean square amplitude) | Normalized % change (0.37 log cd.s/m2) | Normalized % change (0.87 log cd.s/m2) | Normalized % change (1.4 log cd.s/m2) |
| Ischemia (55 min) (n = 6) | 5 ± 1 | 4 ± 1 | 3 ± 1 |
| Post-C (n = 5) | 75 ± 7 | 51 ± 7 | 53 ± 7 |
| Significant p-values | 0.01 | 0.03 | 0.03 |
Histological examination of the retinas 7 d after ischemia showed that the number of RGCs in the ischemic retinae significantly decreased from 12.0 ± 0.8 for Post-C + ischemia (p=0.004; n= 6) to 5.5 ± 0.7 (n=6) in the ischemia only group (Table 4A). The number of cells per unit area (μm2) × 100 for the INL was not significantly changed (Table 4B). The number of cells/area in the ONL for the ischemic retinae significantly decreased from 5.5 ± 0.1 (p=0.01; n=6) for Post-C + ischemia to 4.5 ± 0.1 (n=6) in the ischemia only group (Table 4C).
Table 4.
| A: Number of retinal ganglion cells in the ischemic retinae 7 days after 55 min ischemia alone compared to the number of retinal ganglion cells in the ischemic retinae for Post-C. Ischemia produced a significant decrease in RGCs. | ||
|---|---|---|
| Number of RGCs | p-value vs. Ischemia | |
| 55 min Ischemia | 5.5 ± 0.7 | --- |
| 55 min Ischemia + Post-C | 12.0 ± 0.8 | 0.004 |
| B: Number of INL cells/area (μm2; × 100) in the ischemic retinae 7 days after 55 min ischemia alone compared to the number of cells/area in the ischemic retinae for Post-C. Ischemia produced a non-significant decrease in number of cells in the INL/area. | ||
| Number of INL cells/area | p-value vs. Ischemia | |
| 55 min Ischemia | 2.0 ± 0.1 | --- |
| 55 min Ischemia + Post-C | 2.4 ± 0.1 | 0.08 |
| C: Number of ONL cells/area (μm2; × 100) in the ischemic retinae 7 days after 55 min ischemia alone compared to the number of cells/area in the ischemic retinae for Post-C. Ischemia produced a significant decrease in number of cells in the ONL/area. | ||
| Number of ONL cells/area | p-value vs. Ischemia | |
| 55 min Ischemia | 4.5 ± 0.1 | --- |
| 55 min Ischemia + Post-C | 5.5 ± 0.1 | 0.01 |
Ischemia for 55 min provoked inflammatory changes that were visualized 7 d later in immunostained retinal sections (Fig. 5), including increases in monocyte chemotactic protein (MCP-1, 388 ± 72%; n = 3, P < 0.04 vs normals), a trend for increase in macrophage inflammatory protein (MIP-1α, 378 ± 165%; n = 3), but no significant increases in CD45 antigen (77 ± 30%, NS vs normals, specific for leukocytes). Post-C did not significantly alter any of these changes (data not shown).
Figure 5.

Representative images of MCP-1, MIP-1α, and CD45-stained retinal cryosections comparing normal retinae (left column) and ischemic retinae (right column) at 7 days after ischemia. The RGC layer is at the top of each image.
3.4. Effects of IPC and Post-C combined
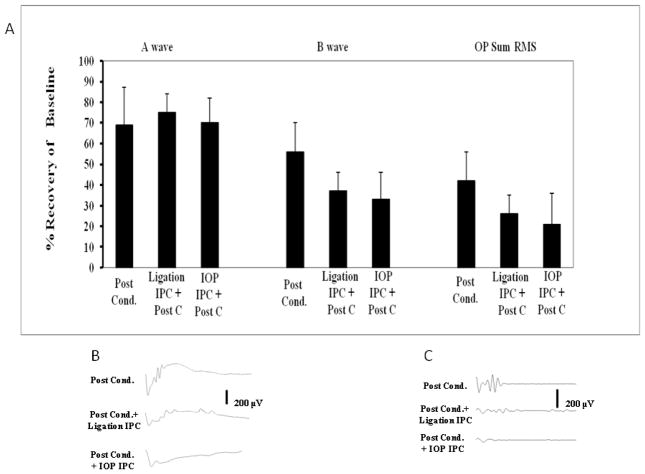
The a-wave recovery at intensity 0.87 log cd.s/m2 was not different between the three groups, 55 min ischemia + Post-C, IOP-IPC + 55 min ischemia + Post-C, and ligation IPC + 55 min ischemia + Post-C (Fig. 6A; Table 5). The b-wave recovery with ligation IPC + Post-C was 39 ± 3%, with IOP IPC + Post-C, 33 ± 7%, as compared to the Post-C group (60 ± 7%; n = 6; but differences were not significant by ANOVA between the three groups, p = 0.43; Fig. 6; Table 5). Recovery of the OP SUM RMS was decreased with ligation IPC + Post-C to 22 ± 3% and decreased with IOP-IPC + Post-C to 21 ± 8% as compared to Post-C (51 ± 7%; Kruskal-Wallis multiple comparison between the three groups not significant, p = 0.38; Fig. 6A; Table 5). Recovery of the waves for the three highest flash intensities is summarized in Table 5.
Figure 6.
A. Combining Post-C and IPC (by ligation method, “Ligation IPC,” n = 8) and by increased IOP, “IOP-IPC, n = 4) did not further enhance recovery of the a- and b-waves or OP at 7 days after 55 min of ischemia, compared to the Post-C group (n = 5) at a representative flash intensity of 0.87 log cd.s/m.2 B. Representative ERG traces. C. Representative OP transformations. RMS = root mean square amplitude of oscillatory potentials.
Table 5.
ERG analysis comparing, at 7 days after ischemia, amplitude of recovery in ischemia + post conditioning and the two different IPC plus post conditioning paradigms with 55 min of ischemia at the three highest flash intensities. These results (mean ± s.e.m.) were corrected for day-to-day variation in the normal eye, and were referenced to the baseline in the ischemic eye. Post-C and IPC were not additive in enhancing recovery after 55 min of ischemia.
| a-wave | Normalized % change (0.37 log cd.s/m2) | Normalized % change (0.87 log cd.s/m2) | Normalized % change (1.4 log cd.s/m2) |
|---|---|---|---|
| Post-C (n = 5) | 109 ± 12 | 72 ± 10 | 84 ± 8 |
| Post-C +Ligation IPC (n = 8) | 90 ± 5 | 78 ± 4 | 81 ± 5 |
| Post-C + IOP IPC (n = 4) | 67 ± 6 | 70 ± 6 | 77 ± 8 |
| Significant p-values | n/a | n/a | n/a |
| b-wave | Normalized % change (0.37 log cd.s/m2) | Normalized % change (0.87 log cd.s/m2) | Normalized % change (1.4 log cd.s/m2) |
| Post-C (n = 5) | 74 ± 11 | 60 ± 7 | 57 ± 6 |
| Post-C +Ligation IPC (n = 8) | 40 ± 3 | 39 ± 3 | 36 ± 3 |
| Post-C + IOP IPC (n = 4) | 28 ± 8 | 33 ± 7 | 37 ± 6 |
| Significant p-values | n/a | n/a | n/a |
| OP (Summed root mean square amplitude) | Normalized % change (0.37 log cd.s/m2) | Normalized % change (0.87 log cd.s/m2) | Normalized % change (1.4 log cd.s/m2) |
| Post-C (n = 5) | 75 ± 7 | 51 ± 7 | 53 ± 7 |
| Post-C +Ligation IPC (n = 8) | 21 ± 4 | 22 ± 3 | 25 ± 4 |
| Post-C + IOP IPC (n = 4) | 27 ± 13 | 21 ± 8 | 21 ± 7 |
| Significant p-values | n/a | n/a | n/a |
Histological examination of the retinas 7 d after ischemia showed that the number of RGCs significantly differed between the 3 groups (Table 6A; Kruskal–Wallis test p= 0.04). The number of RGCs in the ischemic retinae significantly decreased from 12.0 ± 0.8 for Post-C + ischemia to 8.1 ± 0.07 (n=6) for IOP IPC + ischemia + Post-C (p=0.02; n= 4) and 9.3 ± 0.07 for ligation IPC + ischemia + Post-C (p=0.05; n= 6). The number of cells per unit area (μm2) × 100 for the INL (Table 6B; Kruskal–Wallis test p= 0.01) were significantly decreased from 2.4 ± 0.1 (n=6) for Post-C + ischemia to 1.9 ± 0.0 (p=0.01; n=4) for IOP IPC + ischemia + Post-C. The number of cells/area in the INL was not significantly different between Post-C + ischemia and ligation IPC + ischemia + Post-C. Interestingly, the number of cells/area in the INL were significantly different (p=0.01) between IOP IPC + ischemia + Post-C (1.9 ± 0.0; n=4) and ligation IPC + ischemia + Post-C (2.3 ± 0.01; n=6). The number of cells per unit area (μm2) × 100 for the ONL (Table 6C; Kruskal–Wallis test p= 0.28) were not significantly different.
Table 6.
| A: Number of retinal ganglion cells in the ischemic retinae 7 days after Post-C compared to the number of retinal ganglion cells in the ischemic retinae for both forms of IPC + Post-C. IPC + Post-C significantly attenuated the number of RGCs. | |||
|---|---|---|---|
| Number of RGCs | p-value vs. Ischemia + Post-C | p-value vs. IOP IPC + Post-C | |
| 55 min Ischemia + Post-C | 12.0 ± 0.8 | --- | |
| IOP IPC + 55 min Ischemia + Post-C | 8.1 ± 0.7 | 0.02 | --- |
| Ligation IPC + 55 min Ischemia + Post-C | 9.3 ± 0.7 | 0.05 | 0.33 |
| B: Number of INL cells/area (μm2; × 100) in the ischemic retinae 7 days Post-C compared to the number of cells/area in the ischemic retinae for both forms of IPC + Post-C. IPC + Post-C significantly attenuated the number cells in the INL/area. | |||
| Number of INL cells/area | p-value vs. Ischemia + Post-C | p-value vs. IOP IPC + Post-C | |
| 55 min Ischemia + Post-C | 2.4 ± 0.1 | --- | |
| IOP IPC + 55 min Ischemia + Post-C | 1.9 ± 0.0 | 0.01 | --- |
| Ligation IPC + 55 min Ischemia + Post-C | 2.3 ± 0.1 | 0.46 | 0.01 |
| B: Number of ONL cells/area (μm2; × 100) in the ischemic retinae 7 days Post-C compared to the number of cells/area in the ischemic retinae for both forms of IPC + Post-C. There were no significant differences between the groups. | |||
| Number of INL cells/area | p-value vs. Ischemia + Post-C | p-value vs. IOP IPC + Post-C | |
| 55 min Ischemia + Post-C | 5.5 ± 0.1 | --- | |
| IOP IPC + 55 min Ischemia + Post-C | 4.6 ± 0.4 | 0.13 | --- |
| Ligation IPC + 55 min Ischemia + Post-C | 5.4 ± 0.3 | 0.87 | 0.20 |
3.5. Post-C protects against apoptotic cell death
There was a significant difference (Kruskal-Wallis test; p = 0.02) between the three experimental groups studied for the effects of ischemia on apoptotic RGC death (Fig. 7; Table 7). Fluorescent TUNEL analysis of retinae exposed to 55 min of ischemia alone showed that 44.4 ± 10.4% (n = 5) of the RGCs were apoptotic. Post-C reduced TUNEL significantly to 10.1 ± 5.4% (p = 0.02 vs. ischemia; n = 5). The combination of ligation IPC and post-C resulted in a significant increase in apoptotic RGCs to 45.5 ± 10.6% (n = 5) compared to the Post-C group (p = 0.03), to a level no different than the ischemia group (p = 0.47). TUNEL-positive cells co-localized with Thy1-staining (Fig. 7B), indicating apoptotic cell death in RGCs.
Figure 7.
A. Representative fluorescent images of TUNEL in the retina at 24 h after 55 min of ischemia. Using false colors to enhance readability, red is fluorescent TUNEL, and green is DAPI. Arrows denote double-labeled (yellow) TUNEL positive retinal ganglion cells (RGC). There are numerous TUNEL cells with 55 min of ischemia, an effect significantly blunted by Post-C. IPC prior to ischemia together with Post-C following ischemia, increased TUNEL. B. Representative image of TUNEL-positive cells (green) co-localizing with Thy1 staining (red) for the 55 min of ischemia group, indicating the presence of TUNEL in retinal ganglion cells.
Table 7.
Percentage (± s.e.m.) of TUNEL cells in the retinal ganglion cell layer in ischemic retinae 24 h after 55 min of ischemia (n = 5 for each group). Cell nuclei stained green (DAPI), and TUNEL was red. Percent double labeling (yellow) cells was determined relative to the total cell population (green). See Fig. 6 for details. The increase in TUNEL with ischemia was attenuated when Post-C was added 5 min after ischemia ended. Adding prior IPC restored the level of TUNEL to that with ischemia without Post-C.
| % TUNEL cells | p-value vs. Ischemia | p-value vs. Post C | |
|---|---|---|---|
| 55 min Ischemia | 44.4 ± 10.4 | --- | |
| 55 min Ischemia + Post-C | 10.1 ± 5.4 | 0.02 | --- |
| Ligation IPC + 55 min Ischemia + Post-C | 45.5 ± 10.6 | 0.47 | 0.03 |
DISCUSSION
Post-conditioning (Post-C) was functionally and histologically protective in the rat retina following a lengthy ischemic event, and moreover, we showed for the first time that it prevented apoptosis in retinal ganglion cells after ischemia. Inflammatory response after ischemia, characterized by increased retinal inflammation of macrophages and monocytes, was not altered by Post-C. The study showed extensive inflammation after ischemia. This was greater than in previous studies and is not likely due to infection since sterile techniques were used. This may be related to species differences. Functionally, the impact of Post-C to enhance recovery after ischemia was greater than that for IPC, while neither completely protected against ischemic damage under the conditions of this study. Accordingly, we hypothesized that combining ischemic preconditioning with Post-C would be additive in producing a greater degree of recovery after ischemia. In fact, refuting this hypothesis, IPC and Post-C were not additive in enhancing post-ischemic recovery or preventing RGC loss, and when combined, their anti-apoptotic effects were lost. Post-C afforded a greater functionally protective effect on recovery after a longer duration of ischemic insult (55 min) compared to a shorter time (45 min), although histologically, Post-C attenuated loss of RGCs after ischemia in both instances.
It has been recently shown that Post-C was protective in the rat retina when instituted within 60 min after the end of ischemia (Fernandez et al. 2009). Their study revealed that Post-C was effective following both 40 and 60 min of ischemia. A single Post-C stimulus administered for 7 min or 3 to 10-one min pulses of Post-C were protective. Our results differ in that we found that Post-C was more effective in enhancing recovery when instituted after the more severe, longer duration of ischemia (55 vs. 45 min).
Only the functional recovery after ischemia as reflected by the OPs, but not a- and b-waves, was improved with Post-C when ischemia lasted for 45 min. Oscillatory potentials are believed to reflect function of the amacrine cells in the rat retina, and amacrine cells are among the most sensitive of retinal cells to ischemic injury. However, there are differential effects of ischemia on selected amacrine cell populations. It may be that Post-C is able to provide neuroprotection in briefer periods of ischemia only in the most sensitive neuronal cells. That Post-C enhances recovery of retinal function to a greater extent when instituted after more severe or longer duration ischemia suggests that Post-C further accentuates an already present endogenous retinal response to ischemic injury.
We tested the hypothesis that combining IPC (using two different methods) with Post-C would result in greater ischemic tolerance. But we found that the combination was no better than Post-C alone in providing functional protection. Moreover, there was enhanced ischemia-induced RGC loss, and antagonism of the anti-apoptosis effect of Post-C. Possible explanations include that IPC altered the post-ischemic environment that Post-C requires for its neuroprotective effect, or, conversely, Post-C may alter pathways that IPC activated or suppressed earlier. These last two explanations are consistent with the paradoxical activation by IPC of pathways such as oxygen free radicals, and mitogen activated protein kinase p38 (Roth et al. 2003; Dreixler et al. 2009a; Dreixler et al. 2009b; Dreixler et al. 2009c) that have previously been noted to be deleterious after ischemia. In comparing the combination of IPC + ischemia + Post-C to ischemia + Post-C, RGC loss differed between the groups while function did not. It may be that higher variability in the b-wave recovery prevented a statistical determination of a difference, but the histological results showed clear worsening, and support the notion that IPC and Post-C are not additive in producing neuroprotection from ischemia.
The clinical implications of post conditioning have enormous potential for clinical translation. Recent studies in mammalian heart and brain (Mykytenko et al. 2008; Nishino et al. 2008; Wang et al. 2008; Xing et al. 2008; Leconte et al. 2009; Pignataro et al. 2009) as well as this study, and others in the retina (Fernandez et al. 2009) have shown that an easily applied transient ischemic stimulus can be administered after an ischemic event. Clinical benefit of protection via pre-conditioning has obvious temporal limitations, but studies to examine means to prolong the effective time window of Post-C would be worthwhile.
Results of this current study confirm that ischemic post-conditioning is a powerful protective tool against ischemic damage in the rat retina. Moreover, our novel finding was that the combination of both pre- and post-conditioning was not additive and that either IPC attenuated the protection afforded by Post-C or Post-C attenuated the protection afforded by IPC. Further experimentation is underway to examine the possible protective pathways involved in the mechanism of post-conditioning.
Acknowledgments
This research was supported by National Institutes of Health (Bethesda, MD) grants EY10343 (SR), EY10343-15S1 (SR, American Recovery and Reinvestment Act), grant UL1RR024999 to the University of Chicago Institute for Translational Medicine, and by a grant-in-aid (SR) from the Illinois Society for the Prevention of Blindness (Chicago, IL). Immunostained images were generated at the Microscopy Core Facility, supported by the University of Chicago Cancer Research Center and the Digestive Diseases Research Core.
Footnotes
There is no proprietary interest for any of the authors.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bui BV, Edmunds B, Cioffi GA, Fortune B. The gradient of retinal functional changes during acute intraocular pressure elevation. Invest Ophthalmol Vis Sci. 2005;46:202–213. doi: 10.1167/iovs.04-0421. [DOI] [PubMed] [Google Scholar]
- Darling CE, Jiang R, Maynard M, Whittaker P, Vinten-Johansen J, Przyklenk K. Postconditioning via stuttering reperfusion limits myocardial infarct size in rabbit hearts: role of ERK1/2. Am J Physiol. 2005;289:H1618–1626. doi: 10.1152/ajpheart.00055.2005. [DOI] [PubMed] [Google Scholar]
- Dreixler JC, Barone FC, Shaikh AR, Du E, Roth S. Mitogen-activated protein kinase p38alpha and retinal ischemic preconditioning. Exp Eye Res. 2009a;89:782–790. doi: 10.1016/j.exer.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreixler JC, Hagevik S, Hemmert JW, Shaikh AR, Rosenbaum DM, Roth S. Involvement of erythropoietin in retinal ischemic preconditioning. Anesthesiology. 2009b;110:774–780. doi: 10.1097/ALN.0b013e31819c4601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreixler JC, Hemmert JW, Shenoy SK, Shen Y, Lee HT, Shaikh AR, Rosenbaum DM, Roth S. The role of Akt/protein kinase B subtypes in retinal ischemic preconditioning. Exp Eye Res. 2009c;88:512–521. doi: 10.1016/j.exer.2008.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreixler JC, Shaikh AR, Shenoy SK, Shen Y, Roth S. Protein kinase C subtypes and retinal ischemic preconditioning. Exp Eye Res. 2008;87:300–311. doi: 10.1016/j.exer.2008.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettaiche M, Heurteaux C, Blondeau N, Borsotto M, Tinel N, Lazdunski M. ATP-sensitive potassium channels (K(ATP)) in retina: a key role for delayed ischemic tolerance. Brain Res. 2001;890:118–129. doi: 10.1016/s0006-8993(00)03152-8. [DOI] [PubMed] [Google Scholar]
- Faberowski N, Stefansson E, Davidson RC. Local hypothermia protects the retina from ischemia. A quantitative study in the rat. Invest Ophthalmol Vis Sci. 1989;30:2309–2313. [PubMed] [Google Scholar]
- Fernandez DC, Bordone MP, Chianelli MS, Rosenstein RE. Retinal neuroprotection against ischemia-reperfusion damage induced by postconditioning. Invest Ophthalmol Vis Sci. 2009;50:3922–3930. doi: 10.1167/iovs.08-3344. [DOI] [PubMed] [Google Scholar]
- Franco PJ, Fernandez DC, Sande PH, Keller Sarmiento MI, Chianelli M, Saenz DA, Rosenstein RE. Effect of bacterial lipopolysaccharide on ischemic damage in the rat retina. Invest Ophthalmol Vis Sci. 2008;49:4604–4612. doi: 10.1167/iovs.08-2054. [DOI] [PubMed] [Google Scholar]
- Jo N, Wu G-S, Rao NA. Upregulation of chemokine expression in the retinal vasculature in ischemia-reperfusion injury. Invest Ophthalmol Vis Sci. 2003;44:4054–4060. doi: 10.1167/iovs.02-1308. [DOI] [PubMed] [Google Scholar]
- Junk AK, Mammis A, Savitz SI, Singh M, Roth S, Malhotra S, Rosenbaum PS, Cerami A, Brines M, Rosenbaum DM. Erythropoietin administration protects retinal neurons from acute ischemia-reperfusion injury. Proc Natl Acad Sci USA. 2002;99:10659–10664. doi: 10.1073/pnas.152321399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamphuis W, Dijk F, Bergen AAB. Ischemic preconditioning alters the pattern of gene expression changes in response to full retinal ischemia. Mol Vis. 2007a;13:1892–1901. [PubMed] [Google Scholar]
- Kamphuis W, Dijk F, van Soest S, Bergen AA. Global gene expression profiling of ischemic preconditioning in the rat retina. Mol Vis. 2007b;13:1020–1030. [PMC free article] [PubMed] [Google Scholar]
- Leconte C, Tixier E, Freret T, Toutain J, Saulnier R, Boulouard M, Roussel S, Schumann-Bard P, Bernaudin M. Delayed hypoxic postconditioning protects against cerebral ischemia in the mouse. Stroke. 2009;40:3349–3355. doi: 10.1161/STROKEAHA.109.557314. [DOI] [PubMed] [Google Scholar]
- Li B, Roth S. Retinal ischemic preconditioning in the rat: requirement for adenosine and repetitive induction. Invest Ophthalmol Vis Sci. 1999;40:1200–1216. [PubMed] [Google Scholar]
- Manintveld OC, Te Lintel Hekkert M, van den Bos EJ, Suurenbroek GM, Dekkers DH, Verdouw PD, Lamers JM, Duncker DJ. Cardiac effects of postconditioning depend critically on the duration of index ischemia. Am J Physiol. 2007;292:H1551–1560. doi: 10.1152/ajpheart.00151.2006. [DOI] [PubMed] [Google Scholar]
- McCulloch J, Dewar D. A radical approach to stroke therapy. Proc Natl Acad Sci U S A. 2001;98:10989–10991. doi: 10.1073/pnas.211430898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mykytenko J, Reeves JG, Kin H, Wang N-P, Zatta AJ, Jiang R, Guyton RA, Vinten-Johansen J, Zhao Z-Q. Persistent beneficial effect of postconditioning against infarct size: role of mitochondrial K(ATP) channels during reperfusion. Basic Res Cardiol. 2008;103:472–484. doi: 10.1007/s00395-008-0731-2. [DOI] [PubMed] [Google Scholar]
- Nishino Y, Webb IG, Davidson SM, Ahmed AI, Clark JE, Jacquet S, Shah AM, Miura T, Yellon DM, Avkiran M, Marber MS. Glycogen synthase kinase-3 inactivation is not required for ischemic preconditioning or postconditioning in the mouse. Circ Res. 2008;103:307–314. doi: 10.1161/CIRCRESAHA.107.169953. [DOI] [PubMed] [Google Scholar]
- Nonaka A, Kiryu J, Tsujikawa A, Yamashiro K, Nishijima K, Miyamoto K, Nishiwaki H, Honda Y, Ogura Y. Inhibitory effect of ischemic preconditioning on leukocyte participation in retinal ischemia-reperfusion injury. Invest Ophthalmol Vis Sci. 2001;42:2380–2385. [PubMed] [Google Scholar]
- Pignataro G, Scorziello A, Di Renzo G, Annunziato L. Post-ischemic brain damage: effect of ischemic preconditioning and postconditioning and identification of potential candidates for stroke therapy. FEBS J. 2009;276:46–57. doi: 10.1111/j.1742-4658.2008.06769.x. [DOI] [PubMed] [Google Scholar]
- Rosenbaum DM, Rosenbaum PS, Singh M, Gupta G, Gupta H, Li B, Roth S. Functional and morphological comparison of two models to produce transient retinal ischemia in the rat. J Neuro-ophthalmol. 2001;21:62–68. doi: 10.1097/00041327-200103000-00015. [DOI] [PubMed] [Google Scholar]
- Roth S, Dreixler JC, Shaikh AR, Lee KH, Bindokas V. Mitochondrial potassium ATP channels and retinal ischemic preconditioning. Invest Ophthalmol Vis Sci. 2006;47:2114–2124. doi: 10.1167/iovs.05-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth S, Li B, Rosenbaum PS, Gupta H, Goldstein IM, Maxwell KM, Gidday JM. Preconditioning provides complete protection against retinal ischemic injury in rats. Invest Ophthalmol Vis Sci. 1998;39:775–785. [PubMed] [Google Scholar]
- Roth S, Shaikh AR, Hennelly MM, Li Q, Bindokas V, Graham CE. Mitogen-activated protein kinases and retinal ischemia. Invest Ophthalmol Vis Sci. 2003;44:5383–5395. doi: 10.1167/iovs.03-0451. [DOI] [PubMed] [Google Scholar]
- Sakamoto K, Kuwagata M, Nakahara T, Ishii K. Late preconditioning in rat retina: Involvement of adenosine and ATP-sensitive K+ channel. Eur J Pharmacol. 2001;418:89–93. doi: 10.1016/s0014-2999(01)00938-4. [DOI] [PubMed] [Google Scholar]
- Singh M, Savitz SI, Hoque R, Rosenbaum PS, Roth S, Rosenbaum DM. Cell-specific caspase expression by different neuronal phenotypes in transient retinal ischemia. J Neurochem. 2001;77:466–475. doi: 10.1046/j.1471-4159.2001.00258.x. [DOI] [PubMed] [Google Scholar]
- Tang X-L, Sato H, Tiwari S, Dawn B, Bi Q, Li Q, Shirk G, Bolli R. Cardioprotection by postconditioning in conscious rats is limited to coronary occlusions <45 min. Am J Physiol. 2006;291:H2308–2317. doi: 10.1152/ajpheart.00479.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiersch M, Raffelsberger W, Frigg E, Samardzija M, Blank P, Poch O, Grimm C. The hypoxic transcriptome of the retina: identification of factors with potential neuroprotective activity. Adv Exp Med Biol. 2008;613:75–85. doi: 10.1007/978-0-387-74904-4_8. [DOI] [PubMed] [Google Scholar]
- Toprak AB, Ozbilgin K, Toprak V, Tuglu I, Guler C. A histological analysis of the protective effect of ischemic preconditioning in the rat retina. Curr Eye Res. 2002;24:234–239. doi: 10.1076/ceyr.24.3.234.8308. [DOI] [PubMed] [Google Scholar]
- Wang J-y, Shen J, Gao Q, Ye Z-g, Yang S-y, Liang H-w, Bruce IC, Luo B-y, Xia Q. Ischemic postconditioning protects against global cerebral ischemia/reperfusion-induced injury in rats. Stroke. 2008;39:983–990. doi: 10.1161/STROKEAHA.107.499079. [DOI] [PubMed] [Google Scholar]
- Wegener S, Gottschalk B, Jovanovic V, Knab R, Fiebach JB, Schellinger PD, Kucinski T, Jungehulsing GJ, Brunecker P, Muller B, Banasik A, Amberger N, Wernecke KD, Siebler M, Rother J, Villringer A, Weih M. Transient ischemic attacks before ischemic stroke: preconditioning the human brain? A multicenter magnetic resonance imaging study. Stroke. 2004;35:616–621. doi: 10.1161/01.STR.0000115767.17923.6A. [DOI] [PubMed] [Google Scholar]
- Xing B, Chen H, Zhang M, Zhao D, Jiang R, Liu X, Zhang S. Ischemic postconditioning inhibits apoptosis after focal cerebral ischemia/reperfusion injury in the rat. Stroke. 2008;39:2362–2369. doi: 10.1161/STROKEAHA.107.507939. [DOI] [PubMed] [Google Scholar]
- Zhang C, Rosenbaum DM, Shaikh AR, QL, Rosenbaum PS, Pelham DJ, Roth S. Ischemic preconditioning attenuates apoptosis following retinal ischemia in rats. Invest Ophthalmol Vis Sci. 2002;43:3059–3066. [PubMed] [Google Scholar]
- Zhu Y, Zhang L, Gidday JM. Deferroxamine preconditioning promotes long-lasting retinal ischemic tolerance. J Ocul Pharmacol Ther. 2008;24:527–535. doi: 10.1089/jop.2008.0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Zhang Y, Ojwang BA, Brantley MA, Jr, Gidday JM. Long-term tolerance to retinal ischemia by repetitive hypoxic preconditioning: role of HIF-1alpha and heme oxygenase-1. Invest Ophthalmol Vis Sci. 2007;48:1735–1743. doi: 10.1167/iovs.06-1037. [DOI] [PubMed] [Google Scholar]