Abstract
BACKGROUND
Increased dietary sodium has been reported to increase cardiovascular disease (CVD) risk, perhaps through blood pressure (BP) independent vascular remodeling. Carotid intima-media thickness (IMT) is an accepted measure of structural vascular remodeling and a strong predictor of CVD. This study aimed to determine whether urinary sodium is positively associated with carotid IMT in normotensive overweight and obese adults.
METHODS
We evaluated baseline data from 258 participants in the Slow Adverse Vascular Effects (SAVE) Clinical Trial. Urinary sodium was measured from one 24-hour urine collection from each individual. Carotid IMT was measured using high resolution B-mode ultrasonography. Participants were categorized into quartiles of urinary sodium.
RESULTS
There was a significant positive trend with greater IMT associated with increasing urinary sodium quartile in univariate linear regression (P=0.047). This trend was significant when adjusting for age, sex, race, and systolic BP (SBP) (P=0.03) as well as in a fully adjusted model (P=0.04). In pairwise comparisons, the highest urinary sodium quartile had a significantly greater mean IMT (0.62 mm) than the lowest urinary sodium quartile (0.59 mm) after adjustment for age, sex, race, and SBP (P=0.04). This comparison lost significance after the addition of BMI.
CONCLUSIONS
In our community-based sample of normotensive overweight and obese adults, we observed a significant positive trend in carotid IMT with increasing quartile of urinary sodium. If the ongoing clinical trial confirms this relationship between sodium and carotid IMT, it would lend support to efforts to decrease sodium intake in overweight and obese individuals.
Keywords: sodium, obesity, vascular remodeling, carotid IMT
INTRODUCTION
Daily consumption of sodium in most of the western world far exceeds what is necessary. It has been estimated that our current salt consumption is as much as 50 times greater than our evolutionary intake.1 A positive relationship between sodium intake and blood pressure (BP) has been documented in hypertensive and normotensive individuals.2,3 In addition, sodium intake has been suggested to be a risk factor for cardiovascular disease (CVD)3-8 and all cause mortality4, perhaps independently of its effect on BP.6 However, there remains much debate about the usefulness of a population wide reduction in salt intake.7 It appears that subsets of the population, namely African-American, elderly, obese, and hypertensive individuals, have increased sodium sensitivity.7
The potential BP-independent increased CVD risk under a high salt diet may be related to cardiovascular functional and structural changes, through alterations in shear stress and endothelial function.8-10 Such alterations can cause vascular hypertrophy and collagen accumulation10, which increase carotid intima-media thickness (IMT). Carotid IMT is an accepted subclinical atherosclerotic marker and a strong predictor of future clinical cardiovascular events.11 A relationship between urinary sodium and IMT would shed light on the structural response of the carotid wall to excess dietary sodium. Because many mechanisms linking sodium to CVD are potentially BP-independent, it stands to reason that an association between sodium and carotid IMT may be observed in normotensive individuals. The current study population consists of participants in the Slow Adverse Vascular Effects (SAVE) clinical trial, a trial evaluating the relationships between weight loss, dietary sodium, and vascular health. Because all participants are normotensive, we are able to investigate the association between sodium intake and arterial structure independent of BP or BP treatment.
This population also provides a unique opportunity to test the association between sodium intake and IMT in overweight and obese adults, who may be more salt sensitive than lean individuals.7 The purpose of this analysis was to test the hypothesis that sodium intake is positively associated with carotid IMT in normotensive overweight and obese adults.
METHODS
Study Population
This is a cross-sectional analysis of baseline data from participants in the SAVE Study (NCT00366990), a randomized controlled trial assessing the impacts of weight loss, increased physical activity, and reduced sodium intake on vascular health. Moderately overweight or obese (body mass index (BMI) 25-39.9kg/m2) men and women (n=349) aged 20-45 years were recruited from Allegheny County, Pennsylvania. Participants were required to be physically inactive, defined as exercising for <8 months during the past 12 months and for <3 hours a week on average. Participants were excluded if they 1) had diabetes (fasting glucose ≥ 126 mg/dl); 2) were being treated for hypertension or had an average screening and baseline SBP of ≥140 or diastolic blood pressure (DBP) ≥ 90 mmHg; 3) were on cholesterol lowering, anti-psychotic, or vasoactive medications, or using vasoactive devices; 4) were pregnant or breast feeding.
For the current analysis, only those with valid baseline 24-hour urine collections (n=258) were included. All subjects signed informed consent, and the study design was approved by the institutional review board of the University of Pittsburgh (Pittsburgh, PA).
Design and Procedures
All randomized participants completed screening and baseline visits that included self-reported demographic information, self- and interviewer-administered questionnaires, anthropometric measurements, fasting blood draw, 24-hour urine collection, and vascular ultrasound tests.
Carotid Ultrasound
Carotid ultrasound measures and readings were performed at the Ultrasound Research Laboratory of the Department of Epidemiology, University of Pittsburgh, by sonographers using an Acuson Sonoline Antares high resolution duplex scanner (Siemens, Malvern, PA). At baseline, digitized images were obtained from 8 locations (4 locations each from the left and right carotid arteries): the near and far walls of the distal common carotid artery (1 cm proximal to the carotid bulb), the far walls of the carotid bulb (the point in which the near and far walls of the common carotid are no longer parallel, extending to the flow divider), and the internal carotid artery (from the flow divider to 1 cm distal to this point). IMT measures were obtained by electronically tracing the lumen-intima interface and the media-adventitia interface across a 1-cm segment for each of the 8 segments; one measurement was generated for each pixel over the area, for a total of approximately 140 measurements for each segment. The mean of the average readings at all 8 locations was used. The reading software used was the AMS system developed by Dr. Thomas Gustavsson,12 which has an edge detection algorithm that allowed much of the reading to be done automatically. Reproducibility of IMT was excellent with an intraclass correlation coefficient of ≥0.87 between sonographers and ≥0.92 between readers.
24-Hour Urine Collection (Sodium Intake)
Valid 24-hour urine collections had volume between 900 mL and 4000 mL, duration ≥22 hours and ≤26 hours, and total creatinine within the expected range.13 Until March 6, 2009, analytes were measured using an Ortho Vitros 950. Direct potentiometry was used to measure sodium and colorimetry to assess creatinine levels. Afterward, results were determined using a Beckman Coulter DxC 800 instrument employing an indirect ion selective method for sodium and an alkaline picric kinetic method for creatinine.
Blood Assays
All blood assays were performed on fasting blood specimens at the Heinz Laboratory at the University of Pittsburgh’s Graduate School of Public Health.
Total cholesterol (TC) and high density lipoprotein cholesterol (HDL-C) were determined using the enzymatic method of Allain et al.14 HDL-C was determined after selective precipitation by heparin/manganese chloride and removal by centrifugation of very low density lipoprotein and low density lipoprotein cholesterol (LDL-C).15 LDL-C was calculated indirectly using the Friedewald equation. Triglycerides were assessed enzymatically using the procedure of Bucolo et al.16
Serum glucose was determined enzymatically with a procedure similar to that described by Bondar and Mead.17 Insulin was measured using a radioimmunoassay developed by Linco Research, Inc. (St. Charles, MO). Insulin Sensitivity was calculated using the homeostasis model assessment of insulin resistance index (HOMA) derived from fasting insulin and glucose values.18 HOMA (mmol/L × μU/ml) = fasting glucose (mmol/L) × fasting insulin (μU/ml)/22.5.
Leptin, adiponectin, and ghrelin were measured using a radioimmunoassay kit from Linco Research, Inc. Total ghrelin was measured since acylated ghrelin is difficult to measure reliably. Aldosterone was measured using an enzyme-linked immunoassay developed by Diagnostic Systems Laboratories, Inc. (Webster, Texas). C-reactive protein (CRP) was measured using an enzyme-linked immunoassay developed by Alpha Diagnostic International, Inc. (San Antonio, Texas).
Demographic and Physical Measures
Age, race, and smoking status were self-reported at the screening visit. The interviewer-administered Modifiable Activity Questionnaire (MAQ) assessed average physical activity over the past year, expressed as metabolic equivalent tasks (MET)-hours per week. Weight was measured in kilograms using a standard balance scale. Height was measured in centimeters using a calibrated stadiometer. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. Waist circumference was measured in centimeters, against the participant’s skin at the narrowest part of the torso between the ribs and the iliac crest using a non-stretch tape measure. Two measures were performed, and if the second measurement was within 2 centimeters of the first, the mean of the two was used. Otherwise a third measurement was taken and the two closest measurements were averaged.
BP was measured after participants sat quietly for 5 minutes with feet flat on the floor. A standard protocol with a mercury sphygmometer was used. The average of the last 2 of 3 BP measurements taken 30 seconds apart was used.
Statistical Analysis
Descriptive statistics were performed to summarize study variables by urinary sodium quartiles. The statistical differences between urinary sodium quartiles in study variables were assessed using analysis of variance (ANOVA) or χ2 tests as appropriate. Data were presented as median/inter-quartile (IQR) range or mean (SD) for continuous variables, and frequency and percentages for categorical variables.
Covariates of interest were factors known to be associated with either dietary sodium or carotid IMT. Non-normally distributed variables were modeled as continuous variables with necessary transformations to achieve normality. Univariate linear regression was used to determine associations between covariates of interest and study outcome (IMT). An initial multivariate model included age, sex, race (black, non-black), and SBP in order to determine the association between sodium and IMT independent of BP and known demographic factors affecting IMT. Aldosterone and any factors associated with IMT and/or urinary sodium quartile at P<0.20 were then added to the model, and linear trends in mean IMT across quartiles of urinary sodium were examined. Analysis of covariance (ANCOVA) was used to evaluate mean IMT differences between Q1 and other quartiles after adjustment for covariates. Dunnett’s method was used to adjust for multiple pairwise comparisons.
Additional validation analyses were performed. We investigated the association between sodium/creatinine ratio and IMT in the full sample to discern any bias that resulted from excluding participants with invalid urine collections. Sodium/creatinine ratio was evaluated in sex-specific quartiles because of the known sex differences in creatinine excretion.19 Next, all regression analyses were repeated with urinary sodium as a continuous variable. Finally, the regression analyses were repeated using sex-specific sodium quartiles.
Interactions between urinary sodium and age, sex, race, SBP, and BMI were evaluated. Values of P<0.05 were considered statistically significant. Statistical analyses were performed using SAS (Statistical Analysis Software release 9.2, Cary, NC).
RESULTS
258 participants had valid urine collections and were eligible for analysis. The demographic and clinical characteristics of these participants are presented in Table 1. The mean age of the sample was 38.5 (SD 5.8) years. 78.7% of the sample was female (n=203), and 13.2% was black (n=34). 89 (34.5%) individuals had metabolic syndrome, as defined by the AHA/NHLBI criterion.20 The group excluded for invalid urine collection (n=91) had significantly more blacks, fewer smokers, higher fasting glucose, insulin, and HOMA. Sodium/creatinine ratio was similar between the two groups (P=0.13).
Table 1.
Baseline Characteristics by Urinary Sodium Quartile*
| Characteristic | Total (n=258) | Q1 (n=65) | Q2 (n=64) | Q3 (n=64) | Q4 (n=65) | P** |
|---|---|---|---|---|---|---|
|
Range of Urinary Sodium (mEq/24hr) |
48-415 | 48-143 | 144-179 | 180-224 | 225-415 | |
| Age (years) | 38.5 (5.8) | 39.3 (5.6) | 38.7 (5.2) | 37.6 (6.2) | 38.2 (6.0) | 0.40 |
| Women (n, %) | 203 (78.7) | 55 (84.6) | 53 (82.8) | 52 (81.3) | 43 (66.2) | 0.01 |
| Black Race (n, %) | 34 (13.2) | 7 (10.8) | 7 (10.9) | 8 (12.5) | 12 (18.5) | 0.19 |
| Ever Smokers (n, %) | 107 (41.5) | 26 (40.0) | 30 (46.9) | 26 (40.6) | 25 (38.5) | 0.69 |
| BMI (kg/m2) | 32.8 (29.6,35.7) | 30.9 (28.8,35) | 33.1 (29.5,35.7) | 32.6 (29,35.4) | 34.4 (30.8,36.8) | 0.03 |
| Weight (kg) | 92.2 (13.5) | 87.8 (11.5) | 92.4 (12.9) | 90.7 (11.6) | 97.9 (15.6) | 0.0002 |
| Waist Circumference (cm) | 100.2 (10.5) | 97.6 (10.0) | 99.5 (10.8) | 100.0 (9.1) | 103.8 (11.3) | 0.008 |
| SBP (mmHg) | 113.0 (10.2) | 112 (10) | 111 (10) | 113 (10) | 114 (10) | 0.63 |
| DBP (mmHg) | 72.7 (8.6) | 71.9 (8.0) | 72.7 (9.6) | 72.3 (8.8) | 73.8 (70.9) | 0.62 |
| Glucose (mg/dL) | 97.2 (7.7) | 96.4 (7.8) | 98.3 (7.8) | 96.8 (7.8) | 97.2 (7.8) | 0.52 |
| Insulin (μU/mL) | 12.2 (9.4,16.7) | 12.5 (8.8,15.2) | 12.4 (10,18.2) | 12.1 (9.6,16.7) | 12.2 (9.3,16.3) | 0.49 |
| HOMA (mmol/L x μU/mL) | 2.9 (2.2,4.2) | 2.8 (2,3.7) | 3.0 (2.3,4.3) | 2.8 (2.3,4) | 2.9 (2.1,4.1) | 0.40 |
| Total Cholesterol (mg/dL) | 202.0 (177.0,225.0) | 198 (178,218) | 215 (186,241) | 199.5 (176.5,219.5) | 197 (166,223) | 0.02 |
| LDL-C (mg/dL) | 123.0 (100.0,144.0) | 122 (100,142) | 126.5 (107.5,149.5) | 120.5 (107,147) | 120 (90,142) | 0.04 |
| HDL-C (mg/dL) | 51.0 (43.4,60.4) | 50.9 (43.8,58) | 50.6 (44.1,60.8) | 50.5 (43.3,60.8) | 51.6 (42.5,59.3) | 0.96 |
| Triglycerides (mg/dL) | 117 (79,172) | 105 (73,162) | 154 (85,203) | 111 (82,153) | 120 (80,169) | 0.05 |
| CRP (mg/dL) | 2.57 (1.33,5.59) | 2.04 (1.11,3.58) | 3.06 (1.75,7.41) | 3.28 (1.56,5.71) | 2.59 (1.33,5.38) | 0.08 |
| Leptin (ng/mL) | 24.9 (16.6,34.5) | 24.4 (17.5,31.6) | 24.6 (17.9,32) | 26.9 (17.1,37.7) | 24.4 (14.4,34.5) | 0.65 |
| Ghrelin (pg/mL) | 681.5 (545.0,895.5) | 692 (532,1024) | 744 (547,899) | 650 (547,910) | 666.5 (525,825) | 0.46 |
| Adiponectin (μg/mL) | 10.5 (7.8,14.6) | 10.1 (8.3,14.7) | 10.8 (7.7,14) | 10.5 (7.2,14.2) | 11.3 (7.4,15) | 0.97 |
| Aldosterone (pg/mL) | 107.0 (79.1,156.5) | 108 (77,156) | 110 (87,161) | 104 (87,156) | 108 (77,152) | 0.99 |
| Total Physical Activity (MET-hr/wk) |
29.5 (8.0, 92.0) | 38.2 (7.7,92.0) | 26.8 (9.0,82.9) | 20.2 (6.8, 101.9) | 36.6 (10.7, 98.9) | 0.97 |
| Mean IMT (mm) | 0.60 (0.55,0.66) | 0.59 (0.57,0.62) | 0.61 (0.59,0.63) | 0.60 (0.58,0.62) | 0.63 (0.61,0.65) | 0.11 |
Quartiles (Q1-Q4) of urinary sodium and mean (SD) or median (IQR)
P values from ANOVA or χ test after necessary transformations
SBP=systolic blood pressure, DBP=diastolic blood pressure, LDL-C=low density lipoprotein cholesterol, HDL-C=high density lipoprotein cholesterol, HOMA=homeostasis model assessment of insulin resistance, CRP= C reactive protein
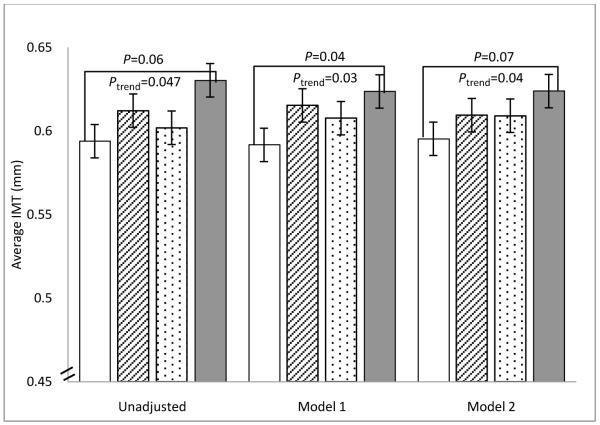
Univariate analysis revealed significant positive associations between urinary sodium quartile and male sex, BMI, weight, waist circumference, TC, and LDL-C (Table 1). In univariate linear regression, age, sex, weight, BMI, waist circumference, SBP, DBP, glucose, HOMA, LDL-C, TC, triglycerides, and adiponectin were associated with carotid IMT (Table 2). We considered three linear regression models: unadjusted, adjusted for age, sex, race, and SBP (Model 1), and a fully adjusted model, which added BMI, aldosterone, LDL-C, triglycerides, HOMA, CRP, adiponectin, and smoking (Model 2). Weight and waist circumference were not included due to multicollinearity. In each model, the increase in IMT with increasing sodium quartile was largest between Q3 and Q4 (Figure 1). Though unadjusted IMT did not significantly differ between the urinary sodium quartiles (P=0.11), a pairwise comparison between Q1 and Q4 was significant due to the increased power of this planned comparison relative to ANOVA. Pairwise comparisons with adjustment for age, sex, race, and SBP (Model 1), revealed that Q4 had a significantly greater IMT than Q1 (P=0.04). In the fully adjusted model (Model 2), this pairwise comparison lost significance. By adding the additional covariates individually to Model 1, BMI proved to be the factor that eliminated the significance of the pairwise comparison. However, there was a significant positive linear trend in IMT with increasing urinary sodium quartile, which was preserved in all three models (Figure 1 and Table 3).
Table 2.
Univariate Associations of Covariates with Carotid Intima-Media Thickness
| Factor | Standardized Parameter Estimate |
Parameter Estimate |
Standard Error |
p |
|---|---|---|---|---|
| Age (years) | 0.486 | 0.012 | 0.001 | <.0001 |
| Female Sex | −0.215 | −0.077 | 0.022 | 0.0006 |
| Black Race | 0.115 | 0.049 | 0.027 | 0.067 |
| BMI (kg/m2)* | 0.262 | 0.334 | 0.077 | <.0001 |
| Weight (kg) | 0.279 | 0.003 | 0.0007 | <.0001 |
| Waist Circumference (cm) | 0.270 | 0.004 | 0.0008 | <.0001 |
| SBP (mm Hg) | 0.240 | 0.003 | 0.001 | 0.0001 |
| DBP (mm Hg) | 0.202 | 0.003 | 0.001 | 0.0011 |
| Glucose (mg/dL) | 0.200 | 0.004 | 0.001 | 0.0013 |
| Insulin (μU/mL)* | 0.112 | 0.002 | 0.001 | 0.18 |
| HOMA (mmol/L × μU/mL)* | 0.140 | 0.043 | 0.019 | 0.025 |
| Total Cholesterol (mg/dL)* | 0.245 | 0.19 | 0.047 | <.0001 |
| LDL-C (mg/dL)* | 0.248 | 0.135 | 0.033 | <.0001 |
| HDL-C (mg/dL)* | −0.074 | −0.045 | 0.038 | 0.24 |
| Triglycerides (mg/dL)* | 0.164 | 0.047 | 0.018 | 0.0089 |
| Leptin (ng/dL)* | −0.070 | −0.017 | 0.015 | 0.26 |
| Ghrelin (pg/mL)* | −0.0062 | −0.002 | 0.024 | 0.92 |
| Adiponectin (μg/mL)* | −0.171 | −0.05 | 0.018 | 0.0061 |
| Aldosterone (pg/mL)* | 0.0049 | 0.002 | 0.02 | 0.94 |
| Ever Smoker | −0.083 | −0.025 | 0.019 | 0.19 |
| Total Physical Activity (MET-hr/wk)** | −0.057 | −0.0022 | 0.0024 | 0.36 |
N=258; Logarithmic transformation of carotid intima-media thickness
Parameter estimates and standard error based on log transformation
Parameter estimates and standard error based on square root transformation
SBP=systolic blood pressure, DBP=diastolic blood pressure, LDL-C=low density lipoprotein cholesterol, HDL-C=high density lipoprotein cholesterol, HOMA=homeostasis model assessment of insulin resistance index
Figure 1. Unadjusted and Adjusted Mean Average IMT by Urinary Sodium Quartile.
Increasing urinary sodium quartile is shown across the horizontal axis for each model. Quartiles of urinary sodium are Q1: 48-143 mEq/24hr, Q2: 144-179 mEq/24hr, Q3: 180-224 mEq/24hr, Q4: 225-415 mEq/24hr. Trend P values are for linear trend in mean carotid intima-media thickness (IMT) by sodium quartile. Pairwise comparison p values are adjusted for multiple comparisons by Dunnett’s method.
Model 1. Mean IMT adjusted for age, sex, race (black, non-black), systolic blood pressure
Model 2. Model 1 + BMI, aldosterone, LDL cholesterol, triglycerides, homeostasis model assessment of insulin resistance index (HOMA), C reactive protein, adiponectin, ever smoker
Model 1: N=258, Model 2: N=253
Table 3.
Multivariate Linear Regression Models for Average Carotid IMT
| Factor | Standardized parameter estimate |
Parameter Estimate |
Standard Error | p value |
|---|---|---|---|---|
| Model 1 (r2=0.34) | ||||
| Male Sex | 0.18 | 0.07 | 0.02 | 0.0007 |
| Age | 0.49 | 0.01 | 0.001 | <.0001 |
| Black Race | 0.14 | 0.06 | 0.02 | 0.008 |
| SBP | 0.17 | 0.002 | 0.0007 | 0.002 |
| Sodium Quartile | 0.11 | 0.01 | 0.007 | 0.03 |
| Model 2 (r2=0.43) | ||||
| Male Sex | 0.11 | 0.04 | 0.02 | 0.06 |
| Age | 0.48 | 0.01 | 0.001 | <.0001 |
| Black Race | 0.13 | 0.06 | 0.02 | 0.01 |
| SBP | 0.10 | 0.001 | 0.0007 | 0.05 |
| Sodium Quartile | 0.11 | 0.01 | 0.007 | 0.04 |
| BMI* | 0.22 | 0.26 | 0.07 | 0.005 |
| CRP* | −0.08 | −0.01 | 0.008 | 0.17 |
| LDL-C* | 0.15 | 0.08 | 0.03 | 0.005 |
| Aldosterone* | −0.02 | −0.006 | 0.02 | 0.71 |
| Triglycerides* | 0.06 | 0.02 | 0.02 | 0.28 |
| HOMA* | 0.06 | 0.02 | 0.02 | 0.29 |
| Adiponectin* | −0.08 | −0.02 | 0.02 | 0.18 |
| Ever Smoker | −0.07 | −0.02 | 0.02 | 0.18 |
Model 1: N=258; Model 2: N=253; Logarithmic transformation of carotid intima-media thickness
Parameter estimates and standard error based on log transformation
SBP=systolic blood pressure, DBP=diastolic blood pressure, LDL-C=low density lipoprotein cholesterol, HDL-C=high density lipoprotein cholesterol, HOMA=homeostasis model assessment of insulin resistance index, CRP=C reactive protein
Because we had accurate urinary sodium data on only 76% of our total baseline sample, we investigated trends in IMT with increasing sodium/creatinine ratio, a measure available for all SAVE participants. Results were similar, with a significant trend in IMT with increasing sodium/creatinine ratio in the fully adjusted model (P=0.02). In another validation analysis, continuous urinary sodium showed a nonsignificant trend with IMT in the unadjusted model (P=0.06), Model 1 (P=0.05), and Model 2 (P=0.07). When sex-specific urinary sodium quartiles were evaluated, the linear trend in IMT was nonsignificant in the multivariate models (Model 1 P=0.08; Model 2 P=0.09). Although sex-specific quartiles are necessary for measures of creatinine excretion, we report sex-pooled analyses for urinary sodium because it showed no significant interaction with sex. In addition, no significant interactions were detected between urinary sodium and age, race, BMI, or SBP in any model.
DISCUSSION
To our knowledge, this is the first study to show a positive relationship between urinary sodium and carotid IMT in normotensive persons. In our community-based sample of normotensive overweight and obese adults, we observed a significant positive trend in carotid IMT with increasing quartile of urinary sodium. This relationship held after adjustments for age, sex, race, BMI, SBP, serum aldosterone, and other cardiovascular risk factors. After adjustment for age, sex, race, and SBP, those in the highest quartile of urinary sodium had a significantly higher mean IMT than those in the lowest quartile. Adjustment for BMI substantially diminished this contrast. The increasing trends in IMT with urinary sodium were similar in models in which continuous urinary sodium or urinary sodium/creatinine quartile was used. Our finding of a positive association between urinary sodium and carotid IMT in normotensive subjects is consistent with the reported relationships between high sodium intake and measures of cardiovascular remodeling such as pulse wave velocity21, left ventricular mass22, and flow-mediated dilation.23
Several hypotheses have been offered to explain the adverse cardiovascular effects of dietary sodium24-26, but the experimental evidence to support these hypotheses remains inadequate. Simon notes two physiologic changes brought about by excessive dietary sodium that may have long-term effects on cardiovascular function and structure.27 First, an increase in cardiac output and regional blood flow dilates the arteries27, and second, an increase in plasma and extracellular sodium concentration increases vascular reactivity and growth.24,25,27
In a study in normotensive rats, inward remodeling of the carotid artery wall compensated for the loss of distensibility induced by dilatation during high sodium intake.28 Studies in hypertensive humans have shown that arterial remodeling with or without wall thickening may compensate for hypertension-induced reduction in distensibility.29,30 In our study, carotid intima-media thickening in individuals with higher sodium consumption could be a result of such compensatory remodeling. Our study consisted of participants with normal BP, but a high salt diet could be sufficient to produce a flow-related increase in shear stress and induce compensatory arterial wall thickening without increasing BP. The small difference in SBP between the highest and lowest urinary sodium quartiles may be explained by the exclusion of individuals with an average BP > 140/90 as well as the fact that a single urine collection is an imperfect measure of habitual dietary sodium consumption.
In addition to flow-related remodeling, the small increase in plasma sodium that occurs during high salt intake can stimulate arterial remodeling.27 A 2 to 5 mmol/L increase in fasting plasma sodium concentration has been observed in humans when sodium intake is increased from 20 mmol/d to 220-250 mmol/d for 7 days.31,32 High plasma sodium can stimulate vascular growth by enhancing trophic responses to angiotensin II, vasopressin, and various growth factors, whose actions involve sodium influx.25 Thus, carotid intima-media thickening in the presence of a high sodium diet may occur as a combination of a compensatory response to increased shear stress and a hypertrophic response of VSMCs to their high sodium environment.
There are several limitations of this study. First, this was a cross-sectional study of baseline measurements. Thus, we could not determine any causal relationship between sodium consumption and IMT. However, because SAVE trial participants will be followed for two years, we will be able to report longitudinal relationships between urinary sodium and IMT. Second, because of the complexities of collecting 24-hour urine, only 76% of subjects had data considered valid for these analyses. However, we obtained similar results when we evaluated sodium/creatinine quartiles for all participants in place of sodium quartiles. We acknowledge the rather weak association between urinary sodium excretion as a continuous variable and carotid IMT. This may be due to a threshold effect, evident by the most marked increase in IMT occurring in the highest quartile of urinary sodium. This effect is not adequately captured by the model with continuous urinary sodium. Finally, we had few blacks in this sample (n=34) and thus were unable to detect a unique relationship in blacks, who are more likely to be salt-sensitive than whites. Nevertheless, this was a study of overweight and obese individuals, who may be more sodium sensitive than normal weight individuals.7 A notable strength of this study is that all participants were normotensive and not on hypertensive or vasoactive medications, enabling us to evaluate the relationship between sodium intake and IMT independent of treatment effects.
Clinical Implications
With the increase in consumption of highly salted processed food, salt intake in much of the world has reached 9-12 g (3.5-4.7 g sodium) per day.33,34 Sodium intake has been proposed as an independent risk factor for CVD and stroke, though not all studies have shown a positive relationship.35 Because carotid IMT is highly predictive of future cerebrovascular and coronary artery events11, our finding of an independent association between urinary sodium and IMT is consistent with the reported relationship between sodium intake and stroke risk6,36, suggesting that decreasing sodium intake in the population would decrease the rate of stroke.
The U.S. Dietary Guideline for sodium intake is less than 2300 mg/day (100 meq/day). It is notable that our second quartile of urinary sodium begins at 144 meq/day, indicating that over 75% of our study population consumes excess sodium. Those individuals in Q4 had a urinary sodium excretion more than two times higher than the current recommended intake and had the most notable increase in IMT. It is probable that individuals in Q4 habitually consumed the most dietary sodium, such that the positive association between carotid IMT and urinary sodium was most evident for Q4 despite our having just one urine collection. If the ongoing trial confirms the relationship of sodium intake with IMT, it would support efforts to decrease sodium intake in overweight and obese individuals.
ACKNOWLEDGMENTS
This independent investigator-promoted research was supported by grants R01 HL077525-01A2 and T32 HL083825-01 from the National Heart, Lung, and Blood Institute of the National Institutes of Health. The authors acknowledge Janice Sabatine, PhD, Avanti Strategies, Cranberry Township PA, for writing and editing assistance.
Footnotes
DISCLOSURE
The authors declare no conflict of interest.
References
- 1.He FJ, MacGregor GA. A comprehensive review on salt and health and current experience of worldwide salt reduction programmes. J Hum Hypertens. 2008;23(6):363–384. doi: 10.1038/jhh.2008.144. [DOI] [PubMed] [Google Scholar]
- 2.Elliott P, Stamler J, Nichols R, Dyer AR, Stamler R, Kesteloot H, Marmot M. Intersalt revisited: further analyses of 24 hour sodium excretion and blood pressure within and across populations. BMJ. 1996;312(7041):1249–1253. doi: 10.1136/bmj.312.7041.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou BF, Stamler J, Dennis B, Moag-Stahlberg A, Okuda N, Robertson C, Zhao L, Chan Q, Elliott P. Nutrient intakes of middle-aged men and women in China, Japan, United Kingdom, and United States in the late 1990s: The INTERMAP Study. J Hum Hypertens. 2003;17(9):623–630. doi: 10.1038/sj.jhh.1001605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.He J, Ogden LG, Vupputuri S, Bazzano LA, Loria C, Whelton PK. Dietary Sodium Intake and Subsequent Risk of Cardiovascular Disease in Overweight Adults. JAMA. 1999;282(21):2027–2034. doi: 10.1001/jama.282.21.2027. [DOI] [PubMed] [Google Scholar]
- 5.He J, Ogden LG, Bazzano LA, Vupputuri S, Loria C, Whelton PK. Dietary Sodium Intake and Incidence of Congestive Heart Failure in Overweight US Men and Women: First National Health and Nutrition Examination Survey Epidemiologic Follow-up Study. Arch Intern Med. 2002;162(14):1619–1624. doi: 10.1001/archinte.162.14.1619. [DOI] [PubMed] [Google Scholar]
- 6.Perry I, Beevers D. Salt intake and stroke: a possible direct effect. J Hum Hypertens. 1996;6:23–25. [PubMed] [Google Scholar]
- 7.Logan A. Sodium sensitivity, not level of salt intake, predicts salt effects. Curr Hypertens Rep. 2000;2(2):115–119. doi: 10.1007/s11906-000-0068-1. [DOI] [PubMed] [Google Scholar]
- 8.Safar ME, Thuilliez C, Richard V, Benetos A. Pressure-independent contribution of sodium to large artery structure and function in hypertension. Cardiovasc Res. 2000;46(2):269–276. doi: 10.1016/s0008-6363(99)00426-5. [DOI] [PubMed] [Google Scholar]
- 9.Sanders PW. Vascular consequences of dietary salt intake. Am J Physiol Renal Physiol. 2009;297(2):F237–F243. doi: 10.1152/ajprenal.00027.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Safar ME. Systolic hypertension in the elderly: arterial wall mechanical properties and the renin-angiotensin-aldosterone system. J Hypertens. 2004;23:673–681. doi: 10.1097/01.hjh.0000163130.39149.fe. [DOI] [PubMed] [Google Scholar]
- 11.Lorenz MW, Markus HS, Bots ML, Rosvall M, Sitzer M. Prediction of Clinical Cardiovascular Events With Carotid Intima-Media Thickness: A Systematic Review and Meta-Analysis. Circulation. 2007;115(4):459–467. doi: 10.1161/CIRCULATIONAHA.106.628875. [DOI] [PubMed] [Google Scholar]
- 12.Wendelhag I, Gustavsson T, Suurkula M, Berglund G, Wikstrand J. Ultrasound measurement of wall thickness in the carotid artery: fundamental principles of a computerized analysing system. Clin Physiol. 1991;11(6):565–577. doi: 10.1111/j.1475-097x.1991.tb00676.x. [DOI] [PubMed] [Google Scholar]
- 13.Ellis D, Lloyd C, Becker DJ, Forrest KYZ, Orchard TJ. The changing course of diabetic nephropathy: Low-density lipoprotein cholesterol and blood pressure correlate with regression of proteinuria. Am J Kidney Dis. 1996;27:809–818. doi: 10.1016/s0272-6386(96)90518-1. [DOI] [PubMed] [Google Scholar]
- 14.Allain CC, Poon LS, Chan CS, Richmond W, Fu PC. Enzymatic determination of total serum cholesterol. Clin Chem. 1974;20(4):470–475. [PubMed] [Google Scholar]
- 15.Warnick GR, Albers JJ. A comprehensive evaluation of the heparin - manganese precipitation procedure for estimating high density lipoprotein cholesterol. J Lipid Res. 1978;19(1):65–76. [PubMed] [Google Scholar]
- 16.Bucolo G, David H. Quantitative Determination of Serum Triglycerides by the Use of Enzymes. Clin Chem. 1973;19(5):476–482. [PubMed] [Google Scholar]
- 17.Bondar RJL, Mead DC. Evaluation of Glucose-6-Phosphate Dehydrogenase from Leuconostoc mesenteroides in the Hexokinase Method for Determining Glucose in Serum. Clin Chem. 1974;20(5):586–590. [PubMed] [Google Scholar]
- 18.Chen H, Sullivan G, Quon MJ. Assessing the Predictive Accuracy of QUICKI as a Surrogate Index for Insulin Sensitivity Using a Calibration Model. Diabetes. 2005;54(7):1914–1925. doi: 10.2337/diabetes.54.7.1914. [DOI] [PubMed] [Google Scholar]
- 19.du Cailar G, Mimran A. Non-pressure-related effects of dietary sodium. Curr Hypertens Rep. 2009;11(1):12–17. doi: 10.1007/s11906-009-0004-y. [DOI] [PubMed] [Google Scholar]
- 20.Grundy SM. Metabolic syndrome scientific statement by the American Heart Association and the National Heart, Lung, and Blood Institute. Arterioscler Thromb Vasc Biol. 2005;25(11):2243–2244. doi: 10.1161/01.ATV.0000189155.75833.c7. [DOI] [PubMed] [Google Scholar]
- 21.Avolio AP, Clyde KM, Beard TC, Cooke HM, Ho KK, O’Rourke MF. Improved arterial distensibility in normotensive subjects on a low salt diet. Arterioscler Thromb Vasc Biol. 1986;6(2):166–169. doi: 10.1161/01.atv.6.2.166. [DOI] [PubMed] [Google Scholar]
- 22.Du Cailar G, Ribstein J, Daures JP, Mimran A. Sodium and left ventricular mass in untreated hypertensive and normotensive subjects. Am J Physiol Heart Circ Physiol. 1992;263(1):H177–H181. doi: 10.1152/ajpheart.1992.263.1.H177. [DOI] [PubMed] [Google Scholar]
- 23.Dickinson KM, Keogh JB, Clifton PM. Effects of a low-salt diet on flow-mediated dilatation in humans. Am J Clin Nutr. 2009;89(2):485–490. doi: 10.3945/ajcn.2008.26856. [DOI] [PubMed] [Google Scholar]
- 24.Friedman S. Sodium in blood vessels. A brief review. Blood Vessels. 1979;16:2–16. doi: 10.1159/000158185. [DOI] [PubMed] [Google Scholar]
- 25.Simon G. Increased vascular wall sodium in hypertension: where is it, how does it get there and what does it do there? Clin Sci. 1990;78(6):533–540. doi: 10.1042/cs0780533. [DOI] [PubMed] [Google Scholar]
- 26.Partovian C, Benetos A, Pommies JP, Mischler W, Safar ME. Effects of a chronic high-salt diet on large artery structure: role of endogenous bradykinin. Am J Physiol Heart Circ Physiol. 1998;274(5):H1423–H1428. doi: 10.1152/ajpheart.1998.274.5.H1423. [DOI] [PubMed] [Google Scholar]
- 27.Simon G. Experimental evidence for blood pressure-independent vascular effects of high sodium diet. Am J Hypertens. 2003;16(12):1074–1078. doi: 10.1016/j.amjhyper.2003.07.019. [DOI] [PubMed] [Google Scholar]
- 28.Simon G, Jaeckel M, Illyes G. Development of structural vascular changes in salt-fed rats. Am J Hypertens. 2003;16(6):488–493. doi: 10.1016/s0895-7061(03)00568-5. [DOI] [PubMed] [Google Scholar]
- 29.Weber R, Stergiopulos N, Brunner HR, Hayoz D. Contributions of Vascular Tone and Structure to Elastic Properties of a Medium-Sized Artery. Hypertension. 1996;27(3):816–822. doi: 10.1161/01.hyp.27.3.816. [DOI] [PubMed] [Google Scholar]
- 30.Barenbrock M, Hausberg M, Kosch M, Golubev SA, Kisters K, Rahn KH. Flow-mediated vasodilation and distensibility in relation to intima-media thickness of large arteries in mild essential hypertension[ast] Am J Hypertens. 1999;12(10):973–979. doi: 10.1016/s0895-7061(99)00083-7. [DOI] [PubMed] [Google Scholar]
- 31.Piccirillo G, Bucca C, Durante M, Santagada E, Munizzi MR, Cacciafesta M, Marigliano V. Heart Rate and Blood Pressure Variabilities in Salt-Sensitive Hypertension. Hypertension. 1996;28(6):944–952. doi: 10.1161/01.hyp.28.6.944. [DOI] [PubMed] [Google Scholar]
- 32.Brown WJ, Jr., Brown FK, Krishnan I. Exchangeable Sodium and Blood Volume in Normotensive and Hypertensive Humans on High and Low Sodium Intake. Circulation. 1971;43(4):508–519. doi: 10.1161/01.cir.43.4.508. [DOI] [PubMed] [Google Scholar]
- 33.Intersalt Cooperative Research Group Intersalt: an international study of electrolyte exccretion and blood pressure. Results for 24 hour urinary sodium and potassium excretion. BMJ. 1988;297:319–328. doi: 10.1136/bmj.297.6644.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.U.S. Department of Agriculture. Agricultural Research Service Nutrient Intakes from Food: Mean Amounts Consumed per individual, One Day, 2005-2006. 2008 www.ars.usda.gov/ba/bhnrc/fsrg.
- 35.Hu G, Qiao Q, Tuomilehto J. Nonhypertensive Cardiac Effects of a High Salt Diet. Curr Hypertens Rep. 2002;4(1):13–17. doi: 10.1007/s11906-002-0047-9. [DOI] [PubMed] [Google Scholar]
- 36.Nagata C, Takatsuka N, Shimizu N, Shimizu H. Sodium Intake and Risk of Death From Stroke in Japanese Men and Women. Stroke. 2004;35(7):1543–1547. doi: 10.1161/01.STR.0000130425.50441.b0. [DOI] [PubMed] [Google Scholar]