Abstract
This fMRI study contrasted case-deviant and letter-deviant forms with familiar forms of the same phonological words (e.g., TaXi and Taksi vs. Taxi) and found, that both types of deviance led to increased activation in a left occipitotemporal region corresponding to the Visual Word Form Area. Case-deviant items, in addition, led to increased activation in a right occipitotemporal region and in a left occipital and a left posterior occipitotemporal region, possibly reflecting the increased demands on letter form coding. For letter-deviant items, in addition to the increased left occipitotemporal activation, a main finding was increased activation primarily in extended left frontal regions, possibly reflecting sublexically mediated access to word phonology. These findings are consistent with general features of cognitive dual-route models of visual word processing. Furthermore, they add support to the main feature of Dehaene et al.’s (2005) neural model of early stages of visual word processing . However, the increased activation found for case-deviant items in the VWFA cannot be immediately reconciled with the assumption of completely abstract case-independent orthographic word codes in the VWFA.
Keywords: Functional MRI, visual word recognition, occipitotemporal cortex, visual word form area, orthographic processing
Introduction
In a recent study we found that orthographically familiar forms of German nouns compared to orthographically unfamiliar forms of the very same phonological words (e.g., Taxi vs. Taksi) led to reduced activation in a region of the left visual ventral pathway, in left inferior parietal regions and in a large left inferior frontal language region (Kronbichler et al., in press). The location of the left occipitotemporal region, which showed an orthographic familiarity effect, roughly corresponded to that of the Visual Word Form Area (VWFA) of Cohen, Dehaene and collaborators (reviewed by Cohen & Dehaene, 2004; McCandliss, Cohen & Dehaene, 2003). In a preceding study, we identified a large left occipitotemporal region, which included the VWFA, to be modulated by the frequency with which the words were encountered in print (Kronbichler et al., 2004). The direction of the modulation corresponded to the orthographic familiarity effect, in that increasing frequency was accompanied by a systematic decrease of left occipitotemporal activation. These findings led us to propose that the left occipitotemporal cortex hosts a visual input lexicon which – in the case of high frequency words and orthographically familiar forms – allows fast assimilation of the letter input by readily available orthographic representations of specific words, and these orthographic representations give direct access to word phonology and meaning. This interpretation was inspired by studies which found that experimentally induced familiarity of pictured faces or objects (by repeated presentations) led to decreased activation in occipitotemporal regions (e.g., Rossion, Schiltz, & Crommelinck, 2003; van Turennout, Ellmore, & Martin, 2000).
An EEG-study from our lab (Sauseng, Bergmann & Wimmer, 2004) provided information on the time-course of visual word processing by finding an ERP divergence from about 200 ms to about 700 ms post-stimulus between familiar and letter deviant forms of the same phonological words. Specifically, familiar forms differed from unfamiliar ones by eliciting a marked positive deflection which peaked around 300 ms post-stimulus (P300) and – following Rudell and Hua (1997) – was interpreted as recognition potential. The unfamiliar forms, from between 200 and 700 ms post-stimulus, elicited a more negative going ERP than familiar forms. However, unexpectedly, unfamiliar case-deviant forms (e.g., TaXi) differed from familiar forms in the same way as letter-deviant forms (e.g., Taksi). We had expected the letter-deviant forms to elicit more mismatch negativity than case-deviant forms, as the former violate the identity of one or two letters of stored orthographic representations, whereas the case-deviant forms violate only superficial letter case information.
We reasoned that despite the unexpected similarity of the ERPs elicited by letter-case format and orthographic format violations, different brain regions may respond to the different types of deviance from the familiar pattern. To examine this expectation, we relied on event-related fMRI. Similar to Sauseng et al. (2004), we contrasted case-deviant and letter-deviant forms with familiar forms of German nouns. The case-deviant forms resulted from changing to upper-case one of the lower-case letters after the first capitalized one (e.g., TaXi, ChAos). This change is a moderate violation of the familiar letter format compared to the usual cAsE-mIxInG, which changes every second letter. The letter-deviant forms resulted from replacing one or two letters of the familiar forms by homophonic ones (e.g., Taxi-Taksi, Chaos-Kaos).
For the letter-deviant forms, we expected replication of the Kronbichler et al. (in press) finding, that is, enhanced activation in a region roughly corresponding to VWFA in the left occipitotemporal cortex, in left inferior parietal and in left inferior frontal/precentral regions. The letter-deviant forms require sublexical processing, that is, the letter string has to be coded as a sequence of graphemes with associations to phonemes which, via assembly, give access to the phonological word. Recognition of the deviance will occur when the phonological word activates the orthographic word representation. Automatic activation of orthographic representations by phonological ones is suggested by findings of orthographic interference on purely phonological tasks. For example, rhyme judgements for auditorily presented words like “bead” and “deed” are slower compared to words like “greed” and “deed” (Seidenberg & Tanenhaus, 1979). The expected enhanced left occipitotemporal activation to the letter-deviant forms may reflect activation of several graphemes compared to activation of a single orthographic word representation in the case of familiar forms. Furthermore, the mismatch between the letter string input and the orthographic representation may contribute to the enhanced activation. The enhanced left inferior parietal and left inferior frontal activation may reflect sublexical phonological processes preceding access to phonological word representations.
For the case-deviant forms (e.g., TaXi), no sublexical processing is expected since the sequence of abstract letter identities should instantiate a single orthographic word representation with a direct association to whole-word phonology. Therefore, we do not expect enhanced activation in the mentioned regions which, in response to letter-deviant forms, are expected to reflect sublexical processing demands. Rather, the expectation was, that regions in the posterior occipitotemporal regions may respond with enhanced activation to the increased demands posed by case-deviant forms. This expectation is derived from a recent elaboration by Dehaene et al. (2005) of the original VWFA hypothesis. This elaboration, based on the results of subliminal priming studies, proposed a hierarchy of increasingly abstract codings in the posterior to anterior direction of the occipital and occipitotemporal cortex.
Non-suprisingly, case-mixing prolongs reading time and was found to reduce perceptibility of briefly presented words (Jordan, Redwood & Patching, 2003). Of interest is, that case-mixing leads to a marked effect of word length (i.e., number of letters) on reading onset latency, and, particularly so, when mixed-case words are presented to the right visual field, so that visual information first arrives in the left hemisphere (e.g., Fiset & Arguin, 1999; Lavidor & Ellis, 2001). This suggests, that left posterior brain regions are specifically tuned to the normal appearance of visual words. The behavioural evidence on which processes are specifically affected by case-mixing is not conclusive (review by Mayall, Humphreys & Olson, 1997). It was hypothesized that the case-mixing effect is due to lateral interference (i.e., the upper-case letters may interfere with the processing of the shapes of the flanking lower-case letters). Other hypotheses are that it reflects the increased number of letter forms which must be considered for coding the visual information, or that it reflects a misleading grouping of same-case letters. Distortion of global word outline is considered an unlikely explanation of the case-mixing effect (e.g., Perea & Rosa, 2002).
As already mentioned, based on the Dehaene et al. (2005) model, we expected case-deviant items to specifically engage posterior bilateral occipitotemporal regions, which are engaged in letter form coding and in the transformation of case-specific letter forms into abstract letter codes. However, a PET study by Mayall et al. (2001) found no effect of case-mixing on activation in any occipital or occipitotemporal region, but found such an effect in a right parietal region previously associated with visual attention. In addition, transcranial magnetic stimulation of this region led to a pronounced negative effect on reading aloud mixed-case words (Braet & Humphreys, 2005; 2006). However, another PET study by Xu et al. (2001) did not find increased right parietal activation, but – roughly corresponding to the Dehaene et al. model – found enhanced activation in a left posterior occipitotemporal and an occipital cluster.
The present fMRI study will provide additional evidence on the issue which brain regions are specifically engaged by letter form coding and by the transformation of case-specific to case-independent codes. We contrast case-deviant forms not only with familiar forms (e.g., TaXi vs. Taxi) but also with letter-deviant forms (e.g., TaXi vs.Taksi). Increased activation of case-deviant forms in relation to letter-deviant forms would be specifically informative because letter-deviant forms can be considered to be more attention-demanding than case-deviant forms. The contrast between case-deviant and familiar forms is of interest for the assumption of the Dehaene et al. (2005) model, that letter-case information is lost in early processing stages located in bilateral occipital and posterior occipitotemporal brain regions. This assumption is based on the results of subliminal priming studies which found a similar reduction of activation in the VWFA when target words were primed by the same word in same-case or in cross-case format (Dehaene et al. 2001; 2004). If these findings generalize to the present study, then one would expect activation of the VWFA to be similar for case-deviant and familiar forms and lower than activation elicited by letter-deviant forms.
Methods
Participants
Participants were 20 university students (13 males, 7 females) in the age range from 19 to 45 years (mean = 29 years). All of them were right-handed, native German speakers and had normal or corrected-to-normal vision. Written informed consent was given by all participants.
Material
For each of 75 German nouns, two deviant forms were created. For the case-deviant items, one of the lower-case letters after the capitalized initial one was changed to upper-case. For the letter-deviant items, one or two letters were replaced by homophonic ones. Examples are Physik – PhySik – Fysik and Boot (boat) – BooT – Boht. The mean frequency of the words in written texts according to the CELEX data base (Baayen, Piepenbrock & van Rijn, 1993) was 15,35 per million (SD= 5.5). Table 1 shows that the letter-deviant items were similar to the familiar ones in terms of length, bigram frequency and number of orthographic neighbours. For assessing the effects of case- and letter-deviance on reading performance, additional 20 adult readers, read aloud lists of all 75 items of each type. Table 1 shows a small increase in reading time from the familiar to the case-deviant items and a large further increase from the case-deviant to the letter deviant items, F(1,19) = 17.38, p < .001, and pairwise comparisons found even the small increase from familiar words to case-deviant items reliable (p < .01). Errors were infrequent and nearly always corrected.
Table1.
Item characteristics [Mean (SD)].
| Characteristics | Familiar/Case-deviant items | Letter-deviant items |
|---|---|---|
| Letter length | 5.56 (1.17) | 5.57 (1.19) |
| Syllable length | 1.89 (0.56) | 1.91 (0.55) |
| Type bigram frequency(per million) | 3408.65 (1981.03) | 3466.16 (1927.47) |
| Number of orthographic neighbors | 1.48 (2.06) | 1.72 (1.91) |
| Reading aloud (ms/word) | 431 (82) / 458 (81) | 549 (152) |
Procedure
The 225 stimuli were presented in three runs, each consisting of 75 items and 25 null-events. Each run included 25 items of each type and within a run the same word was not repeated. Within these constraints, two pseudo-randomized sequences of trials were created and optimized with a genetic algorithm (Wager & Nichols, 2003). Items had to be read silently, but participants were warned that a question mark after an item may prompt articulation which was the case for 4 items per type. Stimuli were displayed for 800 ms. During the interstimulus interval of 3400 ms, a fixation cross was present. Participants were familiarized with the stimuli and the procedure outside the scanner. Stimulus delivery and timing was controlled with Presentation (Neurobehavioral Systems Inc., Albany, CA, USA).
Functional and structural imaging was performed with a Philips Gyroscan NT 1.5 Tesla Scanner (Philips Medical System Inc., Best, The Netherlands). Functional images sensitive to blood oxygen-level dependent (BOLD) contrast were acquired with a T2* weighted GE EPI sequence (TR 2200ms; TE 40 ms; matrix 64 × 64; FOV 220 mm; FA 86°). Twenty-one axial slices with a slice thickness of 6 mm were imaged parallel to the bicomissural line. During each run 194 whole head images were acquired with 6 dummy scans at the beginning. After functional scanning, a high-resolution structural scan (T1-weighted MPRAGE sequence; matrix: 256 × 256; FOV 220 mm; 130 slices; 1.30 mm slice thickness) was acquired to facilitate normalization and localization of functional activations.
fMRI data analysis
Data processing used SPM2 software (The Wellcome Department of Cognitive Neurology, London, UK., http://www.fil.ion.ucl.ac.uk/spm) running in a MATLAB 6.5 environment (Mathworks Inc., Sherbon MA, USA). All functional images were realigned to the first functional image and unwarped, slice time corrected and coregistered to the high resolution structural images. The structural image was normalized to the MNI T1 template image, and the resulting parameters were used for normalisation of the functional images which were resampled to isotropic 3×3×3mm voxels and smoothed with a 6mm FWHM Gaussian kernel. In the subject-specific first level model, each stimulus type was modelled by a canonical hemodynamic response function and its temporal derivative. The 12 stimuli followed by a prompt were modelled separately and treated as covariates of no interest. The functional data from these first level models were high-pass filtered with a cut-off of 128 seconds and corrected for autocorrelation by an AR(1) model (Friston et al., 2002). Statistical analysis was performed within a two-level framework (Holmes & Friston, 1998). First, images for the contrasts of interest were calculated separately for each subject. In the following random-effects analyses, contrast images of each subject were used to allow generalization to the population.
Results
In a first step, each item type separately was contrasted with the fixation baseline which consisted of the interstimulus intervals and the null-events. Because all three item types activated largely the same regions (compared to the fixation baseline), an activation map is only shown for the familiar items. Figure 1A shows activation of bilateral posterior regions (occipital, occipitotemporal, parietal and temporoparietal). There were also extended activations in bilateral frontal regions (inferior frontal, precentral regions and supplementary motor area (SMA)).
Figure 1.
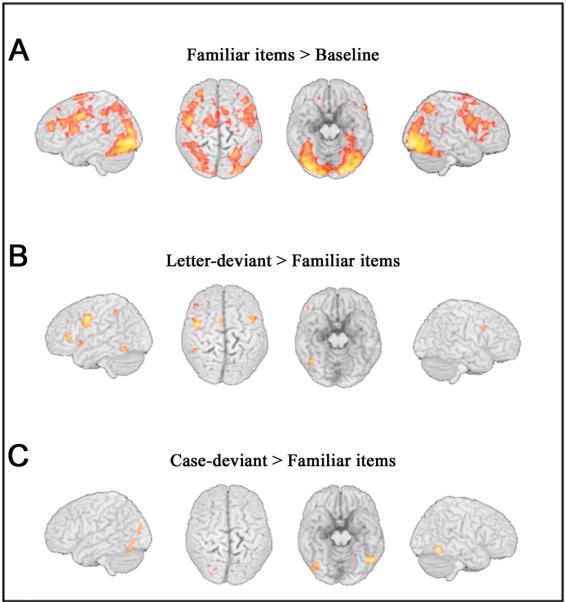
(A) Increased brain activation for familiar items compared to fixation baseline.
(B) Increased brain activation for letter-deviant compared to familiar items baseline.
(C) Increased brain activation for case-deviant compared to familiar items baseline. Regions with increased activation (FDR < .05) are rendered on the surface of a standard brain (L=L).
The following analyses contrasted each deviant item type separately with familiar items using a false discovery rate threshold (FDR; Genovese, Lazar & Nichols, 2002) of q < .05. For these analyses, a reading mask was used. It included all voxels with activation at p < .001, uncorrected, when reading (irrespective of item type) was contrasted with base-line. This mask reduces the multiple comparison problem by restricting comparisons to reading relevant regions, but does not bias the contrasts between item types which are orthogonal to the average effect (Friston et al., 2006). Figure 1B and Table 2 (first section) show that letter-deviant items led to increased brain activity in a region of the left occipitotemporal cortex, in a large left precentral cluster, in left and right inferior frontal regions, in the supplementary motor area (SMA) and in the left inferior parietal lobule. None of these frontal or parietal regions exhibited higher activity for case-deviant compared to familiar items. This latter contrast identified a left and a right occipitotemporal region and additionally a left occipital region (see Figure 1 C and Table 2). With the mentioned FDR-corrected threshold, no region was found with higher activity for familiar compared to deviant items. Furthermore, no region was identified in the contrasts between the two types of deviant items. With an uncorrected threshold of p < .001, a region in the left inferior frontal and precentral cortex (maximum at x= −39, y= 0, z= 33) was identified with higher activation for letter-deviant than case-deviant items. This region corresponds closely to the cluster with higher activity for letter-deviant than familiar items listed in Table 2. With the uncorrected threshold, higher activation for case-deviant compared to letter deviant items was found in a right occipitotemporal region and in left posterior occipitotemporal and occipital regions.
Table 2.
Brain regions showing effects of deviance (FDR < .05) in the reading mask (upper section) and in the occipitpototemporal volume of interest (lower section).
| Region | MNI Coordinates | t | Extent (mm3) | ||
|---|---|---|---|---|---|
| x | y | z | |||
| Effects in reading mask (see text) | |||||
| Letter-deviant > familiar items | |||||
| L occipitotemporal | −48 | −60 | −15 | 4.22 | 351 |
| L precentral | −39 | 0 | 36 | 6.36 | 3024 |
| L inferior frontal | −45 | 33 | 6 | 4.75 | 756 |
| L Suplementary Motor Area (SMA) | −9 | 9 | 57 | 4.49 | 324 |
| L Inferior parietal | −54 | −45 | 51 | 3.96 | 243 |
| R Inferior frontal | 45 | 12 | 36 | 4.58 | 621 |
| Case-deviant > familiar items | |||||
| L occipitotemporal | −42 | −69 | −21 | 4.87 | 243 |
| L posterior occipitotemporal | −48 | −75 | −9 | 4.19 | 216 |
| L middle occipital | −27 | −87 | 27 | 3.98 | 297 |
| R occipitotemporal | 51 | −60 | −12 | 5.63 | 1134 |
| Effects in occipitotemporal volume of interest (see text) | |||||
| Case-deviant > letter deviant | |||||
| L posterior occipitotemporal | −39 | −72 | −18 | 4.16 | 270 |
| R occipitotemporal | 42 | −57 | −9 | 3.29 | 81 |
| Conjuction: letter-deviant > familiar items & case-deviant > familiar items | |||||
| L middle occipitotemporal | −42 | −57 | −15 | 4.16 | 513 |
As the main focus of the present study was on visual word processing in occipitotemporal brain regions, we used a small-volume-correction for the left and right occipitotemporal cortex to increase statistical sensitivity. For this small-volume-correction we combined two cube-sized volumes (one for the left, the other for the right hemisphere), which ranged from x = +/− 48 to +/−32 , y = −86 to −44 and z = −18 to −6, into one volume. This small-volume was based on previous studies (Dehaene et al., 2001; 2004; Jobard et al., 2003; Kronbichler et al., 2004; in press; Xu et al., 2001)
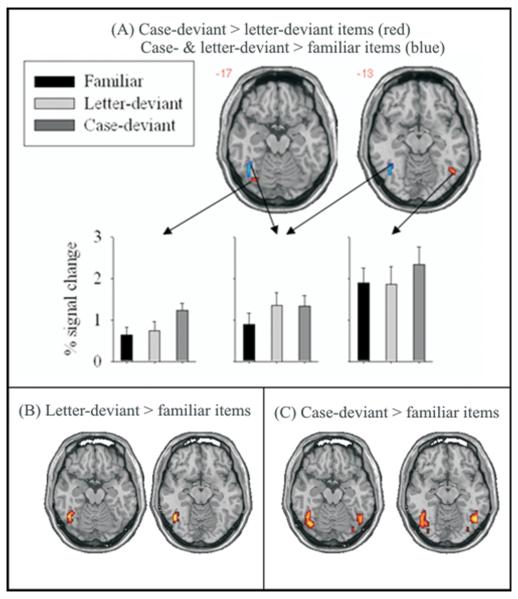
With this small-volume-correction, case-deviant led to higher activity than letter-deviant items in a right occipitotemporal and in a left posterior occipitotemporal region (see Figure 2 and the lower section of Table 2) at an FDR of q < .05. The opposite comparison (i.e., letter-deviant vs. case-deviant items) did not identify any occipitotemporal cluster, even at an uncorrected threshold of p < .01 In a further step, a conjunction analysis (Nichols et al., 2005) was performed to identify occipitotemporal voxels, where each of the two deviant forms led to increased activity compared to familiar items. This analysis, based on an FDR of q < .05 in the occipitotemporal volume of interest, identified a left middle occipitotemporal region (see Figure 2 and Table 2). For this region, and for the two occipitotemporal ones with higher activity to case- than letter-deviant forms, Figure 2 shows mean percentages of signal change (compared to the fixation baseline) for each item type. The signal change values were extracted with the MARSBAR toolbox (Brett et al., 2001). As evident from the means, in the right occipitotemporal and in the left posterior occipitotemporal region, case-deviant items evoked higher activation than letter-deviant and familiar items, whereas in the left middle occipitotemporal region, case-deviant and letter-deviant items elicited about the same increased activity compared to familiar items.
Figure 2.
Axial slices (L=L) showing clusters with increased activation for case-deviant compared to familiar items (red) and increased activation for both deviant item types compared to familiar items (blue) in the occipitotemporal volume of interest (FDR < .05). Mean percentages of signal change for all item types (compared to fixation baseline) in these clusters are shown in the lower section.
The difference between the item-type patterns in the left middle occipitotemporal and in the left posterior occipitotemporal and in the right occipitotemporal regions was confirmed by reliable item type by region interactions in two separate ANOVAs with signal change as dependent variable. In the first ANOVA, the left middle occipitotemporal region was contrasted with the left posterior occipitotemporal region; in the second ANOVA the left middle occipitotemporal region was contrasted with the right occipitotemporal region. In both ANOVAs the item type by region interaction was reliable, Fs(2, 38) > 5.9, ps < .007.
As each phonological word was repeated three times - in one session as familiar, in one as letter-deviant and in one as case-deviant item - one may be concerned that differential repetition effects may partly explain the item type effects. To examine this possibility, we performed a repeated-measures analysis in SPM2 including session as factor in addition to item type. For this analysis we used contrast images for each item type vs. fixation baseline for each session separately. If the item type effects of the previous analyses to some extent were due to differential repetition effects, this analysis should reveal an item type by repetition interaction on brain activity. The critical finding is that none of the regions with differential item type effects listed in Table 1 and shown in Figure 1 and 2, showed an item type by session interaction, even at an uncorrected threshold of p < .05.
Discussion
The present fMRI study was designed as a follow-up to a previous EEG study from our lab (Sauseng et al., 2004) which, surprisingly, found no ERP difference between letter-deviant and case-deviant items. Both types of deviance led to generally increased negativity from about 200 to 700 ms post-stimulus and specifically to an reduced positive recognition potential around 300 ms post-stimulus. We expected that, despite the similar ERP manifestation of orthographic and letter format violation, different brain regions are involved in orthographic word processing and letter format processing. We found support for this expectation. The only brain region with increased activation to both case-deviant and letter-deviant items was located in the left middle occipitotemporal cortex, and this region corresponds roughly to the VWFA (see below for details). The letter-deviant items such as Taksi compared to familiar items – in addition to the increased activation of the VWFA – led to increased activation in an extended left precentral region, in the left SMA, in left and right inferior frontal regions and in a left inferior parietal region. In contrast, the case-deviant items such as TaXi compared to familiar items – in addition to the increased activation of the VWFA – led to increased activation of an extended right occipitotemporal region and of a smaller left occipital and a left posterior occipitotemporal region. The right occipitotemporal and the left posterior occipitotemporal activations elicited by case-deviant items were also identified in the comparison with letter-deviant items when a small-volume-correction was used. This finding rules out that the increased activations to case-deviant items simply reflect prolonged inspection of the letter strings. As noted in the Method section, reading time for letter-deviant items was markedly prolonged compared to reading time for the case-deviant items. In relation to this finding, the observed increased activations to case-deviant items are of particular relevance.
There are plausible interpretations of the different activations in response to the two types of deviance. In terms of the basic features of the dual-route model of visual word processing (Coltheart et al., 2001), the letter-deviant items such as Taksi differ from both familiar items and case-deviant items as they require sublexical orthographic-phonological coding (e.g., grapheme-phoneme coding) for accessing the phonological word and its meaning. This access, in turn, may activate the associated stored orthographic word, which then allows to register the deviance. The increased left inferior frontal activation may reflect phonological sublexical processing (e.g., Fiez et al., 1999; Jobard, Crivello, & Tzourio-Mazoyer; 2003; Mechelli, Gorno-Tempini, & Price, 2003) and the increased left middle occipitotemporal activation may reflect orthographic sublexical coding and the mismatch between orthographic word representation and letter string information. Conversely, the reduced activation to familiar items compared to letter-deviant items in the left middle occipitotemporal cortex may reflect instantiation of a single orthographic word representation by the letter string and the reduced left frontal activation may reflect direct access to a single phonological word presentation. The presently found brain regions (left inferior frontal/precentral and left middle occipitotemporal) with increased activity to letter-deviant items largely correspond to those identified by Kronbichler et al. (in press). However, the extent of the brain regions identified in the previous study were larger than in the present one and this may have to do with task differences. The previous study required explicit phonological lexical decisions (“Does this letter string sound like an existing words?”), whereas the present study required only silent reading. Nevertheless, the correspondence between these studies, despite differing task demands, is encouraging.
As noted in the Introduction, the present case-deviant items such as TaXi constitute a mild form of cAsE-mIxInG which is commonly interpreted to affect letter-shape processing due to an increased number of possible letter-shapes per position or due to lateral interference from the misplaced upper-case letter (Mayall et al., 1997). Consistent with this low-level visual processing interpretation of the case-mixing effect, the case-deviant items (compared to familiar items) were found to lead to increased activation in a right occipitotemporal, a left posterior occipitotemporal and a left occipital region. As noted in the Introduction, the PET study by Mayall et al. (2001) found no effect of case mixing on left or right occipital and occipitotemporal activation. Rather, a case-mixing effect was found in a right parietal region – presumably reflecting increased visual attention. Recent studies using transcranial magnetic stimulation also support a role of the right parietal cortex in reading of case-mixed words (Braet & Humphreys, 2005; 2006). Possibly, our mild form of case-mixing with rather low visual attentional demands was insufficient to led to increased right parietal activation. However, from this hypothesis one would expect the standard form of case-mixing used by Mayall et al. (2001) should be reflected not only in right parietal activation but also in occipital and occipitotemporal activation. This was not the finding of the Mayall et al. (2001) study. The present left occipital activation to case-deviant items corresponds to the PET study by Xu et al. (2001) which found a case-mixing effect in left occipital regions centered y = −82 and −88. However, Xu et al. did not find the right occipitotemporal activation to case-deviant items of the present study. An fMRI study by Polk and Farah (2002) limited image acquisition to the occipitotemporal cortex and found increased activation (compared to same-case consonant strings) in the left occipitotemporal cortex both in response to mixed-case and pure-case words, but, consistent with the present findings, noted a trend towards higher activation for mixed-case words.
As already noted, the present finding that case-deviant items lead to increased activation in right occipitotemporal and in left occipital and posterior occipitotemporal regions is consistent with a low-level visual processing interpretation of the case-mixing effect. These findings are also consistent with the general assumption of neural model of Dehaene et al. (2005) of visual word processing which postulated increasingly abstract coding in the posterior to anterior direction in the occipital and occipitotemporal cortex. Not expected from the Dehaene et al. model is the finding of the present conjunction analysis that both case- and letter-deviant items led to increased activation of a left middle occipitotemporal region at around y = −57 which corresponds to the original VWFA location of Cohen et al. (2002), centered at −43, −54, −12, and is close to the mean coordinates of −44, −58, −15, found in a meta-analysis by Jobard et al. (2003) based on 17 imaging studies on visual word processing. One should note, that the present study cannot shed light on the controversy about whether the VWFA is specialized for visual word processing, as might be suggested by the term VWFA (see Cohen & Dehaene, 2004; Devlin et al., 2006; Price & Devlin, 2003; Xue & Poldrack, in press).
For the middle occipitotemporal region, the Dehaene et al. (2005) model and the original VWFA hypothesis expects that case-specific information is already lost and processing operates on abstract case-independent letter codes. This assumption is based subliminal masked priming studies, which found reduced activation to target words in the VWFA, independent from whether the targets were preceded by the same words in same-case or cross-case format (Dehaene et al., 2001). Cross-case repetition priming in the VWFA was also found when upper- and lower-case of the letters were dissimilar (Dehaene et al., 2004). A straightforward interpretation of the present finding of VWFA activation by both case-deviant and letter-deviant items would assume that the VWFA contains orthographic word representations, which specify the sequence of the letter identities but also the most frequently encountered appearance of the word in terms letter-case information. The deviance of our case- and letter-deviant items from such representations leads to the increased activations. These representation assumption apparently finds no support in the mentioned priming studies. However, intuitively, the assumption that orthographic word representations specify letter identity and letter-case information has appeal. To illustrate, cognitive psychologist would be astonished when confronted with mcclelland instead of McClelland, and proper names in the usual appearance with first letter capitalized were indeed found to be recognized faster than without (Peressotti, Cubelli, and Job, 2003) although the most frequent appearance of Italian words – the context of the Peressotti et al. study – is all letters in lower-case format. These questions on the nature of orthographic word representation may be the focus of further imaging research.
Acknowledgements
This research was supported by grants of the Austrian Science Foundation to Heinz Wimmer and Gunther Ladurner (Grant Numbers P 14494-SPR and P18832-B02). We are grateful to the members of the Department of Radiology for assistance, and to Bettina Lackner and Simon Schindlauer for their help in subject recruitment and data acquisition.
References
- Baayen RH, Piepenbrock R, van Rijn H. The CELEX lexical database (CD-ROM) Linguistic Data Consortium, University of Pennsylvania; Philadelphia, PA: 1993. [Google Scholar]
- Braet W, Humphreys GW. Case mixing and the right parietal cortex: evidence from rTMS. Experimental Brain Research. 2005;168:265–271. doi: 10.1007/s00221-005-0085-z. [DOI] [PubMed] [Google Scholar]
- Braet W, Humphreys G. The “Special Effect” of Case Mixing on Word Identification: Neuropsychological and Transcranial Magnetic Stimulation Studies Dissociating Case Mixing from Contrast Reduction. Journal of Cognitive Neuroscience. 2006;18:1666–1675. doi: 10.1162/jocn.2006.18.10.1666. [DOI] [PubMed] [Google Scholar]
- Brett M, Anton J-L, Valabregue R, Poline J-B. Region of interest analysis using an SPM toolbox. Presented at the 8th International Conference on Functional Mapping of the Human Brain; Sendai, Japan. June 2-6, 2002; 2002. Abstract available on CD-ROM in NeuroImage, Vol 16, No 2. [Google Scholar]
- Cohen L, Dehaene S. Specialization within the ventral stream: the case for the visual word form area. NeuroImage. 2004;22:466–476. doi: 10.1016/j.neuroimage.2003.12.049. [DOI] [PubMed] [Google Scholar]
- Cohen L, Lehericy S, Chochon F, Lemer C, Rivaud S, Dehaene S. Language-specific tuning of visual cortex? Functional properties of the Visual Word Form Area. Brain. 2002;125:1054–1069. doi: 10.1093/brain/awf094. [DOI] [PubMed] [Google Scholar]
- Coltheart M, Rastle K, Perry C, Langdon R, Ziegler J. DRC: A dual route cascaded model of visual word recognition and reading aloud. Psychological Review. 2001;108:204–256. doi: 10.1037/0033-295x.108.1.204. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Cohen L, Sigman M, Vinckier F. The neural code for written words: a proposal. Trends in Cognitive Sciences. 2005;9:335–341. doi: 10.1016/j.tics.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Jobert A, Naccache L, Ciuciu P, Poline JB, Le Bihan D, Cohen L. Letter binding and invariant recognition of masked words - Behavioral and neuroimaging evidence. Psychological Science. 2004;15:307–313. doi: 10.1111/j.0956-7976.2004.00674.x. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Naccache L, Cohen L, Le Bihan D, Mangin JF, Poline JB, Riviere D. Cerebral mechanisms of word masking and unconscious repetition priming. Nature Neuroscience. 2001;4:752–758. doi: 10.1038/89551. [DOI] [PubMed] [Google Scholar]
- Devlin JT, Jamison HL, Gonnerman LM, Matthews PM. The role of the left posterior fusiform gyrus in reading. Journal of Cognitive Neuroscience. 2006;18:911–922. doi: 10.1162/jocn.2006.18.6.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiez JA, Balota DA, Raichle ME, Petersen SE. Effects of lexicality, frequency, and spelling-to-sound consistency on the functional anatomy of reading. Neuron. 1999;24:205–218. doi: 10.1016/s0896-6273(00)80833-8. [DOI] [PubMed] [Google Scholar]
- Fiset S, Arguin M. Case alternation and orthographic neighborhood size effects in the left and right cerebral hemispheres. Brain and Cognition. 1999;40:116–118. [Google Scholar]
- Friston KJ, Glaser DE, Henson RNA, Kiebel S, Phillips C, Ashburner J. Classical and Bayesian inference in neuroimaging: Applications. NeuroImage. 2002;16:484–512. doi: 10.1006/nimg.2002.1091. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Rotshtein P, Geng JJ, Sterzer P, Henson RN. A critique of functional localisers. NeuroImage. 2006;30:1077–1087. doi: 10.1016/j.neuroimage.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15:870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- Holmes AP, Friston KJ. Generalisability, random effects and population inference. NeuroImage. 1998;7:754. [Google Scholar]
- Jobard G, Crivello F, Tzourio-Mazoyer N. Evaluation of the dual route theory of reading: a metanalysis of 35 neuroimaging studies. NeuroImage. 2003;20:693–712. doi: 10.1016/S1053-8119(03)00343-4. [DOI] [PubMed] [Google Scholar]
- Jordan TR, Redwood M, Patching GR. Effects of form familiarity on perception of words, pseudowords, and nonwords in the two cerebral hemispheres. Journal of Cognitive Neuroscience. 2003;15:537–548. doi: 10.1162/089892903321662921. [DOI] [PubMed] [Google Scholar]
- Kronbichler M, Bergmann J, Hutzler F, Staffen W, Mair A, Ladurner G, Wimmer H. Taxi vs. Taksi: Orthographic word recognition in the left ventral occipitotemporal cortex. Journal of Cognitive Neuroscience. doi: 10.1162/jocn.2007.19.10.1584. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronbichler M, Hutzler F, Wimmer H, Mair A, Staffen W, Ladurner G. The visual word form area and the frequency with which words are encountered: evidence from a parametric fMRI study. NeuroImage. 2004;21:946–953. doi: 10.1016/j.neuroimage.2003.10.021. [DOI] [PubMed] [Google Scholar]
- Lavidor M, Ellis AW. Mixed-case effects in lateralized word recognition. Brain and Cognition. 2001;46:192–195. doi: 10.1016/s0278-2626(01)80063-4. [DOI] [PubMed] [Google Scholar]
- Mayall K, Humphreys GW, Mechelli A, Olson A, Price CJ. The Effects of Case Mixing on Word Recognition: Evidence from a PET Study. Journal of Cognitive Neuroscience. 2001;13:844–853. doi: 10.1162/08989290152541494. [DOI] [PubMed] [Google Scholar]
- Mayall K, Humphreys GW, Olson A. Disruption to Word or Letter Processing? The Origins of Case-Mixing Effects. Journal of Experimental Psychology: Learning, Memory and Cognition. 1997;23:1275–1286. doi: 10.1037//0278-7393.23.5.1275. [DOI] [PubMed] [Google Scholar]
- McCandliss BD, Cohen L, Dehaene S. The visual word form area: expertise for reading in the fusiform gyrus. Trends in Cognitive Science. 2003;7:293–299. doi: 10.1016/s1364-6613(03)00134-7. [DOI] [PubMed] [Google Scholar]
- Nichols T, Brett M, Andersson J, Wager T, Poline J-B. Valid conjunction inference with the minimum statistic. NeuroImage. 2005;25:653–660. doi: 10.1016/j.neuroimage.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Perea M, Rosa E. Does “whole-word shape” play a role in visual word recognition? Perception and Psychophysics. 2002;64:785–794. doi: 10.3758/bf03194745. [DOI] [PubMed] [Google Scholar]
- Peressotti F, Cubelli R, Job R. On recognizing proper names: The orthographic cue hypothesis. Cognitive Psychology. 2003;47:87–116. doi: 10.1016/s0010-0285(03)00004-5. [DOI] [PubMed] [Google Scholar]
- Polk TA, Farah MJ. Functional MRI evidence for an abstract, not perceptual, word-form area. Journal of Experimental Psychology: General. 2002;131:65–72. doi: 10.1037//0096-3445.131.1.65. [DOI] [PubMed] [Google Scholar]
- Price CJ, Devlin JT. The myth of the visual word form area. NeuroImage. 2003;19:473–481. doi: 10.1016/s1053-8119(03)00084-3. [DOI] [PubMed] [Google Scholar]
- Rossion B, Schiltz C, Crommelinck M. The functionally defined right occipital and fusiform “face areas” discriminate novel from visually familiar faces. NeuroImage. 2003;19:877–883. doi: 10.1016/s1053-8119(03)00105-8. [DOI] [PubMed] [Google Scholar]
- Rudell AP, Hua J. The recognition potential, word difficulty and individual reading ability: on using event-related potentials to study perception. Journal of Experimental Psychology: Human Perception and Performance. 1997;23:1170–1195. doi: 10.1037//0096-1523.23.4.1170. [DOI] [PubMed] [Google Scholar]
- Sauseng P, Bergmann J, Wimmer H. When does the brain register deviances from standard word spelling?-An ERP study. Cognitive Brain Research. 2004;20:529–532. doi: 10.1016/j.cogbrainres.2004.04.008. [DOI] [PubMed] [Google Scholar]
- Seidenberg MS, Tanenhaus MK. Orthographic Effects on Rhyme Monitoring. Journal of Experimental Psychology-Human Learning and Memory. 1979;5:546–554. van. [PubMed] [Google Scholar]
- Turennout M, Ellmore T, Martin A. Long-lasting cortical plasticity in the object naming system. Nature Neuroscience. 2000;3:1329–1334. doi: 10.1038/81873. [DOI] [PubMed] [Google Scholar]
- Wager TD, Nichols TE. Optimization of experimental design in fMRI: a general framework using a genetic algorithm. NeuroImage. 2003;18:293–309. doi: 10.1016/s1053-8119(02)00046-0. [DOI] [PubMed] [Google Scholar]
- Xu B, Grafman J, Gaillard WD, Ishii K, Vega-Bermudez F, Pietrini P, Reeves-Tyer P, DiCamillo P, Theodore W. Conjoint and Extended Neural Networks for the Computation of Speech Codes: The Neural Basis of Selective Impairment in Reading Words and Pseudowords. Cerebral Cortex. 2001;11:267–277. doi: 10.1093/cercor/11.3.267. [DOI] [PubMed] [Google Scholar]
- Xue G, Poldrack RA. The Neural Substrates of Visual Perceptual Learning of Words: Implications for the Visual Word Form Area Hypothesis. Journal of Cognitive Neuroscience. doi: 10.1162/jocn.2007.19.10.1643. (in press) [DOI] [PubMed] [Google Scholar]