Abstract
Mammalian spermatozoa lose plasma membrane cholesterol during their maturation in the epididymis and during their capacitation in the female reproductive tract. While acceptors such as high-density lipoproteins (HDL) and apolipoproteins A-I (apoA-I) and J have been found in male and female reproductive tracts, transporters that mediate cholesterol efflux from plasma membranes of spermatozoa to such acceptors have not yet been defined. Candidate transporters are members of the ATP-binding cassette (ABC) transporter superfamily including ABCA1, ABCA7, ABCG1 and ABCG4, which have all been implicated in the transport of sterols and phospholipids to apolipoproteins and HDL. Here we show that mouse spermatozoa in the seminiferous tubules and epididymis express ABCA1, ABCA7 and ABCG1, but not ABCG4. Moreover, we show that ABCA1, ABCA7 and ABCG1 antibodies decrease cholesterol efflux from spermatozoa to lipid acceptors apoA-I and albumin and inhibit in vitro fertilization.
Keywords: ATP-binding cassette transporters, cholesterol efflux, in vitro fertilization, sperm, mouse
INTRODUCTION
The loss of cholesterol from the spermatozoa plasma membrane is key to spermatozoa maturation and capacitation, which together render the spermatozoa capable of interacting with an oocyte and inducing the acrosome reaction [1–3]. In the male reproductive tract, the cholesterol acceptors apoA-I and apoJ are both secreted by the epididymis and implicated in spermatozoa maturation [4–6]. However, the significance and role of these epididymal proteins are still poorly understood. ApoA-I and apoJ are also synthesized by epithelial cells of the uterus and oviduct [7, 8]. As components HDL particles, these apolipoproteins are thought to mediate association of HDL particles with spermatozoa plasma membranes and to serve as cholesterol and phospholipid acceptors in the process of lipid efflux. Lipid efflux is considered one of the key events of spermatozoa capacitation that leads to acrosome reaction and fertilization. Apolipoprotein particles enriched with spermatozoa lipids are believed to deliver the cholesterol to principal cells of the epididymis [4, 5, 9] and to epithelial cells of the female reproductive tract where the apolipoprotein particles can be endocytosed via endocytic receptors such as megalin/LRP-2 and cubilin [7].
Although the mechanism by which cholesterol is effluxed from spermatozoa plasma membranes to lipid acceptors is not known, ABC transporters including ABCA1, ABCA7, ABCG1 and ABCG4 represent candidates since they mediate the transport of cholesterol from other types of cells to lipid-poor apoA-I and to lipoprotein particles (e.g., HDL) [10]. Here we evaluated murine spermatozoa for the expression of ABC transporters known to support apolipoprotein-mediated cholesterol release. We also evaluated their roles in the transport of cholesterol from the sperm plasma membrane to lipid-poor apoA-I and albumin and in facilitating fertilization.
MATERIALS AND METHODS
Antibodies
Rabbit ABCA1 antibody was purchased from Novus Biologicals (Littleton, CO). Rabbit antibodies to ABCG1 and ABCG4 were from Alpha Diagnostics International (San Antonio, TX). Monoclonal ABCA7 antibody (KM3096) [11] was provided by Dr. Katzumitsu Ueda (Kyoto University, Japan) and Kyowa Hakko Kogyo Co. Ltd (Tokyo, Japan).
Immunocytochemistry
Testes and epididymides from CD-1 mice (Charles River, Montreal, QC) and 7-week mice homozygous for targeted deletion of ABCA1 [12] (provided by Dr. Yves Marcel, University of Ottawa Heart Institute) were immersed in Bouin’s or in 5% paraformaldehyde. The tissues were embedded in paraffin, sectioned and immunostained with ABCA1, ABCG1 and ABCG4 antibodies as previously described [5, 6]. For ABCA7 staining, sections were blocked with 2.5% horse serum in PBS prior to antibody incubation. Sections were incubated for 30 min with horse anti-rabbit/mouse Biotinylated Universal Antibody (Invitrogen, Burlington, ON) and incubated with Vectastain Elite ABC Reagent (Burlingame, CA).
For confocal microscopy, the caput epididymis was removed and placed in HEPES-buffered Krebs Ringers bicarbonate (KRB-HEPES) and minced. The spermatozoa-containing supernatant was collected, centrifuged at 800 × g at rt for 5 min and the pellet resuspended in 2ml PBS. An aliquot (100µl) of the suspension was fixed on a glass slide with 3.7% formaldehyde for 10 min and blocked with 3% goat serum or with 2% horse serum for 30 min. The slides were incubated with ABCA1, ABCA7 or ABCG1 antibodies or non-immune IgG for 60 min at rt and washed with PBS. The slides were incubated with FITC-conjugated secondary antibodies followed by PBS washing. Nuclei were stained with Hoechst 33342 (Molecular Probe, Eugene, OR).
RNA isolation and RT-PCR
Testes from an adult male CD-1 mouse were removed, washed with Hank's Balanced Salt Solution (HBSS), decapsulated and minced. The seminiferous tubules were then suspended in 10 ml of HBSS containing 0.4 mg/ml collagenase, 0.664 mg/ml DNaseI, 6 mM sodium pyruvate and 2 mM sodium L-lactate and incubated at 37°C for 10 min. Trypsin (18 mg) was added and the suspension incubated for 15 min with agitation. The supernatant, containing germ cells, was collected and subjected to centrifugation at 700×g for 5 min. The pellet containing 90% spermatids was resuspended in HBSS and 2×107 cells extracted using an Oligotex RNA isolation kit (Qiagen). Total RNA was isolated from mouse neonatal brain using Trizol (Invitrogen, Carlsbad, CA). cDNA was made from total RNA (1µg) using an iScript kit (BioRad, Hercules, CA). Primers pairs and cycling conditions used for RT-PCR are shown in Supplemental Table I.
Immunoblot analysis of spermatozoa extracts
The epididymides were separated into caput, corpus and cauda. The tissues were placed in Dulbecco’s Modified Eagle Medium (Invitrogen Corporation, Burlington, ON) containing Complete Protease Inhibitor Cocktail (Roche, Palo Alto, CA), and minced. The spermatozoa-containing supernatant was collected and centrifuged at 500×g, 4°C for 5 min. The spermatozoa were resuspended in 100µl of 1.0% NP40, 154mM NaCl, 0.4mM Tris pH8.0 containing protease inhibitors (Roche). After 30 min incubation, the lysate was centrifuged at 10,000×g, 4°C for 10 min. Aliquots (20µg protein) were subjected to SDS-PAGE (reducing conditions) and transferred to Hybond-ECL membranes (Amersham Biosciences, Piscataway, NJ). Detection was achieved using the ECL+ Western Blotting Detection System (Amersham).
Spermatozoa cholesterol efflux assays
Caput, corpus and cauda epididymides (n=3) were removed and finely chopped and transferred to 5ml of KRB-HEPES. The homogenates were incubated at 37°C for 10 min and the released spermatozoa pelleted by centrifugation at 500×g, for 10 min at 25°C. The pellets were resuspended in 2ml of KRB-HEPES at 1×107 spermatozoa/ml. An aliquot (100µl) of the cell suspension was mixed with 100µl of 50µg/ml human apoA-I in KRB-HEPES plus and minus antibodies to ABCA1 (10µg IgG/ml) or ABCG1 (5µg IgG/ml) and incubated at 37°C and 5%CO2 for 1h. In control reactions, spermatozoa were mixed 1:1 with KRB-HEPES lacking apoA-I. In separate experiments, delipidated BSA was used instead of apoA-I. After 1h incubation, spermatozoa were pelleted at 500×g for 10 min, the supernatant saved and the pellet washed with KRB and resuspended in 140µl of PBS. Aliquots (50µl) of the spermatozoa suspension and supernatant were analyzed for cholesterol using an Amplex Red Cholesterol Kit (Molecular Probes).
Fertilization assays
Epididymides were in M2 capacitating medium (M2) [13] plus and minus ABC transporter antibodies (0.1µg IgG/ml). The contents of the epididymides were squeezed out, using sterile forceps. Spermatozoa were allowed to "swim out" for 10 min at 37°C, 5%CO2. The spermatozoa were overlaid with 2x volume of M2 for 30 min at 37°C, 5%CO2 for “swim-up”. The upper 1/3 of the suspension was taken and adjusted to 1–5×106 spermatozoa/ml for fertilization. Oocytes were isolated from 10-week CD-1 females superovulated by intraperitoneal injection of 7 IU pregnant mare serum gonadotropin (Sigma) followed 24h later with 5.0 IU human chorionic gonadotropin (hCG, Sigma). Oocytes were collected from oviducts into antibody-containing M2 15h after hCG injection. Cumulus oophorus cells were removed by treatment with 0.1% hyaluronidase in M2. The cumulus-free oocytes were rinsed in M2 and groups of 10 oocytes fertilized with spermatozoa at 37°C, 5%CO2. Fertilization was assessed 18h later by counting the number of zygotes as compared to oocytes [14].
Statistical analysis
The Chi-square test was used to test the significance of differences between groups.
RESULTS
ABC transporter expression in the testis
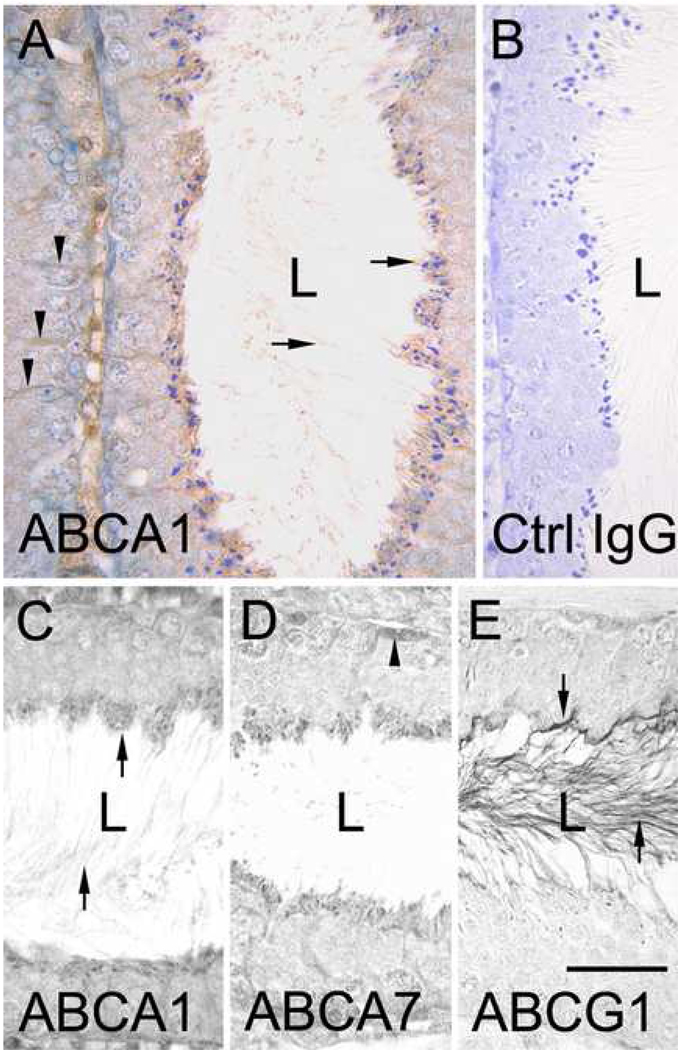
Immunohistological analysis of testicular sections revealed ABCA1 staining of the cytoplasm of Sertoli cells (Fig. 1A, C). ABCA1 was also detected on the head and tail of step 16 spermatids (stage VII–VIII of spermatogenesis) (Fig. 1A, C). By contrast, spermatogonia, spermatocytes and round spermatids (step 1–7) and elongating spermatids (step 8–15), showed no reactivity with ABCA1 antibody (Fig. 1A).
Figure 1. Immunolocalization of ABCA1, ABCA7 and ABCG1 in mouse seminiferous tubules.
A, anti-ABCA1 immunohistochemical labeling of a seminiferous tubule at stage VII of the cycle. Arrows in A points to staining of the heads of late step 16 spermatids (located at apical border) and on the tails of the spermatids in the lumen (L). Arrowheads in A point to anti-ABCA1 staining in Sertoli cells. B, control IgG labeling of a seminiferous tubule. C, D and E, comparative immunolabeling of seminiferous tubules at stage VII using antibodies to ABCA1, ABCA7 and ABCG1, respectively. Arrows in C point to ABCA1 staining of the heads and tails of step 16 spermatids. Arrowheads in D point to anti-ABCA7 staining of a spermatogonium. Arrows in E point to anti-ABCG1 staining of the head and tail (in lumen) of late step 16 spermatids. Bar in A=30µm and applies to A and B. Bar in E=25µm and applies to C–E.
The expression of ABCA7 in testis was similar to that of ABCA1. Sertoli cells exhibited staining, as did the heads and tails of step 16 spermatids, albeit the relative level of ABCA7 staining of the tails was less than that observed for ABCA1. No ABCA7 expression was detected in spermatogonia, spermatocytes or round spermatids (Fig. 1D).
By contrast to ABCA1 and ABCA7, only faint, intermittent ABCG1 immunolabeling was observed in Sertoli cells. While there was pronounced ABCG1 staining in the tails of step 16 spermatids, the heads were negative. No ABCG1 expression was evident in spermatogonia, spermatocytes or round spermatids (Fig. 1E).
No ABCG4 immunoreactivity was detected in the testis. However, the ABCG4 antibody was found to react with other mouse tissues including the brain cortex in which neuronal cells were positive (data not shown). The results of histological analysis of ABC transporter expression in the testis are summarized in Supplemental Table II.
ABCA1, ABCA7, and ABCG1 transcripts were detectable in spermatid RNA preparations, however, ABCG4 RNA was not detectable. The ability of the ABCG4 primers to specifically amplify mouse ABCG4 was verified using brain cDNA. The findings obtained from RT-PCR analysis are consistent with those from immunohistological analyses (Supplemental Fig 1).
ABC transporter expression in the epididymis
Immunostaining of epididymal sections revealed ABCA1 staining of the heads and tails of spermatozoa located throughout the caput, corpus and cauda epididymis (Supplemental Fig. 2A, B). Principal cells of the caput, corpus and cauda epididymis also exhibited a pronounced cytoplasmic ABCA1 immunostaining (Supplemental Fig. 2A–C). A stronger staining was observed along the apical surface at the base of the microvilli (Supplemental Fig. 2A–C, arrows). The degree of ABCA1 immunostaining of principal cells was similar in all three region of the epididymis.
ABCA7 immunostaining was detected on spermatozoa throughout the epididymis. As seen in the testis, ABCA7 was detected on the heads of spermatozoa in the epididymis (Supplemental Fig. 2D–F). Principal cells of the epididymal epithelium were also positive for ABCA7. As for ABCA1, a stronger degree of ABCA7 staining was observed along the apical surface at the base of the microvilli (Supplemental Fig. 2D–F, arrows). The degree of ABCA7 immunostaining of Principal cells was similar in all three regions of the epididymis.
ABCG1 immunolabeling of spermatozoa in the caput and corpus epididymidis was slightly stronger as compared to spermatozoa in the cauda epididymis (Supplemental Fig. 2G,I). Principal cells in the caput exhibited prominent staining of microvilli, with occasional cells showing cytoplasmic staining. Moderate to strong ABCG1 staining was also apparent on the microvilli in the corpus and cauda, but no cytoplasmic staining was observed.
No ABCG4 immunostaining was apparent in any of the three segments of the epididymis (data not shown). The results of histological analysis of ABC transporter expression in the epididymis are summarized in Supplemental Table III.
Localization of ABC transporters in epididymal spermatozoa
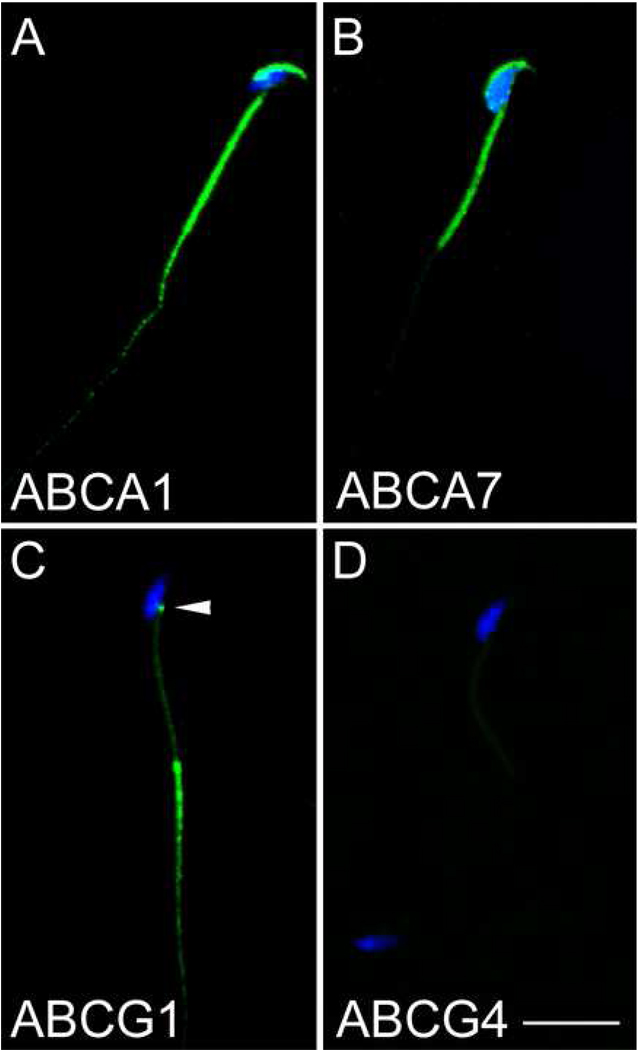
ABCA1 and ABCA7 were detected on the dorsal aspect of sperm heads and on the middle piece of the sperm tail (Fig. 2A and B). Moderate levels of ABCA1 immunolabeling, but no ABCA7 labeling were detected on the first third portion of the principal piece of sperm tails. No ABCG1 immunoreactivity was apparent in either the dorsal aspect of the sperm head or in the middle piece of the sperm tail. However, strong ABCG1 immunolabeling of the principal piece of the sperm tail was observed. Furthermore, ABCG1 immunolabeling was also detected in the capitulum located where the mid piece connects to the ventral region of the sperm head (Fig. 2C arrowhead). No ABCG4 immunolabeling was detectable on spermatozoa (Fig. 2D).
Figure 2. Analysis of ABCA1, ABCA7 and ABCG1 expression in spermatozoa.
A–D, confocal microscopic analysis of ABC transporter expression in spermatozoa. Spermatozoa isolated from the caput epididymis were immunolabeled with antibodies to ABCA1 (A), ABCA7 (B), ABCG1 (C) and ABCG4 (D) and detected with FITC-conjugated anti-IgG (green). Nuclei were stained with Hoechst 33342 (blue). Bar in D=10µm.
ABC transporter expression in sperm preparations
ABCA1, ABCA7 and ABCG1 were detected in extracts of sperm obtained from each region of the epididymis. Consistent with findings from immunocytochemical analyses, relatively lower levels of ABCG1 were detected in membrane extracts of cauda spermatozoa. Also consistent with the immunohistological data was that no ABCG4 was detectable in membrane extracts of epididymal spermatozoa (Supplemental Fig. 3).
Involvement of ABC transporters in spermatozoa cholesterol efflux
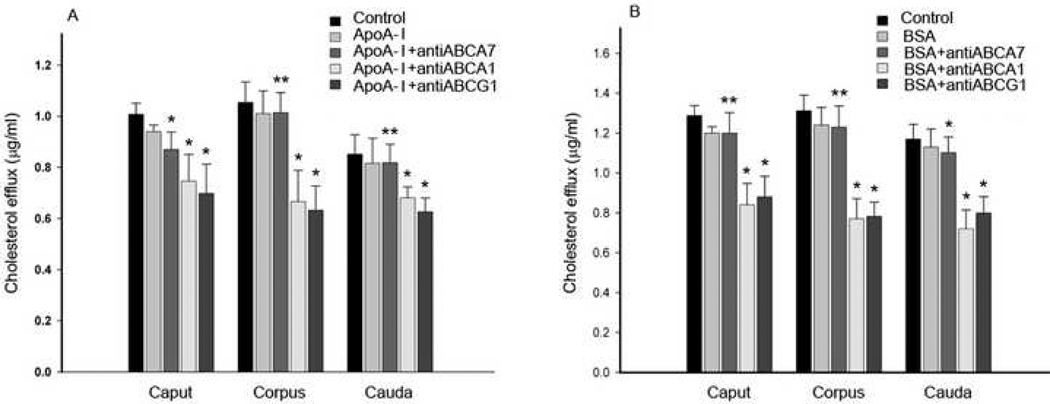
ABCA1, ABCA7 and ABCG1 antibodies were found to effectively inhibit cholesterol efflux to apoA-I (Fig. 3A) and albumin (Fig. 3B) by 15–40%. Inhibition of cholesterol efflux using antibodies to ABCA1, ABCA7 and ABCG1 was achieved using spermatozoa isolated from each of the three segments of the epididymis. The level of inhibition elicited by each of the antibodies was statistically significant (p<0.05) as compared to controls with or without ApoA-I. Consistent with the finding that the ABC antibodies reduced cholesterol efflux were findings that ABC antibody treatment caused an increase in cell-associated cholesterol (7–15% greater compared to spermatozoa treated with control IgG, data not shown).
Figure 3. ABC transporter antibody treatment of spermatozoa affects cholesterol efflux and inhibits in vitro fertilization.
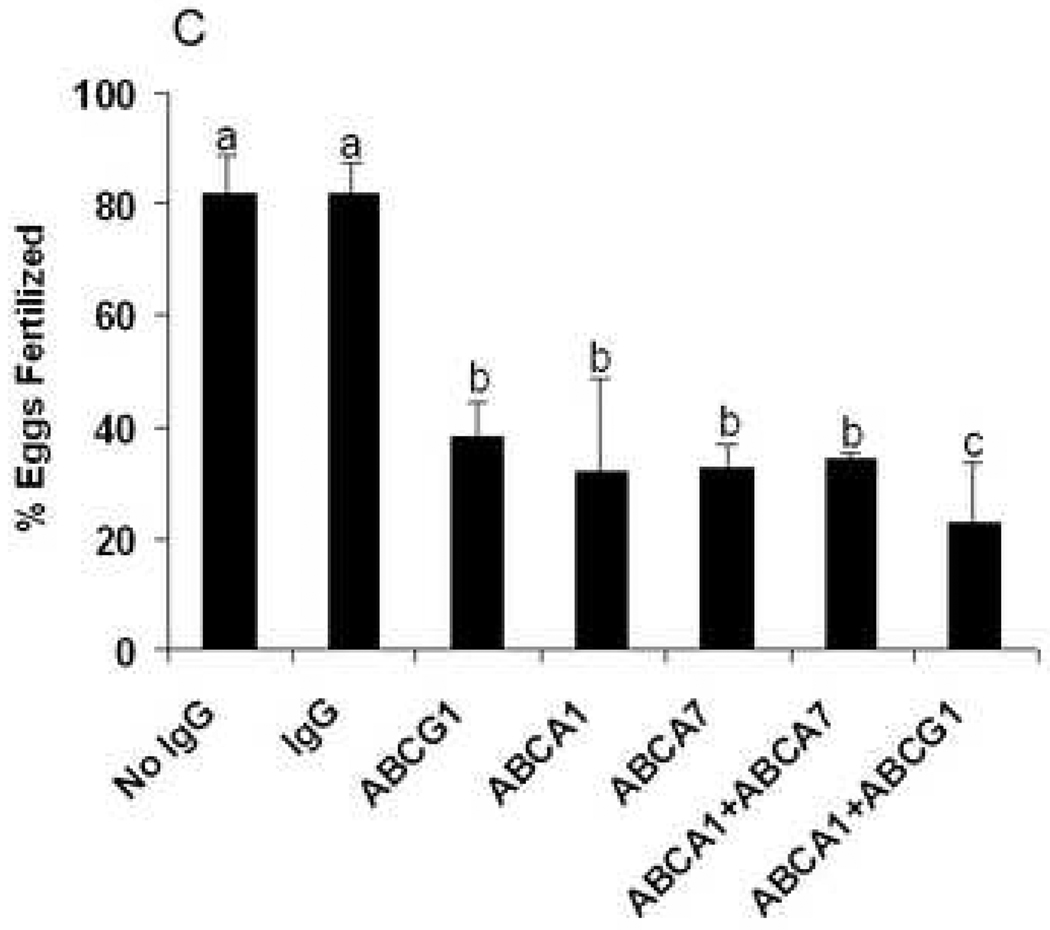
A, levels of spermatozoa cholesterol efflux to apoA-I in the absence or presence of antibodies to ABCA1, ABCA7 or ABCG1. B, levels of spermatozoa cholesterol efflux to BSA in the absence or presence of antibodies to ABCA1, ABCA7 or ABCG1. As controls, spermatozoa were incubated with IgG without apoA-I (Control) or with apoA-I or with BSA alone. * indicates that when compared to both controls the difference is significant (p<0.05). ** indicates that when compared to the IgG control without apoA-I or BSA the difference is significant (p<0.05). C, epididymides spermatozoa were treated with control antibody (IMA32) or antibodies to ABCA1, ABCA7 and ABCG1 (0.1µg IgG/ml), either alone or in combination and incubated with groups of 10 oocytes. Fertilization was assessed 18h later. The plotted values are means from assessments of the indicated number of oocytes for each treatment. The difference between a and b or b and c are significant at the p<0.05 level.
Evaluation of ABCA1, ABCA7 and ABCG1 in fertilization
The level of oocyte fertilization was reduced between 50–70% when spermatozoa were treated with antibodies to ABCA1, ABCA7 or ABCG1 (Fig. 3C). The level of inhibition by each of the antibodies was statistically significant (p<0.05) as compared to the no IgG treatment or treatment with control IgG. Treatment with a combination of ABCA1 plus ABCA7 antibodies or ABCA1 plus ABCG1 antibodies did not produce a significant level of inhibition over that of the individual antibodies.
DISCUSSION
Maturation and capacitation are processes that mammalian spermatozoa must undergo to become fully competent to fertilize an egg. Of critical importance is the loss of cholesterol from the spermatozoa plasma membrane, which destabilizes lipid raft structures in the plasma membrane and initiates protein phosphorylation-based signaling cascades [15]. The loss of cholesterol from the plasma membranes of spermatozoa is mediated by lipid-binding proteins present in epididymal, uterine and oviduct fluids. ApoA-I and apoJ are two major lipid-binding proteins in these fluids, which are constituents of HDL [16]. In the male reproductive tract, apoAI and apoJ are both secreted by epididymal epithelial cells [4] and apoJ was shown to interact with spermatozoa membranes [5, 6]. Both apolipoproteins have also been shown to dissociate from spermatozoa and to be endocytosed by epididymal epithelial cells via the endocytotic receptors megalin and cubilin [4, 5, 9, 17, 18]. ApoA-I and apoJ are also expressed in the female reproductive tract by the uterine and oviduct epithelium [7]. Incubation of spermatozoa with oviductal fluid causes a transfer of cholesterol from spermatozoa to apoA-I-containing HDL in the fluid [19].
Our findings indicate that ABCA1, ABCA7 and ABCG1 are expressed by mouse spermatozoa in the seminiferous tubules and epididymis and that these transporters function to mediate cholesterol efflux from spermatozoa to lipid acceptors. The physiological significance of these transporters is underscored by the finding that antibodies to ABCA1, ABCA7 and ABCG1 inhibit the capacity of spermatozoa to fertilize oocytes. These findings imply that mutation of ABCA1, ABCA7 and ABCG1 transporter genes or other conditions that impair their expression could reduce male fertility. Indeed, ABCA1-deficient mice display a significant reduction (21%) in fertility [12]. In this regard, it is important to note that alterations in Sertoli cell and/or a Leydig cell functions may also be contributing factors given the fact that ABCA1 is also expressed by these cells [12]. Infertility has not been reported in human males carrying mutations in ABCA1 that cause Tangier disease [12, 20, 21]. However, there are no studies of males with ABCA1 mutations that have focused on the status of sperm function by assessing motility, acrosome reaction, sperm-egg binding and fertilization capacity. In addition, there are wide variations in clinical lipid phenotypes observed in individuals with mutations in ABCA1 that may obscure fertility abnormalities. It is also possible that lost of ABCA1 function may be compensated by other ABC transporters. In support of this are our findings showing that ABCG1 expression is increased in both the spermatozoa and epithelial lining of the epidydimides from ABCA1-deficient mice as compared to wild-type mice (Supplemental Fig. 2J–R). Furthermore, other ABC transporters may be involved. For example, ABCA17 is expressed in mouse spermatocytes,[22] however, its role in lipid transport remains to be established.
We also show that ABCA1, ABCA7 and ABCG1 are expressed in a regionally distinct manner in spermatozoa. Treatment of spermatozoa with albumin alters the lipid composition of plasma membrane differently in the major morphological regions [23]. Regional alterations in membrane lipid composition may correlate with regionally distinct responses to lipid acceptor treatments such as induction of the acrosome reaction and hyper flagellar activity. Thus, it may be speculated that the observed regionalization in the distribution of spermatozoa ABC transporters may account for regional changes in membrane lipid composition that are induced by lipid acceptors, such as albumin.
Based on findings from this and other studies we have formulated a model for cholesterol loss by mammalian spermatozoa during maturation and capacitation. The model entails apoA-I and apoJ being synthesized and secreted by the epididymal epithelium [4–6]. The model further predicts that these apolipoproteins bind to ABCA1, ABCA7 and ABCG1 on the epididymal plasma membrane in the caput were transporters translocate cholesterol and phospholipids from plasma membrane to the apolipoproteins bound to the transporters. This results in the formation of nascent HDL particles which then bind to the ABCA1 and ABCA7 on the head and ABCG1 on the tail of spermatozoa and accept cholesterol and phospholipids being effluxed from the plasma membrane. Subsequently, the HDL particles become removed by the epididymal epithelium via receptor-mediated endocytosis by megalin/LRP-2 and cubilin [17, 18]. Once spermatozoa enter the female reproductive tract, an analogous process may occur in which HDL particles shuttle cholesterol and phospholipids from the spermatozoa to the uterine and oviduct epithelium via the action of the ABC transporters, megalin/LRP-2 and cubilin [7], culminating in capacitation of the spermatozoa.
In summary, ABCA1, ABCA7 and ABCG1 are expressed on the surface of mouse spermatozoa. ABCA1, ABCA7 and ABCG1 antibody treatment of immature spermatozoa reduces cholesterol efflux to lipid acceptors and inhibits in vitro fertilization. The findings suggest that these transporters play a role the process of sterol efflux which renders spermatozoa capable of interacting and fertilizing an oocyte.
Supplementary Material
RT-PCR reactions were performed on cDNA templates made from RNA isolated from round and elongating spermatids (S) and neonatal brain (B). The sizes of the amplicons generated in the PCR reactions corresponded to expected values.
A–I show immunostaining of tissues from wild-type mice. A–C, anti-ABCA1 immunohistochemical labeling of caput, corpus and cauda epididymides. D–F, anti-ABCA7 immunohistochemical labeling of caput, corpus and cauda epididymides. G–I, anti-ABCG1 immunohistochemical labeling of caput, corpus and cauda epididymides. Bar in I equals 30 µm and applies to A–H. J–R shows ABCA1, ABCG1 and non-immune IgG immunostaining of tissues from ABCA1-null mice. J–L immunostaining of caput, corpus and cauda epididymides with anti-ABCA1 antibody. M–O immunostaining of caput, corpus and cauda epididymides with anti-ABG1 antibody. P–R immunostaining of caput, corpus and cauda epididymides with non-immune rabbit IgG. Note that while the relative level of ABCA7 immunostaining was not different in epididymal tissues from ABCA1-deficient and wild-type mice (data not shown) there was a greater level of ABCG1 immunoreactivity apparent in both the spermatozoa and the principal cells of the epididymis from ABCA1-deficient as compared to wild-type mice. Bar in R equals 60 µm and applies to J–Q.
Immunoblot analysis of ABCA1, ABCA7 and ABCG1 in spermatozoa membrane extracts. Aliquots of membrane fractions of spermatozoa isolated from the caput, corpus and cauda epididymides were subjected to immunoblot analysis using antibodies to ABCA1, ABCA7, ABCG1 and ABCG4. All samples were subjected to SDS-PAGE under reducing conditions. The immunoreactive polypeptides display Mr values of ~240 kDa, ~250 kDa, and 60 kDa which correspond to the reported sizes of ABCA1, ABCA7 and ABCG1, respectively. As an indication of specificity, immunoreactive bands other than those expected were not apparent using each of the antibodies.
Acknowledgments
Grant support: This work was supported by National Institutes of Health Grant DE14347.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Davis BK, Gergely AF. Studies on the mechanism of capacitation: changes in plasma membrane proteins of rat spermatozoa during incubation in vitro. Biochem Biophys Res Commun. 1979;88:613–618. doi: 10.1016/0006-291x(79)92092-8. [DOI] [PubMed] [Google Scholar]
- 2.Ehrenwald E, Parks JE, Foote RH. Cholesterol efflux from bovine sperm. I. Induction of the acrosome reaction with lysophosphatidylcholine after reducing sperm cholesterol. Gamete Res. 1988;20:145–157. doi: 10.1002/mrd.1120200205. [DOI] [PubMed] [Google Scholar]
- 3.Cross NL, Morales P, Overstreet JW, Hanson FW. Induction of acrosome reactions by the human zona pellucida. Biol Reprod. 1988;38:235–244. doi: 10.1095/biolreprod38.1.235. [DOI] [PubMed] [Google Scholar]
- 4.Law GL, McGuinness MP, Linder CC, Griswold MD. Expression of apolipoprotein E mRNA in the epithelium and interstitium of the testis and the epididymis. J Androl. 1997;18:32–42. [PubMed] [Google Scholar]
- 5.Sylvester SR, Skinner MK, Griswold MD. A sulfated glycoprotein synthesized by Sertoli cells and by epididymal cells is a component of the sperm membrane. Biol Reprod. 1984;31:1087–1101. doi: 10.1095/biolreprod31.5.1087. [DOI] [PubMed] [Google Scholar]
- 6.Sylvester SR, Morales C, Oko R, Griswold MD. Localization of sulfated glycoprotein-2 (clusterin) on spermatozoa and in the reproductive tract of the male rat. Biol Reprod. 1991;45:195–207. doi: 10.1095/biolreprod45.1.195. [DOI] [PubMed] [Google Scholar]
- 7.Argraves WS, Morales CR. Immunolocalization of cubilin, megalin, apolipoprotein J, and apolipoprotein A-I in the uterus and oviduct. Mol Reprod Dev. 2004;69:419–427. doi: 10.1002/mrd.20174. [DOI] [PubMed] [Google Scholar]
- 8.Jaspard B, Fournier N, Vieitez G, Atger V, Barbaras R, Vieu C, Manent J, Chap H, Perret B, Collet X. Structural and functional comparison of HDL from homologous human plasma and follicular fluid. A model for extravascular fluid. Arterioscler Thromb Vasc Biol. 1997;17:1605–1613. doi: 10.1161/01.atv.17.8.1605. [DOI] [PubMed] [Google Scholar]
- 9.Hermo L, Wright J, Oko R, Morales CR. Role of epithelial cells of the male excurrent duct system of the rat in the endocytosis or secretion of sulfated glycoprotein-2 (clusterin) Biol Reprod. 1991;44:1113–1131. doi: 10.1095/biolreprod44.6.1113. [DOI] [PubMed] [Google Scholar]
- 10.Vaughan AM, Oram JF. ABCA1 and ABCG1 or ABCG4 act sequentially to remove cellular cholesterol and generate cholesterol-rich HDL. J Lipid Res. 2006;47:2433–2443. doi: 10.1194/jlr.M600218-JLR200. [DOI] [PubMed] [Google Scholar]
- 11.Ikeda Y, Abe-Dohmae S, Munehira Y, Aoki R, Kawamoto S, Furuya A, Shitara K, Amachi T, Kioka N, Matsuo M, Yokoyama S, Ueda K. Posttranscriptional regulation of human ABCA7 and its function for the apoA-I-dependent lipid release. Biochem Biophys Res Commun. 2003;311:313–318. doi: 10.1016/j.bbrc.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 12.Selva DM, Hirsch-Reinshagen V, Burgess B, Zhou S, Chan J, McIsaac S, Hayden MR, Hammond GL, Vogl AW, Wellington CL. The ATP-binding cassette transporter 1 mediates lipid efflux from Sertoli cells and influences male fertility. J Lipid Res. 2004;45:1040–1050. doi: 10.1194/jlr.M400007-JLR200. [DOI] [PubMed] [Google Scholar]
- 13.Quinn P, Barros C, Whittingham DG. Preservation of hamster oocytes to assay the fertilizing capacity of human spermatozoa. J Reprod Fertil. 1982;66:161–168. doi: 10.1530/jrf.0.0660161. [DOI] [PubMed] [Google Scholar]
- 14.Hogan B, Beddigton R, Costantini F, Lacy E. Manipulating the mouse embryo: A laboratory manual. Second Edition. Cold Spring Harbor Laboratory Press; 1994. [Google Scholar]
- 15.Visconti PE, Galantino-Homer H, Ning X, Moore GD, Valenzuela JP, Jorgez CJ, Alvarez JG, Kopf GS. Cholesterol efflux-mediated signal transduction in mammalian sperm. beta-cyclodextrins initiate transmembrane signaling leading to an increase in protein tyrosine phosphorylation and capacitation. J Biol Chem. 1999;274:3235–3242. doi: 10.1074/jbc.274.5.3235. [DOI] [PubMed] [Google Scholar]
- 16.de Silva HV, Stuart WD, Duvic CR, Wetterau JR, Ray MJ, Ferguson DG, Albers HW, Smith WR, Harmony JA. A 70-kDa apolipoprotein designated ApoJ is a marker for subclasses of human plasma high density lipoproteins. J Biol Chem. 1990;265:13240–13247. [PubMed] [Google Scholar]
- 17.Morales CR, Igdoura SA, Wosu UA, Boman J, Argraves WS. Low density lipoprotein receptor-related protein-2 expression in efferent duct and epididymal epithelia: evidence in rats for its in vivo role in endocytosis of apolipoprotein J/clusterin. Biol Reprod. 1996;55:676–683. doi: 10.1095/biolreprod55.3.676. [DOI] [PubMed] [Google Scholar]
- 18.Van Praet O, Argraves WS, Morales CR. Co-expression and interaction of cubilin and megalin in the adult male rat reproductive system. Mol Reprod Dev. 2003;64:129–135. doi: 10.1002/mrd.10245. [DOI] [PubMed] [Google Scholar]
- 19.Ehrenwald E, Foote RH, Parks JE. Bovine oviductal fluid components and their potential role in sperm cholesterol efflux. Mol Reprod Dev. 1990;25:195–204. doi: 10.1002/mrd.1080250213. [DOI] [PubMed] [Google Scholar]
- 20.Bodzioch M, Orso E, Klucken J, Langmann T, Bottcher A, Diederich W, Drobnik W, Barlage S, Buchler C, Porsch-Ozcurumez M, Kaminski WE, Hahmann HW, Oette K, Rothe G, Aslanidis C, Lackner KJ, Schmitz G. The gene encoding ATP-binding cassette transporter 1 is mutated in Tangier disease. Nat Genet. 1999;22:347–351. doi: 10.1038/11914. [DOI] [PubMed] [Google Scholar]
- 21.Brooks-Wilson A, Marcil M, Clee SM, Zhang LH, Roomp K, van Dam M, Yu L, Brewer C, Collins JA, Molhuizen HO, Loubser O, Ouelette BF, Fichter K, Ashbourne-Excoffon KJ, Sensen CW, Scherer S, Mott S, Denis M, Martindale D, Frohlich J, Morgan K, Koop B, Pimstone S, Kastelein JJ, Genest J, Jr, Hayden MR. Mutations in ABC1 in Tangier disease and familial high-density lipoprotein deficiency. Nat Genet. 1999;22:336–345. doi: 10.1038/11905. [DOI] [PubMed] [Google Scholar]
- 22.Ban N, Sasaki M, Sakai H, Ueda K, Inagaki N. Cloning of ABCA17, a novel rodent sperm-specific ABC (ATP-binding cassette) transporter that regulates intracellular lipid metabolism. Biochem J. 2005;389:577–585. doi: 10.1042/BJ20050159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wolf DE, Hagopian SS, Ishijima S. Changes in sperm plasma membrane lipid diffusibility after hyperactivation during in vitro capacitation in the mouse. The Journal of cell biology. 1986;102:1372–1377. doi: 10.1083/jcb.102.4.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
RT-PCR reactions were performed on cDNA templates made from RNA isolated from round and elongating spermatids (S) and neonatal brain (B). The sizes of the amplicons generated in the PCR reactions corresponded to expected values.
A–I show immunostaining of tissues from wild-type mice. A–C, anti-ABCA1 immunohistochemical labeling of caput, corpus and cauda epididymides. D–F, anti-ABCA7 immunohistochemical labeling of caput, corpus and cauda epididymides. G–I, anti-ABCG1 immunohistochemical labeling of caput, corpus and cauda epididymides. Bar in I equals 30 µm and applies to A–H. J–R shows ABCA1, ABCG1 and non-immune IgG immunostaining of tissues from ABCA1-null mice. J–L immunostaining of caput, corpus and cauda epididymides with anti-ABCA1 antibody. M–O immunostaining of caput, corpus and cauda epididymides with anti-ABG1 antibody. P–R immunostaining of caput, corpus and cauda epididymides with non-immune rabbit IgG. Note that while the relative level of ABCA7 immunostaining was not different in epididymal tissues from ABCA1-deficient and wild-type mice (data not shown) there was a greater level of ABCG1 immunoreactivity apparent in both the spermatozoa and the principal cells of the epididymis from ABCA1-deficient as compared to wild-type mice. Bar in R equals 60 µm and applies to J–Q.
Immunoblot analysis of ABCA1, ABCA7 and ABCG1 in spermatozoa membrane extracts. Aliquots of membrane fractions of spermatozoa isolated from the caput, corpus and cauda epididymides were subjected to immunoblot analysis using antibodies to ABCA1, ABCA7, ABCG1 and ABCG4. All samples were subjected to SDS-PAGE under reducing conditions. The immunoreactive polypeptides display Mr values of ~240 kDa, ~250 kDa, and 60 kDa which correspond to the reported sizes of ABCA1, ABCA7 and ABCG1, respectively. As an indication of specificity, immunoreactive bands other than those expected were not apparent using each of the antibodies.