Abstract
In this study an attempt was made to prepare mucoadhesive microcapsules of gliclazide using various mucoadhesive polymers designed for oral controlled release. Gliclazide microcapsules were prepared using sodium alginate and mucoadhesive polymer such as sodium carboxymethyl cellulose (sodium CMC), carbopol 934P or hydroxy propylmethyl cellulose (HPMC) by orifice-ionic gelation method. The microcapsules were evaluated for surface morphology and particle shape by scanning electron microscope. Microcapsules were also evaluated for their microencapsulation efficiency, in vitro wash-off mucoadhesion test, in vitro drug release and in vivo study. The microcapsules were discrete, spherical and free flowing. The microencapsulation efficiency was in the range of 65–80% and microcapsules exhibited good mucoadhesive property in the in vitro wash off test. The percentage of microcapsules adhering to tissue at pH 7.4 after 6 h varied from 12–32%, whereas the percentage of microcapsules adhering to tissue at pH 1.2 after 6 h varied from 35–68%. The drug release was also found to be slow and extended for more than 16 h. In vivo testing of the mucoadhesive microcapsules in diabetic albino rats demonstrated significant antidiabetic effect of gliclazide. The hypoglycemic effect obtained by mucoadhesive microcapsules was for more than 16 h whereas gliclazide produced an antidiabetic effect for only 10 h suggesting that mucoadhesive microcapsules are a valuable system for the long term delivery of gliclazide.
Key words: controlled release, gliclazide, microcapsules, mucoadhesive
INTRODUCTION
Microencapsulation is a useful method for prolonging drug release from dosage forms and reducing adverse effects (1–3). Recently, dosage forms that can precisely control the release rates and target drugs to a specific body site have made an enormous impact in the formulation and development of novel drug delivery systems. Microparticles are defined as spherical polymeric particles. These microparticle constitutes an important part of these drug delivery systems, by virtue of their small size and efficient carrier characteristics. However, the success of these novel microparticles is limited due to their short residence time at the site of absorption. It would, therefore, be advantageous to have means for providing an intimate contact of the drug delivery system with the absorbing membranes. It can be achieved by coupling bioadhesion characteristics to microparticles and developing novel delivery systems referred to as “bioadhesive microparticles”. Bioadhesive microparticles include microspheres and microcapsules (having a core of the drug) of 1–1000 µm in diameter and consisting either entirely of a bioadhesive polymer or having an outer coating of it, respectively. Bioadhesive microparticles have advantages such as efficient absorption and enhanced bioavailability of drugs owing to their high surface to volume ratio, a much more intimate contact with the mucus layer, and specific targeting of drugs to the absorption site (4–10).
Gliclazide is one of the most frequently used sulfonylureas in the treatment of type II diabetes (11). The conventional formulation required twice daily administration (11). A new once daily gliclazide modified release formulation has been recently introduced (12). In a large randomized study on type II diabetic patients, once daily gliclazide modified release 30–120 mg was found as effective as twice daily gliclazide 80–320 mg in reducing glycosylated haemoglobin (HbA1C), with fewer side effects and less risk of hypoglycemia (12–15). Thus, in this study an attempt was made to prepare oral controlled release mucoadhesive microcapsules of gliclazide utilizing several combinations of mucoadhesive polymers. The effect of formulation factors and processing conditions were studied by determining microencapsulation efficiency, particle size analysis, scanning electron microscopy, FTIR spectroscopic studies, and in vitro release studies. Furthermore, the hypoglycermic effect of prepared gliclazide microcapsules on the diabetic albino rats was determined.
MATERIALS AND METHOD
Materials
Gliclazide was obtained as a gift sample from Lupin Ltd. (Mumbai, India). Sodium CMC (having a viscosity of 1,500–3,000 cps of percent w/v aqueous solution at 25 °C), HPMC (having a viscosity of 50 cps in a 2% w/v aqueous solution at 20 °C), carbopol 934P, sodium alginate and calcium chloride were obtained from Central Drug House (CDH, Mumbai, India). All other reagents used were of analytical grade.
Methods
Preparation of Microcapsules
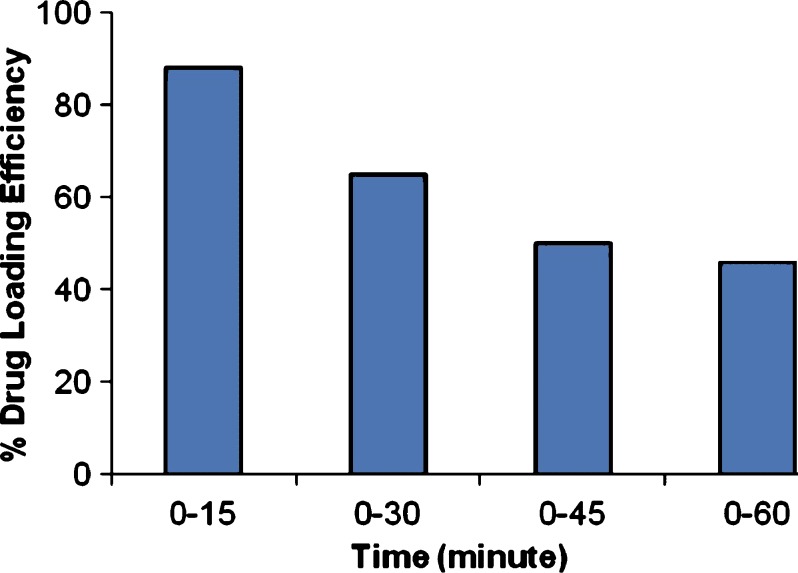
Mucoadhesive microcapsules containing gliclazide were prepared employing sodium alginate in combination with three mucoadhesive polymers—sodium CMC, carbopol 934P and HPMC as coat materials. Orifice-ionic gelation method was employed to prepare the microcapsules (16). Sodium alginate and the mucoadhesive polymer were dissolved in 50 ml of purified water to form a homogenous polymer solution. The active substance gliclazide was added to the polymer solution (in a ratio of gliclazide: polymer solution 1:1) and mixed thoroughly to form a viscous dispersion. The resulting dispersion was then added manually dropwise into calcium chloride (10% w/v) solution (100 ml) through a syringe with a 26 gauge needle. The addition of dispersion in the CaCl2 solution was completed within 3 h of the preparation of the dispersion. The added droplets were retained in the calcium chloride solution for 15 min to complete the curing reaction and to produce spherical rigid microcapsules. The time of gel formation influences the drug loading efficiency and also the cohesion of the gel (17). The drug loading efficiency as a function of microcapsules formation time (time of contact with CaCl2 solution) is reported in Fig. 1. From the graph it is clear that the loading rate of gliclazide decreases as the time of gel formation increases. It can be explained by the fact that the release of the drug starts to occur during the period of gel formation. The time of gel formation also influences the cohesion of the gel. If the structure of the gel is too loose, the polymer network is eroded, consequently the drug releases outside the microcapsules. The released amount is more important when the time of gel formation is short suggesting that the structure of gel is strongly dependent on the contact time between Ca2+ and alginate. In our conditions, 15 min curing time is found optimum for maximum drug loading efficiency. The microcapsules were collected by decantation, and the product thus separated was washed repeatedly with water and dried at 45 °C for 12 h. The microcapsules prepared with their coat composition are listed in Table I.
Fig. 1.
The effect of microcapsule formation time on drug loading efficiency
Table I.
Polymer Admixture Ratios, Amount of Polymer, Gliclazide Microencapsulation Efficiency and Viscosities of Polymer Solutions
| S. No. | Microcapsules | Polymers Admixture Composition (mg/mg) | Viscosity of Polymer Solution | Microencapsulation Efficiency (%) |
|---|---|---|---|---|
| 1. | MC1 | Alginate/sodium CMC (1000:1000) | 1,800 cps | 80.00 (0.20) |
| 2. | MC 2 | Alginate/carbopol (1,000:1,000) | 35,680 cps | 72.00 (1.35) |
| 3. | MC 3 | Alginate/HPMC (1000:1000) | 45.8 cps | 65.50 (0.76) |
| 4. | MC 4 | Alginate/SodiumCMC (1,666.6:333.3) | 1,609 cps | 75.68 (0.80) |
| 5. | MC 5 | Alginate/carbopol (1,666.6:333.3) | 35,670 cps | 73.43 (2.50) |
| 6. | MC 6 | Alginate/HPMC (1,666.6:333.3) | 41.8 cps | 68.20 (2.70) |
| 7. | MC 7 | Alginate/Sodium CMC (1,800:200) | 1,448 cps | 78.00 (2.05) |
| 8. | MC 8 | Alginate: Carbopol (1,800:200) | 35,630 cps | 75.56 (2.72) |
| 9. | MC 9 | Alginate: HPMC (1,800:200) | 37.6 cps | 71.72 (2.23) |
Figures in parentheses are coefficient of variation values
Estimation of Gliclazide
Gliclazide was estimated by ultraviolet visible (UV/Vis) spectrophotometric method (Shimadzu UV–1700) based on the measurement of absorbance at 226.5 nm in phosphate buffer of pH 7.4. The method was validated for linearity, accuracy and precision. The method obeys Beer’s law in the concentration range of 2 to 20 µg/ml.
Microencapsulation Efficiency
An appropriate amount of microcapsules were first crushed and then weighed and suspended in methanol to extract the drug from microcapsules while assuring that there was no loss of material in the process. After 24 h, the filtrate was assayed spectrophotometrically at 229 nm for drug content against methanol as blank. Microencapsulation efficiency was calculated using the formula:
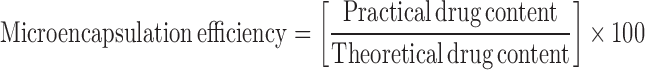 |
Particle Size Analysis
Particle size distribution of the microcapsules was done by sieve analysis procedure. The microcapsules were shaken on a mechanical shaker, using a nest of British standard sieves, for 15 min.
Scanning Electron Microscopy (SEM)
SEM was performed for morphological characterization of microcapsules using scanning electron microscope (SEM—LEICA, 5430, London, U.K). They were mounted directly onto the SEM sample stub using double - sided sticking tape and coated with gold film (thickness, 200 nm) under reduced pressure (0.001 mmHg).
FTIR Spectroscopic Studies
The FTIR spectra were performed on Perkin-Elmer spectrum System one FTIR spectrometer. FTIR spectra of gliclazide, polymers used in the formulation and of microcapsules of gliclazide with polymers were recorded by potassium bromide (KBr) disc method and scanned at the resolution of 4.0 cm−1 over the wave number region 4,000–450 cm−1.
In Vitro Drug Release Study
The drug release was performed using USP 24 (paddle type) apparatus at 37 ± 0.5°C and at 100 rpm in 0.1 N HCl (pH 1.2) and phosphate buffer (pH 7.4) as dissolution medium. Microcapsules 100 mg of gliclazide were used for the test. Five milliliters of sample solution was withdrawn at predetermined time intervals, filtered through a 0.45 µm membrane filter, diluted suitably and analyzed spectrophotometricaly at 226.5 nm. An equal amount of fresh dissolution medium was replaced immediately after withdrawal of test sample. The drug release experiments were conducted in triplicate (n = 3).
Mucoadhesion Testing by In Vitro wash-off Test
The mucoadhesive properties of the microcapsules were evaluated by in vitro wash-off test as reported by Lehr et. al (18). A 2 cm wide and 2 cm long (2 × 2) piece of rat intestinal mucosa was tied onto a glass slide (3 in. long and 1 in. wide) using thread. About fifty microcapsules were spread onto the wet, rinsed, tissue specimen, and allowed to hydrate for 30 s. The prepared slide was hung onto one of the grooves of a USP 24 tablet disintegrating test apparatus. The disintegrating test apparatus was operated such that the tissue specimen was given regular up and down movements in the test fluid at 37 °C contained in one liter vessel of the machine. At the end of 1 h, and at hourly intervals up to 6 h, the machine was stopped and the number of microcapsules still adhering to the tissue was counted. The test was performed at both gastric pH (0.1 N HCl, pH 1.2) and intestinal pH (phosphate buffer, pH 7.4).
In Vivo Test
The approval of the Institutional Animal Ethics Committee was obtained before starting the study. The approval number and date is 716/02/a/CPCSEA and 30/03/2007 respectively. The study was conducted in accordance with standard institutional guidelines. In vivo evaluation studies for gliclazide mucoadhesive microcapsules were performed in diabetic albino rats of either sex, weighing between 260–330 g. After 16 h overnight fast, the experimental animals were made diabetic by single intraperitoneal administration of cold, freshly prepared solution of alloxan (Sigma Chem. Co., St. Louis, USA) at a dose of 150 mg/kg dissolved in 2 mM citrate buffer (pH 3.0). After 1 week, animals with fasting blood glucose of 300 mg/dl or more were considered diabetic and were employed in the study (19,20). The rats were divided randomly into four groups of five rats each and treated as follow: group I was administered with 2 mg/kg body weight of gliclazide solution, group II and III were administered with mucoadhesive microcapsules MC7 and MC8 at a dose equivalent to 2 mg/kg body weight of gliclazide by using oral feeding needle and group IV was administered with marketed conventional gliclazide tablet. Blood samples were withdrawn by the retro orbital puncture at predetermined time at 1 hour intervals up to 24 h, and were analyzed for blood glucose by glucose oxidase and peroxidase (GOD/POD) method using commercial glucose kit.
RESULTS AND DISCUSSIONS
Microcapsules of gliclazide with a coat consisting of sodium alginate and a mucoadhesive polymer-sodium CMC, carbopol 934P or HPMC in 1:1, 5:1 and 9:1 ratio could be prepared by the orifice-ionic gelation method. Microcapsules with a coat of mucoadhesive polymer alone could not be prepared by this method because of their water soluble nature (16).
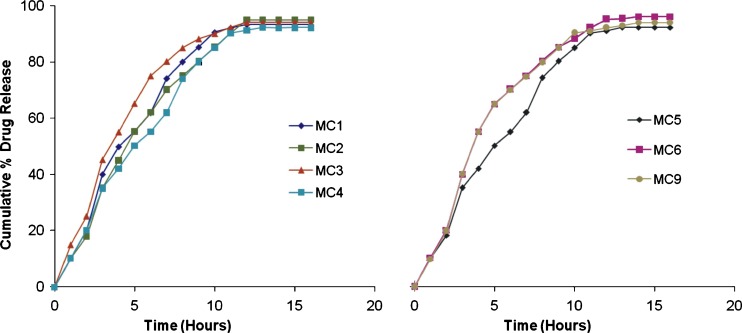
Twenty formulations with different polymers admixtures were prepared. The ratio of mucoadhesive polymer was kept constant and the proportion of only sodium alginate was increased because of the gelling property of alginate to form the beads. These formulations were tested for particle size and an in vitro mucoadhesion test. From the study on these formulations we found that there was no effect of polymer type and ratio mixtures of polymer solution. Only extrusion device and viscosity of the polymer solution affected the particle size. Out of these 20 formulations 9 formulations with alginate: mucoadhesive polymer ratio of 1:1, 5:1 and 9:1 were selected for in vitro study on the basis of there mucoadhesive properties. The mucoadhesion shown by these formulation (ratio 1:1, 5:1 and 9:1) was in the range of 35–68% after 6 h. Since the main aim of our study was to improve the bioadhesive strength of microcapsule so these formulations were selected for the in vitro release study.. From the in vitro release study, formulations MC7 and MC8 have shown good controlled release for more than 16 h and thus they were selected for the in vivo study.
Calibration curve of gliclazide, Y = 0.042X − 0.01 (r = 0.999), showed the good linear relationship with concentration ranged between 2–20 µg/ml. The lowest measurable concentration was 2 µg/ml and percentage coefficient of variation was from 0.2 to 3.5 so the result was sufficiently acceptable.
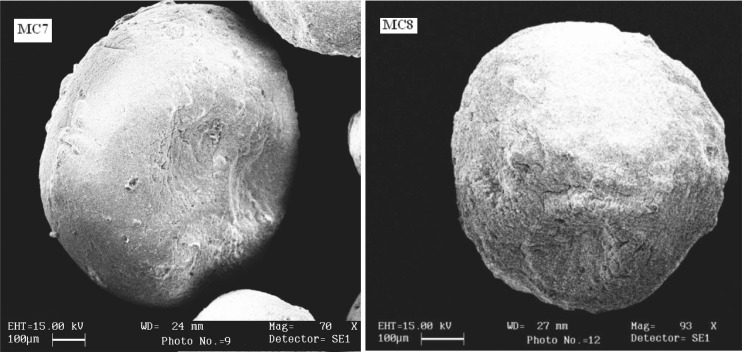
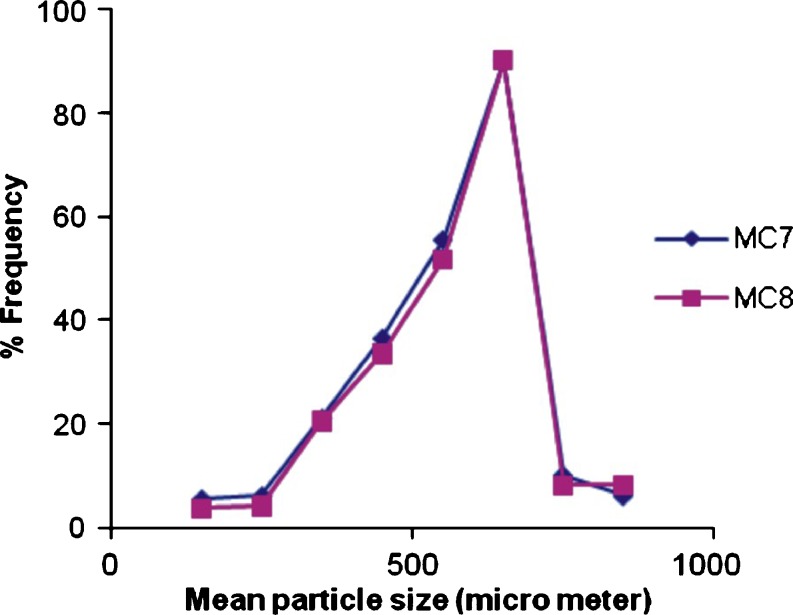
Size of extrusion device and the viscosity of polymer solution were found to affect the particle size of microcapsules prepared by orifice-ionic gelation method. Decreasing the viscosity of polymer solution caused the mean particle size to shift towards a lower particle size. Increasing the viscosity of polymer solution, formed larger droplets and consequently, microcapsules with large particle size. Increasing the size of extrusion device increased the particle size of microcapsules. Needle no. 26 was found suitable for the formulation of microcapsules. The mean particle size was not affected by the polymer type and the ratio of mixture of polymers for all formulations. The SEM photographs (Fig. 2) indicated that the microcapsules were spherical and rough in nature. Surface morphology also revealed presence of cracks on the surface. The arithmetic mean and standard deviation of microcapsule size of batch MC7 and MC8 was found to be 654 ± 52 µm and 682 ± 64 µm respectively. The particle size distribution plot for microcapsule of batch MC7 and MC 8 has been shown in Fig. 3.
Fig. 2.
Scanning electron micrographs of gliclazide microcapsules
Fig. 3.
Particle size distribution plot for gliclazide microcapsules for batch MC7 and MC8
The microencapsulation efficiencies were high for all microcapsules obtained. The micro encapsulation efficiencies were found to be affected by the type of polymer. The micro encapsulation efficiency for sodium alginate–sodium CMC was found higher compared to sodium alginate–HPMC and sodium alginate–carbopol 934P. The microencapsulation efficiencies were found unaffected by the different ratios of polymer mixture. The microencapsulation efficiency was in the range of 65–80% (Table I).
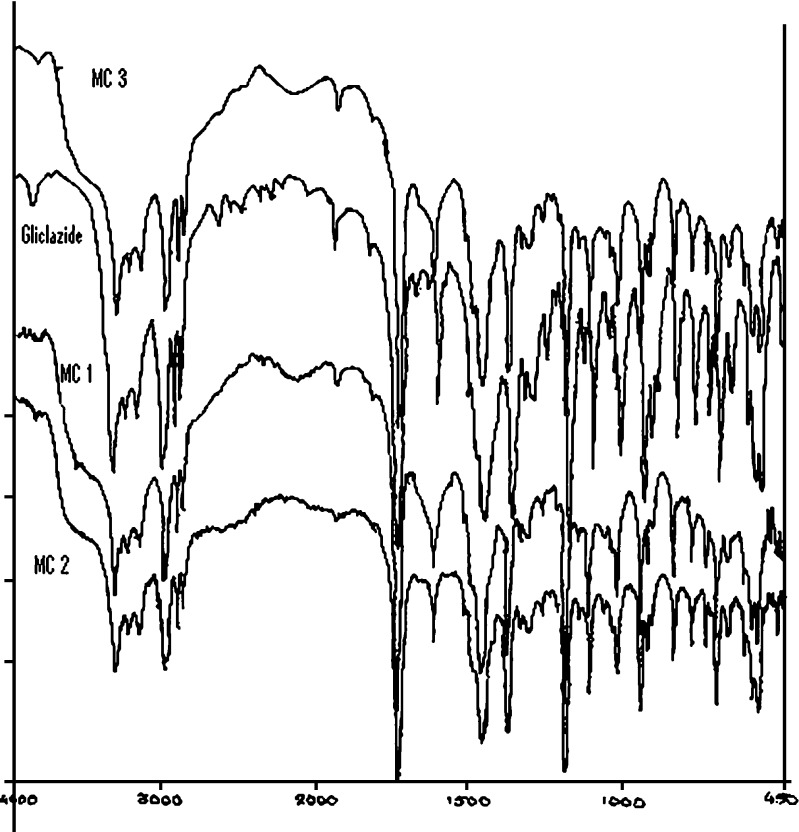
FTIR spectra were employed to confirm the compatibility of gliclazide with various polymers used to prepare the mucoadhesive microcapsules of gliclazide (Fig. 4). The spectrum of gliclazide for carbonyl group showed a sharp concave curve at 1,710 cm−1 was found consistent and unaffected by the various polymers. For the sulphonyl group bands, the spectra of gliclazide are characterized by a symmetric stretching peak at 1,164 cm−1. The symmetric vibration peak of microcapsules presented no shift and no change in frequency. For the amino group, gliclazide demonstrated an evident peak at 3,272 cm−1. No change in the frequency and peak was observed. In short, the microcapsules prepared with different admixture of polymers (sodium alginate–sodium CMC, sodium alginate–carbopol 934P and sodium alginate–HPMC) had significant characters of gliclazide in the FTIR spectrum, suggesting, there were no reactions between the gliclazide and the polymers used.
Fig. 4.
FTIR spectra of microcapsule batch MC3, gliclazide, microcapsule batch MC1 and microcapsule batch MC2
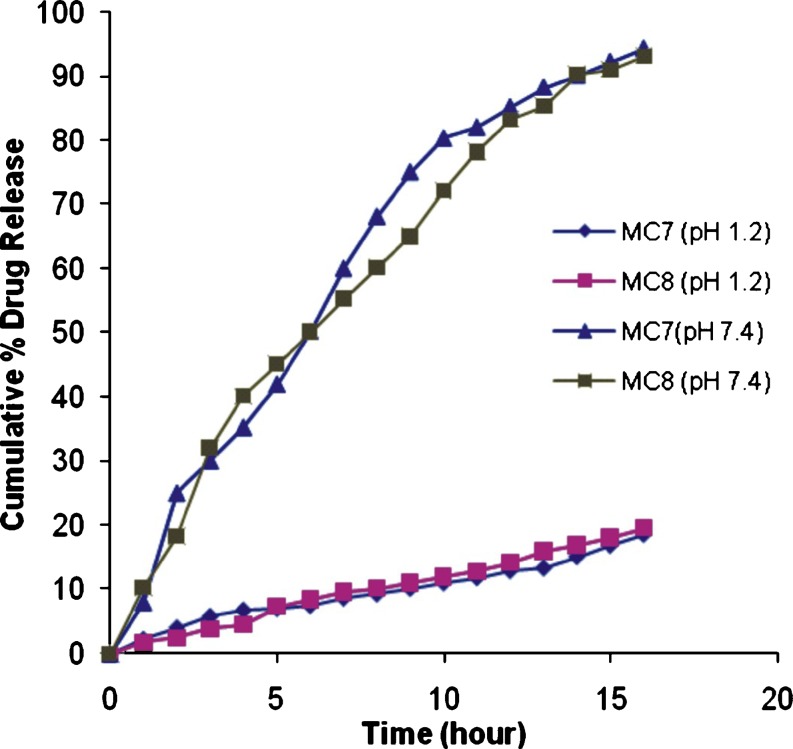
Gliclazide release from the microcapsules was studied at pH 7.4 for 16 h (Figs. 5 and 6) Gliclazide release from microcapsules was slow and depended on the composition of the coat. Microcapsules of alginate–carbopol gave relatively slow release when compared to others. The order of increasing release rate observed with various microcapsules was sodium alginate–carbopol 934 P < sodium alginate–sodium CMC < sodium alginate–HPMC. Gliclazide release from microcapsules MC 7 and MC 8 was slow and extended over a period of 16 h, and these microcapsules were found suitable for oral controlled release formulation (Fig. 6). The microcapsules of batch MC 7 and MC 8 were also evaluated at pH 1.2. Gliclazide release from microcapsules MC7 and MC 8 at pH 1.2 was slow as compared to release at pH 7.4 (Fig. 6). The reason for this low release might be the acid resistant nature of sodium alginate (21) and gliclazide is a weak acid drug and its solubility is higher at high pH, as expected.
Fig. 5.
In vitro release profile of gliclazide mucoadhesive microcapsules of various batches
Fig. 6.
In vitro release profile of gliclazide mucoadhesive microcapsules of batch MC—MC7 and MC8 in phosphate buffer pH 7.4 and 0.1 N HCl pH 1.2
Microcapsules with a coat consisting of sodium alginate and a mucoadhesive polymer exhibited good mucoadhesive properties in the in-vitro wash-off test. The mucoadhesion test for microcapsules was performed at gastric pH 1.2 and intestinal pH 7.4 continuously for 6 h. The wash-off was faster at intestinal pH (7.4) than at gastric pH (1.2). The results of the wash-off test indicated that the microcapsules had fairly good mucoadhesive property. The percentage of microcapsules adhering to tissue at pH 7.4 after 6 h varied from 12 to 32, whereas the percentage of microcapsules adhering to tissue at pH 1.2 after 6 hours varied from 35 to 68 (Table II and III).
Table II.
Percentage of Microcapsules Adhering to Tissue in 0.1 N HCl, pH 1.2
| Microcapsule | Time in hours | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |
| MC 1 | 75 (1.8) | 70 (2.1) | 65 (1.2) | 62 (0.6) | 55 (31) | 51 (1.1) |
| MC 2 | 78 (0.8) | 72 (1.0) | 70 (1.5) | 68 (1.1) | 65 (1.6) | 60 (2.3) |
| MC 3 | 82 (0.9) | 78 (2.1) | 75 (1.1) | 72 (1.6) | 70 (2.0) | 68 (2.3) |
| MC 4 | 70 (1.6) | 68 (1.2) | 60 (2.8) | 55 (2.8) | 50 (1.8) | 42 (3.3) |
| MC 5 | 76 (2.2) | 72 (1.5) | 70 (0.6) | 65 (2.8) | 56 (1.4) | 52 (1.5) |
| MC 6 | 77 (1.7) | 70 (2.1) | 65 (1.7) | 63 (2.8) | 60 (1.6) | 55 (0.7) |
| MC 7 | 78 (1.8) | 68 (2.1) | 65 (0.9) | 52 (3.1) | 48 (2.3) | 42 (1.4) |
| MC 8 | 80 (2.3) | 68 (1.8) | 60 (2.0) | 55 (1.6) | 50 (3.8) | 40 (4.0) |
| MC 9 | 82 (1.1) | 72 (1.1) | 65 (3.3) | 52 (1.3) | 48 (3.1) | 35 (2.8) |
Figures in parentheses are coefficient of variation values
Table III.
Percentage of Microcapsules Adhering to Tissue in Phosphate Buffer pH 7.4
| Microcapsule | Times in hours | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |
| MC 1 | 68 (0.6) | 62 (2.5) | 55 (2.0) | 45 (1.1) | 35 (1.7) | 28 (28) |
| MC 2 | 70 (1.1) | 66 (1.8) | 60 (3.0) | 42 (1.6) | 32 (2.5) | 30 (2.0) |
| MC 3 | 66 (2.1) | 55 (0.9) | 50 (3.6) | 40 (2.0) | 35 (3.1) | 25 (2.4) |
| MC 4 | 70 (1.3) | 62 (1.9) | 55 (2.5) | 50 (2.8) | 45 (1.3) | 32 (3.4) |
| MC 5 | 72 (2.5) | 55 (3.2) | 48 (2.5) | 32 (3.1) | 25 (2.8) | 18 (3.3) |
| MC 6 | 68 (1.6) | 42 (2.6) | 35 (2.2) | 28 (1.4) | 20 (2.0) | 12 (3.3) |
| MC 7 | 71 (2.5) | 54 (0.4) | 36(2.8) | 30 (2.2) | 25 (4.0) | 18 (2.2) |
| MC 8 | 76 (0.8) | 65 (2.3) | 55 (2.0) | 42 (0.9) | 35 (2.8) | 25 (0.8) |
| MC 9 | 70 (1.6) | 52 (1.7) | 45 (1.7) | 35 (1.7) | 20 (3.0) | 12 (1.6) |
Figures in parentheses are coefficient of variation values
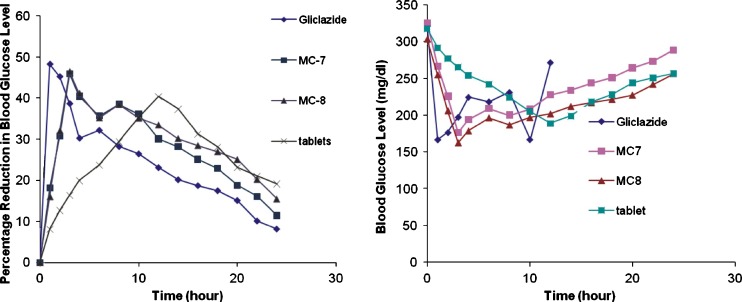
In vivo studies in diabetic albino rats were performed with microcapsules of batch MC 7 and MC 8. The drug was administered at a dose equivalent to 2 mg/kg body weight of gliclazide. Pure gliclazide was administered in a suspension form at the same dose. When pure gliclazide solution was administered, a rapid reduction in blood glucose level was observed and maximum reduction of 48.24% was observed within 1 h after oral administration. Blood glucose levels were recovered to the normal level in 14 h (Fig. 7). A blood glucose level of 70–99 mg/dl is considered normal level. In the case of gliclazide mucoadhesive microcapsules, the reduction in blood glucose levels was slow and reached maximum reduction within 3 h after oral administration. This reduction in blood glucose level was sustained over longer periods of time. A 25% reduction in blood glucose level is considered a significant hypoglycemic effect. Significant hypoglycemic effect was maintained from 0.5 to 10 h after oral administration of gliclazide, whereas in case of mucoadhesive microcapsules of gliclazide MC 7 significant hypoglycemic effect was maintained for a period of 2 to 16 h, and 2–20 h in case of MC 8. The sustained hypoglycemic effect observed over a longer period of time in the case of mucoadhesive microcapsules is due to the slow release and absorption of gliclazide over longer period of time. Gliclazide sustained release formulation is significantly more effective than the immediate release gliclazide formulation in reducing blood glucose levels and side effects.
Fig. 7.
Comparative in vivo study of gliclazide microcapsules of batch MC7 and MC8 with pure glicalzide and marketed gliclazide tablet
CONCLUSIONS
The spherical microcapsules with a coat consisting of alginate and a mucoadhesive polymer (sodium CMC, carbopol 934P or HPMC) could be prepared by an orifice-ionic gelation process. The microcapsules exhibited good mucoadhesive properties in an in vitro test. Gliclazide release from these mucoadhesive microcapsules was slow and extended over longer periods of time (12–16 h) and depended on compositions of the coat. The in vivo study demonstrated significant blood glucose reducing activity of mucoadhesive microcapsules of gliclazide. Developed mucoadhesive microcapsules are suitable for controlled release effect after oral administration of gliclazide.
References
- 1.Kristmundsottir T., Ingvarsdotir K. Ibuprofen microcapsules: The effect of production variables on microcapsule properties. Drug Dev Ind Pharm. 1990;20:769–778. doi: 10.3109/03639049409038330. [DOI] [Google Scholar]
- 2.Bolourtchian N., Karimi, Aboofazeli R. Preparation and characterization of ibuprofen microspheres. J Microencapsul. 2005;22:529–538. doi: 10.1080/02652040500161941. [DOI] [PubMed] [Google Scholar]
- 3.Bakan J. A. Microencapsulation. In: Lachman L., Liberman H. A., editors. The Theory and practice of Industrial pharmacy. 2. Mumbai, India: Varghese; 1991. pp. 412–428. [Google Scholar]
- 4.Vasir J. K., Tambwekar K., Garg S. Bioadhesive microspheres as a controlled drug delivery system. Int J Pharm. 2003;255:13–32. doi: 10.1016/S0378-5173(03)00087-5. [DOI] [PubMed] [Google Scholar]
- 5.Chowdary K. P. R., Rao Y. S. Mucoadhesive microspheres for oral controlled drug delivery. Biol Pharm Bull. 2004;27:717–1724. doi: 10.1248/bpb.27.1717. [DOI] [PubMed] [Google Scholar]
- 6.Tabassi S. A. S., Razavi N. Preparation and characterization of albumin microspheres encapsulated with propranolol HCl. DARU. 2003;11:137–141. [Google Scholar]
- 7.Haznedar S., Dortunc B. Preparation and in vitro evaluation of Eudragit microspheres containing acetazolamide. Int J Pharm. 2004;269:131–140. doi: 10.1016/j.ijpharm.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 8.Chowdary K. P. R., Rao Y. S. Preparation and evaluation of mucoadhesive microcapsules of indomethacin. Saudi Pharm J. 2003;11:97–103. [Google Scholar]
- 9.Bogataj M., Mrhar A., Korosec L. Influence of physiochemical and biological parameters on drug release from microspheres adhered on vesical and intestinal mucosa. Int J Pharm. 1999;177:211–220. doi: 10.1016/S0378-5173(98)00341-X. [DOI] [PubMed] [Google Scholar]
- 10.Chickering D. E., Mathiowitz E. Bioadhesive microspheres I.A novel electrobalance-based method to study adhesive interactions between individual microspheres and intestinal mucosa. J Control Release. 1995;34:251–261. doi: 10.1016/0168-3659(95)00011-V. [DOI] [Google Scholar]
- 11.D Tripathi K. Essentials of Medical Pharmacology. 4. New Delhi, India: Jaypee Brothers, Medical Publishers; 1999. pp. 276–278. [Google Scholar]
- 12.Scherathaner G., et al. Guide study: double - blind comparison of once - daily gliclazide MR and glimepiride in type 2 diabetic patients. Eur J Clin Invest. 2004;34:535–542. doi: 10.1111/j.1365-2362.2004.01381.x. [DOI] [PubMed] [Google Scholar]
- 13.Wangnoo S. K. Treatment of type 2 diabetes with gliclazide modified release 60 mg in the primary care setting of India. Int J Diab Dev Countries. 2005;25:50–54. doi: 10.4103/0973-3930.26751. [DOI] [Google Scholar]
- 14.Yong J. F., Wei G. L., Lu R., Liu C. X., Zheng B. Z., Feng P. Bioavailability of gliclazide sustained release tablet in healthy volunteers. Asian J Pharmcodyn Pharmucokin. 2006;2:153–160. [Google Scholar]
- 15.Arno E. A., Anand P., Bhaskar K., Ramchandran S., Saravanan M., Vinod R. Eudragit NE 30D based metformin/gliclazide extended release tablets: formulation, characterisation and in vitro release studies. Chem Pharm Bull. 2002;5011:1495–1498. doi: 10.1248/cpb.50.1495. [DOI] [PubMed] [Google Scholar]
- 16.Chowdary K. P. R., Rao Y. S. Design and In vitro and in vivo evaluation of Mucoadhesive microcapsules of glipizide for oral controlled release A technical note. AAPS Pharm Sci Tech. 2003;4:1–6. doi: 10.1208/pt040339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.M. G. Sankalia, R. C. Mashru, R. C. Sankaliax, V. B. Sutariya. Papain Entrapment in alginate beads for stability improvement and site-specific delivery: Physicochemical characterization and factorial optimization using neural network modeling. AAPS PharmSciTech 6(2): article 31 (2005). http://www.aapspharmscitech.org. [DOI] [PMC free article] [PubMed]
- 18.Lehr C. M., Bowstra J. A., Tukker J. J., Junginger H. E. Intestinal transit of bioadhesive microspheres in an in situ loop in the rat. J Control Release. 1990;13:51–62. doi: 10.1016/0168-3659(90)90074-4. [DOI] [Google Scholar]
- 19.Mukhtar H. M., Ansari S. H., Ali M., Bhat Z. A., Naved T. Effect of aqueous extract of Cyamopsis tetragonoloba Linn. beans on blood glucose level in normal and alloxan-induced diabetic rats. Indian J Exp Biol. 2004;42:1212–1215. [PubMed] [Google Scholar]
- 20.Satyanarayana S., Kilari E. K. Influence of nicorandil on the pharmacodynamics and pharmacokinetics of gliclazide in rats and rabbits. Mol Cell Biochem. 2006;291:101–105. doi: 10.1007/s11010-006-9202-y. [DOI] [PubMed] [Google Scholar]
- 21.Yoo S. H., Song Y. B., Chang P. S., Lee H. G. Microencapsulation of α tocopherol using sodium alginate and its controlled release properties. Int J Biol Macro. 2006;38:25–30. doi: 10.1016/j.ijbiomac.2005.12.013. [DOI] [PubMed] [Google Scholar]