Abstract
This investigation was undertaken to evaluate practical feasibility of site specific pulmonary delivery of liposomal encapsulated Dapsone (DS) dry powder inhaler for prolonged drug retention in lungs as an effective alternative in prevention of Pneumocystis carinii pneumonia (PCP) associated with immunocompromised patients. DS encapsulated liposomes were prepared by thin film evaporation technique and resultant liposomal dispersion was passed through high pressure homogenizer. DS nano-liposomes (NLs) were separated by ultra centrifugation and characterized. NLs were dispersed in phosphate buffer saline (PBS) pH 7.4 containing different carriers like lactose, sucrose, and hydrolyzed gelatin, and 15% l-leucine as antiadherent. The resultant dispersion was spray dried and spray dried formulation were characterized to ascertain its performance. In vitro pulmonary deposition was assessed using Andersen Cascade Impactor as per USP. NLs were found to have average size of 137 ± 15 nm, 95.17 ± 3.43% drug entrapment, and zeta potential of 0.8314 ± 0.0827 mV. Hydrolyzed gelatin based formulation was found to have low density, good flowability, particle size of 7.9 ± 1.1 μm, maximum fine particle fraction (FPF) of 75.6 ± 1.6%, mean mass aerodynamic diameter (MMAD) 2.2 ± 0.1 μm, and geometric standard deviation (GSD) 2.3 ± 0.1. Developed formulations were found to have in vitro prolonged drug release up to 16 h, and obeys Higuchi's Controlled Release model. The investigation provides a practical approach for direct delivery of DS encapsulated in NLs for site specific controlled and prolonged release behavior at the site of action and hence, may play a promising role in prevention of PCP.
Key words: dapsone, dry powder inhaler, nano-liposomes, Pneumocystis carinii pneumonia, spray drying
INTRODUCTION
Pneumocystis carinii pneumonia (PCP) is a most common life threatening opportunistic infection associated with immunodeficiency (1), and persons with human immunodeficiency virus infection, which represents the population with the greatest number of cases of PCP reported each year. Early in the AIDS epidemic, it was estimated that >80% of all AIDS patients would develop PCP and is most frequent cause of death in this group (2–4). Three treatments are routinely used to prevent PCP: trimethoprim sulfamethoxazole (Cotrimaxazole) (TMP/SMX), pentamidine aerosol, and oral Dapsone (DS) and all carry the risk of serious side effects or treatment failure. DS is an antibiotic belonging to sulphone group, widely used for prevention of PCP, has been the drug of choice for those who cannot tolerate TMP/SMX (3). The DS is easily available at economical cost to patients. DS is a safer drug and has been studied less but may be as effective in prevention of PCP (5). Conventional administration of DS is associated with number of adverse effects such as anemia, stomach upset, nausea, leg or back pain, headache, dizziness, or peripheral neuropathy etc (3). The oral delivery of DS in the treatment of PCP leads to lower concentration at site of action, inefficient management of disorders, low therapeutic index and critical side effects. As P. carnii organism are largely confined to the alveolar space, direct aerosolized liposomal delivery of DS will provide an site-specificity with prolonged pharmacological response, improve therapeutic efficacy and reduced extra-pulmonary side effects and hence, may prove promising in management of PCP. The utilization of liposomes for aerosol delivery has many potential advantages, including universal carrier suitability for most lipophilic drugs, aqueous compatibility, sustained release or depot and intracellular delivery (6). Dry powder inhaler formulations (DPIs) stand out because of the stability of drugs and formulations among pulmonary drug delivery systems (7). Liposomal drug DPIs have many advantages for pulmonary administration specifically with respect to the controlled delivery, increased potency, reduced toxicity, can uniformly deposit drugs locally, propellant free, patient compliance, high dose carrying capacity, stability, patent protection (8–16).
Therefore, the objective of this investigation was to encapsulate DS within nanoliposomes (NLs), incorporate NLs into DPIs using spray drying technique and to evaluate in vitro lung deposition of the developed DPIs in this investigation for drug release, and pulmonary deposition in lung model. It was hypothesized that spray dried NLs DPIs will provide stable formulation, high aerosolization efficiency to deep lungs, prolonged drug release, slow systemic dilution, and avoid macrophage uptake of encapsulated drug by carrier based delivery of nano-range liposomes. Hence, it is expected to provide synergistic combinations of localized site specific and sustained action of DS within the lungs to achieve an effective concentration for prolonged period of time in lungs; thereby may play a promising role in prevention of PCP and reduce associated systemic toxicities.
MATERIALS AND METHODS
Materials
DS of 99.5% purity was received as a gift from Atul Ltd, Atul, Gujarat, India, Dipalmitoyl phosphotidylcholine (DPPC) was obtained from Lipoid (Lipoid GmBH, Ludwigshafen, Germany). The cholesterol (CHOL) and Triton X-100 were purchased from S. D. Fine chemicals (Vadodara, India). l-leucine was received as a gift from Alembic limited (Vadodara, India). Hydrolyzed gelatin was received as a gift sample from Croda Healthcare (East Yorkshire, UK). Dialysis Bag (mol. cut off weight 10,000) was obtained from Sigma Chemical Co. (Milwaukee WI 53209, USA). Quali-V®, a hydroxypropyl methylcellulose (HPMC) capsule of size 2 was generous gift samples of Shionogi Qualicaps, S.A., Spain. Inhalator brev I. S. F. was provided by Panacea Biotec Ltd, Lalru, Punjab, India for this research studies. All chemicals were of an analytical grade or spectroscopic grade and used as received.
Preparation of DS Loaded Liposomes
NLs of DS were prepared by thin film evaporation technique (17). Drug-to-lipid molar ratios of 1:2 was used in preparation of NLs. Briefly, 200 mg of DS, DPPC and CHOL (7:3) were dissolved in a mixture of methanol and chloroform (2:1 v/v) and subjected to dry thin film formation in Rotaevaporator at 100 rpm, for 1 h under 20″ of Hg at 60°C. Resultant lipid films were put into vacuum desiccators in the presence of dry silica gel for 4 h to remove chloroform. Liposomes were hydrated using 20 ml of PBS pH 7.4 for 1 h and liposomal dispersion was passed through high-pressure homogenizer (Emulsiflex®-C5, Avestin Inc., Canada) pre-heated to 60°C for 3 cycles at 10,000 psi. Resultant nano-size dispersion was centrifuged at 30,000 rpm, 20°C for 20 min using ultracentrifuge (Sigma, 3K 30, Osterode, GmBH) to yield NLs pellets and washed twice with 25 ml PBS pH 7.4. The percent drug entrapment within NLs and un-entrapped drug content from supernatant were analyzed by HPLC method.
Spray Drying of Nano-Liposomal Dispersion
The NLs equivalent to 50 mg of DS were dispersed separately in 200 ml PBS containing 20 mg/ml of carrier (lactose/sucrose/hydrolyzed gelatin), and 15% w/w of leucine was added to above dispersions. Similarly, 50 mg of plain DS was dissolved in 200 ml methanolic PBS containing lactose (20 mg/ml) and 15% of l-leucine. Dispersions were filtered through 1 μm filter and spray dried using spray-dryer (LSD-48, JISL, Mumbai, India) having pneumatically atomized through a 0.7-mm nozzle. The spray drying was performed using feed pump rate of 1.0 ml/min, 100% aspiration, atomization air at 2.5 k/cm2, inlet temperature 100 ± 5°C with an outlet temperature of 60°C to 65°C. The spray dried powders equivalent to 500 ± 20 μg of DS were filled in a size 2 Quali-V® capsule.
Assay
A modified USP 27 HPLC method was used for estimation of DS during different stages of formulation development and validated for accuracy, precision and reproducibility. The equivalent quantity of NLs or spray dried formulations containing 500 μg of DS were dispersed in deionized water and resultant centrifuged NLs pellets were solubilized in 0.1% Triton X-100 in methanol followed by dilution with mobile phase and filtered through a 0.22 μm syringe filter before sample injections. The drug content was determined using a Dionex HPLC system (Dionex Soffron GmbH, Germany) with 20-μl loop injector and UV detection at 295 nm. The separation was carried out on a C18 column (Thermo Electron Corporation, USA) having 4.6 by 25 cm (internal diameter) and particle size of 10 μm. The mobile phase comprised of water/acetonitrile (65:35 v/v) with 5 mM ammonium acetate with 0.1% formic acid and run at a flow rate of 0.5 ml/min. The calibration curve of DS was prepared in each assay in a concentration range from 100 to 600 ng/ml. The correlation coefficient of more than 0.9997 was observed for HPLC method and showed acceptable results for accuracy, precision and reproducibility.
Particle Size and Zeta Potential Measurement
The size of DS NLs was measured by dynamic light scattering using Malvern Zetasizer 3000 HS (Malvern Instruments Inc., UK) at a 90° scattering angle. The zeta potential was calculated by Smoluchowski's equation from the electrophoretic mobility of DS-loaded liposomes at 25°C (18). The particle sizes of spray dried formulations were assessed by dispersing them in isopropyl alcohol to achieve an obscuration between 10% and 20% at a stirring speed of 1,000 rpm based on laser diffraction using Malvern MasterSizer SM 2000K (Malvern Instruments Inc., UK). The results were for volume mean diameter (VMD), which is the diameter where 50% of the distribution is above and 50% is below. Span value is defined from the polydispersibility of powder.
Percent Drug Entrapment/Retention
For estimation of percent drug entrapped within DS NLs, the NLs were solubilized in 0.1% Triton X-100 in methanol. The drug retained in spray dried formulation was determined after rehydration of powder equivalent to 500 ± 20 μg of DS in PBS followed by separated of NLs using centrifugation at 35,000 rpm, 20°C for 20 min. Resultant NLs were solubilized in 0.1% Triton X-100 in methanol followed by dilution with mobile phase for HPLC analysis.
Solid State Characterization
Tapped density of spray dried powder was assessed using 10 g of sample by 500 mechanical taps in a measuring cylinder. The pile was carefully built up by dropping the material through a funnel till the tip of the funnel (height, 2 cm) and the angle of repose was calculated by inversing tangentially the ratio of height and radius of the formed pile. The Carr's compressibility index was calculated. The residual water content of the developed formulations (50 mg) was determined using Automatic Karl-Fischer Titrator (Chemito CL 48885, India). Commercially available pyridine free reagent was standardized with known quantity of water (250 mg).
Surface Morphology and Topographical Features
The surface morphology was examined by scanning electron microscopy (SEM) (JSM-5610 LV, JEOL, Japan). Powder samples were adhered to sample stubs using double sided adhesive tape, and then viewed using an accelerating voltage of 15 kV at the magnification of ×250 to ×5,000. The surface texture and topographical parameters such as roundness, aspect ratio, fractional dimension, heterogeneity and clumpiness of developed formulations were assessed using Image analysis software Image Proplus 5.0 (Media Cybernetics, USA) (15). Image analysis of the SEM pictures was conducted on a fixed area selected on the particle flat base in order to avoid tilting angle shadow effect.
X-ray Diffraction Studies
The X-ray diffraction studies were carried out using X ray diffractometer (Zydus Cadila Ltd, Ahmedabad) at a scan speed of 2°/min. The X-ray diffraction procedure to estimate the degree of crystallinity was based upon the measurement of the total scattering and the scattering from the crystalline region of formulations.
In Vitro Release Studies
In vitro release studies of formulations and spray dried plain DS with lactose (SDSL) were evaluated in a customized and validated diffusion cell across cellophane membrane (10,000 MCO) for 18 h using 50 ml of mixture of phosphate buffer saline (PBS) and methanol (6:3) as diffusion medium at 37°C. The diffusion cell was validated for the hydrodynamic characteristics using the benzoic acid disc method prior to experimentation (19). The formulations equivalent to 15 doses; 500 × 15 μg of DS were dispersed in 1 ml of PBS. Formulations to be compared were separately transferred to the donor compartment and stirred at 50 rpm while the receptor compartment was stirred at 100 rpm. 1ml of the sample was withdrawn from the receptor compartment at definite time intervals and replaced with fresh medium. DS content in aliquots were evaluated using HPLC method and the graph was plotted with the percent drug released vs time. To ascertain the drug release kinetics and mechanism, the data were treated with following equations:
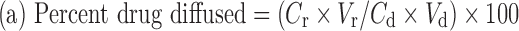 |
1 |
Where, Cr = conc. of drug in receptor compartment, Vr = volume of the receptor compartment, Cd = conc. of drug in donor compartment and Vd = volume of donor compartment.
(b) Kinetics of release: the order of drug release was determined by performing regressions over the mean values of percent drug release vst and percent drug release vs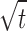 .
.
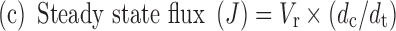 |
2 |
Where, Vr = volume of receptor compartment and (dc/dt) = rate of change of concentration,
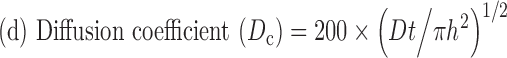 |
2 |
Where, h = thickness of the membrane (0.02 cm), t = time (s), and Dc = diffusion coefficient (cm2/s).
Characterization of Aerosol Performance
Aerosol performance of formulations was assessed using an eight stage, nonviable Anderson Cascade Impactor with a preseparator (Graseby-Andersen, Atlanta, USA) operating at an airflow rate of 28.3 lpm. The impaction plates were pre-coated with a 1.5% w/v of HPMC (4,000 cps) gel in water to overcome particle bounce and re-entrainment phenomenon. Quali-V® capsule containing powder equivalent to 500 ± 20 μg of DS were aerosolized using Inhalator brev I. S. F at 28.3 lpm. Ten capsules were actuated for each impaction with each capsule for 10 s. The drug content deposited in different parts such as induction port, preseparator, individual impaction plates, and powder remaining in capsule and inhaler device was rinsed with methanol and subjected to HPLC analysis. From drug deposition data the emitted dose, fine particle dose, FPF, MMAD, and GSD were calculated according to USP 27 NF 22.
Statistical analysis
Each batch was prepared six times and data from all experiments were expressed as the mean ± standard deviation (SD). Data were compared using ANOVA and Student's t-test and P < 0.05 was considered significant.
RESULT AND DISCUSSIONS
DPPC, a saturated phospholipid was used in this investigation due to its advantages such as stability and biocompatibility as it is an indigenous lung surfactant (20–21). DS loaded NLs were found to have average size of 137 ± 15 nm, zeta potential of 0.8314 ± 0.0827 mV, span value of 0.52, and drug percent entrapment of 95.17 ± 3.43%. Among recent developments techniques of DPIs, the spray drying technique offers a number of potential advantages over freeze drying technique (22–24). Nano-size range of liposome helps to achieve its uniform distribution in the bulk of dry powder formulations. Spray drying process parameters plays an vital role on properties DPIs such as particle size, shape, topographical features, density, moisture content, and drug retention (22,25). Hence, process parameters were optimized for development of DPIs and operating parameters were kept constant during preparation of all DPIs. The spray dried powder yield was observed in between 60% and 70%. The compositions and abbreviations for different spray dried formulations were represented in Table I. Spray dried NLs with lactose (SLDPIL), spray dried NLs with hydrolyzed gelatin (SLDPIH), and spray dried NLs with sucrose (SLDPIS) were found to have VMD of 8.9 ± 1.1, 7.9 ± 1.1, and 11.2 ± 1.3 μm respectively, whereas SDSL was found to have VMD of 9.4 ± 1.3 μm (Table II).
Table I.
Composition of Spray Dried Liposomal Formulations
| Spray Dried Plain Dapsone with Lactose (SDSL) | Spray Dried Liposomal Dry Powder Inhaler with Hydrolyzed Gelatin (SLDPIH) | Spray Dried Liposomal Dry Powder Inhaler with Sucrose (SLDPIS) | Spray Dried Liposomal Dry Powder Inhaler with Sucrose (SLDPIL) |
|---|---|---|---|
| Dapsonea | Dapsone loaded NLsa | Dapsone loaded NLsa | Dapsone loaded NLsa |
| Lactoseb | Hydrolyzed gelatinb | Sucroseb | Lactoseb |
| l-leucinec | l-leucinec | l-leucinec | l-leucinec |
a50 mg of drug or equivalent
b20 mg/ml
c15% w/w
Table II.
Summary Report of Particle Size Distribution and Drug Retention of Spray Dried Formulations
| Formulations | Particle Size VMD (μm) | Span | Drug Retention (%) |
|---|---|---|---|
| SDSL | 9.4 ± 1.1 | 1.4 ± 0.5 | 98.5 ± 1.1 |
| SLDPIH | 7.9 ± 1.1 | 1.4 ± 0.3 | 97.9 ± 1.0 |
| SLDPIS | 11.2 ± 1.3 | 1.6 ± 0.6 | 97.8 ± 0.9 |
| SLDPIL | 8.9 ± 1.1 | 1.3 ± 0.1 | 98.4 ± 1.0 |
Mean ± SD, n = 3
SDSL: spray dried plain dapsone with lactose, SLDPIH: spray dried liposomal dry powder inhaler with hydrolyzed gelatin, SLDPIS: spray dried liposomal dry powder inhaler with sucrose, SLDPIL: spray dried liposomal dry powder inhaler with lactose
A non significant effect (P > 0.05) of operational parameters was observed on particle size distribution of developed DPIs using different carriers. Above 97% of drug retention was observed with spray dried formulations (Table II). Recently, various novel techniques for development of aerodynamically light particles have been studied which resulted in maximum drug deposition in to the deep lung (25–28). Formulations having a tap density less than 0.4 g/cm3 and relatively large mean diameter between 5 and 30 μm, but possess MMAD in the range of 1 to 5 μm. The SLDPIH was found to have the lowest density (0.14 ± 0.06 g/cc), good flowability (Angle of repose 26. 3 ± 1.5°, Carr's compressibility index 36.8 ± 1.5%), and low residual water content of 2.3 ± 0.4. The SLDPIL found to have density of 0.18 ± 0.03 g/cc, angle of repose of 31. 5 ± 1.5°, Carr's compressibility index of 34.9 ± 2.0%, and residual water content of 4.5 ± 0.6, and SLDPIS possess density of 0.21 ± 0.04 g/cc, angle of repose of 33.6 ± 1.6°, Carr's compressibility index of 31.5 ± 2.1%, and residual water content of 5.2 ± 0.9. Developed NLs DPIs were found to have low density, good flowability and low residual moisture content as is observed from Table III. The developed DS NLs DPIs found to have particles size >5 μm and a tap density less than 0.4 g/cm3 suggestive of formation of aerodynamically light particles. Such DPIs were reported to be more capable of escaping inertial and gravitational deposition in the oropharyngeal region, and site specifically targeted to the deep lung or airways (25).
Table III.
Solid State Characterization and Residual Water Content of Spray Dried Formulations
| Formulations | Tapped density (g/cc) | Angle of Repose (°) | Carr's compressibility index | Residual water content (%) |
|---|---|---|---|---|
| SDSL | 0.80 ± 0.20 | 47.8 ± 2.2 | 25.1 ± 2.5 | 6.2 ± 0.9 |
| SLDPIH | 0.14 ± 0.06 | 26. 3 ± 1.5 | 36.8 ± 1.5 | 2.3 ± 0.4 |
| SLDPIS | 0.21 ± 0.04 | 33.6 ± 1.6 | 31.5 ± 2.1 | 5.2 ± 0.9 |
| SLDPIL | 0.18 ± 0.03 | 31. 5 ± 1.5 | 34.9 ± 2.0 | 4.5 ± 0.6 |
Mean ± SD, n = 3
SDSL: spray dried plain dapsone with lactose, SLDPIH: spray dried liposomal dry powder inhaler with hydrolyzed gelatin, SLDPIS: spray dried liposomal dry powder inhaler with sucrose, SLDPIL: spray dried liposomal dry powder inhaler with lactose
Phospholipids were component of pulmonary surfactants and were reported to facilitate droplet formation in the atomization step of spray drying, decrease particle surface energy, powder cohesiveness, and reduce residual water content (28). An amino acid, l-leucine, was used as anti-adherent to prevent tendency of the particles to bond strongly and result into light particles with good flow behavior, deaggregation properties, and dose reproducibility of DPIs. In this investigation, an attempt was made to develop aerodynamically light particles of liposomal DS having a tap density below 0.4 g/cc, mean geometrical diameter above 5 μm and mean MMAD of the particles is between 2 and 3 μm. The Formation of aerodynamically light particles may attribute to inclusion of NLs and l-leucine as antiadherent. Due to the hygroscopic behavior of sucrose, spray drying with sucrose resulted into DPI with higher moisture content and larger particle size among all formulations, this report is in consistence with earlier findings (28).
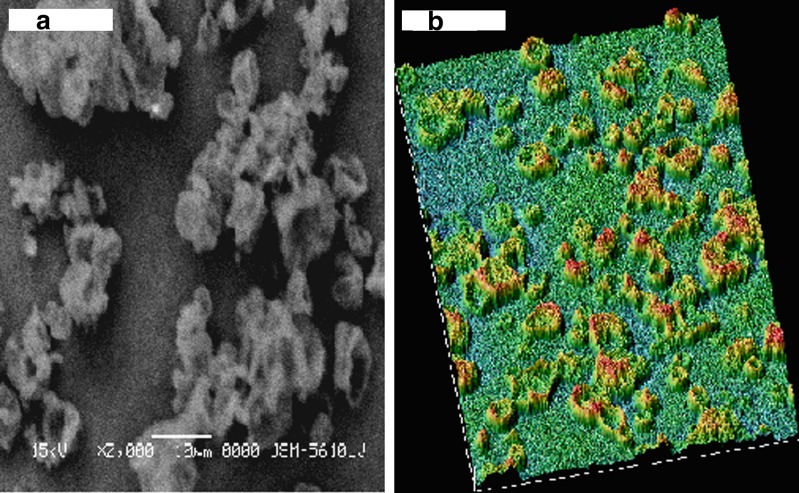
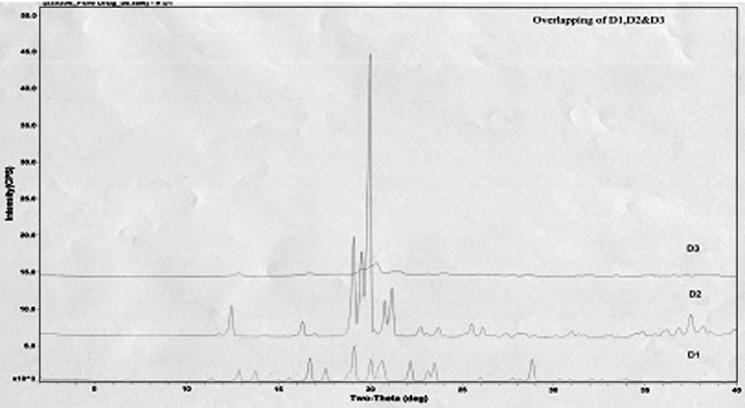
SEM reveals smooth and porous surface of SLDPIH (Fig. 1a) and Fig. 1b suggest varying degrees of surface roughness at different locations on surface and porous nature of spray dried formulation. The surface fractal dimension, which represents degree of particle surface corrugation (29) was determined from the texture of the images of powder surfaces. The results indicate that a SLDPIH showed lower roughness among developed formulations. Higher value of heterogeneity and clumpiness for SLDPIL and SLDPIS also suggest high degree of roughness in contrast to SLDPIH (Table IV). These variations were probably related to the composition and kinetics of particle formation. The X ray diffraction pattern for SLDPIH showed weak diffraction peaks and lower degree of crystallinity compared to pure drug and physical mixture suggestive of amorphous nature of formulation (Fig. 2).
Fig. 1.
a Scanning electron microphotograph and b surface texture analysis of hydrolyzed gelatin based spray dried formulation
Table IV.
Topographical Features of Spray Dried Formulations Measured by SEM Image Analysis
| Formulations | Roundness | Aspect Ratio | Fractal Dimension | Heterogeneity | Clumpiness |
|---|---|---|---|---|---|
| SLDPIH | 0.981 ± 0.09 | 1.046 ± 0.249 | 0.957 ± 0.076 | 0.319 ± 0.064 | 0.197 ± 0.054 |
| SLDPIS | 1.286 ± 0.08 | 1.348 ± 0.471 | 1.217 ± 0.090 | 0.710 ± 0.07 | 0.270 ± 0.080 |
| SLDPIL | 1.098 ± 0.05 | 1.275 ± 0.276 | 1.007 ± 0.081 | 0.508 ± 0.064 | 0.216 ± 0.041 |
Mean ± SD, n = 10
SLDPIH: spray dried liposomal dry powder inhaler with hydrolyzed gelatin, SLDPIS: spray dried liposomal dry powder inhaler with sucrose, SLDPIL: spray dried liposomal dry powder inhaler with lactose
Fig. 2.
X-ray diffractogram of D1 pure drug, D2 additives, and D3 SLDPIH
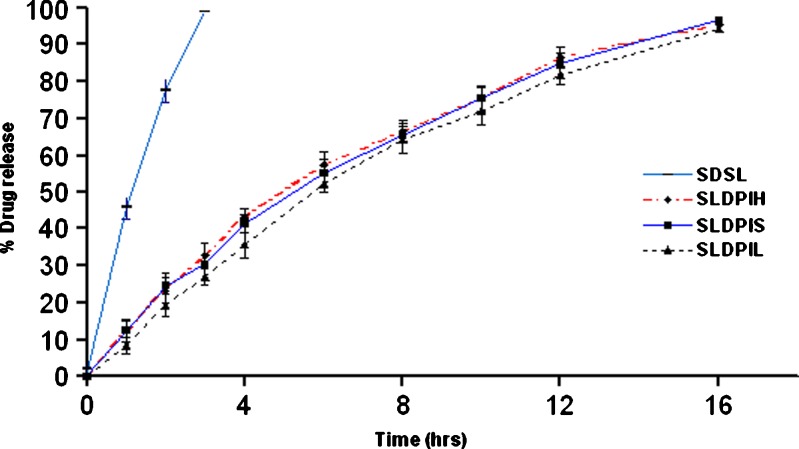
Statistically significant difference (P < 0.01) was observed in between in vitro release patterns of spray dried DPIs and SDSL. The regression coefficients values of formulations were found in the range of 0.9420–0.9615 suggesting a linear relationship between percent drug diffused and 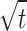 . The release pattern follows Higuchi's controlled release model and release rate found to be close to first order kinetics. A non-significant difference (P > 0.01) was observed in release pattern among the all developed formulations. In vitro drug release studies showed 90% drug release within 3 h form SDSL and 16 h from SLDPIH, SLDPIS, and SLDPIL respectively (Fig. 3). The mean flux values and diffusion coefficient of the SDSL was found to be 4.7 times and 3.62 times higher than those of SLDPIH, 4.86 times and 3.69 times higher than those of SLDPIS, and 5.07 times and 3.80 times higher than those of SLDPIL respectively (Table V). This is suggestive of prolonged drug release DS from developed formulations and may play a promising in prevention of PCP.
. The release pattern follows Higuchi's controlled release model and release rate found to be close to first order kinetics. A non-significant difference (P > 0.01) was observed in release pattern among the all developed formulations. In vitro drug release studies showed 90% drug release within 3 h form SDSL and 16 h from SLDPIH, SLDPIS, and SLDPIL respectively (Fig. 3). The mean flux values and diffusion coefficient of the SDSL was found to be 4.7 times and 3.62 times higher than those of SLDPIH, 4.86 times and 3.69 times higher than those of SLDPIS, and 5.07 times and 3.80 times higher than those of SLDPIL respectively (Table V). This is suggestive of prolonged drug release DS from developed formulations and may play a promising in prevention of PCP.
Fig. 3.
In vitro release pattern of developed NLs DPIs and plain dapsone DPIs
Table V.
In vitro Drug Release Parameters Regression Coefficient of the Line of Percent Drug Released Vs Square Root of Time, Mean Flux and Diffusion Coefficient of Spray Dried Formulations
| Formulations | Regression Coefficient (r 2) | Mean Flux (μg/min) | Diffusion coefficient (cm2/s) |
|---|---|---|---|
| SDSL | – | 195.2 | 2.52 E-03 |
| SLDPIH | 0.9420 | 41.5 | 6.96 E-04 |
| SLDPIS | 0.9533 | 40.2 | 6.82 E-04 |
| SLDPIL | 0.9615 | 38.5 | 6.62 E-04 |
SDSL: spray dried plain dapsone with lactose, SLDPIH: spray dried liposomal dry powder inhaler with hydrolyzed gelatin, SLDPIS: spray dried liposomal dry powder inhaler with sucrose, SLDPIL: spray dried liposomal dry powder inhaler with lactose
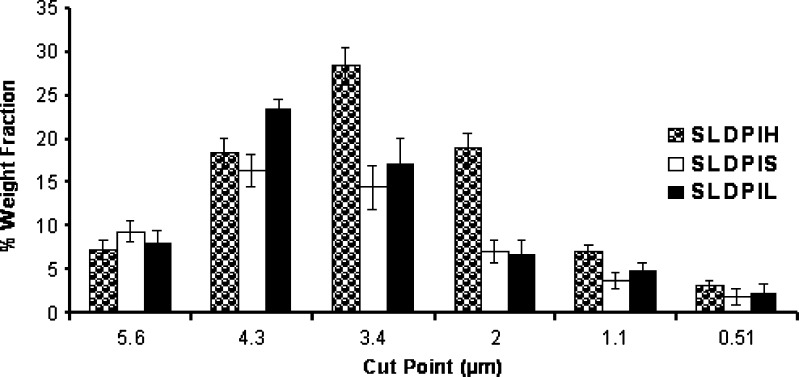
The efficiency of powder recovery from the cascade impaction test was found to be >93%. The weight fraction according to the size distribution of the aerosolized particles from DPIs was graphically presented in Fig. 4. Each bar represents the powder of certain sizes collected on a defined stage of the Andersen cascade impactor. Significant difference (P < 0.05) was observed in the aerodynamic diameter distribution among the developed DPIs. Shift in the deposition pattern of SLDPIH towards the lower stages of the impactor (stages 3–7) was also been noticed (Fig. 4). Maximum FPF of 75.6 ± 1.6% was observed with SLDPIH as compared to SLDPIS of 55.9 ± 1.7% and SLDPIL of 62.8 ± 2.8% (Table VI). SLDPIH found to have 1.34 times and 1.20 times enhanced FPF compared to SLDPIS and SLDPIL respectively (Table VI and Fig. 4). SLDPIH found to have MMAD of 2.2 ± 0.1 and GSD of 2.3 ± 0.1. The emitted dose percentage was ranged from 55.9 ± 1.7% for SLDPIS to 87 ± 2% for SLDPIH (Table VI). Statistically significant change (P < 0.05) in the FPF was observed in the in vitro aerosolization performance among developed formulations. SLDPIH exhibited the best aerosol powder performance in this investigation in terms of emitted dose, MMAD, FPF, homogeneous size distribution among developed DPIs. Promising aerosolization results of SLDPIH may be contributing to formation of aerodynamic light and large particles, low density, good flow properties, and lower moisture content as evident from Tables II and III.
Fig. 4.
In vitro pulmonary deposition pattern of different spray dried formulations. Each bar represents the average of six repeats and the error bars refer to the SD
Table VI.
In vitro Aerosol Deposition Data of Spray Dried Formulations
| Formulations | Emitted dose (%) | Fine particle fraction (%) | Mean median aerodynamic diameter (μm) | GSD |
|---|---|---|---|---|
| SLDPIH | 87 ± 2 | 75.6 ± 1.6 | 2.2 ± 0.1 | 2.3 ± 0.1 |
| SLDPIS | 67 ± 4 | 55.9 ± 1.7 | 2.8 ± 0.1 | 2.9 ± 0.2 |
| SLDPIL | 78 ± 2 | 62.8 ± 2.8 | 2.5 ± 0.2 | 2.6 ± 0.2 |
Mean ± SD, n = 6
SLDPIH: spray dried liposomal dry powder inhaler with hydrolyzed gelatin, SLDPIS: spray dried liposomal dry powder inhaler with sucrose, SLDPIL: spray dried liposomal dry powder inhaler with lactose
CONCLUSIONS
Developed aerodynamically light and porous spray dried formulation of DS loaded NLs showed enhanced deep lung deposition (FPF > 75%). The prolonged in vitro drug release up to 16 h was observed with developed formulations compared to 3 h for plain drug spray dried with lactose. Hence, the developed spray dried formulations may play a promising role in management of PCP by providing prolonged site-specific local action, necessitating less frequent administration and/or dose of DS and eventually, reduced associated systemic toxicities.
ACKNOWLEDGMENTS
The authors are thankful to Indian Council of Medical Research (ICMR), New Delhi, India for providing funding to the research project and Technology Information and Forecasting Council's (TIFAC) Centre of Relevance and Excellence in New Drug Delivery System. The authors are thankful to Sun Pharmaceutical Advanced Research Center (SPARC), Vadodara, India, Zydus Cadila Ltd, Ahmedabad, India and Panacea Biotec Ltd, Lalru, India for availing facilities for part of research work.
REFERENCES
- 1.Ashley E. A., Johnson M. A., Lipman M. C. I. Human immunodeficiency virus and respiratory infection. Curr. Opin. Pulm. Med. 2000;6:240–245. doi: 10.1097/00063198-200005000-00013. [DOI] [PubMed] [Google Scholar]
- 2.Murray J. F., Mills J. Pulmonary infectious complications of human immunodeficiency virus infection. Part I. Am. Rev. Respir. Dis. 1990;141(5 Pt 1):1356–1372. doi: 10.1164/ajrccm/141.5_Pt_1.1356. [DOI] [PubMed] [Google Scholar]
- 3.Fishman J. A. Treatment of infection due to Pneumocystis carinii. Antimicrob. Agents Chemother. 1998;42(6):1309–1314. doi: 10.1128/aac.42.6.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gallant J. E., Chaisson R. E., Moore R. D. The effect of adjunctive corticosteroids for the treatment of Pneumocystis carinii pneumonia on mortality and subsequent complications. Chest. 1998;114(5):1230–1231. doi: 10.1378/chest.114.5.1258. [DOI] [PubMed] [Google Scholar]
- 5.Mathew B. S., Grossman S. A. Pneumocystis carinii pneumonia prophylaxis in HIV negative patients with primary CNS lymphoma. Cancer Treat. Rev. 2003;29:105–119. doi: 10.1016/S0305-7372(03)00002-1. [DOI] [PubMed] [Google Scholar]
- 6.Waldrep J. C. New aerosol drug delivery systems for the treatment of immune-mediated pulmonary diseases. Drugs Today. 1998;34(6):549–561. doi: 10.1358/dot.1998.34.6.485253. [DOI] [PubMed] [Google Scholar]
- 7.Ashurst S., Malton A., Prime D., et al. Latest advances in the development of dry powder inhaler. Pharm. Sci. Tech. Today. 2000;3(7):246–256. doi: 10.1016/S1461-5347(00)00275-3. [DOI] [PubMed] [Google Scholar]
- 8.Letsou G. V., Safi H. J., Reardon M. J., et al. Pharmacokinetics of liposomal aerosolized cyclosporine A for pulmonary immunosuppression. Ann. Thorac. Surg. 1999;68:2044–2048. doi: 10.1016/S0003-4975(99)01183-2. [DOI] [PubMed] [Google Scholar]
- 9.Chougule M. B., Padhi B., Misra A. N. Nanoliposomal dry powder inhaler formulation of Amiloride hydrochloride. J. Nanosci. Nanotechnol. 2006;6:3001–3009. doi: 10.1166/jnn.2006.405. [DOI] [PubMed] [Google Scholar]
- 10.Lo Y., Tsai J., Kuo J. Liposomes and disaccharides as carriers in spray-dried powder formulations of superoxide dismutase. J. Control. Release. 2004;94:259–272. doi: 10.1016/j.jconrel.2003.09.019. [DOI] [PubMed] [Google Scholar]
- 11.Lu D., Hickey A. J. Liposomal dry powders as aerosols for pulmonary delivery of proteins. AAPS PharmSciTech. 2005;6(4):E641–E648. doi: 10.1208/pt060480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vermehren C., Frokjaer S., Aurstad T., et al. Lung surfactant as a drug delivery system. Int. J. Pharm. 2006;307(1):89–92. doi: 10.1016/j.ijpharm.2005.10.029. [DOI] [PubMed] [Google Scholar]
- 13.Shah S. P., Misra A. N. Development of liposomal Amphotericin B dry powder inhaler formulation. Drug Deliv. 2004;11(4):247–253. doi: 10.1080/10717540490467375. [DOI] [PubMed] [Google Scholar]
- 14.Joshi M., Misra A. N. Disposition kinetics of ketotifen from liposomal dry powder for inhalation in rat lung. Clin. Exp. Pharmacol. Physiol. 2003;30(3):153–156. doi: 10.1046/j.1440-1681.2003.03813.x. [DOI] [PubMed] [Google Scholar]
- 15.Chougule M. B., Padhi B., Misra A. N. Nano-liposomal dry powder inhaler of tacrolimus: preparation, characterization and pulmonary pharmacokinetics. Int. J. Nanomed. 2007;2(4):1–17. [PMC free article] [PubMed] [Google Scholar]
- 16.Joshi M. R., Misra A. Liposomal budesonide for dry powder inhaler: preparation and stabilization. AAPS PharmSciTech. 2001;2(4):25. doi: 10.1208/pt020425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.New R. R. C. Preparation of liposomes. In: New R. R. C., editor. Liposomes a practical approach. New York: Oxford University Press; 1990. [Google Scholar]
- 18.Mu L., Feng S. S. Fabrication, characterization and in vitro release of paclitaxel (Taxol) loaded poly (lactic-co-glycolic acid) microspheres prepared by spray drying technique with lipid/cholesterol emulsifiers. J. Control Release. 2001;76:239–254. doi: 10.1016/S0168-3659(01)00440-0. [DOI] [PubMed] [Google Scholar]
- 19.Mojaverian J., Rosen W. A., Vadino S., et al. In-vivo/in-vitro correlation of four extended release formulations of pseudoephedrine sulfate. J. Pharm. Biomed. Anal. 1997;15:439–445. doi: 10.1016/S0731-7085(96)01834-1. [DOI] [PubMed] [Google Scholar]
- 20.Kharasch V. S., Sweeney T. D., Fredberg J., et al. Pulmonary surfactant as a vehicle for intratracheal delivery of technetium sulfur colloid and pentamidine in hamster lungs. Am. Rev. Respir. Dis. 1991;144(4):909–913. doi: 10.1164/ajrccm/144.4.909. [DOI] [PubMed] [Google Scholar]
- 21.Davidson W. J., Dorscheid D., Spragg R. Exogenous pulmonary surfactant for the treatment of adult patients with acute respiratory distress syndrome: results of a meta-analysis. Crit. Care. 2006;10:R41. doi: 10.1186/cc4851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elversson J., Millqvist-Fureby A., Alderborn G., et al. Droplet and particle size relationship and shell thickness of inhalable lactose particles during spray drying. J. Pharm. Sci. 2003;92((4)):900–910. doi: 10.1002/jps.10352. [DOI] [PubMed] [Google Scholar]
- 23.Nguyen X. C., Herberger J. D., Burke1 P. A. Protein powders for encapsulation: a comparison of spray-freeze drying and spray drying of darbepoetin alfa. Pharm. Res. 2004;21(3):507–514. doi: 10.1023/B:PHAM.0000019306.89420.f0. [DOI] [PubMed] [Google Scholar]
- 24.Bosquillon C., Rouxhet P. G., Ahimou F., et al. Aerosolization properties, surface composition and physical state of spray-dried protein powders. J. Control Release. 2004;99(3):357–367. doi: 10.1016/j.jconrel.2004.07.022. [DOI] [PubMed] [Google Scholar]
- 25.Vanbever R., Mintzes J., Wan J., et al. Formulation and physical characterization of large porous particles for inhalation. Pharm. Res. 1999;16:1735–1742. doi: 10.1023/A:1018910200420. [DOI] [PubMed] [Google Scholar]
- 26.Tsapis N., Bennet D., Jackson B., et al. Trojan particles: large porous carriers of nanoparticles for drug delivery. Proc. Nat. Acad. Sci. U. S. A. 2002;99:12001–12005. doi: 10.1073/pnas.182233999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Steckel H., Brandes H. G. A novel spray-drying technique to produce low density particles for pulmonary delivery. Int. J. Pharm. 2004;278:187–195. doi: 10.1016/j.ijpharm.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 28.Lo Y., Tsai J., Kuo J. Liposomes and disaccharides as carriers in spray-dried powder formulations of superoxide dismutase. J. Control Release. 2004;94:259–272. doi: 10.1016/j.jconrel.2003.09.019. [DOI] [PubMed] [Google Scholar]
- 29.Chew N. Y. K., Tang P., Chan H. K., et al. How much particle surface corrugation is sufficient to improve aerosol performance of powders? Pharm. Res. 2005;22:148–152. doi: 10.1007/s11095-004-9020-4. [DOI] [PubMed] [Google Scholar]