Abstract
There is a critical need for development of novel delivery systems to facilitate the translation of nucleic acid-based macromolecules into clinically-viable therapies. The aim of this investigation was to develop and evaluate a novel nanoparticles-in-microsphere oral system (NiMOS) for gene delivery and transfection in specific regions of the gastrointestinal (GI) tract. Plasmid DNA, encoding for the enhanced green fluorescent protein (EGFP-N1), was encapsulated in type B gelatin nanoparticles. NiMOS were prepared by further protecting the DNA-loaded nanoparticles in a poly(epsilon-caprolactone) (PCL) matrix to form microspheres of less than 5.0 μm in diameter. In order to evaluate the biodistribution following oral administration, radiolabeled (111In-labeled) gelatin nanoparticles and NiMOS were administered orally to fasted Balb/C mice. The results of biodistribution studies showed that, while gelatin nanoparticles traversed through the GI tract fairly quickly with more than 54% of the administered dose per gram localizing in the large intestine at the end of 2 h, NiMOS resided in the stomach and small intestine for relatively longer duration. Following oral administration of EGFP-N1 plasmid DNA at 100 μg dose in the control and test formulations, the quantitative and qualitative results presented in this study provide the necessary evidence for transfection potential of NiMOS upon oral administration. After 5 days post-administration, transgene expression in the small and large intestine of mice was observed. Based on these results, NiMOS show significant potential as novel gene delivery vehicle for therapeutic and vaccination purposes.
Key words: gene delivery, GI tract, NiMOS, non-viral, transfection
INTRODUCTION
In the past few years, significant advances in molecular engineering and biotechnology have resulted in the discovery of large number of nucleic acid-based macromolecules for therapeutic and vaccination purpose. One of the major problems associated with these molecules is limited bioavailability upon systemic administration, which is further reduced when attempting oral delivery. The oral route however, offers a very convenient method of drug delivery and has remained as a forerunner amongst all of the other routes of administration because of high patient compliance and an opportunity for repeated administration. The gastrointestinal (GI) tract offers an interesting target for gene therapy as a patient-friendly non-invasive route that also exhibits features such as large surface area presented by the gut epithelium for uptake and expression of DNA resulting in local sustained expression of therapeutic proteins, allows access to the luminal side of the intestine for treatment of regional disorder and can also offer long lasting therapeutic gene expression achieved due to the presence of a large number of stem cells in the intestinal crypts (1).
Several investigators have examined the potential of oral plasmid DNA based therapeutics for local gastrointestinal disorders and food allergies. The GI tract also serves as the entry portal for many pathogens and also provides a very convinient route for vaccine administration (2,3). Using DNA-based vaccines, it is possible to establish an immunological barrier against pathogens entering via the mucosal membrane (4–6). and have been reported previously (7–9). However, oral gene delivery for efficient and sustained expression remains the most challenging because of various anatomical (mucus and epithelial layer) and physiological barriers (varying pH, degradative enzymes) that are exhibited by the GI tract (10).
A major shift has been observed away from viral vectors and towards the use of non-viral vectors for systemic and local delivery of nucleic acid therapeutics in the last decade. Among the non-viral vectors, significant research and development effort has focused on use of polymeric systems that include nanoparticles and microspheres, for delivery of nucleic acids therapeutics (11,12). Judicious selection of polymeric materials can lead to development of nano- and micro-particle systems, which are useful in oral administration of genetic material (13). By controlling the microsphere size, in the range of 1–5 μm, these systems can deliver a variety of therapeutic and immunogenic agents, including peptides and proteins, for uptake by the M-cells lining the Peyer`s patch in the small intestine (14). Several groups have examined the use of oral vaccination strategies based on administration of biodegradable polymeric microsphere formulations to generate mucosal and systemic immunity (1,4–7,15).
Previously, the formulation optimization of nanoparticles-in-microsphere oral system (NiMOS) using a “double emulsion-like” technique has been reported (16). NiMOS are formulated to encapsulate gelatin or other nanoparticulate system within a biodegradable poly(epsilon-caprolactone) (PCL) microsphere matrix. In another study, the biodistribution and transfection ability of this system using plasmid DNA CMV-βgal and EGFP-N1 encoding β-galactosidase and green fluorescent protein, respectively upon oral administration in fasted Wistar rats was reported (17). This study describes the biodistribution and quantitative gene transfection results in Balb/C mice upon oral administration of reporter plasmid DNA encapsulated in NiMOS. Oral distribution and absorption studies were carried out using indium-111 labeled gelatin nanoparticles encapsulated in NiMOS in fasted Balb/C mice. Quantitative gene transfection studies in the GI tract were performed with plasmid DNA vector EGFP-N1, which encodes for enhanced green fluorescent protein.
MATERIALS AND METHODS
Materials
Type-B gelatin (MW 40,000–50,000) of 225 bloom strength, with 100–115 mM of free carboxylic acid per 100 g of protein, and an isoelectric point of 4.7–5.2 was obtained from Sigma Chemical Company, (St. Louis, MO). Poly(epsilon-caprolactone) (PCL) (MW 10,000–20,000 as verified by gel permeation chromatography) was purchased from Polysciences, Inc. (Warrington, PA). Poly(vinyl alcohol) (PVA) (Celvol 205, degree of hydrolysis 88%, MW 31,000–50,000) was purchased from Celanese Inc. (Dallas, TX). Sodium metabisulfite and reagent grade dichloromethane were purchased from Fisher Scientific (Fairlawn, NJ). Reagent grade ethanol was obtained from Acros Chemicals (Parsipanny, NJ). For in vitro release study bovine pancreatic protease with an activity of 6.9 units/mg was obtained from Sigma Chemical Company (St. Louis, MO) and bovine pancreatic lipase with an activity of 16 units/mg was purchased from ICN Biomedicals (Aurora, OH). Diethylenetriaminepentaacetate-anhydride (DTPA-A), 4-(2-hydroxyethyl)-1-piperazine-ethanesulfonic acid (HEPES), sodium citrate, and sodium chloride were also purchased from Fisher Scientific (Fair Lawn, NJ). The radioactive isotope, indium-111 chloride (t1/2 = 2.8 days and specific activity 380–415 Ci/mg), was purchased from Perkin Elmer Life Sciences (Wellesley, MA). Plasmid DNA vector, enhanced green fluorescent protein (EGFP-N1) were propagated and purified by Elim Biopharmaceuticals, Inc (Hayward, CA). ELISA kit was obtained from Pierce Biotechnology (Rockford, IL). Detection antibody conjugated to alkaline phosphatase was obtained from Novus Biologicals (Littleton, CO). All other reagents and chemicals were of analytical grade. Aqueous solutions were prepared exclusively in deionized distilled water (Nanopure II, Barnstead/Thermolyne, Dubuque, IA).
Formulation of Nanoparticles-in-Microsphere Oral System (NiMOS)
Gelatin nanoparticles encapsulating plasmid DNA encoding β-galactosidase or enhanced green fluorescent protein, respectively, were prepared by the ethanol precipitation method under controlled conditions of temperature and pH as described previously (18,19). The lyophilized gelatin nanoparticles were further used for preparation of NiMOS according to an optimized protocol for formation of 2–5 μm NiMOS as described previously (16).
Characterization of Gelatin Nanoparticles and NiMOS
Particle Size Analysis
Freshly-prepared suspension of gelatin nanoparticles was analyzed for particle size and size distribution by a light scattering method using the Brookhaven Instrument’s (Holtsville, NY) 90Plus® ZetaPALS particle size analyzer as described previously (16). The particle size analysis was carried out at 90° scattering angle and a temperature of 25 °C.
Similarly, freshly prepared NiMOS were characterized for particle size and size distribution using a Multisizer™ 3, Beckman Coulter (Fullerton, CA) instrument as described previously (16). All measurements of particle size determination for NiMOS were performed at room temperature.
Scanning Electron Microscopy
Plasmid DNA-containing gelatin nanoparticles and NiMOS were separated from the suspension by centrifugation and lyophilized. The freeze-dried particles were mounted on an aluminum sample mount and sputter coated with gold-palladium to minimize surface charging. The sputter coated samples were then observed for surface morphology with a Hitachi S-4800 (Pleasanton, CA) field emission scanning electron microscope.
DNA Loading Studies
Gelatin nanoparticles and NiMOS containing pEGFP-N1 were prepared as described above. Loading capacity and efficiency of plasmid DNA in gelatin nanoparticle formulation was determined using PicoGreen® dye and in exactly the same manner as mentioned previously (20).
In Vitro Release Studies
One-hundred milligrams of NiMOS encapsulating pEGFP-N1 in gelatin nanoparticles were placed in an Eppendorf tubes and 1.5 mL of PBS or PBS containing lipases (0.5 mg/mL) and protease (0.2 mg/mL) was added. Lipase and protease were added to the release medium in order to evaluate the release of plasmid DNA in the presence of PCL- (lipase) and gelatin- (protease) degrading enzymes. These tubes were then placed on a Lab-Line® temperature-controlled rocking incubator and the release studies were performed at 37 °C. At pre-determined time intervals, the tubes were removed from the incubator and centrifuged at 5,000 rpm for 10 min. A volume of 500 μL was drawn from the supernatant and replaced with same volume of either buffer system to maintain a constant volume and sink condition. The amount of plasmid DNA released at each time interval was determined by the PicoGreen® dsDNA assay method.
Biodistribution Studies in Balb/c Mice
In order to determine the fate of gelatin nanoparticles and NiMOS formulations in vivo upon oral administration, each was labeled with indium-111 (111In). Gelatin nanoparticles, in the absence of plasmid DNA, were prepared in the same way as described previously (20,21). The 111In-labeled gelatin nanoparticles were then used to formulate NiMOS as described previously. All of the animal studies described below were performed according to the experimental protocol approved by the Institutional Animal Care and Use Committee, the Radiation Safety Committee, and the Office of Environmental Health and Safety at Northeastern University (Boston, MA). Female Balb/C mice, 8–10 weeks of age and weighing approximately 18–20 g, were purchased from Taconic Farms (Hudson, NY). The animals were allowed to acclimate for at least 48 h before any experiments were performed on them.
The animals were fasted for 24 h before administration and housed on raised wire gauge platforms in polycarbonate cages during the period of study. A dose of 2.0 microCuries (μCi) of 111In-labeled gelatin nanoparticles dispersed in 1.5 mL of saline was administered orally using a gavage needle and syringe to one group of animals. Another group of 24-h fasted animals received 2.0 μCi dose of 111In-labeled gelatin nanoparticles encapsulated in NiMOS. At specific time points of 1, 2, 4, and 6 h after administration, each group of 4 rats were sacrificed by CO2 inhalation. GI tissue samples including the stomach, small intestine, and large intestine were harvested. In addition, blood samples and liver tissues were obtained to determine systemic absorption, if any. The radioactivity accumulated in each of these fluid or tissues was determined with a Wallac 1470 Wizard™ automatic gamma counter. Weight of each fluid or tissue was measured and the corresponding radioactivity from that sample was recorded. The counts-per-minute readings obtained from the gamma counter was converted into microCi of radioactivity using calibration standards for 111In. The concentrations of gelatin nanoparticles and NiMOS in all of the samples were expressed as percent injected dose per gram of fluid or tissue.
Quantitative and Qualitative In Vivo Green Fluorescent Protein Expression
Animals were fasted for 24 h before administration and housed on raised wire gauge platforms in polycarbonate cages during this period. The animals were divided into 4 groups each containing 4 mice. One group of animal received 0.5 mL saline orally, second group was administered orally with naked pEGFP-N1, the third group of animals were administered orally using a gavage needle with gelatin nanoparticles suspended in 0.5 mL of saline corresponding to a dose of 100 μg of pDNA as determined from loading studies and the last group of animals received NiMOS encapsulating pEGFP-N1 corresponding to 100 μg of pEGFP-N1.
Animals were sacrificed at day 5 after administration by carbon dioxide inhalation and gastrointestinal organs including stomach, small intestine, large intestine and liver were harvested. All the tissues were processed in the same manner to prepare them for determination of protein expression by sandwich ELISA. Briefly, 0.5 mL of tissue protein extraction buffer with protease inhibitor was added to the Eppendorf microcentrifuge tube containing the tissue. After homogenizing the tissue with tissue protein extraction buffer, samples were centrifuged at 10,000 rpm for 20 min. After centrifugation the supernatant was carefully removed from the tube and 200 μL of it was added to 96-well microplate pre-coated with polyclonal antibody against GFP, Reacti-Bind™ Anti-GFP plates purchased from Pierce Biotechnology (Rockford, IL), and incubated overnight at 4 °C. After incubation, the plates were washed several times with phosphate buffered saline containing 0.05% Tween® - 20 (PBS-T) and to this was added 100 μL secondary polyclonal antibody against GFP conjugated to enzyme alkaline phosphatase and incubated for 2 h at room temperature. Upon binding of secondary antibody, 100 μL (20 mM) of p-nitrophenolphosphate (PNPP), substrate for alkaline phosphatase, purchased from Roche Diagnostic Corporation (Indianapolis, IN), was added to plate and incubated at room temperature for 1 h. The enzyme reaction was stopped by adding equal volume of 0.75 M sodium hydroxide solution. Finally, sample absorbance was measured from the plate at 409 nm using Bio-Tek Synergy™ HT Multi-Detection Microplate reader (Winooski, Vermont) which was converted to amount of GFP. The samples were also analyzed for total protein content using NanoOrange™ protein quantitation kit, Invitrogen (Carlsbad, CA) as per manufacturer guidelines. Amount of GFP expressed in tissue was reported as micrograms of GFP per milligram of total protein.
Qualitative GFP expression was determined by fluorescent microscopy of the tissue sections from gastrointestinal organs harvested from animals administered with control and NiMOS formulation (20). Briefly, the isolated organs were washed with PBS and stored overnight in 2.0% (v/v) formaldehyde solution in PBS. After this step, the tissues were again washed twice with PBS to remove excess formaldehyde before transferring them in 30% (w/v) solution of sucrose in PBS and stored at 4 °C. The fixed tissues were frozen and placed in tissue-Tek-OCT and 10-μm sections were obtained with a cryostat maintained at −20 °C. The tissue sections were mounted on a clean glass slide, washed with PBS to remove tissue-Teck-OCT, and observed with a Olympus IX71 fluorescence microscope equipped with Hamamatsu ORCA-285 digital camera.
RESULTS AND DISCUSSION
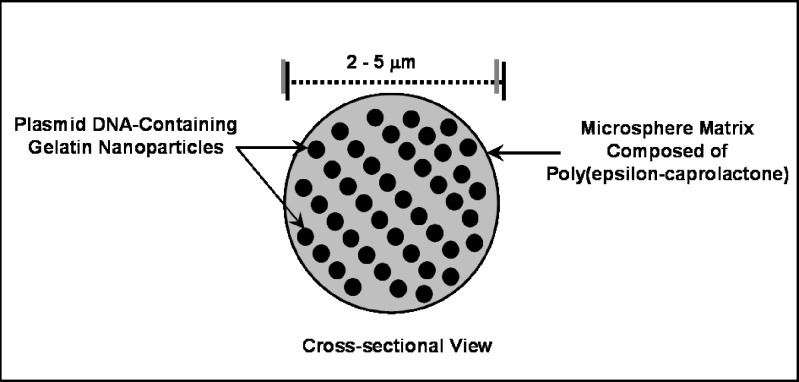
The NiMOS formulation is composed of type B gelatin nanoparticles encapsulated in PCL matrix to form microspheres of less than 5 μm in diameter (Fig. 1). Gelatin nanoparticles encapsulating plasmid DNA were formulated using a solvent displacement technique and has been previously reported by our lab (16,18,21). The “double-emulsion like” technique was employed to encapsulate these gelatin nanoparticles into the microsphere matrix (16). Local delivery of nucleic acid encoding for therapeutic protein/enzyme or DNA vaccine can be achieved by using the appropriate size of NiMOS. Previously, the in vivo transfection potential of NiMOS has been reported by us in fasted Wistar rats using plasmid DNA vectors CMVβ-gal and EGFP-N1, encoding for bacterial β-galactosidase and green fluorescent protein, respectively and provided qualitative evidence for transfection potential of NiMOS (20).
Fig. 1.
Schematic illustration showing the concept of nanoparticles-in-microsphere oral system (NiMOS)
Particle Size Characterization and Scanning Electron Microscopy
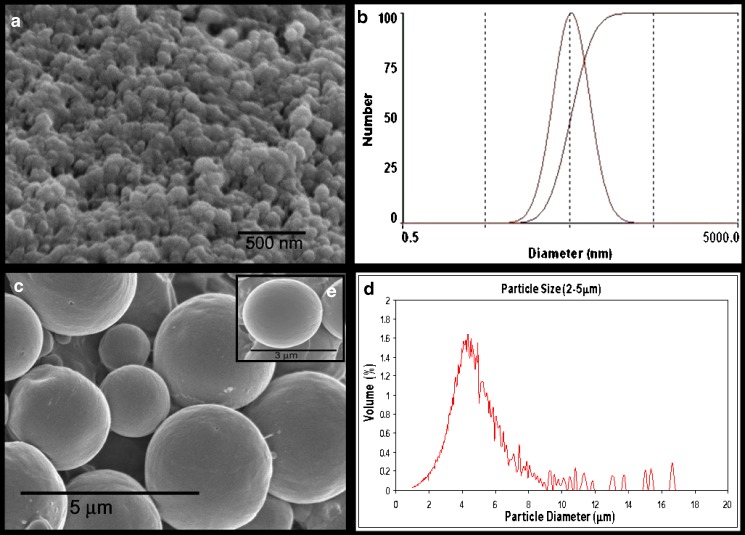
The use of solvent displacement technique has consistently resulted in the formation of gelatin nanoparticles encapsulating plasmid DNA of approximately 150 nm (80–300 nm) as determined by with 90Plus® ZetaPALS particle size analyzer which determines particle size based on dynamic light scattering principle. Particle size of NiMOS, on the other hand, was determined using a Multisizer3™ which measures particles size based on the change in electrical resistance. NiMOS prepared by “double emulsion like technique” were observed to have a particle size range of 2–5 μm. Figure 2 shows the particle size distributions as well as the SEM images of gelatin nanoparticles and NiMOS. As seen from the SEM, NiMOS formed were fairly uniform spherical microparticles with smooth surface morphology. Higher magnification SEM image, Fig. 2 inset, illustrates that the gelatin nanoparticles were encapsulated in the PCL matrix and not adsorbed to the surface. If the nanoparticles were on surface of NiMOS, the surface morphology would have been fairly rough.
Fig. 2.
Scanning electron microscopy (SEM) image a and particle size analysis b of gelatin nanoparticles. SEM image c and particles size analysis d of NiMOS. The inset shows higher magnification SEM image of individual NiMOS e
Plasmid DNA Encapsulation and Release
NiMOS in this study were prepared by encapsulating enhanced green fluorescent protein expressing plasmid DNA (EGFP-N1, 4.7 kb) in the gelatin nanoparticles. Based on the PicoGreen® assay used for quantitation of double stranded DNA, at 0.5% (w/w) concentration of added plasmid DNA, the average loading efficiency was found to be greater than 93% in the gelatin nanoparticles. The final DNA loading efficiency in NiMOS was measured to be 46% of the added DNA.
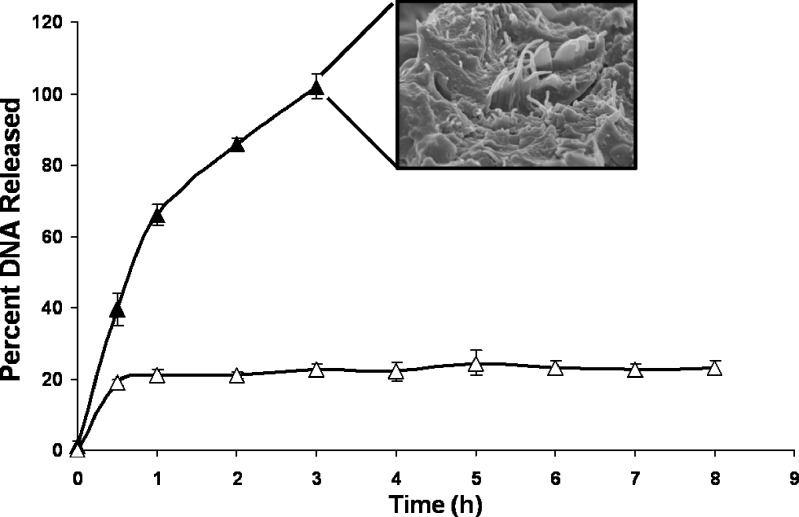
For the in vitro DNA release studies, NiMOS formulation was incubated in the absence and presence of lipase and protease. Since the encapsulated plasmid DNA is fairly large, no release of encapsulated plasmid DNA occurring by passive diffusion was anticipated. Lipase is known to degrade the PCL matrix and protease will degrade the gelatin matrix, thus releasing the encapsulated plasmid DNA. As shown in Fig. 3, there was relatively small percentage (<20%) of DNA released from the NiMOS in the absence of the enzymes. In addition, even after incubating the NiMOS for up to 8 h in PBS at 37 °C, the percent release did not change. This shows that the encapsulated DNA was protected by the PCL and gelatin matrices in the absence of the degrading enzymes. On the other hand, when the two enzymes were present in PBS, more than 65% of the encapsulated plasmid DNA was released in the first hour and the entire fraction was released within 3 h of incubation. This is also seen in the SEM image of NiMOS taken after 100% release. The image clearly shows degradation of polymers in presence of lipase and protease.
Fig. 3.
Plasmid DNA release from NiMOS in the absence (open triangles) and presence (filled triangles) of 0.2 mg/mL protease and 0.5 mg/mL lipase in phosphate-buffered saline (pH 7.4) at 37 °C. The inset shows SEM image of NiMOS at the end of release study
The results of in vitro release in the absence and presence of enzymes clearly support our concept that the plasmid DNA was encapsulated in the gelatin nanoparticles, which were further protected by PCL in NiMOS formulation. In the presence of proteolytic degrading enzymes in the stomach, the PCL shell is expected to provide the necessary protection. Release of gelatin nanoparticles is expected to occur only in the small and large intestines.
Biodistribution after Oral Administration in Balb/C Mice
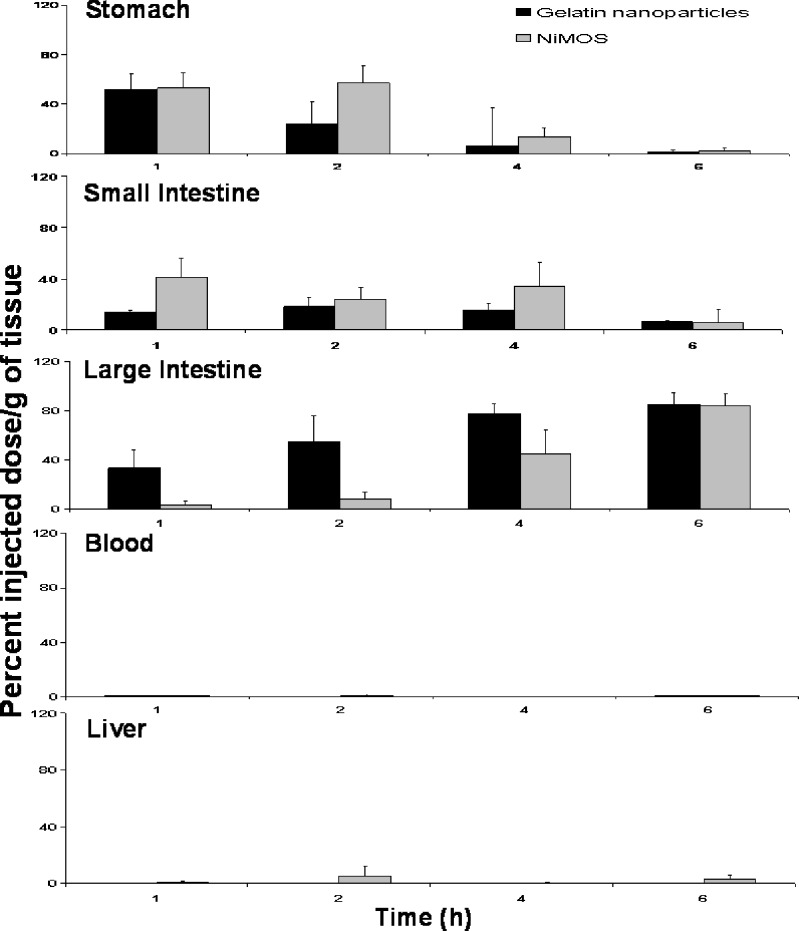
For the biodistribution studies with 111In-labeled gelatin nanoparticles alone or after encapsulation in NiMOS, the radiolabel was covalently attached to the nanoparticle surface through DTPA linker. After oral administration in conscious 24-h fasted female Balb/C mice, the distribution profile determined based on radioactivity values in each tissue. As shown in Fig. 4, gelatin nanoparticles rapidly cleared the stomach and small intestine and were predominantly found in the large intestine. After 1 h of administration, more than 32% of the administered dose per gram was in the large intestine with this amount increasing to 54% at the end of 2 h. Correspondingly, minimal radioactivity was recovered from the stomach and small intestine at 2, 4 and 6 h after administration. In addition, no significant systemic absorption of the 111In-labeled gelatin nanoparticles as the radioactivity levels in the blood and liver were negligible. From the biodistribution profile, it seemed that delivery of unprotected plasmid DNA-containing gelatin nanoparticles would not result in efficient transfection upon oral administration due to the rapid degradation, which may result in premature release of DNA and its degradation before the nanoparticles are internalized by enterocytes.
Fig. 4.
Gastrointestinal distribution following oral administration of 111In-labeled gelatin nanoparticles and 111In-labeled gelatin nanoparticles encapsulated in the NiMOS in 24-h fasted female Balb/C mice
In contrast, when the 111In-labeled gelatin nanoparticles were encapsulated in NiMOS and administered orally to conscious 24-h fasted mice, a different biodistribution pattern was observed as shown in Fig. 4. NiMOS exhibited a very controlled passage in the gastrointestinal tract with more than 55% of the administered dose residing in the small intestine during the same period and very little (less than 10%) of the administered dose was seen in the large intestine. In addition, about 25% of the administered dose per gram of NiMOS was in the small intestine remained there for up to 4 h post-administration. At later time points, even with NiMOS, a large percentage of administered dose was found to be localized in the large intestine. However, in this case, there is possibility that certain fraction of the NiMOS in the large intestine at 4 and 6 h time points remained intact and could potentially transfect the encapsulated plasmid DNA. Similar to gelatin nanoparticles, there was no significant systemic absorption of NiMOS based on measurements of radioactivity in the blood and the liver. The longer residence of NiMOS in the small and large intestine as compared to the un-encapsulated gelatin nanoparticles may translate into enhanced transfection efficiency of the plasmid DNA.
In Vivo Transfection Studies in Balb/C Mice
The transfection potential of reporter plasmids expressing enhanced green fluorescent protein (EGFP-N1) upon oral administration was assessed by administering the control and NiMOS formulations in 24-h fasted female Balb/C mice.
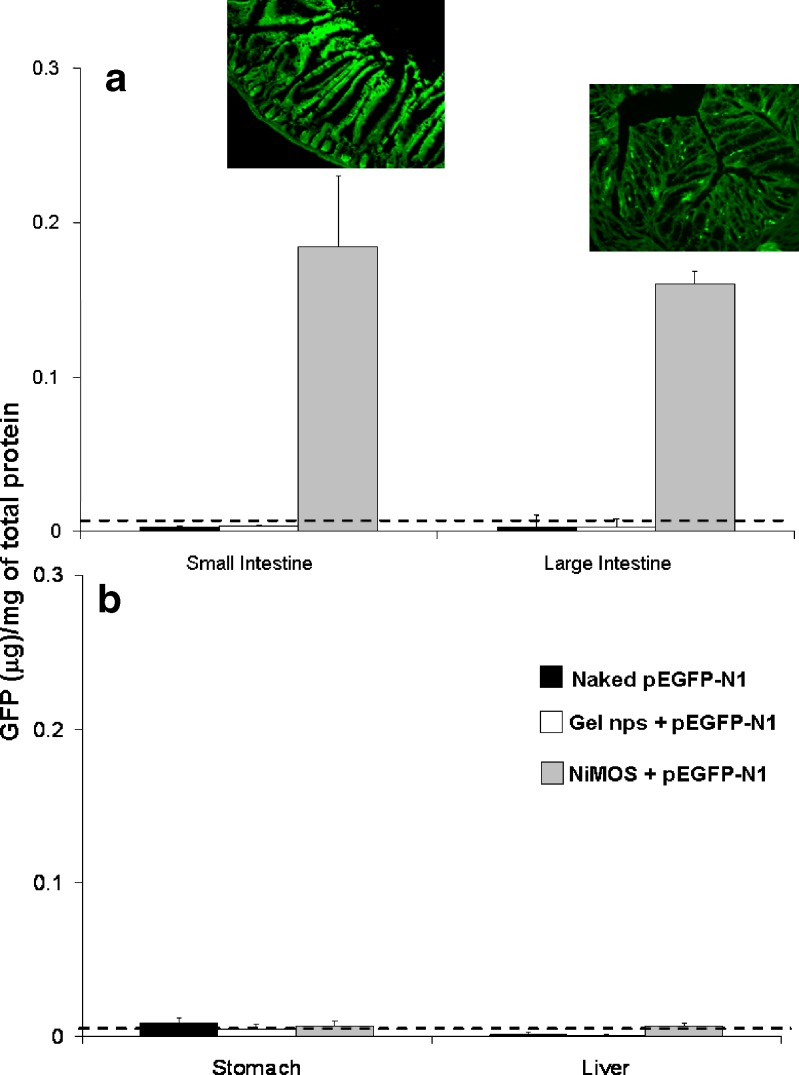
Since the expressed green fluorescent protein is localized in the cell and is not expected to be intrinsically present in Balb/C mice, enhanced green fluorescent protein (EFGP-N1) plasmid DNA was used and administered to the control. Also, NiMOS formulation encapsulating pEGFP-N1 was administered to 24-h fasted female Balb/c mice. After 5 days post-administration of 100°μg plasmid DNA dose as naked plasmid DNA in saline, gelatin nanoparticles, and in NiMOS, the tissues samples including stomach, small intestine, large intestine and liver were harvested and processed to obtain the expressed protein for quantification by sandwich ELISA. Based on ELISA results, shown in Fig. 5, administration of naked EGFP-N1 plasmid in saline or in gelatin nanoparticles did not show transfection as determined by ELISA. Both naked plasmid DNA and the one encapsulated in gelatin nanoparticles could not withstand the harsh environment of the GI tract to allow for any transgene expression. However, when the EGFP-N1 plasmid DNA was administered in NiMOS formulation, there was considerable enhancement of green fluorescent protein production as seen in Fig. 5-a after 5 days. Since transgene protein expression was seen until 5 days post administration (0.183 and 0.167 μg of GFP/mg of total protein in small and large intestine, respectively), NiMOS might be capable of transfecting the lumen enterocytes and as such, would provide a relatively longer lasting transfection as compared to epithelial cells. The dotted line in the figure represents the background as determined from tissues of animal administered with saline alone. To demonstrate the ability of NiMOS to be target specific i.e. small and large intestine only, stomach and liver tissues were also assayed by ELISA to determine the amount of GFP expressed in them. As seen in Fig. 5-b, no GFP was detected by ELISA in stomach and liver tissues 5 days post administration.
Fig. 5.
Quantitative green fluorescent protein (GFP) expression in the small and large intestinal tracts a along with stomach and liver b. The inset shows fluorescent microscopy images of GFP expression in intestinal cryosections
This result was further supported by qualitative tissue section images that were observed under fluorescent microscope. Based on epi-fluorescence images administration of EGFP-N1 plasmid in saline or in gelatin nanoparticles did not show any transfection and the tissue sections emitted similar fluorescence as the untreated control (data not shown). Both naked plasmid DNA and the one encapsulated in gelatin nanoparticles could not withstand the harsh environment of the GI tract to allow for any transgene expression. However, when the EGFP-N1 plasmid DNA was administered in NiMOS formulation (Fig. 5-a), there was considerable enhancement of green fluorescent protein production as shown by the fluorescence signal after 5 days from small and large intestine, both. Further, no fluorescent signal was recorded from non-target organs, stomach and liver for any of groups. Interestingly, the fluorescence signal from NiMOS transfected tissue sections was localized in the luminal enterocytes and as such, would provide a relatively longer lasting transfection as compared to epithelial cells.
CONCLUSIONS
NiMOS composed of biocompatible and biodegradable polymers (i.e., gelatin and PCL) can potentially form an ideal system for delivery of nucleic acids, which are highly unstable in the harsh environment of the GI tract. The specificity of the NiMOS system to transfect only the intestinal tract and not stomach provides an opportunity for delivery of therapeutic protein which can exert a local therapeutic effect.
ACKNOWLEDGEMENTS
The authors are grateful to David Nyugen in Professor Robert Langer`s lab at MIT (Cambridge, MA) for the use the Coulter particle size analysis instrument. Scanning electron microscopy was performed at the Nano-Instrumentation Facility of Northeastern University (Boston, MA).
REFERENCES
- 1.Ponchel G., Irache J. Specific and non-specific bioadhesive particulate systems for oral delivery to the gastrointestinal tract. Adv. Drug Deliv. Rev. 1998;2–3:191–219. doi: 10.1016/S0169-409X(98)00040-4. [DOI] [PubMed] [Google Scholar]
- 2.Bowersock T. L., Hogenesch H., Suckow M., Porter R. E., Jackson R., Park H., Park K. Oral vaccination with alginate microsphere systems. J. Control. Release. 1996;2–3:209–220. doi: 10.1016/0168-3659(95)00155-7. [DOI] [Google Scholar]
- 3.Mestecky J., Moldoveanu Z., Novak M., Huang W. Q., Gilley R. M., Staas J. K., Schafer D., Compans R. W. Biodegradable microspheres for the delivery of oral vaccines. J. Control. Release. 1994;13:131–141. doi: 10.1016/0168-3659(94)90160-0. [DOI] [Google Scholar]
- 4.Donnelly J. J., Wahren B., Liu M. A. DNA vaccines: progress and challenges. J. Immunol. 2005;2:633–639. doi: 10.4049/jimmunol.175.2.633. [DOI] [PubMed] [Google Scholar]
- 5.Dubensky T. W., Liu M. A., Ulmer J. B. Delivery systems for gene-based vaccines. Mol. Med. 2000;9:723–732. [PMC free article] [PubMed] [Google Scholar]
- 6.Horner A. A., Nguyen M. D., Ronaghy A., Cinman N., Verbeek S., Raz E. DNA-based vaccination reduces the risk of lethal anaphylactic hypersensitivity in mice. J. Allergy Clin. Immunol. 2000;2:349–356. doi: 10.1067/mai.2000.107933. [DOI] [PubMed] [Google Scholar]
- 7.Jones D. H., Corris S., McDonald S., Clegg J. C., Farrar G. H. Poly(DL-lactide-co-glycolide)-encapsulated plasmid DNA elicits systemic and mucosal antibody responses to encoded protein after oral administration. Vaccine. 1997;8:814–817. doi: 10.1016/S0264-410X(96)00266-6. [DOI] [PubMed] [Google Scholar]
- 8.Roy K., Mao H. Q., Huang S. K., Leong K. W. Oral gene delivery with chitosan–DNA nanoparticles generates immunologic protection in a murine model of peanut allergy. Nat. Med. 1999;4:387–391. doi: 10.1038/7385. [DOI] [PubMed] [Google Scholar]
- 9.K. Roy, H. Shau-Ku, H. Sampsom, and K. Leong. Oral Delivery of Nucleic Acid Vaccines by Particulate Complexes. US 6475995 B1. November 5, 2002.
- 10.Martien R., Loretz B., Schnurch A. B. Oral gene delivery: design of polymeric carrier systems shielding toward intestinal enzymatic attack. Biopolymers. 2006;4:327–336. doi: 10.1002/bip.20521. [DOI] [PubMed] [Google Scholar]
- 11.Amiji M. M. Polymeric Gene Delivery ed. Boca Raton, CA: CRC press; 2005. [Google Scholar]
- 12.Bhavsar M. D., Shenoy D. B., Amiji M. M. Polymeric nanoparticles for delivery in the gastro-intestinal tract. In: Torchilin V. P., editor. Nanoparticulates as Drug Carriers. London: Imperial College Press; 2006. pp. 609–648. [Google Scholar]
- 13.Bhavsar M. D., Amiji M. M. Polyermic nano- and microparticle technologies for oral gene delivery. Expert Opin. Drug Deliv. 2007;4:197–213. doi: 10.1517/17425247.4.3.197. [DOI] [PubMed] [Google Scholar]
- 14.Guliyeva U., Oner F., Ozsoy S., Haziroglu R. Chitosan microparticles containing plasmid DNA as potential oral gene delivery system. Eur. J. Pharm. Biopharm. 2006;1:17–25. doi: 10.1016/j.ejpb.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 15.Loretz B., Foger F., Werle M., Bernkop-Schnurch A. Oral gene delivery: Strategies to improve stability of pDNA towards intestinal digestion. J. Drug Target. 2006;5:311–319. doi: 10.1080/10611860600823766. [DOI] [PubMed] [Google Scholar]
- 16.Bhavsar M. D., Tiwari S. B., Amiji M. M. Formulation optimization for the nanoparticles-in-microsphere hybrid oral delivery system using factorial design. J. Control. Release. 2006;2:422–430. doi: 10.1016/j.jconrel.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 17.Bhavsar M. D., Amiji M. M. Gastrointestinal distribution and in vivo transfection studies with nanoparticles-in-microsphere oral system (NiMOS) J. Control. Release. 2007;119:339–348. doi: 10.1016/j.jconrel.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 18.Kaul G., Amiji M. Cellular interactions and in vitro DNA transfection studies with poly(ethylene glycol)-modified gelatin nanoparticles. J. Pharm. Sci. 2005;1:184–198. doi: 10.1002/jps.20216. [DOI] [PubMed] [Google Scholar]
- 19.Kaul G., Amiji M. Tumor-targeted gene delivery using poly(ethylene glycol)-modified gelatin nanoparticles: in vitro and in vivo studies. Pharm. Res. 2005;6:951–961. doi: 10.1007/s11095-005-4590-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bhavsar M. D., Amiji M. M. Gastrointestinal distribution and in vivo transfection studies with nanoparticles-in-microsphere oral system (NiMOS) J. Control. Release. 2007;3:339–348. doi: 10.1016/j.jconrel.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 21.Kommareddy S., Amiji M. Biodistribution and pharmacokinetic analysis of long-circulating thiolated gelatin nanoparticles following systemic administration in breast cancer-bearing mice. J. Pharm. Sci. 2007;2:397–407. doi: 10.1002/jps.20813. [DOI] [PubMed] [Google Scholar]