Abstract
This study aimed at developing a topical formulation of lapachol, a compound isolated from various Bignoniaceae species and at evaluating its topical anti-inflammatory activity. The influence of the pharmaceutical form and different types of emulsifiers was evaluated by in-vitro release studies. The formulations showing the highest release rate were selected and assessed trough skin permeation and retention experiments. It was observed that the gel formulation provided significantly higher permeation and retained amount (3.9-fold) of lapachol as compared to the gel-cream formulation. Antinociceptive and antiedematogenic activities of the most promising formulation were also evaluated. Lapachol gel reduced the increase in hind-paw volume induced by carrageenan injection and reduced nociception produced by acetic acid (0.8% in water, i.p.) when used topically. These results suggest that topical delivery of lapachol from gel formulations may be an effective medication for both dermal and subdermal injuries.
Key words: gel, gel-cream, in-vitro permeation, lapachol
INTRODUCTION
Lapachol [2-hydroxy-3-(3methyl-2-butenyl)-1,4-naphtoquinone] is a compound isolated from various species of Bignoniaceae. It is a yellow powder, weakly acidic, highly lipophilic, with limited solubility in water, but very soluble in alkaline solutions (1–3). In clinical studies based on the literature, the topical anti-inflammatory efficacy of that drug was proven in preclinical and clinical studies (1–6). Moreover, in some preliminary studies of galenic development, lapachol was incorporated into gel formulations and assessed by in-vitro release and permeation tests that demonstrated its viability in such forms (7,8). Besides, experimental studies on its sensitization capacity showed that lapachol used alone has not sensitized guinea pigs, and this advantage, in addition to other previously mentioned factors, has made it an alternative choice to treat topical inflammatory diseases (9).
Emulsions, vehicles extensively used to deliver various drugs to the skin, have a high ability to penetrate the skin. In addition, the formulator can control the viscosity, appearance, and degree of greasiness of cosmetic or dermatological emulsions. Gels for dermatological use have also several favorable properties such as being thixotropic, greaseless, easily spreadable, easily removable, emollient, nonstaining, compatible with several excipients, and water-soluble or miscible. Emulgels are emulsions, either of the oil-in-water or water-in-oil type, which are gelled by mixing with a gelling agent. This kind of formulation has a high patient acceptability since it possess the previously mentioned advantages of both emulsions and gels. Hence, emulgels have been used as vehicle to deliver various drugs to the skin (8,10–13).
Thus, based on the above-mentioned information and considering the cutaneous route as an alternative and, on various instances, as a complement of other administrations routes, the aim of this study was to compare a lapachol gel-cream to lapachol gel. In addition, the in-vivo anti-inflammatory activity of the most promising formula was tested.
MATERIALS AND METHODS
Chemicals
Lapachol was obtained in the Pharmaceutical Laboratory of Pernambuco State (LAFEPE-Brazil). Glycerin, triethalonamine, ethanol, acetic acid [high-performance liquid chromatography (HPLC) grade], and methanol (HPLC grade) were obtained from Merck & Co. Inc (USA). Carragenaan was supplied by Sigma (USA). Propylparaben, methylparaben, edetic acid, and isodecyl oleate were supplied by Henrifarma (Brazil); glyceryl monostearate (Cithrol GMS®) by CRODA (Brazil), Carbopol ultrez® by BF Goodrich Company (Cleveland, Ohio) and the other excipients by Galena (Brazil).
Animals
Male and female mice, Mus musculus (20–33 g), and Wistar rats (160–280 g) were originally obtained from the Department of Antibiotics, CCS/UFPE, Recife, Brazil. The animals were housed, acclimatized in polypropylene cages at 25 ± 2 °C with 12/12 light/dark cycles and maintained with food and water ad libitum. Animal experiments were performed according to the Principles for Laboratory Animal Care (NIH publication #85-23, revised in 1985).
Topical Formulation
Gel and gel-cream formulations used in this study are demonstrated in Table I.
Table I.
Composition of Different Formulations Analyzed In This Study
| Constituents | Formulations (%) | ||
|---|---|---|---|
| F1 | F2 | F3 | |
| Lapachol | 0.5 | 0.5 | 0.5 |
| Cetostearyl alcohol | 6.0 | – | – |
| Cetomacrogol 1000 | 3.0 | 3.0 | – |
| Glyceryl monostearate | – | 8.0 | – |
| Carbopol ultrex | 0.3 | 0.3 | 0.5 |
| Methylparaben | 0.15 | 0.15 | 0.15 |
| Propylparaben | 0.05 | 0.05 | – |
| Edetic acid | 0.1 | 0.1 | – |
| Mineral oil | 5.0 | 5.0 | – |
| Isodecyl oleate | 5.0 | 5.0 | – |
| Tween 80 | – | – | 0.025 |
| Triethanolamine | pH 8.0 | pH 8.0 | pH 8.0 |
| Ethanol | 14.0 | 14.0 | 14.0 |
| Glycerin | 6.0 | 6.0 | 6.0 |
| Water distilled to | 100.0 | 100.0 | 100.0 |
Diclofenac formulation (Cataflan emulgel®) from Novartis Biociências S.A. (Brazil) was used as a reference standard in the Writhing Test.
Gel-Cream Preparation (F1 and F2)
Carbopol, edetic acid and methylparaben were previously dispersed in water. The oily phase was prepared by mixing emulsifiers, oils and propylparaben. Two phases were heated to approximately 70 °C and then the oily phase was poured into the aqueous phase under 3000 rpm agitation. The preparation was neutralized using triethanolamine. Lapachol was dispersed into a glycerin:ethanol solution, poured onto the preparation and mixed after room temperature was reached.
Gel Preparation (F3)
Carbopol was previously dispersed in water. Tween 80® was added to the preparation and mixed at 3000 rpm. Lapachol was dispersed into glycerin:ethanol solution, poured onto the preparation and mixed to homogenization at the same speed. The preparation was neutralized using triethanolamine (8).
The pH value was determined after dissolving or dispersing 0.5 g of each formulation in 50 g of distilled water (13,14).
Determination of Apparent Viscosity
A rotation viscometer (Rheology International, Shannon Ltd., Ireland) was used to measure viscosity. The apparent viscosity of the gel-cream and gel formulations was determined at 25 °C using spindle 07 at 50 rpm. Each sample was then measured three times and the results were averaged.
“In-Vitro” Release and Permeation Studies
The study was performed using Franz diffusion cells with available diffusional area of 1.15 cm2, and the volume of the receptor compartment was 5.5 mL. Approximately 550 μg of lapachol was placed in the donor compartment and the receptor compartment was filled with phosphate buffer solution (pH 7.0), maintained at 37 ± 1 °C and stirred by using magnetic stirring bars (300 rpm).
For in-vitro release studies, artificial cellulose membranes (pore size 0.44 μm) obtained from Schleicher & Schuell (Whatman®, UK) were soaked in the same buffer solution for 24 h before mounting on the diffusion cells. At 0.5, 1, 2, 3, 4 and 6 h, all the receptor liquid was withdrawn for ensuring “sink conditions”, and the volume of the liquid was replaced immediately. Then, the samples were filtered and lapachol concentrations were assayed by HPLC.
In the permeation studies, pig ear skin obtained from a local slaughterhouse was adequately prepared for the experiment. The skin was removed from the cartilage of the outer region by a scalpel, and the sub-cutaneous fat was removed. Pieces of full-thickness skin were kept and stored for a period not exceeding 4 weeks at −20 °C until use (15). The removed skin was put on the diffusion cells with the dermis side in contact with the receptor compartment. At 2, 4, 6, 8, 12 and 24 h, the samples were collected and processed according to release studies using artificial membranes. All concentrations were determined by HPLC, model HP 1100 with a UV detector (λ = 278), automatic injector and an RP-18.250 × 4 mm (Shimadzu®) column. The column mobile phase was methanol: 5% acetic acid (80:20); 1 mL/min of flow was utilized, and the injection volume was 20 μl (16).
The drug permeated or released per unit area was calculated and plotted against time. The steady-state flow (Jss) was calculated from the slope and the x-intercept of the linear portion was fitted through regression analysis. The experiments were performed in triplicate.
Skin Retention Studies
To proceed with the skin retention study, the methodology employed was adapted from De Rosa et al. (17), where the skin was removed and rinsed with distilled water and then carefully dried with absorbent paper. The stratum corneum (SC) was removed by the tape-stripping method involving the removal of approximately 15 strips, using adhesive tape. A volume of 4 ml methanol was added to the tapes for extracting ATRA, which were mixed for 1 min, sonicated for 30 min and then filtered using a 0.45 μm membrane. The resulting filtrate assayed by CLAE (17).
The remaining tissue was weighed, homogenized in 5 mL methanol, sonicated for 30 s, centrifuged and filtered. The samples were assayed by HPLC.
Rat-Paw Edema Test
Antiedematogenic activity was evaluated by the rat-paw edema test according to a previously modified method (18,19). Wistar rats (n = 5) were randomized and assigned to the experimental groups. Lapachol formulation (15 mg/kg) was applied to shaved back skin of rats and the site of application gently massaged so as to enable good distribution and penetration of the delivered drugs in the skin. After treatment, the animals were housed individually and 30 min later, 100 μL of 1% carrageenan (w/v) in saline solution was injected into the plantar surface of the right paw.
Evaluation of swelling was performed by the displacement technique using a calibrated glass tube immediately before and at 1, 2, 3, 4, 5 and 6 h after the injection of carrageenan (20).
Writhing Test
The antinociceptive effect was evaluated by the writhing test in mice (n = 6), induced by acetic acid 0.8% (0,1 mL/10 g; i.p.) (21,22). The lapachol formulation (15 mg/kg) was administered 30 min before the nociceptive agent. Five minutes after acetic acid administration, the number of writhes was observed for a period of 20 min. Diclofenac (30 mg/kg) was used as test standards.
Statistical Analysis
All data are presented as arithmetic mean values ± standard deviation (X ± SD). Significance of differences was analyzed by using F and Student’s T Tests, and P < 0.05 was considered to be significant.
RESULTS AND DISCUSSION
Viscosities Determination
The recorded viscosities of the different lapachol formulations at both low and high shear rates are collected in Table II, which shows that the glyceryl monostearate-based gel-cream formulations (F2) possessed considerably higher viscosities than the others (F1 and F3).
Table II.
Release Parameters of Tested Formulations (n = 3, mean values ± SD)
| Formulations | Released Amount (%) | Flux J (μg/cm2 h−1) | Apparent Viscosity (poise) |
|---|---|---|---|
| F1 | 24.63 ± 3.96 | 11.95 ± 0.02 | 671.9 ± 4.20 |
| F2 | 38.95 ± 3.58 | 20.65 ± 0.60 | 132.6 ± 18.10 |
| F3 (gel) | 64.24 ± 5.31 | 51.66 ± 1.83 | 95.30 ± 4.80 |
“In-Vitro” Release Studies
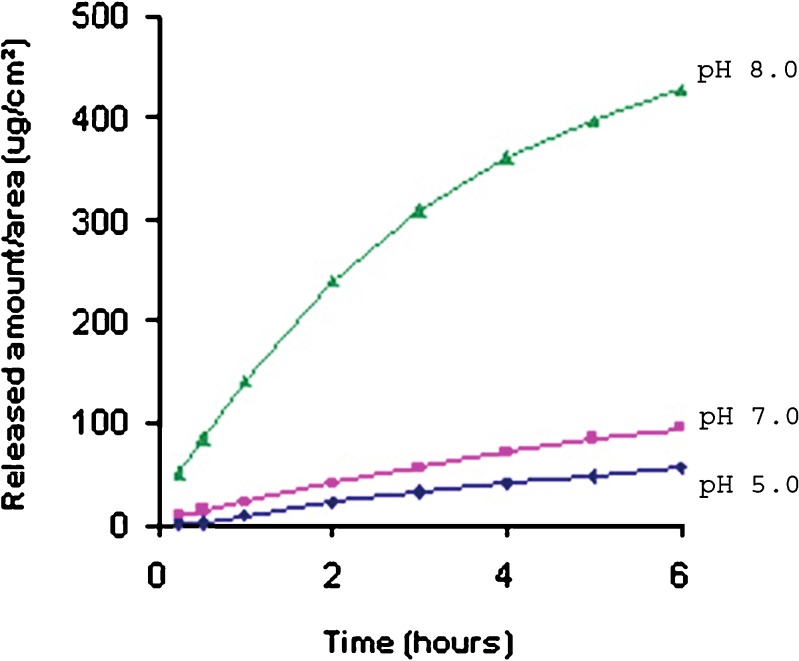
Most drugs are found in the form of strong to moderately weak electrolytes, thus promoting the balance of ionized and non-ionized molecules. pH acts according to the drug’s pKa, and these forms generally present different permeation behavior, but, in general, it is the non-ionized form that presents better permeability in most studies (23). However, some studies have demonstrated that an increase in drug solubilization through its ionization can provide an increase in permeation and drug flow (24–26). Lapachol is a weak acid compound which is very soluble in alkaline solutions. Previous studies in our laboratory revealed that gel preparations with pH 8.0 (ionized form) showed higher release and permeation rates than did gel preparations with pH 7.0 and 5.0 (Fig. 1) (8). Thus, all preparations utilized in this study had pH 8.0 in order to prevent the interference of this parameter in drug permeation.
Fig. 1.
Release profiles of the lapachol gel obtained in an earlier study (15)
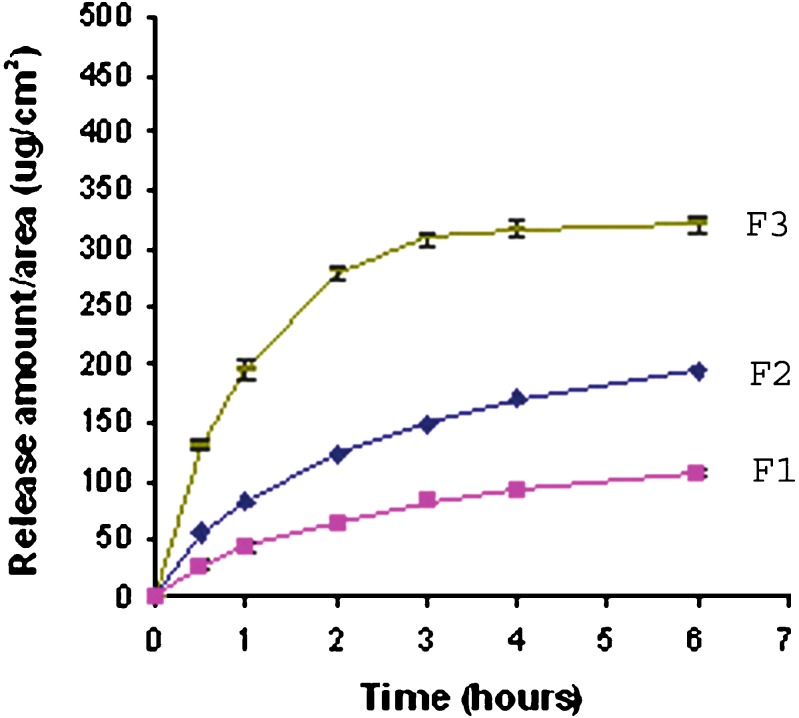
The in-vitro release profiles of lapachol from its various gel-cream formulations are presented in Fig. 2. In general, it can be observed that the drug release from its gel formulation was higher than that from all gel-cream formulations. The drug release from formulations can be ranked in the following order: F1 < F2 << F3, and the amounts of the drug released after 24 h and flux are presented in Table II. Thus, the greatest drug release was observed with F3 formulations (lapachol gel).
Fig. 2.
Release profiles of the gel (F3) and gel-cream formulations (F1 and F2)
An important parameter that might have influenced drug release was the viscosity of formulations. In general, the higher viscosity of topical formulation, the lower drug release rate (10,13). Viscosity seems to influence drug release from different pharmaceutical forms. Gel formulation, which presented the lowest viscosity, showed the highest drug release rate.
On the other hand, viscosity was not shown to influence drug release from gel-cream formulations since formulation F2 (higher viscosity) presented higher drug release than did formulation F1 (lower viscosity).
Since lapachol is a lipophilic compound, emulsified formulation could increase its retention in the formulation. This was observed in formulations F1 and F2 when compared to formulation F3 (gel). Moreover, the emulsifier type seems to interfere in the drug release rate. It was observed that the release rate significantly increased when cetostearyl alcohol was replaced with glyceryl monostearate. The presence of glyceryl monostearate in the preparations seemed to improve lapachol release. As a result, formulation gel-cream F2 was assessed trough retention and penetration studies and compared with gel formulation (F3).
Skin-Retention and Permeation Studies
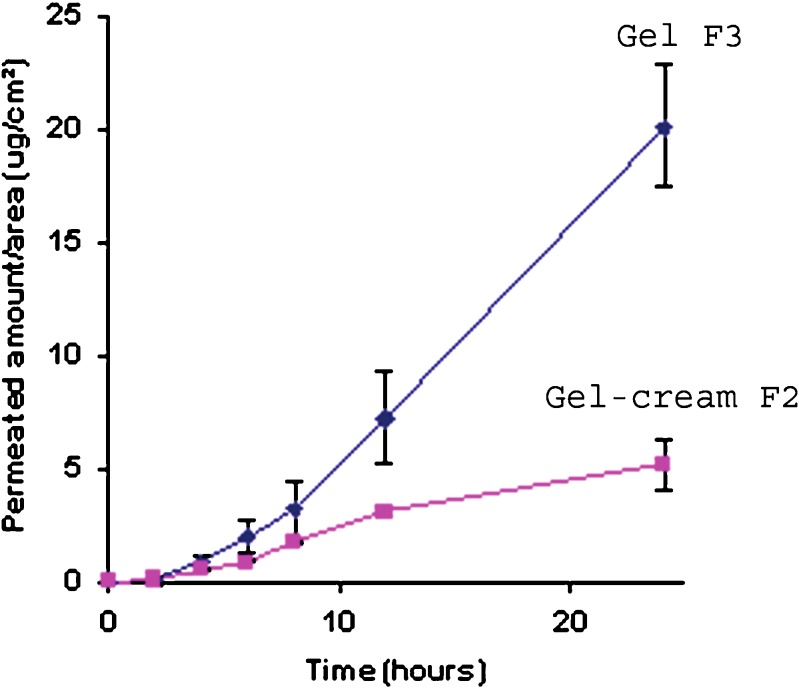
The results obtained in permeation studies using pig-ear skin are presented in Fig. 3. The permeation rate of a topical drug may be influenced by drug-vehicle, drug-skin, and vehicle-skin interactions. In the clinical assessment of a topical agent, the vehicle may significantly affect drug release and skin penetration, thereby altering biological activity (29–33).
Fig. 3.
Permeation profiles of lapachol from gel (F3) and gel-cream (F2) formulations
Emulsions, and consequently, gel-creams have an ability to penetrate the skin, and these kinds of formulation could promote the penetration of lapachol, but that did not occur in this case. Skin permeation studies have shown that the permeated amount of lapachol from gel formulations was 3.9-fold higher than that of gel-cream formulations, reinforcing the hypothesis that lapachol has more affinity with emulsifying preparations, thus retarding the release rate of the drug.
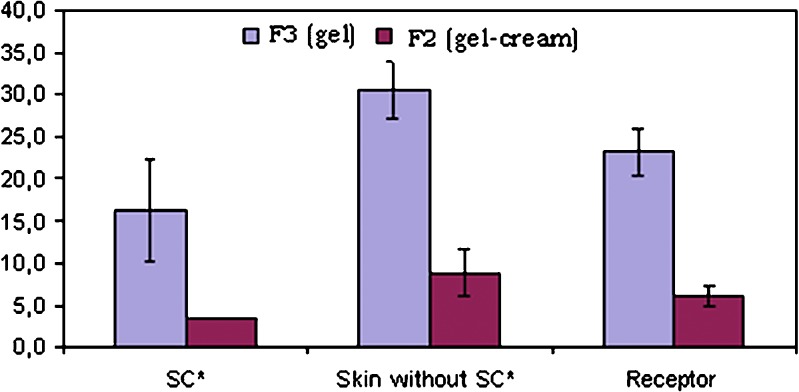
In skin retention studies, the affinity of lapachol with skin was observed, since retention of that drug in the skin-layer occurred in both formulations (Fig. 4). In both formulations, it was observed that approximately 67% of the drug was retained in the skin layers. On the other hand, only about 33% of the drug was found in the receptor liquid.
Fig. 4.
Distribution of lapachol in pig-ear skin layers after 24 h (asterisks, stratum corneum)
The amount of drug released from gel which was retained in the skin was also significantly (3.9-fold) higher than that from gel-cream. Then, gel formulation seems to be the most appropriate formulation, since it provides the highest penetration flux and also provides the highest values for drug retained in the skin.
Evaluation of Antiedematogenic Activity
Carrageenan-induced paw inflammation has been accepted as a useful phlogistic tool for investigating anti-inflammatory agents and has been used to test the antiedematogenic effect of various substances (21,34).
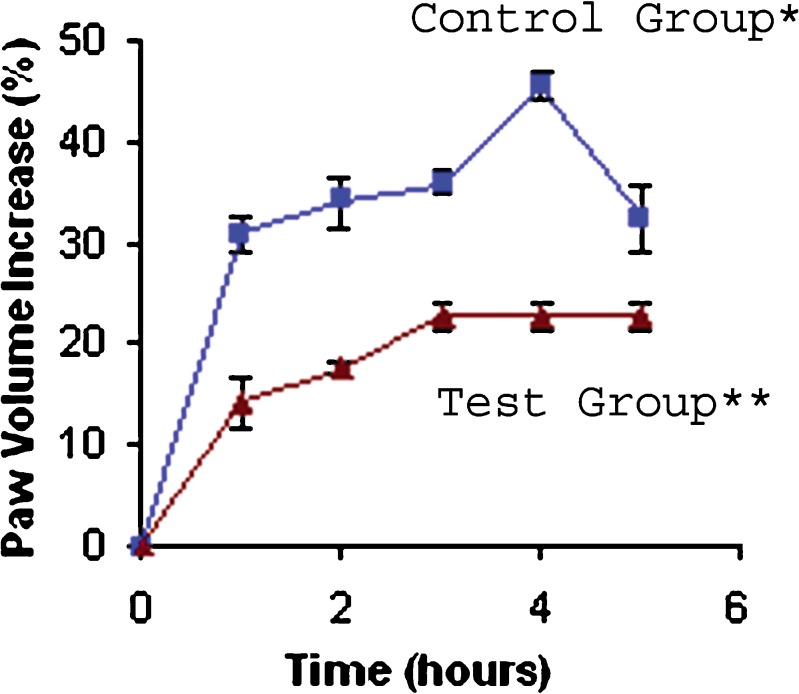
Topical administration of lapachol gel showed significant antiedematogenic activity (Table III and Fig. 5). There was a reduction in carrageenan-induced rat paw edema at 0.5% lapachol gel over a period of 5 h (ANOVA; P < 0.05).
Table III.
Antiedematogenic Activity of Lapachol Gel Formulation in Carragenaan-induced Rat Paw Edema (n = 6, mean values ± SD)
| Treatment | Percentage Inflammation After Carragenaan Injection (%) | ||||
|---|---|---|---|---|---|
| 1 h | 2 h | 3 h | 4 h | 5 h | |
| Control | 30.79 ± 1.59a | 34.02 ± 2.47a | 35.84 ± 1.09a | 45.43 ± 1.41a | 32.48 ± 3.27a |
| Lapachol gel | 14.15 ± 2.30a,b | 17.63 ± 0.66 a,b | 22.64 ± 1.47 a,b | 22.64 ± 1.47 a,b | 22.64 ± 1.47 a,b |
aValues significantly different from zero time (before treatment)
bValues significantly different from control value at P < 0.05, ANOVA
Fig. 5.
Percentage increase in paw volume in function of time after subplantar injection of carrageenan. Results are the mean ± S.D. for six rats. P < 0.05 as compared with control
The maximal antiedematogenic activity was 50.16% at 4 h, after carrageenan administration, for lapachol gel. In an earlier study, Almeida et al. (1990) demonstrated significant lapachol antiedematogenic action of 76% and 85% for doses of 100 and 500 mg/kg orally administered in Wistar rats, respectively, 4 h after the injection of carrageenan (4). Although these results were better than those from topical lapachol used in this study, the gel formulation dose (15 mg/kg) was much lower than that needed to obtain the antiedematogenic activity mentioned above (100 and 500 mg/kg).
In general, three distinct phases are involved in acute vascular response. In the first phase, simultaneous release of histamine and serotonin occurs. In the second phase kinin, such as bradykinin, release occurs. And in the terminal phase, prostaglandins release takes place (21,35).
In this study, the time interval in which antiedematogenic activity took place indicates action against the release of histamine, serotonin and kinins (18,36).
Evaluation of antinociceptive activity
Topical administration of lapachol gel also showed significant antinociceptive activity (Table IV). When performing a writhing test, results showed notable differences in the frequency between the tested groups (ANOVA; P < 0.05). Lapachol applied topically effectively reduced acetic acid effect.
Table IV.
Effect of Control, Lapachol Gel and Diclofenac Formulation at the Writhes Induced by Acetic Acid 0.8% in Mice, Expressed as Number of Stretches (n = 6, mean values ± SD)
| Time (min) | Control | Gel Formulation | Diclofenac Formulation |
|---|---|---|---|
| 0–5 | 15.17 ± 1.22 | –a | –a |
| 5–10 | 56.00 ± 2.19 | 9.67 ± 0.95b | 12.50 ± 0.92b |
| 10–15 | 97.00 ± 3.18 | 20.50 ± 0.67b | 27.50 ± 2.58b |
| 15–20 | 132.17 ± 2.84 | 30.00 ± 1.37b | 40.33 ± 2.66b |
aThere was not stretches in this time
bSignificantly different from control value at P < 0.05, ANOVA
Antinociceptive activity was 77.30 and 69.49% for lapachol gel and diclofenac formulation, respectively, indicating that topical lapachol gel (0.5%) proved to be more efficient than others.
CONCLUSION
Gel formulation provided a significantly higher skin-retained and permeated (3.9-fold) amount of lapachol as compared to gel-cream. Moreover, lapachol gel presented significant antiedematogenic and antinociceptive activities when used topically. Hence, these results suggest that the topical delivery of lapachol from gel formulations can be an effective medication for topical injuries.
Acknowledgements
The authors thank LAFEPE, CAPES and MCT/CNPq for financial support.
References
- 1.Araújo E. L., Alencar J. R. B., Rolim Neto P. J. Lapachol: segurança e eficácia na terapêutica. Rev. Bras. Farmacogn. 2002;12:57–59. doi: 10.1590/S0102-695X2002000300028. [DOI] [Google Scholar]
- 2.Fonseca S. G. C., Braga R. M. C., Santana D. P. Lapachol. chemistry, pharmacology and assay methods. Rev. Bras. Farm. 2003;84:9–16. [Google Scholar]
- 3.Lui C. Y., Ayeni A. A., Gyllenhaal C., Groves M. J. Some formulation properties of Lapachol, A potential oncolytic agent of natural origin. Drug. Dev. Ind. Pharm. 1985;11:63–79. doi: 10.3109/03639048509057698. [DOI] [Google Scholar]
- 4.Almeida E. R., Silva A. A., Santos E. R., Lopes C. A. C. Antiinflammatory action of lapachol. J. Ethnopharmacol. 1990;29:239–241. doi: 10.1016/0378-8741(90)90061-W. [DOI] [PubMed] [Google Scholar]
- 5.Wanick M. C., Bandeira J. A., Fernandes R. V. Ação antiinflamatória e cicatrizante do extrato hidroalcoólico do Líber do Pau d’arco Roxo (Tabebuia avellanedae), em pacientes portadores de Cervicites e Cérvico-vaginites. Revista do instituto de Antibióticos. 1970;10:41–46. [Google Scholar]
- 6.Duarte D. S., Dolabela M. F., Salas C. E., et al. Chemical characterization and biological activity of Macfadyena unguis-cati (Bignoniaceae) J. Pharm. Pharmacol. 2000;52:347–352. doi: 10.1211/0022357001773904. [DOI] [PubMed] [Google Scholar]
- 7.Santos E. R., Prista L. V. N., Lobo J. M. S., Santos D. G. Estudos de difusão cutânea do Lapachol. I – Ensaios in vitro. Rev. Port. Farm. 1991;41:15–19. [Google Scholar]
- 8.Lira A. A. M., Sester E. A., Abreu L. R., Silva L. B. L., Santana D. P. Preliminary development of lapachol gel: in vitro premeation study. Brazilian Journal of Pharmaceutical Sciences. 2004;40:35–41. [Google Scholar]
- 9.Schulz K. H., Garbe I., Hausen B. M., Simatupang M. H. The sensitizing capacity of naturally occurring quinones. Experimental studies in guinea pigs. I. Naphthoquinones and related compounds. Arch. Dermatol. Res. 1977;258:41–52. doi: 10.1007/BF00582866. [DOI] [PubMed] [Google Scholar]
- 10.Abd El-Bary A., Shalaby S., Abd El-Aal S. Formulation and stability of chloramphenicol gel and emulgel. Bull. Fac. Pharm. 2001;39:89–99. [Google Scholar]
- 11.Zhang X. L., Zhao R., Qian W. Preparation of an emulgel for treatment of aphthous ulcer on the basis of carbomers. Chin. Pharm. J. 1995;30:417–418. [Google Scholar]
- 12.Hamza Y. E., Molokhia A. M., Soliman I. I., Ahmed F. H., Soliman N. A. Formulation and evaluation of topical preparations containing phenol and local vesicants. Az. J. Pharm. Sci. 2002;29:412–432. [Google Scholar]
- 13.M. I. Mohamed. Optimization of Chlorphenesin Emulgel Formulation. AAPS Journal [serial online] 6(3) 2004 article 26. DOI 10.1208/aapsj060326. [DOI] [PMC free article] [PubMed]
- 14.Ho H., Huang F. C., Sokoloski T. D., Sheu M. T. The influence of cosolvents on the in vitro percutaneous penetration of diclofenac sodium from a gel system. J. Pharm. Pharmacol. 1994;46:636–642. doi: 10.1111/j.2042-7158.1994.tb03873.x. [DOI] [PubMed] [Google Scholar]
- 15.Trotta M., Ugazio U., Peira E., Pulitano C. Influence of ion pairing on topical retinoic acid from microemulsions. J. Control. Rel. 2003;86:315–321. doi: 10.1016/S0168-3659(02)00416-9. [DOI] [PubMed] [Google Scholar]
- 16.Fonseca S. G. C., Silva L. B. L., Castro R. F., Santana D. P. Validação de metodologia analítica para doseamento de soluções de lapachol por CLAE. Quim Nova. 2004;27:157–159. [Google Scholar]
- 17.De Rosa F. S., Tedesco A. C., Lopez R. F. V., Pierre M. B. R., Lange N., Marchetti J. M., Rotta J. C. G., Bentley M. V. L. B. In vitro skin permeation and retention of 5-aminolevulinic acid ester derivatives for photodynamic therapy. J. Control Rel. 2003;89:261–269. doi: 10.1016/S0168-3659(03)00125-1. [DOI] [PubMed] [Google Scholar]
- 18.Mujumdar A. M., Misar A. V. Anti-inflammatory activity of Jatropha curcas roots in mice and rats. J. Ethnopharmacol. 2004;90:11–15. doi: 10.1016/j.jep.2003.09.019. [DOI] [PubMed] [Google Scholar]
- 19.Porzio S., Caselli G., Pellegrini L., Pallottini V., Del Rosário M., Coppola A., Boltri L., Gentile M., Clavenna G., Melillo G. Efficacy of a new topical gel-spray formulation of ketoprofen lysine salt in the rat: percutaneous permeation in vitro and in vivo and pharmacological activity. Pharmacol. Research. 1998;37:41–47. doi: 10.1006/phrs.1997.0260. [DOI] [PubMed] [Google Scholar]
- 20.Chin C. T., Chun C. L. Anti-inflammatory effects of Taiwan folk medicine ‘Teng-Khia-U’ on carrageenan-and adjuvant-induced paw edema in rats. J. Ethnopharm. 1999;64:85–89. doi: 10.1016/s0378-8741(98)00108-1. [DOI] [PubMed] [Google Scholar]
- 21.Miranda F. G. G., Vilar J. C., Alves I. A. N., Cavalcanti S. C. H., Antoniolli R. R. Antinociceptive and antiedematogenic properties and acute toxicity of Tabebuia avellanedae Lor. ex Griseb. inner bark aqueous extract. BMC Pharmacol. 2001;1:6. doi: 10.1186/1471-2210-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Collier H. O., Dinneen L. C., Johnson C. A., Schneider C. The abdominal constriction response and its suppression by analgesic drugs in the mouse. Br. J. Pharmacol. 1968;32:295–310. doi: 10.1111/j.1476-5381.1968.tb00973.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chien Y. W. Novel Drug Delivery Systems. New York, NY: Marcel Dekker; 1992. [Google Scholar]
- 24.Kushla G. P., Zatz J. L. Influence of pH on lidocaine penetration through human and hairless mouse skin in vitro. Int. J. Pharm. 1991;71:167–173. doi: 10.1016/0378-5173(91)90387-4. [DOI] [Google Scholar]
- 25.Zatz J. L. Fundamental of transdermal controlled drug administration: Physicochemical considerations. Drug Dev. Ind. Pharm. 1983;9(4):561–577. doi: 10.3109/03639048309044693. [DOI] [Google Scholar]
- 26.Swarbrick J., Lee G., Brom J., Gensmantel N. P. Drug permeation through human skin II: Permeability of ionizable compounds. J. Pharm. Sci. 1984;73:1352–1355. doi: 10.1002/jps.2600731006. [DOI] [PubMed] [Google Scholar]
- 27.Gwak H. S., Chun I. K. Effects of vehicles and penetration enhancers on the in vitro percutaneous absorption of tenoxican through hairless mouse skin. Int. J. Pharm. 2002;236:57–64. doi: 10.1016/S0378-5173(02)00009-1. [DOI] [PubMed] [Google Scholar]
- 28.Kemken J., Ziegler A., Müller B. W. Influence of supersaturation on the pharmacodynamic effect of Bupranolol after dermal administration using microemulsions as vehicle. Pharm. Res. 1992;9:554–558. doi: 10.1023/A:1015856800653. [DOI] [PubMed] [Google Scholar]
- 29.T. Higuchi. In vitro drug release from ointments and creams. In: Brandau R, Lippold BH, eds. Dermal and Transdermal Absorption. Stüttgart, Germany: Wissenschaftliche Verlagsgesellschaft; 1982:90–100.
- 30.El Laithy H. M., El-Shaboury K. M. F. The Development of Cutina Lipogels and Gel Microemulsion for Topical Administration of Fluconazole. AAPS PharmSciTech [serial online] 2002;3:E35. doi: 10.1208/pt030435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Coldman M. R., Poulsen B. J., Higuchi T. Enhancement of percutaneous absorption by the use of volatile, nonvolatile systems as vehicles. J. Pharm. Sci. 1969;58:1098–1102. doi: 10.1002/jps.2600580912. [DOI] [PubMed] [Google Scholar]
- 32.Ostrenga J., Steinmetz C., Poulsen B. J. Significance of vehicle composition I: relationship between topical vehicle composition, skin penetrability, and clinical efficacy. J. Pharm. Sci. 1971;60:1175–1179. doi: 10.1002/jps.2600600812. [DOI] [PubMed] [Google Scholar]
- 33.Ostrenga J., Haleblian J., Poulsen B. J., Ferrell B., Mueller N., Shastri S. Vehicle design for a new topical steroid, fluocinonide. J. Invest. Dermatol. 1971;56:392–399. doi: 10.1111/1523-1747.ep12261282. [DOI] [PubMed] [Google Scholar]
- 34.Winter C. A., Risley E. A., Nuss G. W. Carrageenan-induced edema in hind paw of the rat as a assay for antiinflammatory drugs. Proc. Soc. Exp. Biol. Med. 1962;111:547. doi: 10.3181/00379727-111-27849. [DOI] [PubMed] [Google Scholar]
- 35.Di Rosa M., Giround J. P., Willoughby P. A. Studies on the mediators of acute inflammatory response induced in rat in different sites by carrageenan and tupentine. J. Pathol. Bacteriol. 1971;104:5–29. doi: 10.1002/path.1711040103. [DOI] [PubMed] [Google Scholar]
- 36.Just M. J., Recio M. C., Giner R. M., Cullar M. J., Manez S., Bilia A. R. Anti-inflammatory activity of unusual Lupane saponins from Bupleurum fruticescens. Planta Med. 1998;64:404–407. doi: 10.1055/s-2006-957469. [DOI] [PubMed] [Google Scholar]