INTRODUCTION
The physiological constraints imposed by the protective mechanisms of the eye lead to low absorption of drugs and a short duration of the therapeutic effect on ocular drug delivery. Upon instillation of the eye drops only 1–10% of the drug is bioavailable while the rest is drained out of the eye through lacrimal secretions [1]. To overcome this problem various approaches have been reported, such as ointments, inserts and aqueous gels, to increase the ocular residence time of topically applied medication.
Controlled drug delivery to the eye offer several advantages over conventional therapies like drug solutions or suspensions as eye drops [2,3]. Ophthalmic inserts offer many advantages over conventional dosage forms, like increased ocular residence, possibility of releasing drugs at a slow and constant rate, accurate dosing, and exclusion of preservatives, increased shelf life and reduced systemic absorption [4–6]. Several reports revealed improved ocular therapy by ophthalmic inserts. Frequency of instillation of gentamycin sulfate was reduced by a long-acting ophthalmic insert [7]. O-butyryl ester prodrug of tilisolol and the O-palmitoyl ester prodrug of tilisolol were incorporated into an ophthalmic insert to control drug release [8]. Di Colo G. and co-researcher [9] observed respective contributions of diffusion and erosion to the release mechanism of drugs, namely prednisolone, oxytetracycline hydrochloride and gentamycin sulphate through erodible ophthalmic inserts based on poly(ethylene oxide).
Acyclovir is a polar drug with short plasma half life of 2–3 h [10], therefore 4–5 times application is required when administered as ophthalmic ointment. Also, about 95% of the drug is drained out due to high tear turn-over via nasolacrimal drainage leading to ineffective therapy. Several approaches have been used to improve ocular bioavailabilty of acyclovir. Nanospheres of poly-d,l-lactic acid loaded with acyclovir were prepared and characterized for effect of different formulation parameters. Nanospheres showed a sustained acyclovir release, were highly tolerated by the eye and were able to increase the aqueous humor levels of acyclovir to improve the pharmacokinetic profile [11]. Fresta et al [12] prepared acyclovir-loaded polyethyl-2-cyanoacrylate (PECA) nanospheres by an emulsion polymerization process in the micellar phase and coated with polyethylene glycol (PEG). Acyclovir-loaded PEG-coated PECA nanospheres were compared with an aqueous solution of the drug for drug levels in aqueous humor. The acyclovir-loaded PEG-coated PECA nanospheres showed 25-fold increase in drug levels in aqueous humor compared with the free drug or the physical mixture.
In the present investigation an attempt has been made to prepare ocular inserts of acyclovir capable of releasing drug continuously at controlled rate for 5 days.
MATERIALS AND METHODS
Materials
Acyclovir was gift from Borroughs Welcome Ltd., India, marketed ophthalmic ointment OCUVIR® (3% acyclovir w/w ointment) was purchased from FDC, India. Sodium alginate (Loba chemicals, India), Eudragit RL 100 and RS 100 (Pharmax, India) and dibutyl phthalate (S.D. Fine Chemicals Ltd., India) were used for formulation. All the solvents used were of either analytical or HPLC grade.
Preparation of Ocular Inserts
Ocular inserts of reservoir type were prepared with a reservoir film of sodium alginate and rate controlling film surrounding reservoir of Eudragit. Placebo films were first prepared of sodium alginate and Eudragit to fix the type and percent of plasticizer for optimum film characteristics. Placebo films of sodium alginate were prepared using two plasticizers; PEG 200 and PEG 400 in different concentrations (16, 32 and 48% w/w of polymer) with 2% sodium alginate. PEG 400 as 48% w/w of sodium alginate gave best film, thus fixed for formulation of drug loaded reservoir films. Placebo Eudragit films were prepared using dibutylphthalate as a plasticizer in concentrations 5, 8.5,10, 15 and 17% w/w of polymer with 10% polymer (4:1 Eudragit RL 100/RS100). Dibutylphthalate in concentration 15% w/w of polymer provided best film and therefore selected for formulation of drug containing ocular inserts.
Drug loaded reservoir films were prepared by suspending different concentrations of sodium alginate along with drug and PEG 400 in 7 ml of isotonic phosphate buffer (pH 7.4). The polymeric dispersions were poured into a glass ring of area 28.3 cm2 placed in Teflon coated Petridish and thereafter solvent was allowed to evaporate under 36 ± 2°C and 30 ± 0.5% RH in a temperature and humidity controlling chamber (SECOR India, Delhi, India) for 20 h (Table I).
Table I.
Composition of Reservoir Films
| Ingredients | Weights in mg Per Film | ||||
|---|---|---|---|---|---|
| A | B | C | D | E | |
| Acyclovir | 86.0 | 86.0 | 86.0 | 86.0 | 86.0 |
| Sodium alginate | 100 | 120 | 148 | 180 | 250 |
| PEG-400 | 48.4 | 57.0 | 71.0 | 86.0 | 120 |
PEG-400 added as plasticizer in the concentration 48% w/w of sodium alginate; area of the film was 28.3 cm2
Rate controlling films were prepared by dissolving Eudragit RS 100 and Eudragit RL 100 in different ratios as shown in Table II along with 15% of dibutylphthalate in ethanol/acetone (60/40) mixture by weight of polymer. Films were prepared in the same way as reservoir film under 25 ± 2°C, 55 ± 0.5 % RH for 12 h.
Table II.
Composition of Rate Controlling Membrane
| Ingredients | Weights in mg Per Film | ||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| Eudragit RL100 | 250 | 400 | 400 | 350 | 250 |
| Eudragit RS 100 | – | – | 100 | 150 | 250 |
Dibutylphthalate added as plasticizer in the concentration 15% w/w of polymer (Eudragit); area of the film was 28.3 cm2
Elliptical shaped ocular inserts of area 0.65 cm2 were cut out of medicated reservoir film with the help of stainless steel die and were placed between two rate controlling membranes of Eudragit. The two rate controlling membranes containing reservoir film between them were placed over a beaker saturated with ethanol/acetone vapors (60:40) for 1–2 minutes. This procedure resulted in sealing of the two rate controlling membranes containing the medicated reservoir film in between.. Finally the reservoirs surrounded by rate controlling membrane were cut by stainless steel die of surface area 0.7 cm2, packed in polyethylene laminated aluminum foil and sterilized by 2.5 Mrads dose of gamma rays for 18 h (Thirty eight ocular inserts were formed per ring). Each ocular insert contained 2 mg drug, and had length; 13.1 mm and width 5.4 mm. Ocular inserts were stored in an air-tight container below 25 ± 2°C.
Evaluation of Ocular Inserts
Drug Content
The optimized ocular insert of the drug was powdered in the mortar and dissolved in isotonic phosphate buffer (pH 7.4) and volume was made up to 100 ml. The solution was filtered and analyzed spectrophotometrically at 252 nm (Beckman, DU 64 Spectrophotometer, USA). Results are shown in Table III.
Table III.
Physical Characteristics of Reservoir and Rate Controlling Films
| Parameters tested | Sodium Alginate Reservoir Films | Rate Controlling Eudragit Films | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| A | B | C | D | E | 1 | 2 | 3 | 4 | 5 | |
| Percentage elongation at break | 10 ± 1.52 | 13 ± 0.57 | 14 ± 1.15 | 18 ± 0.53 | 14 ± 2.5 | 18 ± 1.2 | 18 ± 2.0 | 20 ± 1.0 | 25 ± 0.5 | 19 ± 1.5 |
| Drug Content (mg) per reservoir | 1.85 ± 0.55 | 1.90 ± 0.50 | 1.95 ± 0.32 | 2.01 ± 0.02 | 2.10 ± 0.22 | – | – | – | – | – |
| Thickness(μm) | 150 ± 1.0 | 150 ± 1.0 | 151 ± 1.3 | 155 ± 0.5 | 156 ± 0.5 | 48 ± 2.0 | 47 ± 1.5 | 50 ± 1.0 | 50 ± 1.5 | 50 ± 2 |
Results are mean of five observations ± standard deviation; values are rounded up to nearest value
Interaction Studies
Interaction studies were conducted to investigate any interaction between drug and polymer as well as to study the effect of gamma radiation on acyclovir. Pure drug, unsterilized and sterilized medicated ocular inserts and placebo films were analysed by assay (mentioned before), UV scanning (between 200–500 nm) and infrared analysis in the range of 400–4,000 cm−1 by KBr disc method (Hitachi, Japan).
In Vitro Drug Release
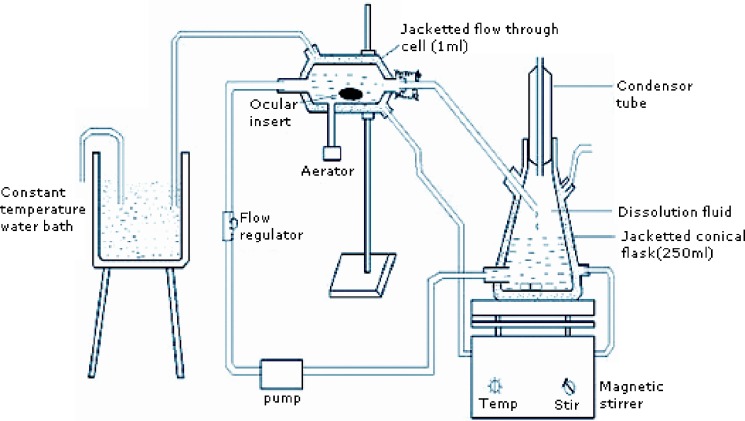
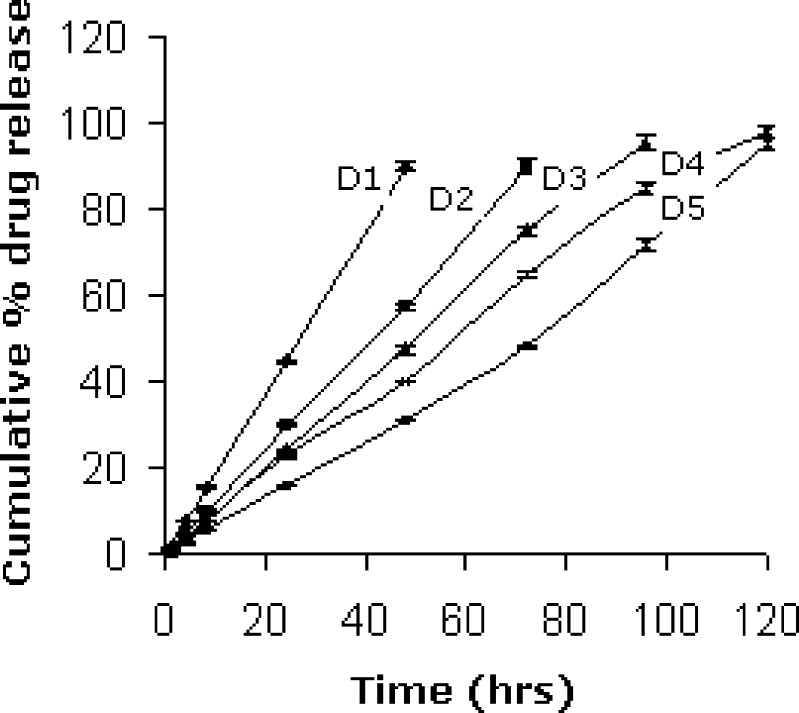
An in vitro apparatus similar to Ali and Sharma [13, 14] was modified to perform release studies. The apparatus consisted of jacketed flow-through cell and the jacketed flask, dissolution medium was allowed to flow via the pump to the jacketed flow through cell and then back to the flask as shown in Fig. 1. The flow was adjusted to 0.28 ml per minute to simulate tear turn over. The ocular insert was placed in the jacketed flow through cell and the whole assembly was maintained at 33 ± 2 °C by circulating hot water through the jacket. Samples were withdrawn at appropriate time intervals up to 120 h and analyzed spectrophotometrically at 252 nm (Fig. 2).
Fig. 1.
Sketch of flow-through apparatus
Fig. 2.
In vitro release of acyclovir through different ocular inserts
In Vivo Drug Release
Approval for the use of animals in the study was obtained from the Jamia Hamdard Animal Ethics Committee ( Hamdard University, New Delhi, India). Twenty rabbits of either sex weighing 2.8 to 4.1 kg were used to measure the in vivo release of the drug in the eye. The rabbits were housed singly in restraining boxes during the experiment and allowed the food and water ad libitum. Free leg and eye movement was allowed.
The ocular inserts were inserted in both eyes of animals. Four ocular inserts (two animals) were removed at each 24 h time point till 5 days, assayed and amount of drug remaining in each ocular insert was determined. Cumulative % drug released in vivo was calculated.
Estimation of Drug in Aqueous Humor
Thirty healthy albino rabbits (1.5–2.0 kg) were selected. Both eyes were utilized, two groups were formed. One group containing 10 animals was treated with ophthalmic ointment and another group containing 20 animals was treated with ocular inserts. Aqueous humor acyclovir levels were monitored at 0.5, 1, 2, 4 and 8 h after a single instillation of 0.5 g of 3% ophthalmic ointment into the conjuctival sac. Aqueous humor was analysed in another group at 0.5, 1, 2, 4, 8, 24, 48, 72, 96 and 120 h after administration of ocular inserts into the cul de sac. Two animals (four eyes) were used for each time point for each acyclovir formulation tested. The rabbits were anaesthesized by i.v. injection of ketamine (25 mg/kg) and through the limbus 200 μl aqueous humor was withdrawn with 26 gauge needle [15]. After being vortexed for 30 sec and centrifuged for 15 minute at 2000 rpm, 20 μl of the sample was directly injected in to the chromatographic system (Perkin Elmer, Massachusetts, USA).
The column was RP Hypersil C18 (250 × 4.6 mm i.d., particle size 5 μm); mobile phase, sodium acetate (0.7 M; pH 6.0 with acetic acid). The flow rate was adjusted to 1.0 ml min−1 and the eluent was monitored using UV detector at 252 nm [16]. The quantity of drug was estimated from the calibration curve prepared using spiked aqueous humor between concentration 0.5 to 12 μg/ml.
Stability Studies
Stability studies were conducted by storing ocular inserts under 40 ± 0.5°C and 75 ± 5% RH for 180 days. The samples were tested for drug content after 0, 30, 60, 90 and 180 days respectively.
RESULTS AND DISCUSSION
Characterization of Ocular Inserts
As acyclovir is polar and it was not possible to have common solvent for the drug and rate controlling polymer Eudragit to form the film, reservoir type of delivery system was approached which consisted of the drug containing sodium alginate reservoir interspersed between the two rate controlling membranes of Eudragits. Isotonic phosphate buffer (pH 7.4) was found suitable for fabrication of reservoir film because the drug was found to be more soluble in IPB pH 7.4 than water. Film D containing 2.57% w/v of sodium alginate was found most suitable for preparation of ocular inserts because of the maximum percentage elongation at break (18 ± 0.57%) and uniform drug content.
In Vitro Release
Release of the drug increased with the increase in amount of Eudragit RL 100 and decreased with increased Eudragit RS 100 due to higher water permeability of Eudragit RL 100 which contains 10% of functional quaternary ammonium groups and lower water permeability of Eudragit RS 100 having only 5% of functional quaternary ammonium groups. Ocular insert D4 containing Eudragit RL 100/RS 100 in the ratio 3.5: 1.5 gave 98% drug release in 120 h and therefore selected for in vivo studies. The release profiles of formulations were treated with Peppas equation and values for slope were in the range 0.96 to 1.04. Slope values near to 1 describes a case II transport mechanism, characterized by a linear time dependence in both the amount of drug diffused and the penetrating swelling front. Drug release from such devices follows constant zero-order kinetics [17].
Interaction Studies
Strip-packed ocular inserts were sterilized by Gamma rays for 18 h and tested for sterility and effect of gamma rays. All the ocular inserts passed test for sterility. No interaction between drug and polymer and interaction due to sterilization by gamma radiation was found when tested by UV absorption studies and IR spectrometry.
The UV absorption spectra for pure drug, formulations before and after sterilization showed similar pattern and λmax at 252 nm. Polymer solution did not show absorption at λmax of drug suggesting lack of interference with UV absorption of drug. Principal peaks of acyclovir at 1,717 cm−1 for c=o stretching and at 1,632 cm−1 for −NH group were retained in IR spectra after sterilization. Thus no irradiated product was formed within the ocular insert. The IR spectra of placebo films showed no matching peaks with the drug and hence not interfered with the drug chemically.
In Vivo Release Study and Estimation of Drug in Aqueous Humor
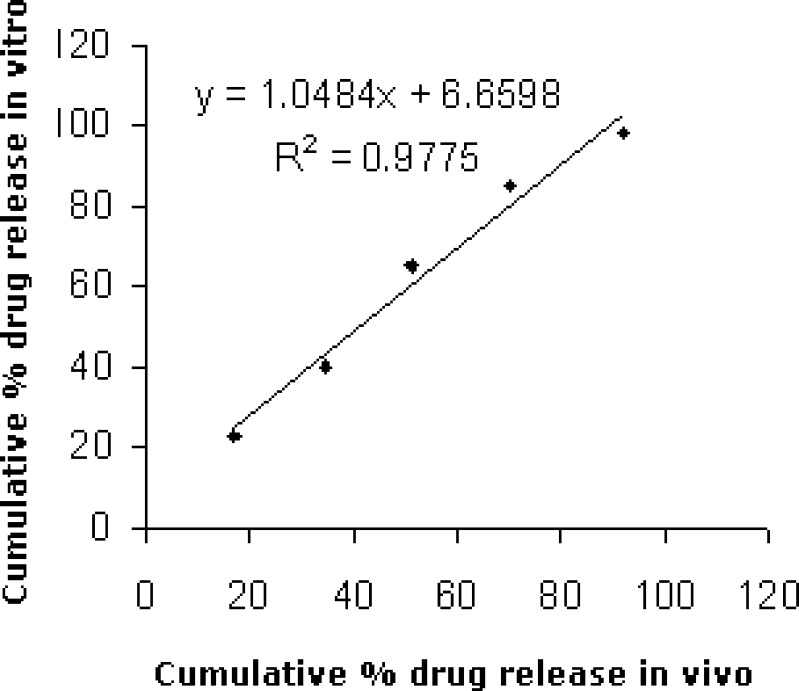
Scatter diagram (Fig. 3) between cumulative percent drug released in vivo and in vitro for formulation D4 showed high values of correlation (0.977). Zero order release rate constant found by in vivo studies (0.77) was close to that found by in vitro studies (0.84).
Fig. 3.
Scatter diagram between cumulative percent drug release in vitro and in vivo of formulation D4
On administration of ocular insert D4, it was found that concentration of acyclovir in aqueous humor reached above the reported minimum effective concentration of 1.7 μg/ml [18] after 8 h and lasted for 5 days. However in case of the ophthalmic ointment maximum acyclovir concentration (1.8 ± 1.2 μg/ml) reached within 1 hr and the drug content in aqueous humor was not detected after 4 h (Table IV). Thus, ocular insert was found to overcome the disadvantage of the see-saw pattern of ophthalmic ointment by maintaining almost constant amount of drug for longer period of time. However to compensate the initial lag time, fast releasing formulation should be first administered and then the ocular insert. No significant change was found in drug content after stability study for 180 days.
Table IV.
Concentration of Acyclovir in Aqueous Humor after Single Instillation of Acyclovir Ointment and Acyclovir Ocular Insert D4
| Time (Hrs) | Ocular Insert (μg/ml) | Ointment (μg/ml) |
|---|---|---|
| 0.5 | n.d. | 0.85 ± 0.4 |
| 1 | n.d. | 1.8 ± 1.2 |
| 2 | n.d. | 1.23 ± 1.1 |
| 4 | 0.52 ± 0.2. | 0.58 ± 0.5 |
| 8 | 1.52 ± 0.9 | n.d. |
| 24 | 1.91 ± 0.8 | n.d. |
| 48 | 2.36 ± 1.5 | n.d. |
| 72 | 2.38 ± 1.2 | n.d. |
| 96 | 2.03 ± 1.2 | n.d. |
| 120 | 1.68 ± 1.1 | n.d. |
Values are mean ± SD of results from four eyes; n.d. means not detectable
SUMMARY AND CONCLUSION
Reservoir type ocular inserts comprising reservoir film of sodium alginate and rate controlling membrane consisting of different Eudragit RL 100: RS 100 ratios were prepared by film casting technique on Teflon coated petri dishes and tested for drug content, physical characteristics, interaction between drug and polymers due to sterilization by gamma radiations and in vitro drug release. Reservoir film containing 2.5% sodium alginate and 48% PEG 400 by weight of polymer as plasticizer was considered best for formulation of ocular insert owing to maximum percentage elongation at break (18 ± 0.57). Based on in vitro drug release studies formulation containing 3.5:1.5 of Eudragit RL 100/RS 100 (D4) was found better than other formulations with 98% drug release in 120 h therefore, selected as optimized and subjected to in vivo and stability studies. High in vitro–in vivo release correlation (0.977) was observed for the formulation. Concentration of acyclovir in aqueous humor reached above the reported minimum effective concentration of 1.7 μg/ml after 8 h and remained almost constant up to 5 days however, acyclovir concentration could not be detected after 4 h on administration of 3% ophthalmic ointment. Ocular inserts were stable with no sign of interaction due to gamma radiation and demonstrated controlled release of acyclovir for 5 days.
Thus In vivo studies conclusively demonstrates capability of ocular insert consisting rate controlling membrane of Eudragit RS 100 and Eudragit RL 100 in controlling release of acyclovir to the eye. Therefore, administration of the ocular insert with an immediate release ointment would probably offset the initial lag time and maintain almost constant concentration in aqueous humor.
References
- 1.Desai S.D., Blanchard J. Ocular drug formulation and delivery. In: Swarbrick J., Boylar J., editors. Encyclopedia of Pharmaceutical Technology, Vol. 3. New York: Marcel Dekker; 1994. pp. 43–76. [Google Scholar]
- 2.Schoenwald R. D. Ocular drug delivery, pharmacokinetic considerations. Clin. Pharmacokinet. 1998;18:255–269. doi: 10.2165/00003088-199018040-00001. [DOI] [PubMed] [Google Scholar]
- 3.Lee V. H. L. Precorneal, corneal and postcorneal factors. Drugs Pharm. Sci. 1993;58:59–81. [Google Scholar]
- 4.Attis M. A., Kassem M. A., Safewat S. In vivo performance of [3H] dexamethasone ophthalmic film delivery systems in the rabbit eye. Int. J. Pharm. 1988;47:21–30. doi: 10.1016/0378-5173(88)90211-6. [DOI] [Google Scholar]
- 5.Barath S., Hiremath S. R. Ocular delivery system of perfloxacin mesylate. Pharmazie. 1999;54:33–38. [PubMed] [Google Scholar]
- 6.Humo L. R., Lee H. K., Benedetti L., Sanagiri Y. D., Topp E. M., Stella V. J. Ocular sustained delivery of prednisolone using hyaluronic acid benzyl ester films. Int. J. Pham. 1994;111:293–298. [Google Scholar]
- 7.Gurtler F., Kaltsatos V., Boisrame B., Gurny R. Long-acting soluble bioadhesive ophthalmic drug insert (BODI) containing gentamycin for veternary use : optimization and clinical investigation. J. Control. Release. 1995;33:231–236. doi: 10.1016/0168-3659(94)00096-D. [DOI] [Google Scholar]
- 8.Kawakami S., Nishida K., Mukai T., Yamamura K., Nakamura J., Sakaeda T., Nakashima M., Sasaki H. Controlled release and ocular absorption of tilisolol utilizing ophthalmic insert-incorporated lipophilic prodrugs. J. Control. Release. 2001;70:255–263. doi: 10.1016/S0168-3659(01)00441-2. [DOI] [PubMed] [Google Scholar]
- 9.Di Colo G., Zambito Y. A study of release mechanisms of different ophthalmic drugs from erodible ocular inserts based on poly(ethylene oxide) Eur. J. Pharm. Biopharm. 2002;54:193–199. doi: 10.1016/S0939-6411(02)00086-3. [DOI] [PubMed] [Google Scholar]
- 10.Sean C Sweetness(Ed). Martindale: The Complete Drug Reference.33rd ed, Vol.I, The Pharmaceutical Press, London; 2002:605–648.
- 11.Giannavola C., Bucolo C., Maltese A., Paolino D., Vandelli M.A., Puglisi G., Lee V. H., Fresta M. Influence of preparation conditions on acyclovir-loaded poly-d,l-lactic acid nanospheres and effect of PEG coating on ocular drug bioavailability. Pharm. Res. 2003;20:584–590. doi: 10.1023/A:1023290514575. [DOI] [PubMed] [Google Scholar]
- 12.Fresta M., Fontana G., Bucolo C., Cavallaro G., Giammona G., Puglisi G. Ocular tolerability and in vivo bioavailability of poly(ethylene glycol) (PEG)-coated polyethyl-2-cyanoacrylate nanosphere-encapsulated acyclovir. J. Pharm. Sci. 2001;90:288–297. doi: 10.1002/1520-6017(200103)90:3<288::AID-JPS4>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 13.Ali A., Sharma S. N. Fabrication of a through-flow apparatus for in-vitro determination of drugs from ophthalmic preparations. Indian Drugs. 1992;29:157–160. [Google Scholar]
- 14.Ali A., Sharma N. Cromolyn sodium in the treatment of allergic conjunctivitis caused by congress grass. Ind. J. Hosp. Pharm. 1991;28:163–169. [Google Scholar]
- 15.Castela N., Vermerie N., Chast F., Saubegeon-Martre H., Denis J., Godard V., Goldschmidt P., Pouliquen Y. Gancyclovir ophthalmic gel in herpes simplex virus rabbit keratitis: Intraocular penetration and efficacy. J. Ocul. Pharmacol. 1994;10:439–451. doi: 10.1089/jop.1994.10.439. [DOI] [PubMed] [Google Scholar]
- 16.Genta I., Conti B., Perugini P., Pavanetto F., Spadaro A., Puglisi G. Bioahesive microsheres for ophthalmic administration of acyclovir. J. Pharm. Pharmacol. 1997;49:737–742. doi: 10.1111/j.2042-7158.1997.tb06103.x. [DOI] [PubMed] [Google Scholar]
- 17.Rao V., Shyale S. Preparation and evaluation of ocular inserts containing norfloxacin. Turk. J. Med. Sci. 2004;34:239–246. [Google Scholar]
- 18.Wagstaff A. J., Faulds D., Goa K. L. Acyclovir: a reappraisal of its antiviral activity, pharmacokinetic properties and therapeutic efficacy. Drug. 1994;47:153–205. doi: 10.2165/00003495-199447010-00009. [DOI] [PubMed] [Google Scholar]