INTRODUCTION
Tablet coating is perhaps one of the oldest pharmaceutical processes still in existence (1). It offers many benefits namely: improving the aesthetic qualities of the dosage form, masking unpleasant odour or taste, easing ingestion, improving product stability and modifying the release characteristics of the drug. It is widely used in enteric coating, controlled release system and osmotic pump systems (2).
Enteric coated dosage forms are designed to resist the destructive action of the gastric fluid and to disintegrate in the higher pH environment of the intestinal fluid. Polymer for enteric coating can be applied to solid dosage forms (i.e. granules, pellets, or tablets) from aqueous solutions of alkali salts, or organic solvent solutions. The most commonly used pH-sensitive enteric coating polymers today include: cellulose acetate phthalate (CAP), cellulose acetate trimellitate (CAT), hydroxypropyl methyl cellulose phthalate (HPMCP) and methacrylic acid copolymers (3). In recent years, acrylic copolymers have evolved as the most preferred materials for designing enteric coating formulations in terms of performance and global acceptability (1).
Aqueous enteric film coatings have been used widely in recent years. Many of these systems are pseudolatex dispersions of polymers such as CAP, latex dispersions of methacrylic acid copolymers and aqueous solutions of alkali salts (4).The advantages of an aqueous based coating system have been recognized. This is derived from the drawbacks of organic solvents including pollution, explosion hazards, and solvent toxicity. Especially there are risks for operators (5).
Almost all nonsteroidal anti-inflammatory drugs irritate gastric mucosa and enhance ulceration by blocking protective action of the prostaglandins on gastric mucosa, causing ulcer formation not only in stomach but also in lower part of oesophagus and in duodenum too (6).
The most important side effects of flurbiprofen include peptic ulceration, hemorrhage and perforation (7).The aim of current investigation was to develop an enteric coated flurbiprofen tablet formulation to minimize the gastric intolerance caused by flurbiprofen on long term use and to improve patient compliance by utilizing Acryl-Eze. Acryl-Eze is an optimized, one-step, pigmented, aqueous acrylic system for the application of an enteric film coating for oral solid dosage forms. Acryl-Eze combines the benefits and performance of a globally accepted enteric polymer (Eudragit® L100-55) with a fully formulated coating system, providing significant time savings in both development and production. Acryl-Eze has further been optimized for either tablet or multiparticulate applications (8). Eudragit® L100-55 is a copolymer of methacrylic acid and ethyl acrylate (1:1 ratio) which meets the USP definition for Methacrylic Acid Copolymer Type C. L100-55 is a re-dispersible powder produced by spray drying Eudragit® L30-D. Film coatings prepared from such dispersions are resistant to gastric juice but readily dissolve at a pH above 5.5 (9). EudragitL100-55 has been used as a coating polymer in several studies (10,11,12).
MATERIAL AND METHOD
Materials
Flurbiprofen (C15H13FO2) was a gift from Platinum Pharmaceuticals (Pvt.) Limited, Pakistan, microcrystalline cellulose (Avicel PH 102; FMC Corp, USA), crospovidone (ISP Technologies, INC, Wayne, NJ) and magnesium stearate (Merck, KgaA, Darmstadt, Germany) were used as directly compressible excipients. Sodium hydroxide (Merck, KgaA, and Darmstadt, Germany) monobasic potassium phosphate (Merck, KgaA, Darmstadt, Germany) and all the other chemicals used were of analytical grade and obtained from commercial sources. The enteric coating material was methacrylic acid copolymer based pigmented Acryl-Eze 93092157 Yellow and the subcoat material was Opadry YS-1-7027 White, all by Colorcon (Verna, Goa, India).
Preparation of Flurbiprofen (50 mg) Tablet
All the ingredients (excipients drug) were properly weighed and sieved through a 20 mesh screen. Avicel PH 102, flurbiprofen and crospovidone were mixed homogenously for 5 min by tumbling action in a poly bag of suitable size. Finally magnesium stearate was added for lubrication and mixed for additional 5 min till a fine blend of ingredients was obtained. The tablets were manufactured on a single punch tablet machine (KORSCH Erweka, Frankfurt, Germany) using round biconcave punches 6.5 mm in diameter, at a compaction force of 40–60 N. All the formulations were prepared with the same blending time and same compression conditions.
Optimization of Flurbiprofen Formulation
For optimization purposes, nine different formulations of flurbiprofen 50 mg tablets were developed. The tablets were prepared using the composition as given in [Table I]. Avicel PH 102 (X1), crospovidone (X2) and magnesium stearate (X3) were selected as independent variables and the response was studied on tablet weight (Y1), hardness (Y2), thickness (Y3), diameter (Y4), friability (Y5), disintegration time (Y6), assay (Y7) and dissolution (Y8).
Table I.
Composition of Flurbiprofen 50 mg Tablets
| Ingredients (mg/tablet) | Batch Code | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| F1 | F2 | F3 | F4 | F5 | F6 | F7 | F8 | F9 | |
| Avicel PH 102 | 75 | 75 | 75 | 75 | 75 | 75 | 65 | 55 | 85 |
| Crospovidone | 5 | 5 | 5 | 10 | 7.5 | 2.5 | 10 | 10 | 10 |
| Magnesium stearate | 5 | 2.5 | 7.5 | 5 | 5 | 5 | 5 | 5 | 5 |
| Total tablet weight (mg) | 135 | 132.5 | 137.5 | 140 | 137.5 | 132.5 | 130 | 120 | 150 |
Evaluation of Flurbiprofen Tablet Core
After compression, tablets of all the formulations were evaluated for different parameters. Weight variation was determined using electronic balance (Mettler Toledo, B204-S (College) Switzerland)). Tablet thickness and diameter was measured using vernier caliper. Friability was determined using Roche type friabilator (H.Jurgens, GmbH & Co., Bremen, Germany) whereas tablet hardness was determined using a hardness tester (OSK Fujiwara Hardness Tester, Tokyo, Japan). A model ZT-2 disintegrator tester (Erweka, Husenstamn, Germany) was used for disintegration testing. A dissolution station (Erweka DT 700, Husenstamn, Germany) was used for drug release testing. The paddle was operated at 50 rpm using 900 ml pH 7.2 phosphate buffer maintained at 37 °C (13). Tablet assay was determined using UV Spectrophotometer (UV 150-02, double beam spectrophotometer, Shimadzu Corp., Kyoto, Japan) at 247 nm (14). The formulations were also statistically analyzed for weight variation, hardness, thickness and diameter using Shewhart quality control charts (15).
Choice of the Optimized Formulation
Formulation 7 was selected as an optimized formulation after comparative evaluation of all necessary parameters for enteric coating.
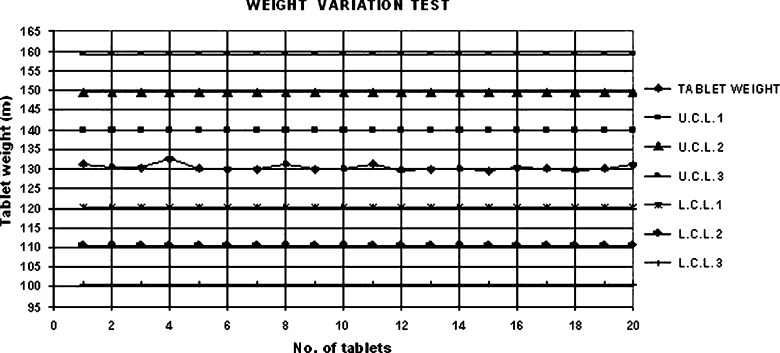
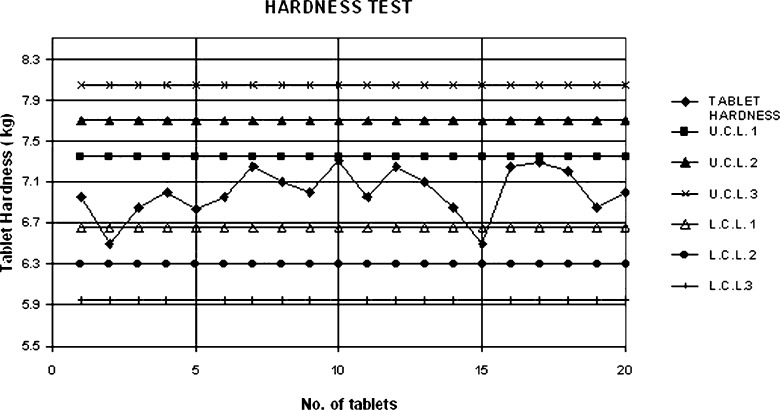
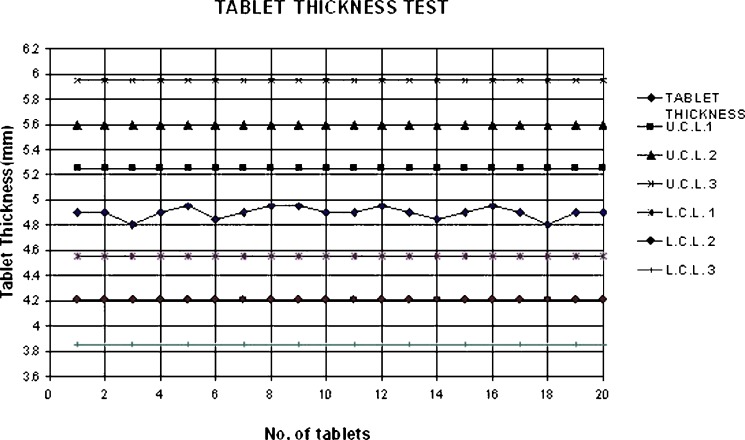
Shewhart quality control charts for weight variation, tablet hardness and thickness of formulation 7 are given in Figs. 1, 2 and 3.
Fig. 1.
Shewhart control chart for weight variation (formulation 7)
Fig. 2.
Shewhart control chart for tablet hardness (formulation 7)
Fig. 3.
Shewhart control chart of tablet thickness (formulation 7)
Enteric Film Coating of Tablets
The compressed tablets were subcoated using Opadry dispersion prepared in 600 ml of organic solvent (343 ml of methylene chloride and 257 ml of ethanol). The subcoating was followed by aqueous enteric coating with Acryl-Eze. Both coating dispersions were prepared according to technical document provided by the Colorcon (16,17) [Table II].
Table II.
Dispersion Preparation Parameters
| Parameters | Subcoat layer | Acryl-Eze |
|---|---|---|
| Theoretical weight gain (%) | 2 | 8–10 |
| Dispersion solid content (%) | 15 | 20 |
| Powder (g) | 30 | 80 |
| Organic solvent (ml) | 600 | N/A |
| Deionize water (g) | N/A | 320 |
| Total Dispersion (ml) | 630 | 400 |
The tablet coating was performed in 12 in coating pan (Erweka GmbH., type UG, Frankfurt, Germany) using external spray gun, dryer and air compressor (Type H-2, No. 311319, Meiji air compressor Mfg.Co.Ltd. Osaka, Japan).The pan load was 1 kg. Tablets were prewarmed at reduced coating speed to 40 °C. Warm air was introduced into the coating pan (up to 50–55 °C) during the entire coating process. The subcoat was applied at a 2% weight gain, followed by Acryl-Eze at a weight gain of 8–10%. The coating process parameters were set as recommended by the manufacturer for enteric coating system (18).
Disintegration Test of Enteric Coated Tablets
The disintegration time of enteric coated flurbiprofen 50 mg tablets was determined according to the procedure reported in USP 28 NF 23<701> (13). Six tablets of flurbiprofen enteric coated tablets were weighed individually and placed in acid phase (0.1 N HCl) for 2 h in a USP basket rack assembly (Erweka ZT-2, Husenstamn, Germany) after which they were removed and inspected for cracking or disintegration. The same tablets were then placed in phosphate buffer, pH 6.8 and observed for disintegration.
Dissolution Test of Enteric Coated Tablets
The dissolution test for enteric coated tablets was performed as mentioned in USP 28 NF 23<724> (13).Drug release was measured in a USP dissolution bath (Erweka DT 700, Husenstamn, Germany) using apparatus II at 50 rpm. The dissolution medium were 1000 ml of 0.1 N HCl and pH 6.8 phosphate buffer maintained at 37 ± 0.5 °C. The tablets were placed in acidic medium for 2 h and in basic medium for 1 h. Samples were assayed spectrophotometrically (UV 150-02, Double beam spectrophotometer, Shimadzu Corp., Kyoto, Japan) at 247 nm.
Stability Study
Samples of Flurbiprofen enteric coated tablets were blister packed using packing machine (Ulhmann-200, Germany) in 1×10’s using aluminum foil (Heuch folien GMBH & G, KG, Germany) and PVDC (Perlin converting AG., Switzerland). These samples were then subjected for stability study according to ICH guidelines (19). Tests were conducted at room temperature (RT) and accelerated stability conditions. The samples were designated as time 0, 3, 6, 9 and 12 month for RT and 0, 1, 2, 3 and 6 month for accelerated studies. Samples designed for RT storage were kept at 30 ± 2 °C and 65 ± 5% relative humidity (RH). The samples in the accelerated stability study were kept at 40 ± 2 °C and 75 ± 5% RH in humidity chamber (Binder GMBH Bergster, Tullingen, Germany). Samples were tested for its appearance, disintegration, dissolution and assay using the previously described procedure.
RESULTS AND DISCUSSION
Flurbiprofen tablet cores were successfully prepared by direct compression method using avicel PH 102 as filler/binder, crospovidone as disintegrating agent and magnesium stearate as lubricant. Tablets were compressed without any problem and the prepared tablets were free from defects such as capping and lamination. The formulations had good flow resulting in low tablet weight variation. Tablets of high mechanical strength (high breaking force and low friability) were manufactured. Results of uncoated flurbiprofen tablet evaluation of all the formulations are shown in Table III. Physical appearance, hardness, friability, weight variation, and drug content evaluation of different tablet formulations were found to be satisfactory under pharmacopoeial standards of tablet evaluation. It was observed that the incorporation of superdisintegrant ‘crospovidone’ greatly improve the disintegration time of all the formulations. Invitro disintegration study reveals that the tablets were completely disintegrated within few seconds.
Table 3.
Evaluation Parameters of Different Formulations
| Batch Code | Wt. of 20 Tabs. (mg) | Friability (%) | Disint. Time (s) | Diameter (mm) | Thickness (mm) | Hardness (kg.) | Diss. Test (%) | Assay 247 nm (%) |
|---|---|---|---|---|---|---|---|---|
| F1 | 135.08 ± 0.223 | 0.071 | 25 | 6.495 ± 0.015 | 4.872 ± 0.061 | 6.990 ± 0.098 | 94.08 | 97.56 |
| F2 | 132.42 ± 0.912 | 0.156 | 22 | 6.495 ± 0.015 | 4.897 ± 0.041 | 5.959 ± 0.073 | 92.56 | 96.95 |
| F3 | 137.07 ± 0.744 | 0.150 | 28 | 6.492 ± 0.018 | 4.910 ± 0.030 | 6.977 ± 0.119 | 95.59 | 95.27 |
| F4 | 140.23 ± 0.668 | 0.142 | 18 | 6.492 ± 0.018 | 4.920 ± 0.034 | 8.027 ± 0.175 | 94.84 | 96.64 |
| F5 | 137.40 ± 0.587 | 0.071 | 21 | 6.492 ± 0.018 | 4.905 ± 0.042 | 6.525 ± 0.277 | 93.32 | 97.40 |
| F6 | 132.42 ± 0.315 | 0.155 | 36 | 6.495 ± 0.015 | 4.900 ± 0.039 | 7.230 ± 0.249 | 96.35 | 97.86 |
| F7 | 130.42 ± 0.706 | 0.251 | 11 | 6.492 ± 0.018 | 4.897 ± 0.044 | 6.998 ± 0.232 | 99.39 | 99.69 |
| F8 | 120.42 ± 0.758 | 0.080 | 11 | 6.495 ± 0.015 | 4.837 ± 0.042 | 7.867 ± 0.251 | 96.96 | 98.32 |
| F9 | 150.29 ± 0.906 | 0.208 | 10 | 6.495 ± 0.015 | 5.707 ± 0.037 | 5.083 ± 0.308 | 95.90 | 97.10 |
| Limits | ±7.5% | <than 1% | <then 15 min | ±5% | ±5% | 6–10 | ≥75 | 92.5–107.5 |
The results obtained from the evaluation of tablet characteristics were utilized in the selection of optimized formulation. Formulation 7 was found to have best results according to the optimization criteria with dissolution up to 99.39% in basic medium and assay results up to 99.69%. It was finally chosen for the preparation of enteric coated formulation. Tablets used in functional film coating process must be sufficiently robust to withstand mechanical stresses and to exhibit a very low potential for erosion and edge chipping. Any defects in the tablet core may result in localized weakness of functional film.
Tablet coating was carried out in conventional coating pan using spray coating technique. Spray applications enables finely atomized droplets of the coating solution to be delivered across the surface of the moving tablet mass in a manner that achieves uniform coverage while preventing adjacent tablets from sticking together as the coating solution is rapidly dried. The coating process was conducted under specific conditions with a continuous supply of warm air. Parikh et al. (20) in 1993 investigated three independent coating process variables namely percent solids content in the coating polymeric dispersion, inlet-air temperature, and spray rate of the polymeric dispersion to determine their effect on the performance characteristics of tablets coated with a plasticized aqueous ethylcellulose dispersion (Sure-lease) in a fluid-bed equipment. Inlet-air temperature was found to be the most critical process variable for all the three response variables studied. It was concluded that lower rates of drug release from the coated tablets may be obtained by using high inlet-air temperature and low spray rate of the polymeric dispersion during coating.
A subcoating process may be used to strengthen friable cores before the application of the functional coat but is not desirable in terms of process time and complexity. Subcoats may also be necessary to prevent interaction between the drug substance and the coating formulation ingredients. In the present study subcoating was carried out using Opadry resulting in white and smooth tablets with improved stability. Final enteric coating was done with Acryl-Eze. The aqueous dispersion was prepared in one step by adding fully formulated dry powder in water with the aid of high shear mixer. Enteric coating with Acryl-eze was completed in a short period of time and the coated tablets had no obvious defects or signs of peeling or chipping.
The enteric coated tablets were subjected to both tablet disintegration and dissolution test. Results showed that there were no signs of cracking, peeling or disintegration in 0.1 N HCl however the coated tablets were completely disintegrated in 5–7 min in pH 6.8 phosphate buffer.
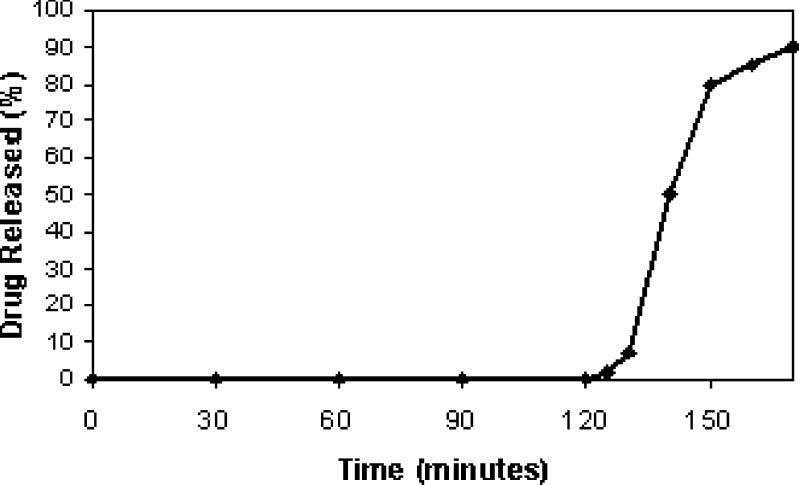
Drug release met the criteria outlined in this study. i.e. not less than 75% dissolved after 45 min in buffer, pH 6.8. Figure 4 illustrates the release of flurbiprofen from enteric coated tablets.
Fig. 4.
Drug release from Flurbiprofen tablets in 0.1 N HCl and pH 6.8 phosphate buffer
Results of stability test were satisfactory showing no significant change in the color, appearance and disintegration of coated tablet. Tablet dissolution and assay results were within acceptable limits. [Table IV]
Table 4.
Stability Testing of Flurbiprofen 50 mg Tablets
| Stability Test Conditions | Time (months) | Dissolution (%) | Assay(%) |
|---|---|---|---|
| RT | 0 | 98.9 | 99.6 |
| 3 | 98.5 | 98.5 | |
| 6 | 98.4 | 98.39 | |
| 9 | 98.3 | 98.3 | |
| 12 | 98.1 | 98.2 | |
| Accelerated | 0 | 98.9 | 99.6 |
| 1 | 98.3 | 98.9 | |
| 2 | 98.2 | 98.7 | |
| 3 | 98.1 | 98.5 | |
| 6 | 98.0 | 98.3 |
SUMMARY AND CONCLUSIONS
A delayed release flurbiprofen (50 mg) formulation was developed using Opadry/Acryl-Eze system. The core formulation yielded mechanically strong tablets with low weight variation. Aqueous enteric coating was successfully conducted under lab-scale facilities. Opadry/Acryl-Eze system provides acceptable enteric performance in 0.1 N HCl and pH 6.8 phosphate buffer. The system also provides the additional advantage of being fully pigmented.
It is thus concluded that aqueous enteric coating with Opadry/Acryl-Eze system is an easy and economical approach for preparing delayed release formulations.
Acknowledgement
The authors wish to thank Platinum Pharmaceuticals (Pvt.) Limited for providing active material Flurbiprofen and Colorcon Asia Pacific for providing Acryl-Eze and Opadry.
References
- 1.Porter S. C. Remington, The Science and Practice of Pharmacy. 21. New York: Lippincott William and Wilkins; 2006. Coating of pharmaceutical dosage form; pp. 929–933. [Google Scholar]
- 2.Missaghi S., Fassihi R. A novel approach in the assessment of polymeric film formation and film adhesion on different pharmaceutical substrates. AAPSPharmSciTech. 2004;5:E29. doi: 10.1208/pt050229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guo H. X., Heinamaki J., Yliruusi J. Diffusion of a freely water soluble drug in aqueous enteric coated pellets. AAPSPharmSciTech. 2002;3:E16. doi: 10.1208/pt030216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wheatley T. A., Steuernagel C. R. Latex emulsion for controlled drug delivery. In: Mcginity J. W., editor. Aqueous Polymeric Coatings for Pharmaceutical Dosage Forms. New York: Marcel Dekker Inc; 1997. pp. 11–13. [Google Scholar]
- 5.Baudoux M., Dechesne J. P., Delattre L. Film coating with enteric polymers from aqueous dispersions. Pharm. Technol. Int. 1990;12:18–26. [Google Scholar]
- 6.Derle D. V., Gujar K. N., Sagar B. S. H. Adverse effects associated with the use of nonsteroidal anti-inflammatory drugs: An overview. Indian J. Pharm. Sci. 2006;68:409–414. doi: 10.4103/0250-474X.27809. [DOI] [Google Scholar]
- 7.C. Dollery, A. Boobis, M. Rawlins, and S. Thomas (eds.). In Therapeutic Drugs, 2nd ed., Churchill Livingstone, Edinburgh, 1991, pp. F126–F128.
- 8.Colorcon. Oral Modified Release System, Acryl-Eze Sales Brochure. Available at: http://www.colorcon.com/pharma/mod_rel/acryl_eze/lit/acryl_sales_new.pdf. Accessed: August 28, 2007.
- 9.Colorcon. Oral Modified Release System, Performance characteristics of Acryl-Eze, a new, fully formulated, acrylic based, enteric film coating system. Poster Reprint, Controlled Release Society. June 26, 2001. Available at: http://www.colorcon.com/pharma/mod_rel/acryl_eze/lit/perf_char_nw.pdf. Accessed 29 August, 2007.
- 10.Sauer D., Zheng W., Coots L. B., McGinity J. W. Influence of processing parameters and formulation factors on the drug release from tablets powder-coated with Eudragit® L 100-55. Eur. J. Pharm. Biopharm. 2007;67:464–475. doi: 10.1016/j.ejpb.2007.02.021. [DOI] [PubMed] [Google Scholar]
- 11.Law D., Zhang Z. Stabilization and target delivery of Nattokinase using compression coating. Drug Dev. Ind. Pharm. 2007;33:495–503. doi: 10.1080/03639040601050247. [DOI] [PubMed] [Google Scholar]
- 12.Thoma K., Bechtold K. Influence of aqueous coatings on the stability of enteric coated pellets and tablets. Eur. J. Pharm. Biopharm. 1999;47:39–50. doi: 10.1016/S0939-6411(98)00086-1. [DOI] [PubMed] [Google Scholar]
- 13.United States Pharmacopeia-28. Rockville, MD: Convention; 2004:866. Also pp. 2417–2418.
- 14.British Pharmacopoeia (B.P.) London, UK: Stationary Office, London; 2005.
- 15.Bolton S., Bon C. Quality control. In: Swarbrick J., editor. Pharmaceutical Statistics: Practical and Clinical Applications. 4. NewYork: Marcel Dekker Inc; 2004. pp. 373–375. [Google Scholar]
- 16.Colorcon. Oral Modified Release System, Preparation and Use Guide lines, ACRYL-EZE. 2006. Available at: http://www.colorcon.com/pharma/mod_rel/acryl_eze/lit/prep_use_new.pdf. Accessed January 15, 2007.
- 17.Colorcon. Film Coatings, Preparation and Use Guide lines, Opadry. 2006. Available at: http://www.colorcon.com/pharma/film_coat/pharma_coatings/opadry/lit/reconstitution_oy_type2.pdf. Accessed January 15, 2007
- 18.Colorcon. Oral Modified Release System, Coating Parameters, ACRYL-EZE. 2006. Available at: http://www.colorcon.com/pharma/mod_rel/acryl_eze/lit/coating_params.pdf. Accessed January 17, 2007.
- 19.ICH Guidelines, Stability testing of new drug substances and products, Q1A(R2) Step 4 version, dated 6 February 2003. Available at: http://www.ich.org/cache/compo/363-272-1.html#Q1C
- 20.Parikh N. H., Porter S. C., Rohera B. D. Aqueous ethylcellulose dispersion of ethylcellulose I. Evaluation of coating process variables. Pharm. Res. 1993;10:525–534. doi: 10.1023/A:1018989717297. [DOI] [PubMed] [Google Scholar]