INTRODUCTION
Under certain circumstances prolonging the gastric retention of a delivery system is desirable for achieving greater therapeutic benefit of the drugs. For example, drugs that are absorbed in the proximal part of the gastrointestinal tract (1), and drugs that are less soluble in or are degraded by the alkaline pH may benefit from prolonged gastric retention (2). In addition, for local and sustained drug delivery to the stomach and proximal small intestine to treat certain conditions, prolonged gastric retention of the therapeutic moiety may offer numerous advantages including improved bioavailability and therapeutic efficacy, and also the possible reduction of dose size (3). Gastroretentive drug delivery systems (GRDDS) can remain in the gastric region for several hours and hence significantly prolong the gastric residence time of drugs.
Various GRDDS reported in literatures provide controlled delivery of drugs in matrix and/or reservoir type systems. The release of drugs from reservoir/matrix type systems is affected by hydrodynamic conditions of the absorption site and also results in bioavailability fluctuation due to gastric pH variation.
Osmotic drug delivery system (ODDS) utilizes the principles of osmotic pressure for controlled delivery of drugs (4). Drug release from these systems is independent of pH and other physiological parameters to a large extent and exhibit significant in vitro–in vivo correlation (5). Drug delivery from ODDS follows zero-order kinetics hence provides better control over in-vivo performance.
Ranitidine hydrochloride (RH) is a histamine H2-receptor antagonist. It is widely prescribed in active duodenal ulcers, gastric ulcers, Zollinger–Ellison syndrome, gastroesophageal reflux disease, and erosive esophagitis (6). The recommended adult oral dosage of ranitidine is 150 mg twice daily or 300 mg once daily. The effective treatment of erosive esophagitis requires administration of 150 mg of ranitidine four times a day (6). A frequent dosage schedule for patients often leads to poor patient compliance; thus a sustained release dosage form of RH is desirable. The short biological half-life of drug (2.5–3 h) also favours for development of a sustained release formulation.
A traditional oral sustained release formulation of RH releases most of the drug at the colon; due to less solubility of RH in small intestine, thus the drug have absorption window in the colon or in stomach. Ranitidine is absorbed only in the initial part of the small intestine and has 50% absolute bioavailability (6). Moreover, colonic metabolism of ranitidine is partly responsible for the poor bioavailability of ranitidine from the colon (7). These properties of RH do not favour the traditional approach to sustained release delivery. Hence, clinically acceptable sustained release dosage forms of RH prepared with conventional technology may not be successful.
With all these considerations in mind, we designed floating osmotic drug delivery system (FODDS) of RH. FODDS consists of an osmotic core (containing drug, osmotic agent and excipients), an inner semipermeable membrane (SPM), and an outer compression coating of gelling agent containing gas generating agent and an orifice drilled through both membranes for delivery of drug. When system comes in contact with gastric environment, gas generating agent, generate CO2 by reacting with the surrounding fluid, the gas generated is trapped and protected within the gel (formed by hydration of gelling agent), thus decreasing the density of tablet. As the density of tablet falls below 1 (density of water), the tablet becomes buoyant. At the same time, the osmotic core also draws surrounding fluid across semipermeable membrane because of osmotic pressure gradient and form saturated solution of the drug. This pressure is relieved by the flow of saturated solution of drug through the delivery orifice.
MATERIALS AND METHODS
Materials
Ranitidine hydrochloride (99.9% purity) was a gift sample from Cadila Pharmaceuticals Ltd, Ahmedabad, India. HPMC-K4M, Sodiumcarboxymethylcellulose (SCMC—high viscosity grade) and HPMC-K100 were obtained as a gift samples from Torrent research centre, Ahemdabad, India. Following chemicals and excipients were purchased from commercial sources and were used as such: cellulose acetate (39.8% acetylation), polyvinyl pyrrolidone (PVP), dextrose, microcrystalline cellulose (MCC), magnesium stearate, talc, sodium chloride (all from CDH Delhi, India), acetone, dibutyl phthalate (both from S.D. Fine Chemicals, Mumbai, India), ZANTAC®-300 mg (Retail Pharmacy).
Methods
Design Parameters
By using the pharmacokinetic parameters of RH a dose structure was designed for controlled delivery of RH by using following equation (8).
 |
1 |
Where D0 is the dose, Cp is therapeutic drug plasma level, ClT is clearance total and T is dosing interval. Therapeutic range for RH is reported to be 0.05–1 μg/ml (9) and desired steady state concentration of RH for 150 mg (b.i.d.) dose is 0. 495 μg/ml. By taking steady state concentration as desired therapeutic plasma level following values were proposed; (1) sustaining dose 300 mg (2) zero-order release rate 21.8 mg/h (3) dosing interval of 12 h. By plotting the cumulative zero-order release rate (21.8 mg/h) versus time a desired release profile was generated which was used as target release profile for developed formulations (Fig. 4).
Fig. 4.
Release profiles of promising developed ODDS coated with CT3 in comparison with theoretically desired release profile. Bars represent ±SD (n = 3)
Preparation of Core Tablets
Before initiating formulation development, compatibility of RH with different excipients was tested using the techniques of DSC (DU-PONT, Model 9900, U.S.A) and FT-IR (SHIMADZU, Model 8400S, Tokyo, Japan). Excipients used in the final formulation were found to be compatible with RH. Core tablets of RH were prepared by direct compression and batch size was kept as 100 tablets. Formula of different core formulations of RH is listed in Table I. RH was mixed with HPMC (K-100), Sodium carboxy methyl cellulose (SCMC) for 10 min. After passing this mixture through #30 mesh sieve, osmotic agent (dextrose), and PVP (as a binder) were added in geometric dilution and mixing continued for additional 10 min. To this mixture, #60 mesh sieve passed talc and magnesium stearate were added and mixing continued for another 10 min. The blend was then compressed into tablets having average weight of 500 mg using a single station tablet punching machine (Manesty E-2, London, U.K.) fitted with 10 mm round standard concave punches. The punched tablets were of 6 ± 0.38 kg/cm2 hardness on Monsanto hardness tester. The drug content of the tablets was found to be within the limit of 97.98–102.36%.
Table I.
Formula for Different Batches of Core Formulation
| Ingredients (mg/tablet) | Batch number | |||
|---|---|---|---|---|
| I | II | III | IVa | |
| Ranitidine hydrochloride | 300 | 300 | 300 | 300 |
| Dextrose | 95 | 95 | 95 | 95 |
| HPMC K-100:SCMC (1:1) | – | 55 | 75 | 95 |
| MCC | 75 | 20 | – | – |
| PVP | 24 | 24 | 24 | 24 |
| Talc | 4 | 4 | 4 | 4 |
| Magnesium stearate | 2 | 2 | 2 | 2 |
| Density (g/cm3)b | 1.431 | 1.485 | 1.502 | 1.542 |
HPMC Hydroxypropyl methyl cellulose, SCMC sodium carboxy methyl cellulose, MCC microcrystalline cellulose, PVP polyvinylpyrrolidone
aBatch with tablet weight of 520 mg
bMeasured on compression coated tablet
Preparation of Osmotic Drug Delivery System (ODDS)
ODDS were prepared by coating of core tablets with a SPM in a conventional laboratory coating pan (Scientific instrument, New Delhi, India) with outer diameter of 10 cm fitted with three baffles placed at an angle of 120°. Cellulose acetate (2.5% w/w) with dibutyl phthalate (insoluble plasticizer, 15% w/w of total solid cellulose acetate) dissolved in acetone was used as coating solution. Coating process was done on a batch of 100 tablets; pan speed was maintained at 20 rpm and hot air inlet temp. was kept at 38–42 °C. The manual coating procedure based on intermittent spraying and coating technique was used with spray rate of 4–5 ml/min (10). Coat weight and thickness were controlled by the volume of coating solution consumed in coating process. Coating was continued until desired coat thickness was obtained on the core tablets.
Preparation of Floating Osmotic Drug Delivery System (FODDS)
FODDS were prepared by compression coating of ODDS with a gelling agent (HPMC-K4M) containing gas generating agent (Sodium bicarbonate) Table II. About one third quantity of coating formulation is placed in die cavity (11 mm diameter), the ODDS (10 mm diameter) was carefully positioned in the centre of the die cavity and was then filled with the remainder of the coat formulation. It was then compressed around the core tablet using 11 mm round concave punches at an applied force so as to give 900 μm thickness to compression coat (11). An appropriate size orifice (500 μm) was made on one face of all coated tablets using microdrill (Kamlesh Engineers, Udaipur, India) through SPM and compression coat (11). In all cases coated tablets were dried at 50 °C for 10 h before further evaluation.
Table II.
Formula of Compression Coating Used in Coating of All the Developed Four Batches of ODDS
| Ingredients (mg/tablet) | Coat code | ||
|---|---|---|---|
| CT1 | CT2 | CT3 | |
| HPMC-K4M | 205 | 195 | 185 |
| SBC | 40 | 50 | 60 |
| Talc | 5 | 5 | 5 |
HPMC Hydroxypropyl methyl cellulose, SBC sodium bicarbonate
Evaluation of Developed Formulations
Evaluation of Core and Coated Tablets
The core and coated tablets were evaluated for weight variation and thickness of SPM and compression coat. Thickness and diameter of the core and coated tablets was measured using screw gauze (Ultra Science Aid, Mumbai, India). Hardness of randomly selected tablets was tested using hardness tester (Monsanto hardness tester, Campbell Electronics, Mumbai, India). Friability of core tablets and FODDS was carried out on a Roche Friabilator (Electrolab, Mumbai, India) for 20 accurately weighed tablets.
Drug Content Uniformity
For content uniformity testing, accurately weighed 10 tablets (of all batches) were dissolved in 500 ml of distilled water. The samples were sonicated for 30 min and filtered through 0.45 μm nylon membrane filter. The filtered samples, after appropriate dilution with mobile phase, were analyzed at 315 nm spectrophotometrically.
In-vitro Drug Release Study
The developed formulation of RH were subjected to release studies (n = 3) using USP-XXIV dissolution apparatus Type II (Campbell Electronics, Mumbai, India) at 50 rpm. Dissolution media used was simulated gastric fluid (SGF, pH 1.2, 900 ml) maintained at 37 ± 0.2 °C. The samples (5 ml) were withdrawn at different time intervals and replaced with equivalent volume of fresh medium. The dissolution samples, after filtration through 0.45 μm nylon membrane filters, were analyzed using a validated UV spectrophotometric method at 315 nm (UV/VIS spectrophotometer, JASCO 7800, Japan). Experimental results were expressed as a mean ± SD. Student’s t-test was performed to determine the level of significance. Difference was considered to be statistically significant at p < 0.05.
Further in order to study the effect of pH on drug release, release studies of the developed formulations were also carried out according to pH change method (12) (For initial 2 h in pH 1.2, next 2 h in pH 4.5, 2 h in pH 6.8 and finally for 2 h in pH 7.4).
Floating Properties
The time FODDS took to emerge on the dissolution medium surface (floating lag time) and the time the tablet constantly floated on the water surface (duration of floating) were evaluated in a dissolution vessel (apparatus USP-XXIV type II) filled with 500 ml of simulated gastric fluid (pH 1.2) without pepsin at 37 ± 0.5 °C, with paddle rotation of 100 rpm (13). The measurement was carried out for each batch of tablet (n = 6).
Scanning Electron Microscopy (SEM) Studies
In order to study the effect of compression force during compression coating on surface morphology of SPM of ODDS, the surface of SPM of ODDS both before and after compression coating was studied using scanning electron microscope (SEM). The samples were placed on a spherical brass stub (12 mm diameter) with a double backed adhesive tape. The mounted samples were sputter coated for 5 to 10 min with gold using fine coat ion sputter (JEOL, JFC-1100, Japan) and examined under SEM (JEOL, JSM-6100, Japan).
Kinetics of Drug Release
The drug release data observed from the developed formulations were fitted to various mathematical models (zero-order, first-order, and Higuchi) in order to describe the kinetics of drug release. Smallest value of sum of squared residuals (SSR), Akaike information criterion (AIC) and best goodness-of-fit test (R2) were taken as criteria for selecting the most appropriate model.
Accelerated Stability Studies
Optimized formulations of RH were packed in strips of 0.04 mm thick aluminum foil laminated with polyvinyl chloride (PVC). The packed formulations were stored in international conference of harmonization (ICH) certified stability chambers (Narang Scientific work, New Delhi, India) maintained at 40 °C and 75% relative humidity for 3 months. The samples were withdrawn periodically and evaluated for drug content, hardness, floating lag time, duration of floating and in-vitro drug release studies.
RESULTS AND DISCUSSION
Preliminary Trials
RH is a basic drug with pKa values of 8.64 and, 2.23 hence it is freely soluble in acidic pH (6). Freely soluble drug generally demonstrate uncontrolled and high release rate from elementary ODDS (14). Hydrophilic polymers (HP) are frequently added to the core to form polymeric matrix, and can also be used to retard the release rate of highly water soluble drug from ODDS to get desired zero-order release rate (15,16).
Inclusion of HP (HPMC K-100 and SCMC in present study) is expected to control the drug release from the core by forming the hydrophilic gel matrix. Batch II, III, and IV were prepared containing 11%, 15%, and 18.26% w/w of HP, coated with 80 μm SPM thickness and further compression coated with CT3 coded as Batch-II/80/CT3, Batch-III/80/CT3, Batch-IV/80/CT3 respectively. In-vitro release of all three batches were compared with Batch-I (without HP polymer) coated with 80 μm SPM thickness and further compression coated with CT3 coded as Batch-I/80/CT3 in Fig. 1. It was found that with increase in concentration of hydrophilic polymers in the core there was significant (p < 0.05) decrease in the rate and extent of RH release. Batches II/80/CT3, III/80/CT3 and IV/80/CT3 showed more controlled and prolonged drug release as compared to Batch-I/80/CT3, which delivered more that 80% RH within 5 h. The difference in mean dissolution time for 50% drug release (MDT50%) between batches (1.380, 2.747, 3.258, 3.792 h for batch-I/80/CT3, II/80/CT3, III/80/C3, and IV/80/CT3 respectively) was found to be statistically significant (p < 0.05).
Fig. 1.
Profiles showing effect of hydrophilic polymer on RH release from developed FODDS (with 80 μm SPM thickness). Bars represent ±SD (n = 3)
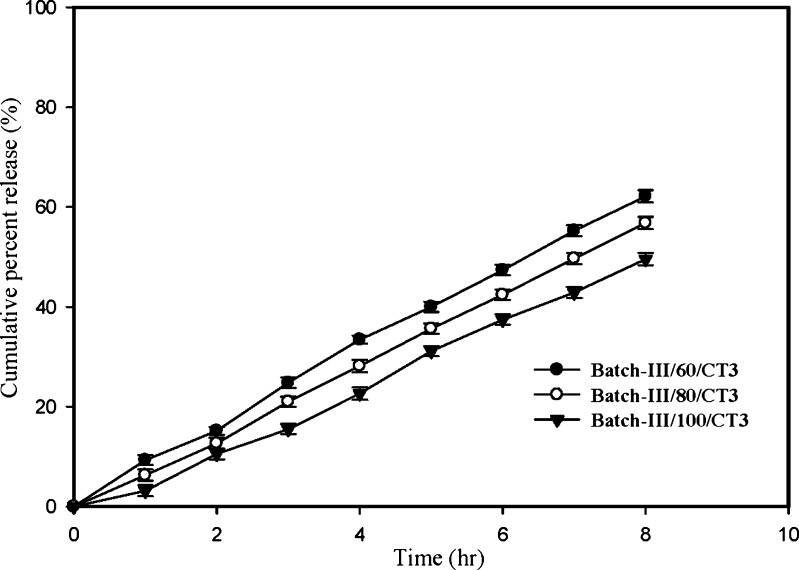
Core formulation of Batch-III was coated with SPM to give different coat thickness (60, 80, and 100 μm) and further compression coated with CT3 coded as Batch-III/60/CT3, Batch-III/80/CT3 and Batch-III/100/CT3 respectively. Release profiles of these formulations were compared in Fig. 2. It is clearly evident that with increase in the SPM thickness the rate and extent of RH release were significantly (p < 0.05) decreased. The MDT50% between different formulations (2.762, 3.258, 3.812 h for formulation with SPM thickness of 60, 80, 100 respectively) was found to be statistically significant (p < 0.05).
Fig. 2.
Profiles showing the effect of SPM thickness on RH release from developed FODDS. Bars represent ±SD (n = 3)
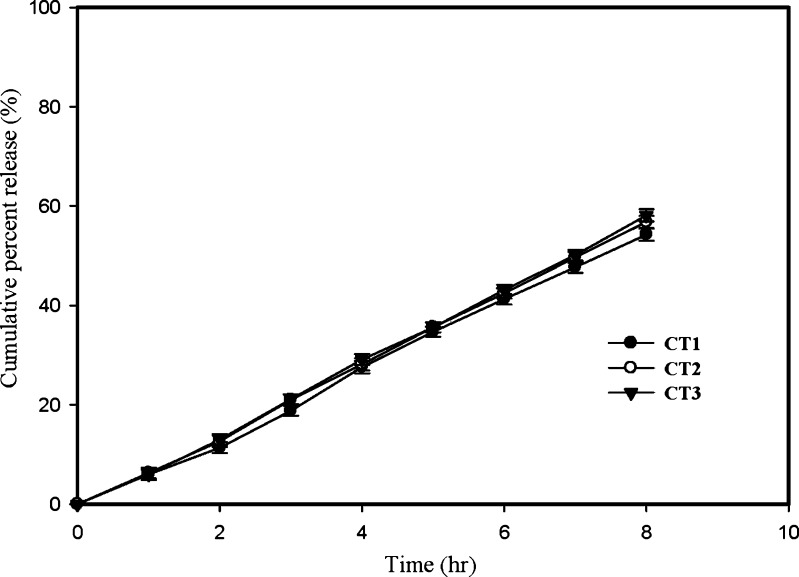
Further release profiles of Batch-III formulations (with 80 μm SPM thickness) compression coated with three different coating composition (Table II) containing three different levels of SBC (16, 20, 24% w/w, coded as CT1, CT2, CT3) were compared in Fig. 3. It is evident from the release profiles that three different concentrations of SBC in compression coating produced insignificant (p > 0.05) effect on rate and extent of drug release. The time for 50% drug release (t50%) for CT1, CT2 and CT3 were found to be 7.28, 6.94, 7.11 h respectively. This is in contradiction to earlier reports of GRDDS, where SBC decreases the drug release (17). SBC when present in the core makes an alkaline environment in and around the core of the formulation which decreases the solubility of those drugs which are highly soluble in acidic environment like Ranitidine. But in this study SBC is since present in compression coat, outside SPM, which was totally separated from core of formulation, hence pH within the core remains unaltered and resulted in no change in drug release. SBC can alter the pH of surrounding fluid outside the FODDS, however RH release from ODDS is independent of pH.
Fig. 3.
Profiles showing the effect of gas generating agent (SBC) on RH release from developed FODDS. Bars represent ±SD (n = 3)
To study the effect of pH on RH release, release study of Batch-III/80/CT3 (with 80 µm thickness of SPM and coated with compression coatings CT3) was conducted according to pH change method. There was insignificant effect (p > 0.05) of different pH of release medium on RH release from developed FODDS when compared to in-vitro data of Batch-III/80/CT3 in pH 1.2. The t50% for pH 1.2, 4.5, 6.8 and 7.4 were found to be 6.96, 7.10, 7.24 h respectively.
To study the effect of hydrodynamic conditions on RH release, release study of Batch-III/80/CT3 was carried out at three different rotational speeds (50, 100, 150 rpm) of paddle. There was insignificant effect of rotational speed (p > 0.05) on RH release from developed formulations when all the three release profiles were compared. The t50% for rotation speed 50, 100 and 150 rpm were found to be 6.96, 7.14, 7.22 h respectively.
Floating Properties
All the developed FODDS floated on the surface of dissolution medium for more than 12 h. When Batch-III (with 80 μm thickness of SPM) coated with all three compression coatings coded as Batch III/80/CT1, Batch III/80/CT2 and Batch III/80/CT3 respectively was tested for floating properties. The Batch III/80/CT1 showed maximum floating lag time (61 s) followed by Batch-III/80/CT2 (49 s) and Batch-III/80/CT3 (37 s). The compression coating composition CT3 was having highest concentration of SBC which generated more CO2 hence resulted in rapid floatation. When all the four Batches of ODDS (with 80 μm thickness of SPM) were compression coated with composition CT3, Batch-I showed minimum floating lag time (21 s) followed by Batch-II (32 s), III (37 s), and IV (46 s). As MCC has a porous structure and may have less density, which help the tablet to float much earlier, hence Batch-I showed minimum floating lag time. Whereas in other batches lag phase was more as they were having low/no concentration of MCC.
Performance Evaluation
To evaluate the performance of developed FODDS release profiles of promising batches (Batch II, III, IV with SPM thickness of 80 μm, and compression coat CT3, coded as II/80/CT3, III/80/CT3, and IV/80/CT3) was compared with marketed immediate release (ZANTAC®) formulation of RH and with theoretical desired release profile in Fig. 4. It is clearly evident that developed FODDS provided more controlled and prolonged drug release as compared to marketed formulation of RH. Drug release from Batch III/80/CT3 was found closest to desired release profile. The f1 and f2 values of Batch-III/80/CT3 were found to be 5.71 and 88.31 respectively, taking desired release profile as reference indicating no difference in drug release between Batch-III/80/CT3 and reference. This formulation also showed minimum floating lag time (37 s) out of all promising formulations hence was selected as the optimized formulation.
The optimized formulation was evaluated for various pharmacopoeial and non-pharmacopoeial tests, results of which are listed in (Table III). The dissolution data of all the promising formulations (II/80/CT3, III/80/CT3, and IV/80/CT3) were fitted to zero-order kinetic, the optimized formulation (Bacth-III/80/CT3) showed highest value of coefficient of determination (R2 = 9994) followed by Batch-II/80/CT3 (R2 = 9962) and Batch-III/80/CT3 (R2 = 9862) showing its superiority on other formulations.
Table III.
Properties of Core Tablets, and Coated Tablets of the Optimized Formulation (Batch-III/80/CT3)
| Parameters | Value ± SD |
|---|---|
| Tablet weight (mg, n = 10) | |
| Core tablet | 500 ± 5.58 |
| SPM coated tablet | 514 ± 4.48 |
| Compression coated tablet | 764 ± 4.54 |
| Thickness (mm, n = 10) | |
| Core tablet | 5.75 ± 0.04 |
| SPM coated tablet | 5.84 ± 0.02 |
| Compression coated tablet | 6.74 ± 0.02 |
| Diameter (mm, n = 10) | |
| Core tablet | 10.01 ± 0.11 |
| SPM coated tablet | 10.09 ± 0.08 |
| Compression coated tablet | 10.99 ± 0.12 |
| Hardness (kg/cm2) | |
| Core tablet | 6.12 ± 1.12 |
| SPM coated tablet | 10.24 ± 1.48 |
| Friability (%) | |
| Core tablet | 0.096 |
| Compression coated tablet | 0.089 |
| Content uniformity (%, n = 10) | 102.26 ± 2.24 |
| Thickness of compression coat (μm, n = 10) | 900 ± 0.04 |
SPM Semipermeable membrane
Scanning Electron Microscopy (SEM) Studies
SPM of ODDS of optimized Batch-III/80/CT3 both before and after the compression coating was studied under scanning electron microscope (Fig. 5). There was no significant change in surface morphology of the SPM, after compression coating, indicating that process of compression coating does not deform SPM.
Fig. 5.
SEM micrograph showing the SPM surface morphology of FODDS of Batch III (80 µm SPM thickness) a before and b after compression coating
Kinetic and Mechanism of Drug Release
Dissolution data of the optimized formulation was fitted to various mathematical models (18) (zero-order, first-order, and Higuchi) Table IV. Drug release from optimized formulations (Batch-III/80/CT3) fitted well into zero-order kinetics confirming that the release from formulation is close to desired release.
Table IV.
Fitting Drug Release Data of the Optimized Formulation (Batch-III/80/CT3) According to Various Mathematical Models
| Model | Parameters used | ||||||
|---|---|---|---|---|---|---|---|
| R 2 | r | Intercept | Slope | k | SSR | AIC | |
| Zero-order | 0.9991 | 0.9996 | −0.16916 | 7.34 | 20.87 | 19.25 | 12.86 |
| First-order | 0.7762 | 0.8810 | 1.7949 | 0.41 | 0.41 | 220.25 | 32.52 |
| Higuchi model | 0.9107 | 0.9543 | 9.8954 | 21.37 | 21.37 | 157.25 | 29.82 |
R 2 Goodness of fit, r correlation coefficient, SSR sum of squares of residuals, AIC Akaike information criteria, k release rate constant for respective models (k 0 in mg/h, k 1 in h−1, and k H in %/h1/2 for zero-order, first order, and Higuchi rate equations respectively)
The optimized formulation was subjected to short term stability studies at 40 °C and 75% relative humidity for 3 months. Samples withdrawn after 3 months showed no significant change in physical properties, drug content, hardness, floating lag time, duration of floating and in-vitro release characteristics.
SUMMARY AND CONCLUSION
Drug release from developed formulations was directly influenced by concentration of hydrophilic polymers in core and thickness of SPM but remain unaffected by pH, hydrodynamic condition of release medium and amount of gas generating agent (SBC) in compression coat. All the formulations showed floating lag time of less than 2 min (desired) and remain floated for up to 12 h. Floating lag time was directly related to amount of SBC in compression coat and inversely related to the density of the FODDS. Batch-III/80/CT3 was selected as optimized formulation based on best correlation with desired release profile and lower floating lag time of 37 s. Optimized formulation was found to be stable when stored at 40 °C and 75% relative humidity for 3 months.
References
- 1.Rouge N., Buri P., Doelker E. Drug absorption sites in the gastrointestinal tract and dosage forms for site-specific delivery. Int. J. Pharm. 1996;136:117–139. doi: 10.1016/0378-5173(96)85200-8. [DOI] [Google Scholar]
- 2.Fell J. T., Whitehead L., Collet H. Prolonged gastric retention using floating dosage forms. Pharm. Technol. 2000;24:82–90. [Google Scholar]
- 3.Fell J. T. Delivery systems for targeting to specific sites in the gastrointestinal tract. J. Pharm. Pharmacol. 1999;51(Suppl):41. [Google Scholar]
- 4.Verma R. K., Mishra B., Garg S. Osmotically controlled oral drug delivery. Drug Dev. Ind. Pharm. 2000;26:695–708. doi: 10.1081/DDC-100101287. [DOI] [PubMed] [Google Scholar]
- 5.Kumar P., Singh S., Rajinikanth P. S., Mishra B. An overview of osmotic pressure controlled release formulation. J. Pharm. Res. 2006;5:34–45. [Google Scholar]
- 6.Grant S. Ranitidine: an updated review of its pharmacodynamic and pharmacokinetic properties and therapeutic use in peptic ulcer and other allied diseases. Drugs. 1989;37:801–870. doi: 10.2165/00003495-198937060-00003. [DOI] [PubMed] [Google Scholar]
- 7.Basit A. W., Lacey L. F. Colonic metabolism of ranitidine: implications for its delivery and absorption. Int. J. Pharm. 2001;227:157–165. doi: 10.1016/S0378-5173(01)00794-3. [DOI] [PubMed] [Google Scholar]
- 8.Sinchaipanid N., Pongwai S., Limsuwan P., Mitrevej A. Design of salbutamol EOP tablets from pharmacokinetic parameters. Pharm. Dev. Tech. 2003;8:135–142. doi: 10.1081/PDT-120018479. [DOI] [PubMed] [Google Scholar]
- 9.Regenthal R., Krueger M., Koeppel C., Preiss R. Drug levels: therapeutic and toxic serum/plasma concentrations of common drugs. J. Clin. Monit. 1999;15:529–544. doi: 10.1023/A:1009935116877. [DOI] [PubMed] [Google Scholar]
- 10.Ozdemir N., Sahin J. Design of a controlled release osmotic pump system of ibuprofen. Int. J. Pharm. 1997;158:91–97. doi: 10.1016/S0378-5173(97)00250-0. [DOI] [Google Scholar]
- 11.Krishnaiaha Y. S. R., Satyanarayana V., Bhaskar P. R., Karthikeyan R. S. Studies on development of oral colon targeted drug delivery systems for metronidazole in treatment of amoebiasis. Int. J. Pharm. 2002;236:43–55. doi: 10.1016/S0378-5173(02)00006-6. [DOI] [PubMed] [Google Scholar]
- 12.Verma R. K., Garg S. Development and evaluation of osmotically controlled oral drug delivery systems of glipizide. Eur. J. Phar. Biopharm. 2004;57:513–525. doi: 10.1016/j.ejpb.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 13.Srivastava A. K., Wadhwa D., Ridhurkar D., Mishra B. Oral sustained delivery of Atenolol from floating matrix tablets—formulation and in-vitro evaluation. Drug. Dev. Ind. Pharm. 2005;31:367–374. doi: 10.1081/ddc-54313. [DOI] [PubMed] [Google Scholar]
- 14.Theeuwes F. Elementary osmotic pump. J. Pharm. Sci. 1975;64:1987–1991. doi: 10.1002/jps.2600641218. [DOI] [PubMed] [Google Scholar]
- 15.Prabakaran D., Singh P., Kanaujia P., Vyas S. P. Effect of hydrophilic polymers on the release of Diltiazem hydrochloride from elementary osmotic pump. Int. J. Pharm. 2003;259:173–179. doi: 10.1016/S0378-5173(03)00230-8. [DOI] [PubMed] [Google Scholar]
- 16.Alderman D. A. A review of cellulose ethers in hydrophilic matrices for oral controlled-release dosage forms. Int. J. Pharm. Technol. Prod. Manuf. 1984;5:1–9. [Google Scholar]
- 17.Chavanpatil M., Jain P., Chaudhari S., Shear R., Vavia P. Development of sustained release gastroretentive drug delivery system for ofloxacin: in vitro and in vivo evaluation. Int. J. Pharm. 2005;304:178–184. doi: 10.1016/j.ijpharm.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 18.Costa P., Lobo J. M. S. Modeling and comparison dissolution profiles. Int. J. Pharm. 2001;13:123–133. doi: 10.1016/s0928-0987(01)00095-1. [DOI] [PubMed] [Google Scholar]