Abstract
The purpose of this study was to prepare and evaluate layered matrix tablets of propranolol HCl containing HPMC and phytowax as matrix component using direct compression technique. Layering with this polymeric matrix could prolong the release of drug and shift the release pattern approach to zero order as described from the least square curve fitting. Increasing the amount of coating layer could apparently prolong the drug release. The longer lag time of drug release from one planar apparently when the amount of coating layer was increased. HPMC concentration and compression force did not affect the drug release from this three-layer tablet. The drug release from this three-layer tablet was influenced by hydrodynamic force. An increase in stirring rate was a corresponding increasing in the release rate. From photoimage and SEM, gel mass of HPMC was increased with time during dissolution and covered the core surface, therefore dissolved drug molecules were allowed to diffuse out from the core through the polymer network of gel layer containing the porous structure. This suggested that HPMC and phytowax could be fabricated into the layered matrix tablet exhibiting sustained drug release.
KEY WORDS: drug release, HPMC, layered matrix, variables
INTRODUCTION
Geometric modulation of matrix systems has been employed to achieve a zero order drug release behavior. The proper technique relies on the fabrication of three-layer matrix tablets. Linear release profiles could be obtained by applying hydrophilic barrier layers on both faces of the hydrophobic matrix tablet, or by applying a hydrophilic barrier layer on one face and a hydrophobic barrier layer on the other face of the matrix tablet (1). Polymer used in this system plays the important role in the modulation of drug release. Polymers with higher gelling ability potentially sustain or control the release of water soluble drug, whereas polymers with a weak gel forming ability lack this potential (2). The barriers made of hydroxypropyl methylcellulose (HPMC) seem more efficient in reducing drug delivery than poly(ethylene oxide) (PEO) barriers, and their efficiency in drug release modulation depended on the viscosity generated from polymer (3). Layering with polymeric matrix containing chitosan and xanthan gum could prolong the drug release in HCl buffer pH 1.2 and shift the release pattern approach to zero order (4). In addition, increasing the amount of this matrix component in coating layer or in core tablet could apparently prolong the drug release. The swellable barrier exhibited stronger modulation efficiency and was more suitable to modify the release of highly soluble drugs, whilst the erodible barriers exhibited a time-dependent coating effect that provided better control the release of sparingly soluble drugs (5).
Several variables, such as the presence of diluents and compression force could modify the drug release from the simple monolithic matrices (6,7). The varying loading dose of diltiazem HCl in three-layer tablets (prepared using polyethylene oxide combined with HPMC K4M) did not affect the drug release behavior (8). However, an increase in stirring rate during dissolution of those systems enhanced the drug release rate with the linearity of release profiles remained unaltered. Availability of a drug for gastrointestinal absorption from developed dosage forms is reflected by in vitro dissolution rate. Variables, such as agitation intensity, hydrodynamic force and hydrodynamic condition influence the drug dissolution rate. As the agitation intensity of the paddle or the basket increased, the percent drug dissolved from tablets and mass transfer coefficient were increased, but the film thickness was decreased (9). Emphasis has been on the type of polymer and diluent used, with scant attention given to study the effect of amount of coating barrier, compression force and pH of dissolution media on release characteristic of three-layer tablets.
The current investigation dealt with evaluating the effect of the coating barrier amount, the concentration of polymer, the compression force and the pH of dissolution media on physical properties and release of propranolol HCl from three-layer tablet comprising HPMC K15M and phytowax.
MATERIALS AND METHODS
Materials
Hydroxypropyl methylcellulose (HPMC) type K 15 M, lot no. PH26012N31, (Dow Chemical, USA) was used as received. Propranolol hydrochloride (batch no. 941002) was purchased from China National Chemical Imp. & Exp., Shanghai, China. Lactose was purchased from Wyndale, Hawera, New Zealand. Magnesium stearate (lot no. MAF 07, P. C. Drug Center Co.) was passed through sieve no. 80 mesh before used. Phytowax (Phytowax Olive 14L 48, hydrogenated myristyl esters of olive oil), purchased from Sophim, Parc de la Cassine, France, was passed through sieve No. 60 before use. All other reagents were of analytical grade.
Preparation of Matrix Tablets
The single layer 200-mg tablets containing propranolol HCl were prepared by direct compression. The concentration of drug was kept constantly at 80-mg/tablet. To make the powder mixture, the 40%w/w drug, 23%w/w phytowax and 35%w/w HPMC were mixed with 2%w/w magnesium stearate for 10 min using mortar and pestle. Then the blended powder of 200-mg was compressed into a tablet at a compression force of 1.5 tons using 12 mm round, flat and plain punches using a hydraulic press (Carver Press, WI, USA). For layered tablets, there was a barrier layer on upper or lower part of core tablet. Layered matrix tablets were prepared by adding the preweighed amount of the powder mixture without drug (containing 2% magnesium stearate) in the die cavity and the preweighed amount of the powder mixture with drug was placed over the first layer compressed with a 1.5-tons force using a hydraulic press to obtain the two-layer tablet. The dwell time after target pressure achieved was 10 s. To prepare the three-layer tablet, the preweighed amount of the powder mixture without drug was placed over the well spread second layer and was compressed with a 1.5-tons force. The three-layer tablets containing phytowax with various amount of HPMC were prepared. The amount of HPMC was also varied from 0 to 35% of the matrix component in three-layer tablet preparation. The three-layer tablets with different amount of barrier (100, 150 and 200-mg for each side of barrier) were prepared using the formulation containing HPMC 35%. The compression force was also varied with 1, 1.5, 1.75 and 2 tons in this study. For the formulations, the number after H (HPMC) of the first term of formula represents the percentage of HPMC, the number of the second term of formula represents the number of layer (L) of tablet; and the first and third number in the third term multiply with 100 represents the amount (mg) of each barrier layer and the second number in the third term multiply with 100 represents the amount (mg) of core tablet (Table I).
Table I.
Physical Properties of the Prepared Tablets
| Tablet | Weight ± SD (mg) | Thickness ± SD (mm) | Friability (%) | Hardness ± SD (Kp) |
|---|---|---|---|---|
| H35% 1L | 200.6 ± 6.2 | 1.45 ± 0.02 | 1.91 | 2.54 ± 0.70 |
| H35% 2L | 401.0 ± 6.5 | 3.19 ± 0.04 | 0.74 | 3.70 ± 0.21 |
| H35% 3L 1/2/1 | 403.3 ± 8.5 | 3.18 ± 0.20 | 0.62 | 3.21 ± 0.31 |
| H35% 3L 1.5/2/1.5 | 495.1 ± 5.9 | 3.60 ± 0.23 | 1.01 | 4.19 ± 0.27 |
| H35% 3L 2/2/2 | 602.7 ± 10.1 | 4.38 ± 0.09 | 0.58 | 4.43 ± 0.19 |
| H0% 3L 1/2/1 | 402.3 ± 6.0 | 3.44 ± 0.27 | 0.22 | 4.11 ± 0.23 |
| H15% 3L 1/2/1 | 396.9 ± 6.0 | 3.12 ± 0.11 | 0.71 | 4.55 ± 0.45 |
| H25% 3L 1/2/1 | 404.6 ± 6.1 | 3.23 ± 0.09 | 1.61 | 3.81 ± 0.38 |
| H35% 3L 2/2/2 1 ton | 601.8 ± 4.6 | 4.49 ± 0.05 | 1.32 | 3.85 ± 0.11 |
| H35% 3L 2/2/2 1.5 tons | 602.7 ± 10.1 | 4.38 ± 0.09 | 0.58 | 4.43 ± 0.19 |
| H35% 3L 2/2/2 1.75 tons | 608.6 ± 6.3 | 4.26 ± 0.11 | 0.49 | 4.87 ± 0.10 |
| H35% 3L 2/2/2 2 tons | 603.6 ± 5.3 | 4.02 ± 0.06 | 0.33 | 5.01 ± 0.12 |
Evaluation of Matrix Tablets
The hardness of tablets was determined using a hardness tester (Pharmatest, USA). The tablet thickness was measured using a thickness tester (Teclock, Japan). The friability was determined as the percent weight loss from 20 tablets. Twenty tablets were weighed and rotated for 100 revolutions in 4 min in a friabilitor (Yieheng Engineering, Bangkok, Thailand). A test of drug release was undertaken using a dissolution apparatus (type 1; Prolabo, France) with the basket method at 100 rpm. A 900-ml distilled water equilibrated at 37°C was used as dissolution fluid. Samples were collected at specific time intervals and assayed by a UV-Vis spectrophotometer (Perkin-Elmer, Germany) at a wavelength of 320 nm. To study the effect of the dissolution fluid on release behavior, the drug release tests in both distilled water and phosphate buffer pH 6.8 were also undertaken. For the dissolution test with pH change, the drug released in HCl buffer pH 1.2 was conducted for 1 1/2 h. Then the pH was increased to 6.8 by adding 4.6 g sodium hydroxide, 3.06 g monobasic potassium phosphate and 4.005 g dibasic sodium phosphate. The operation was continued until completing 26 h. During the drug release studies, the tablets were observed for physical integrity. To study the effect of hydrodynamic force on drug release, the tablets were tested for dissolution at different basket rotational speed of 50, 100 and 150 rpm. To study the drug release from one planar surface of tablets with different amount of barrier (100, 150 and 200-mg), these tablets except only upper flat planar of tablet were imbedded into melt hard paraffin which was placed in plastic cylinder. After solidification of hard paraffin, the prepared devices containing single planar area of barrier layer were introduced into the basket of the dissolution apparatus for exposure to the distilled water and tested for drug dissolution.
Dissolution Profile Fitting
Least square fitting the experimental dissolution data (cumulative drug release >10% and up to 80%) to the mathematical equations (power law and zero order) was carried out using a nonlinear computer programme, Scientist for Windows, version 2.1 (MicroMath Scientific Software, Salt Lake City, UT, USA). The coefficient of determination (r2) was used to indicate the degree of curve fitting. Goodness-of-fit was also evaluated using the Model Selection Criterion (MSC) (10), given below.
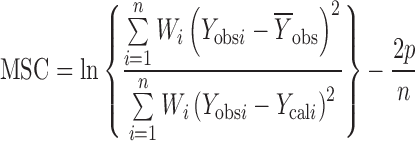 |
Where Yobsi and Ycali are observed and calculated values of the i-th point, respectively, and Wi is the weight that applies to the i-th point, n is number of points and p is number of parameters.
Photoimages of Layered Matrix Tablets during Release Test
Photoimages of layered matrix tablets before and after dissolution were taken at different times using Sony digital camera (Cyber-shot DSC-W50, Bangkok, Thailand) to compare their characteristics.
Scanning Electron Microscopy (SEM)
At that specified time intervals (1, 6, 12 and 26 h after dissolution testing), the tablets were removed from dissolution fluid and then were freeze-dried using a freeze dryer (type 77560-01, Labconco, Missouri, USA). The surface and cross sectional topography of the tablets was characterized under a scanning electron microscope (Maxim2005, Cam scan, UK).
RESULTS AND DISCUSSION
The Physical Properties of Matrix Tablets
The data on weight, thickness, friability and hardness of the core and the layered tablets are presented in Table I. The hardness of tablet was increased as the amount of coating layer was increased. The hardness of three-layer tablets tended to increase and the friability decreased as the compression force was increased. The lowest % friability was found in case of three-layer tablet containing phytowax and drug without HPMC (H0% 1/2/1). Under compression force, the powder could be embedded into this wax matrix and therefore the friability was rather low. Since this production was one step manufacturing process, the commercial products can be scaled up by the high speed three-layer tableting machines.
In Vitro Drug Release from The Prepared Tablets
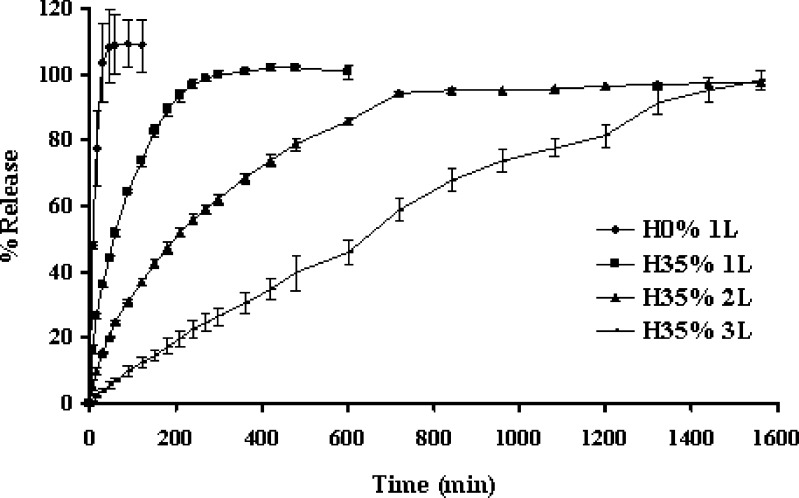
The immediate release of propranolol HCl was attained from a single layer tablet without HPMC as shown in Fig. 1. This indicated that the high water soluble drug could rapidly diffuse from this compressed matrix. An addition of 35% HPMC resulted in slower rate and extended release of the drug but there was the drug release of 100% over 4 h. This slow release was due to the formation of a thick gel structure that delayed drug release from the tablet matrix, where hydration of the individual HPMC particles resulted in extensive swelling. Greater retardation of drug release was observed in two-layer and three-layer tablets. The drug release rate from three-layer tablet was obviously lower than that of two-layer tablet and single layer tablet, respectively. High gelling formation with the sticky property of HPMC promoted the good adhesion between layers during immersion of the layer tablets in a dissolution medium. Coating layer played importance role on retardation of drug release, since this layer diminished the contact area of core matrix to the dissolution medium. Therefore one of the benefits of using the three-layer tablet was to reduce dose dumping for high solubility drug substances. The compression with polymeric layers on both sides of tablet could prolong drug release and modify the drug release to achieve constant release rate. Zero-order release could be qualitatively explained by assuming that the decreasing release rate at late state from the lateral surface of the middle layer was balanced by the delayed diffusion through the two laminated faces as a result of increasing polymer hydration/dissolution over time. The initial drug release was the diffusion of dissolved drug molecules through cylindrical side surface of the tablet. The coated layers were designed to initially delay the hydration rate of the middle layer. The external layers would disappear gradually at disproportionate rates, creating more surface area for drug diffusion, thus there was the counterbalancing of the reduction of diffusing surface area due to the erosion as well as the increase in diffusional path-length due to continuous system swelling (8).
Fig. 1.
Dissolution profiles of propranolol HCl from single layer tablets without HPMC (H0% 1L); or containing 35% HPMC as single (H35% 1L); two (H35% 2L) and three (H35% 3L) layer tablets in distilled water at rotational speed of 100 rpm. Each point represents the mean ± SD, n = 3
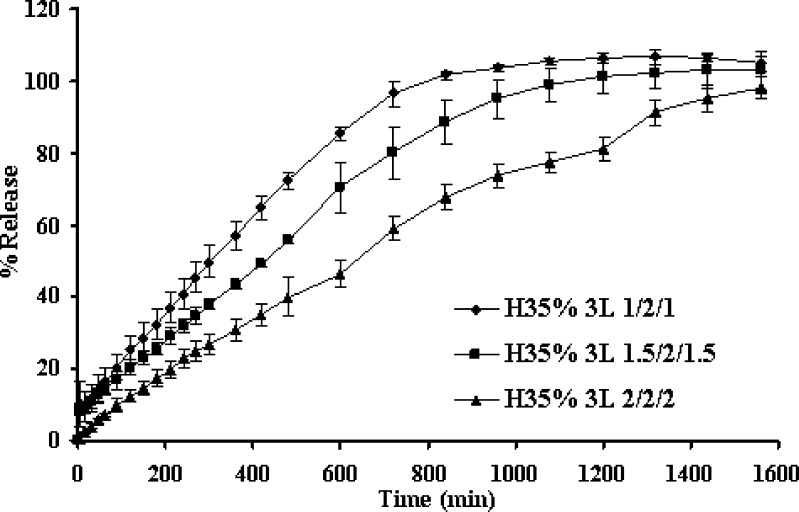
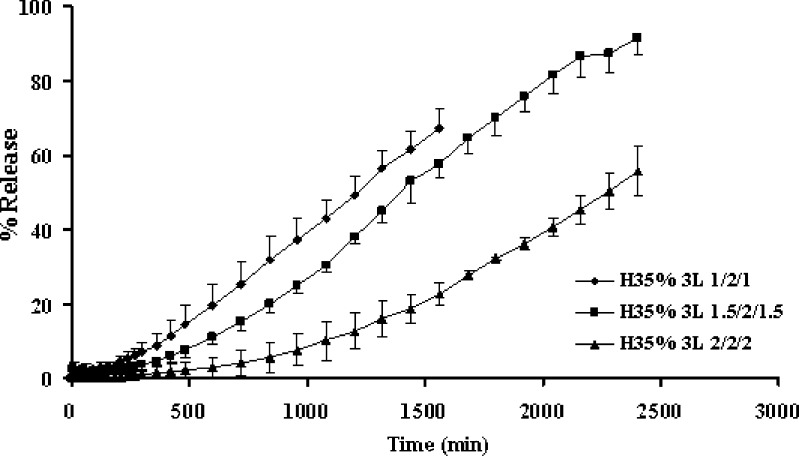
Increasing amount of barrier apparently retarded the drug release as shown in Fig. 2. Drug release from the middle layer could be modified by the delayed diffusion from the two coated surfaces as a result of simple diffusion. From one planar drug release study, apparent lag time was observed for drug release of three-layer tablets. The lag time of drug release tended to prolong as the amount of barrier was increased as shown in Fig. 3. Similar result was reported on the release of this drug from layered matrix comprising chitosan and xanthan gum (4). The result suggested that the barrier layer plays a significant role in modifying the lag time and the drug release. The increase in matrix quantity of barrier resulted to a substantial increase of the lag time. Therefore, the apparent release rate was determined by the summation of release rates from the lateral surface and from two coated surfaces, which would vary, depending on the formulation variables of the barrier layer including thickness.
Fig. 2.
Effect of coating layer amount on dissolution profiles of propranolol HCl from three-layer tablets in distilled water at rotational speed of 100 rpm. Each point represents the mean ± SD, n = 3
Fig. 3.
Dissolution profiles of propranolol HCl from one planar of the three-layer tablets containing different amount of upper and lower barriers in distilled water at rotational speed of 100 rpm. Each point represents the mean ± SD, n = 3
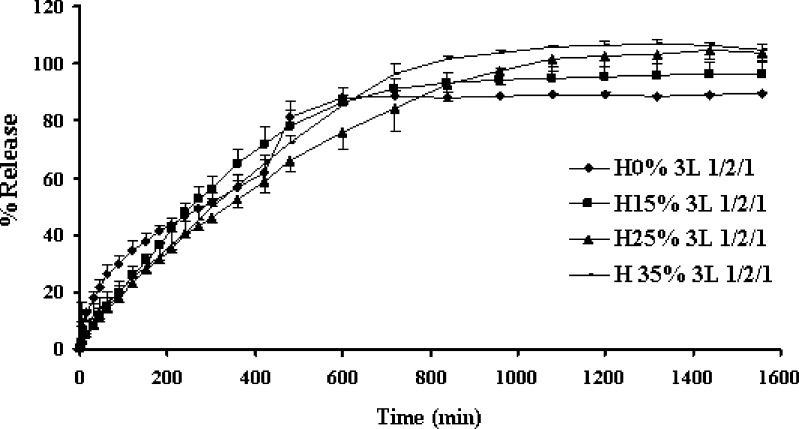
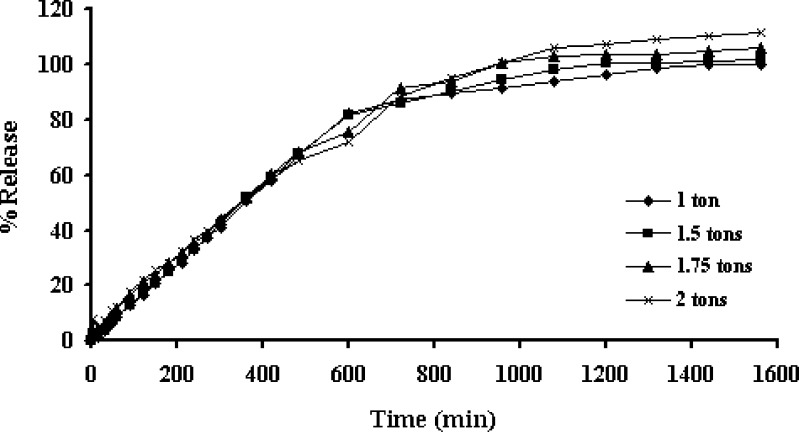
There was no significant difference of drug release from tablets containing different amount of HPMC and compression force (Figs. 4 and 5, respectively). The alteration of compression force did not affect the drug release from three-layer tablet, since the polymeric matrix could hydrate and form gel rapidly to modulate the drug release, the difference of distance between particles of polymers due to compression could not significantly affect the drug diffusion. Porosity of the hydrated matrix did not depend on the initial porosity, the compression force posed to have little influence on drug release (11). Some researchers also previously mentioned that compression force did not influence the system release performances even at very low force levels (12).
Fig. 4.
Effect of the amount of HPMC on the dissolution profiles of propranolol HCl from the three-layer tablets containing the 100-mg upper and lower barriers in distilled water at rotational speed of 100 rpm. Each point represents the mean ± SD, n = 3
Fig. 5.
Effect of compression force on the dissolution profiles of propranolol HCl from the three-layer tablets containing the 200-mg upper and lower barriers in distilled water at rotational speed of 100 rpm. Each point represents the mean ± SD, n = 3
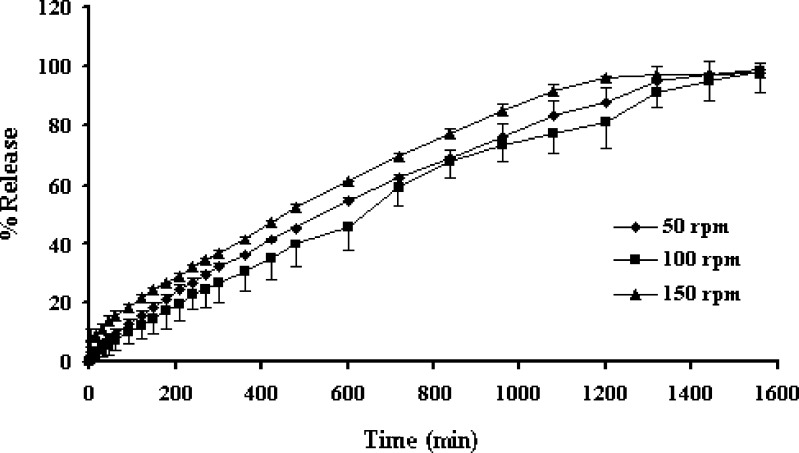
Availability of a drug for gastrointestinal absorption from developed dosage forms is normally reflected by in vitro dissolution rate. Variables, such as agitation intensity, hydrodynamic force and hydrodynamic condition influence the drug dissolution rate (9). Increasing the basket rotational speed increased the drug dissolution from three-layer tablets as presented in Fig. 6. This study demonstrated that hydrodynamic stress had an effect on dissolution rate, mass transfer rate, and film thickness underlying the dissolution process. As the stirring speed was excessive, the stagnant boundary layer effect was no longer the rate-limiting step in drug transport (8,13). In addition, an increase of mass transfer rate and a decrease of film thickness as the increment of basket rotation speed has been reported (9). Although the erosion based on the low viscosity grade of the material was strongly influenced by agitation, the polymer particle erosion was only moderately affected by the hydrodynamic stress (14). Owing to the coating by layering both sides with strong gelling layer containing HPMC, the influence of hydrodynamic force on drug dissolution from the three-layer tablet was minimized. The decrease of influence of hydrodynamic force on drug dissolution of tablet by using a high gel forming polymer as matrix component has been reported (15).
Fig. 6.
Effect of rotational speed on the dissolution profiles of propranolol HCl from the three-layer tablets containing the 200-mg upper and lower barriers in distilled water. Each point represents the mean ± SD, n = 3
By comparison, the drug release from the three-layer tablets in distilled water was faster than that in HCl buffer pH 1.2, pH change and phosphate buffer pH 6.8, respectively, (data not shown). This indicated that this drug release was pH dependent. Propranolol HCl, an anionic basic drug with a pKa of 9.45, should be more soluble in acidic environment than in neutral and alkaline environment, respectively. However, this anticipation was not found, the drug solubility in deionized water was greater than that in acid and basic dissolution fluids respectively. The decline of solubility in HCl buffer pH 1.2 was attributed to the common-ion (chloride) effect, which provided an unexpected trend in solubility of this medicament in the presence of chloride ion in this acidic medium. Therefore, the release of propranolol in HCl buffer pH 1.2 was slower than that in distilled water. This evidence was also noted by Rekhi et al. (16) and Takka et al. (17). In addition, the pH dependence on dissolution of this drug from matrices containing HPMC and carbopol was reported (18).
Tablet Morphology
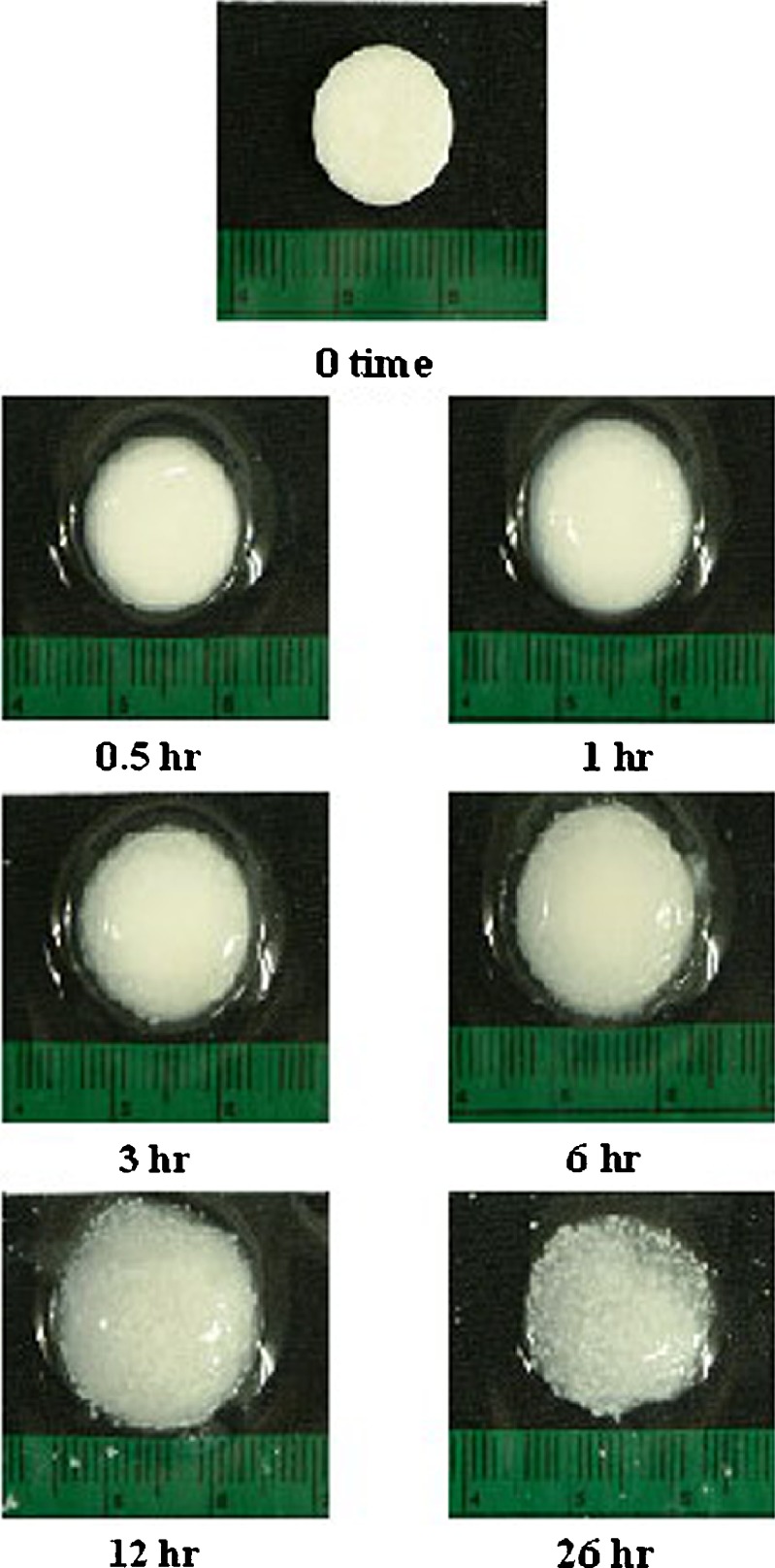
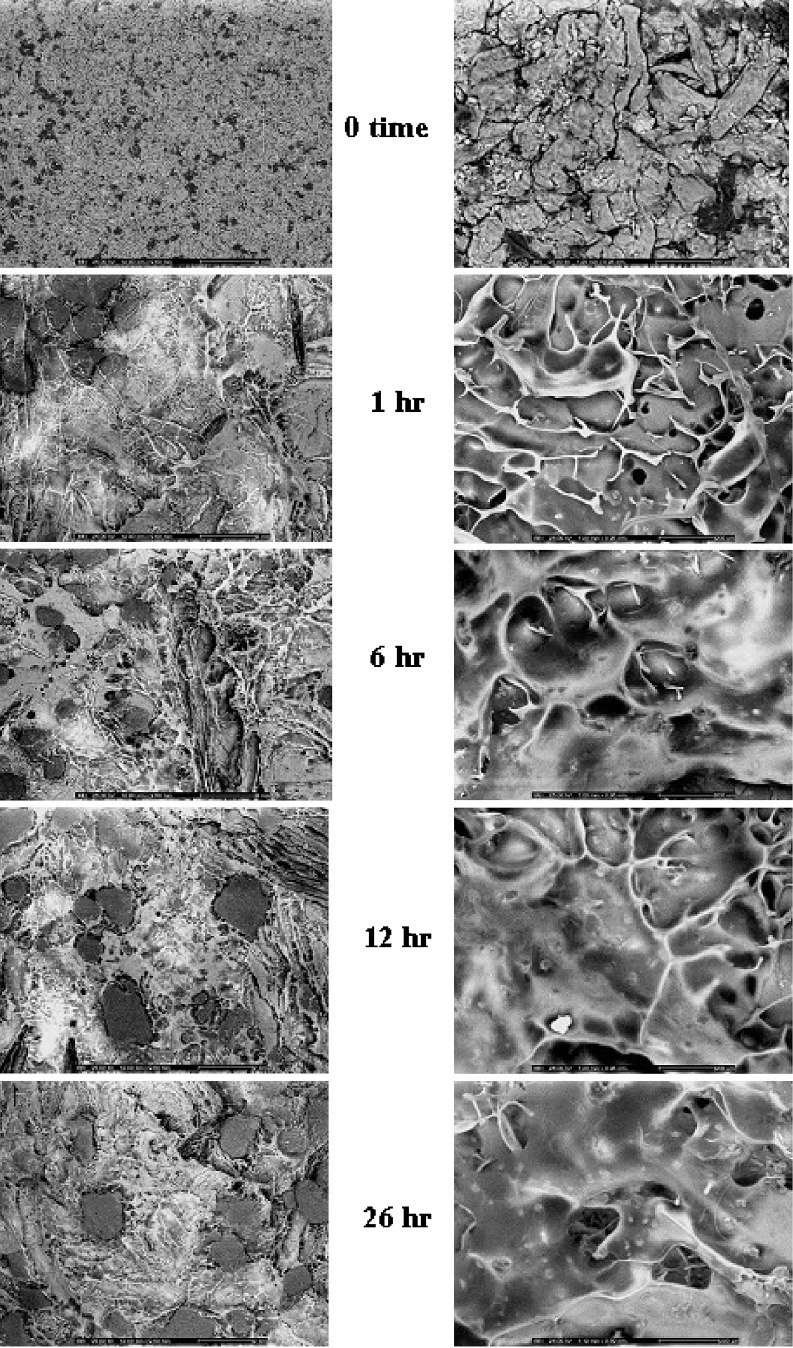
Figure 7 shows photoimages of the three-layer tablet containing 100-mg upper and lower barriers prepared with compression force of 1.5 tons before and after dissolution at different time intervals. The gel was formed around the tablet after dissolution and the dimension of tablet was increased with time. However, the tablet dimension was decreased after dissolution for 26 h and the wax particles found within the gel after dissolution for 12 and 26 h. This evidence indicated the erosion of a polymeric gel with time and the dispersed insoluble wax particles were observed in the remained gel structure. The SEM images of the dried tablet surfaces show a degree of mechanical interlocking of the polymers and drug imbedded in wax matrix as shown in Fig. 8a, b. The hydrated tablets after freeze drying exhibited apparently swollen surface as seen from high magnification of observation. Gel layer is formed by the polymer relaxation upon absorption of water. Owing to the partial hydration of polymer, the characteristic of gel layer on the surface of the tablet after dissolution for 1 hr was different from that of the tablet after that time. This indicated that the gel mass was increased with time during dissolution test and gradually covered the core surface. The dissolved drug molecules were allowed to diffuse out from the core through the polymer network of gel layer containing the porous structure. The insoluble wax particles appeared after covered polymeric gel was eroded and the gel structure was thinner with time as shown in Fig. 8. This evidence also indicated the erosion of HPMC gel mass from coating layer with time.
Fig. 7.
Photoimages of the three-layer tablet containing 100-mg upper and lower barriers after dissolution at different time intervals
Fig. 8.
Surface topography of the three-layer tablets containing 100-mg upper and lower barriers after dissolution at different time intervals at magnification of 10 (left) and 100 (right)
Dissolution Profile Fitting
The large value of the coefficient of determination (r2) or model selection criteria (MSC) indicated a superiority of the dissolution profile fitting to mathematical equations. The estimate parameters from curve fitting to power law and zero order equations are shown in Table II. Fitting experimental drug dissolution profiles to the power law equation provided high r2 (0.9944 to 0.9997) and MSC (4.75 to 7.85) indicating a superiority of this model. The n values from the fitting of almost drug dissolution profiles were close to 1.00, indicating the nearly zero order release kinetic. The curve fitting to zero order kinetic gave the large r2 and MSC values (Table II, Zero order equation). These results suggested that the developed three-layer tablets showed zero-order or Case II release. Lower n values of drug release from the single and double layer tablets signified that the mechanism of drug released from these tablets was different from that of the three-layer tablets. The values of the kinetic constant (k) were in accordance with the values of n, the diffusional exponent, with k having lower values when the transport mechanism was Case II and higher values for formulations that released the drug by Fickian diffusion. The alterations of these estimated parameters as mentioned above were previously reported (19,20). The increased amount of coating layer or compression force resulted in the trend of decreasing rate of drug release as presented in Table II (Zero order equation). The rate of drug release into distilled water was higher than that into HCl buffer pH 1.2, pH change and phosphate buffer pH 6.8, respectively (data not shown). Owing to pH dependent solubility of this drug as described above, the drug release from this three-layer tablet in phosphate buffer pH 6.8 and pH change system was rather far from zero order kinetic release.
Table II.
Estimate Parameters from Curve Fitting of Drug Dissolution in Distilled Water to Power Law Equation and Zero Order Equation
| Tablet | r 2 | MSC | k ± SD *10−3 | n ± SD |
|---|---|---|---|---|
| Power law equation | ||||
| H35% 1L 100 rpm | 0.9995 | 6.65 | 55.43 ± 6.14 | 0.5377 ± 0.0212 |
| H35% 2L 100 rpm | 0.9990 | 6.55 | 30.93 ± 1.75 | 0.5275 ± 0.0096 |
| H35% 3L 50 rpm | 0.9997 | 7.82 | 4.65 ± 0.37 | 0.7447 ± 0.0114 |
| H35% 3L 100 rpm | 0.9955 | 5.00 | 3.84 ± 0.42 | 0.7818 ± 0.0154 |
| H35% 3L 150 rpm | 0.9994 | 7.04 | 2.10 ± 0.95 | 0.8520 ± 0.0625 |
| H35% 3L 1/2/1 | 0.9997 | 7.85 | 2.16 ± 0.22 | 0.9293 ± 0.0156 |
| H35% 3L 1.5/2/1.5 | 0.9989 | 6.46 | 0.66 ± 0.18 | 1.0621 ± 0.0382 |
| H35% 3L 2/2/2 | 0.9955 | 5.00 | 3.84 ± 0.42 | 0.7818 ± 0.0154 |
| H0% 3L 1/2/1 | 0.9983 | 5.96 | 38.97 ± 3.02 | 0.4545 ± 0.0133 |
| H15% 3L 1/2/1 | 0.9982 | 5.84 | 8.59 ± 1.49 | 0.7378 ± 0.0279 |
| H25% 3L 1/2/1 | 0.9992 | 6.73 | 7.47 ± 0.81 | 0.7249 ± 0.0169 |
| H35% 3L 1/2/1 | 0.9997 | 7.85 | 2.16 ± 0.22 | 0.9293 ± 0.0156 |
| H35% 3L 2/2/2 1 ton | 0.9994 | 6.84 | 1.47 ± 0.32 | 0.9924 ± 0.0364 |
| H35% 3L 2/2/2 1.5 tons | 0.9955 | 5.00 | 3.84 ± 0.42 | 0.7818 ± 0.0154 |
| H35% 3L 2/2/2 1.75 tons | 0.9944 | 4.75 | 3.52 ± 1.30 | 0.8465 ± 0.0109 |
| H35% 3L 2/2/2 2 tons | 0.9991 | 6.53 | 1.93 ± 0.48 | 0.9358 ± 0.0346 |
| Zero order equation | ||||
| H35% 1L 100 rpm | 0.9698 | 3.00 | 44.13 ± 0.32 | |
| H35% 2l 100 rpm | 0.9990 | 6.55 | 30.93 ± 1.75 | |
| H35% 3L 50 rpm | 0.9940 | 4.86 | 0.74 ± 0.02 | |
| H35% 3L 100 rpm | 0.9939 | 4.83 | 0.80 ± 0.01 | |
| H35% 3L 150 rpm | 0.9946 | 5.00 | 0.71 ± 0.02 | |
| H35% 3L 1/2/1 | 0.9994 | 7.09 | 1.36 ± 0.10 | |
| H35% 3L 1.5/2/1.5 | 0.9987 | 6.38 | 1.01 ± 0.09 | |
| H35% 3L 2/2/2 | 0.9939 | 4.83 | 0.80 ± 0.01 | |
| H0% 3L 1/2/1 | 0.9620 | 3.00 | 1.22 ± 0.07 | |
| H15% 3L 1/2/1 | 0.9895 | 4.24 | 1.58 ± 0.04 | |
| H25% 3L 1/2/1 | 0.9901 | 4.33 | 1.18 ± 0.03 | |
| H35% 3L 1/2/1 | 0.9994 | 7.09 | 1.36 ± 0.10 | |
| H35% 3L 2/2/2 1 tons | 0.9993 | 7.00 | 1.39 ± 0.11 | |
| H35% 3L 2/2/2 1.5 tons | 0.9939 | 4.83 | 0.80 ± 0.01 | |
| H35% 3L 2/2/2 1.75 tons | 0.9915 | 4.49 | 1.27 ± 0.03 | |
| H35% 3L 2/2/2 2 tons | 0.9883 | 4.16 | 1.17 ± 0.04 | |
CONCLUSIONS
Layering with polymeric matrix comprising HPMC and phytowax could prolong the release of propranolol HCl and shift the release pattern approach to zero order. Increasing the amount of matrix component in coating layer could apparently prolong the drug release. The drug release from this three-layer tablet was influenced by hydrodynamic force and pH of dissolution medium. The alteration of concentration of HPMC and compression force did not affect the drug release from this three-layer tablet.
Acknowledgements
This research work was kindly supported by Silpakorn University Research and Development Institute and Faculty of Pharmacy, Silpakorn University. The author would like to thank Prof. Dr. Garnpimol C. Ritthidej and Dr. Wisit Tangkeangsirisin for their help and expertise.
References
- 1.Chidambaram N., Porter W., Flood K., Qiu Y. Formulation and characterization of new layered diffusional matrices for zero-order sustained release. J. Control Rel. 1998;52:149–158. doi: 10.1016/S0168-3659(97)00207-1. [DOI] [PubMed] [Google Scholar]
- 2.Siahi M. R., Barzegar-Jalali M., Monajjemzadeh F., Ghaffari F., Azarmi S. Design and evaluation of 1- and 3-layer matrices of verapamil hydrochloride for sustaining its release. AAPS PharmSciTech. 2005;6(4):E626–632. doi: 10.1208/pt060477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maggi L., Bruni R., Conte U. High molecular weight polyethylene oxides (PEOs) as an alternative to HPMC in controlled release dosage forms. Int. J. Pharm. 2000;195:229–238. doi: 10.1016/S0378-5173(99)00402-0. [DOI] [PubMed] [Google Scholar]
- 4.Phaechamud T., Ritthidej G. C. Sustained-release from layered matrix system comprising chitosan and xanthan gum. Drug Dev. Ind. Pharm. 2007;33:595–605. doi: 10.1080/03639040601015521. [DOI] [PubMed] [Google Scholar]
- 5.Conte U., Maggi L. Modulation of the dissolution profiles from Geomatrix multi-layer matrix tablets containing drugs of different solubility. Biomaterials. 1996;17:889–896. doi: 10.1016/0142-9612(96)83284-4. [DOI] [PubMed] [Google Scholar]
- 6.Ford J. L., Rubinstein H. M., McCaul F., Hogan J. E. Importance of drug type, tablet shape and added diluents on drug release kinetics from hydroxypropylmethylcellulose matrix tablets. IL Farmaco. 1987;40:223–234. [Google Scholar]
- 7.Lotfipour F., Nokhodchi A., Saeedi M., Norouzi M., et al. The effect of hydrophilic and lipophilic polymers and fillers on the release rate of atenolol from HPMC matrices. IL Farmaco. 2004;59:819–825. doi: 10.1016/j.farmac.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 8.Yang L., Fassihi R. Examination of drug solubility, polymer types, hydrodynamics and loading dose on drug release behavior from a triple-layer asymmetric configuration delivery system. Int. J. Pharm. 1997;155:219–229. doi: 10.1016/S0378-5173(97)00164-6. [DOI] [Google Scholar]
- 9.Wu Y., Kildsig D. O., Ghaly E. S. Effect of hydrodynamic environment on tablets dissolution rate. Pharm. Dev. Tech. 2004;9(1):25–37. doi: 10.1081/PDT-120027415. [DOI] [PubMed] [Google Scholar]
- 10.MicroMath Scientist Handbook Rev. 7EEF. MicroMath, Salt Lake City, 1995, p. 467.
- 11.Velasco M. V., Ford J. L., Rowe P., Rajabi-Siahboomi A. R. Influence of drug:hydroxypropylmethylcellulose ratio, drug and polymer particle size and compression force on the release of diclofenac sodium from HPMC tablets. J. Control Rel. 1999;57:75–85. doi: 10.1016/S0168-3659(98)00110-2. [DOI] [PubMed] [Google Scholar]
- 12.Conte U., Maggi L., Colomba P., La Manna A. Multi-layered hydrophilic matrices as constant release devices (GeomatrixTM Systems) J. Control Rel. 1993;26:39–47. doi: 10.1016/0168-3659(93)90207-L. [DOI] [Google Scholar]
- 13.El-Gazayerly O. N. Release of pentoxifylline from xanthan matrix. Drug Dev. Ind. Pharm. 2003;29(2):241–246. doi: 10.1081/DDC-120016732. [DOI] [PubMed] [Google Scholar]
- 14.Zuleger S., Fassihi R., Lippold B. C. Polymer particles erosion controlling drug release. II. Swelling investigations to clarify the release mechanism. Int. J. Pharm. 2002;247:23–37. doi: 10.1016/S0378-5173(02)00362-9. [DOI] [PubMed] [Google Scholar]
- 15.Susanne Z., Fassihi R., Lippold B. C. Polymer particle erosion controlling drug release. II. Swelling investigations to clarify the release mechanism. Int. J. Pharm. 2002;247:23–37. doi: 10.1016/S0378-5173(02)00362-9. [DOI] [PubMed] [Google Scholar]
- 16.Rekhi G. S., Mendes R. W., Porter S. C., Jambhekar S. S. Aqueous polymeric dispersion for controlled drug delivery Wuster process. Pharm. Tech. 1989;13:112–126. [Google Scholar]
- 17.Takka S., Rajbhandari S., Sakr K. Effect of anionic polymers on the release of propranolol hydrochloride from matrix tablets. Eur. J. Pharm. Biopharm. 2001;52:75–82. doi: 10.1016/S0939-6411(01)00147-3. [DOI] [PubMed] [Google Scholar]
- 18.Perez-Marcos B., Ford J. L., Armstrong D. J., Elliott P. N., Rostron C., Hogan J. E. Influence of pH on the release of propranolol hydrochloride from matrices containing hydroxypropylmethylcellulose K4M and carbopol 974. J. Pharm. Sci. 1996;85:330–334. doi: 10.1021/js950359z. [DOI] [PubMed] [Google Scholar]
- 19.Ritger P. L., Peppas N. A. A simple equation for description of solute release II. Fickian and anomalous release from swellable devices. J. Control. Release. 1987;5:37–42. doi: 10.1016/0168-3659(87)90035-6. [DOI] [PubMed] [Google Scholar]
- 20.Nerurkar J., Jun H. W., Price J. C., Park M. O. Controlled-release matrix tablets of ibuprofen using cellulose ethers and carrageenans: effect of formulation factors on dissolution rates. Eur. J. Pharm. Biopharm. 2005;61:56–68. doi: 10.1016/j.ejpb.2005.03.003. [DOI] [PubMed] [Google Scholar]