Abstract
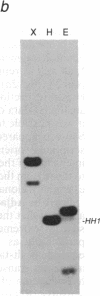
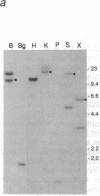
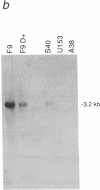
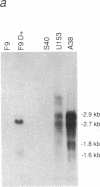
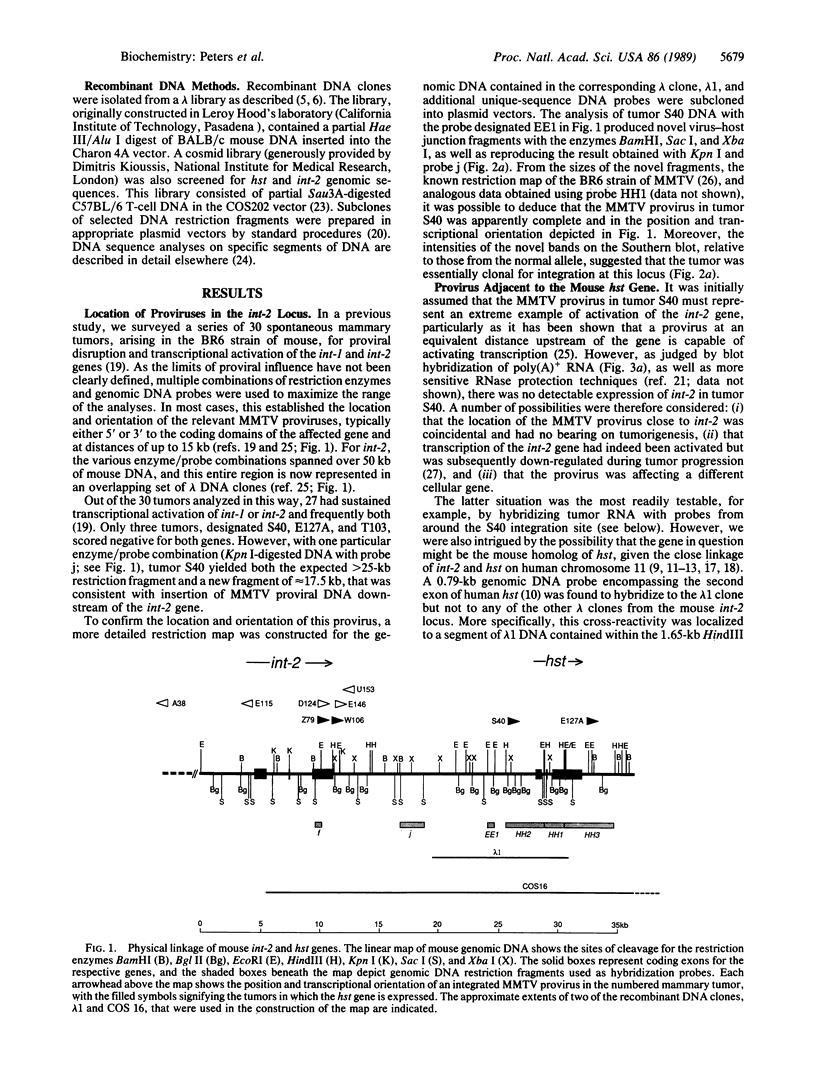
The fibroblast growth factor-related protooncogenes, int-2 and hst/k-FGF, are within 17 kilobase pairs of one another on mouse chromosome 7 and are in the same transcriptional orientation. Approximately 70% of tumors induced in BR6 mice by mouse mammary tumor virus have proviral insertions adjacent to the int-2 gene. We find that the murine homolog of the hst/k-FGF gene can also be transcriptionally activated by the insertion of mouse mammary tumor virus DNA either upstream or downstream of the gene. In most tumors, only one of these adjacent genes is activated, but in some cases both genes are expressed. One of the hst-expressing tumors also has a virally activated int-3 gene. At least five distinct cellular genes (int-1, -2, -3, -4, and hst/k-FGF) can therefore contribute, either singly or in concert, to the development of histologically indistinguishable mammary tumors in mice infected by mouse mammary tumor virus.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham J. A., Whang J. L., Tumolo A., Mergia A., Friedman J., Gospodarowicz D., Fiddes J. C. Human basic fibroblast growth factor: nucleotide sequence and genomic organization. EMBO J. 1986 Oct;5(10):2523–2528. doi: 10.1002/j.1460-2075.1986.tb04530.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adelaide J., Mattei M. G., Marics I., Raybaud F., Planche J., De Lapeyriere O., Birnbaum D. Chromosomal localization of the hst oncogene and its co-amplification with the int.2 oncogene in a human melanoma. Oncogene. 1988 Apr;2(4):413–416. [PubMed] [Google Scholar]
- Brookes S., Smith R., Thurlow J., Dickson C., Peters G. The mouse homologue of hst/k-FGF: sequence, genome organization and location relative to int-2. Nucleic Acids Res. 1989 Jun 12;17(11):4037–4045. doi: 10.1093/nar/17.11.4037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey G., Smith R., McGillivray D., Peters G., Dickson C. Characterization and chromosome assignment of the human homolog of int-2, a potential proto-oncogene. Mol Cell Biol. 1986 Feb;6(2):502–510. doi: 10.1128/mcb.6.2.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delli Bovi P., Basilico C. Isolation of a rearranged human transforming gene following transfection of Kaposi sarcoma DNA. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5660–5664. doi: 10.1073/pnas.84.16.5660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delli Bovi P., Curatola A. M., Kern F. G., Greco A., Ittmann M., Basilico C. An oncogene isolated by transfection of Kaposi's sarcoma DNA encodes a growth factor that is a member of the FGF family. Cell. 1987 Aug 28;50(5):729–737. doi: 10.1016/0092-8674(87)90331-x. [DOI] [PubMed] [Google Scholar]
- Dickson C., Peters G. Potential oncogene product related to growth factors. 1987 Apr 30-May 6Nature. 326(6116):833–833. doi: 10.1038/326833a0. [DOI] [PubMed] [Google Scholar]
- Dickson C., Smith R., Brookes S., Peters G. Tumorigenesis by mouse mammary tumor virus: proviral activation of a cellular gene in the common integration region int-2. Cell. 1984 Jun;37(2):529–536. doi: 10.1016/0092-8674(84)90383-0. [DOI] [PubMed] [Google Scholar]
- Gallahan D., Callahan R. Mammary tumorigenesis in feral mice: identification of a new int locus in mouse mammary tumor virus (Czech II)-induced mammary tumors. J Virol. 1987 Jan;61(1):66–74. doi: 10.1128/jvi.61.1.66-74.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallahan D., Kozak C., Callahan R. A new common integration region (int-3) for mouse mammary tumor virus on mouse chromosome 17. J Virol. 1987 Jan;61(1):218–220. doi: 10.1128/jvi.61.1.218-220.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huebner K., Ferrari A. C., Delli Bovi P., Croce C. M., Basilico C. The FGF-related oncogene, K-FGF, maps to human chromosome region 11q13, possibly near int-2. Oncogene Res. 1988;3(3):263–270. [PubMed] [Google Scholar]
- Kioussis D., Wilson F., Daniels C., Leveton C., Taverne J., Playfair J. H. Expression and rescuing of a cloned human tumour necrosis factor gene using an EBV-based shuttle cosmid vector. EMBO J. 1987 Feb;6(2):355–361. doi: 10.1002/j.1460-2075.1987.tb04762.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lidereau R., Callahan R., Dickson C., Peters G., Escot C., Ali I. U. Amplification of the int-2 gene in primary human breast tumors. Oncogene Res. 1988 Feb;2(3):285–291. [PubMed] [Google Scholar]
- Moore R., Casey G., Brookes S., Dixon M., Peters G., Dickson C. Sequence, topography and protein coding potential of mouse int-2: a putative oncogene activated by mouse mammary tumour virus. EMBO J. 1986 May;5(5):919–924. doi: 10.1002/j.1460-2075.1986.tb04304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore R., Dixon M., Smith R., Peters G., Dickson C. Complete nucleotide sequence of a milk-transmitted mouse mammary tumor virus: two frameshift suppression events are required for translation of gag and pol. J Virol. 1987 Feb;61(2):480–490. doi: 10.1128/jvi.61.2.480-490.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen C., Roux D., Mattei M. G., de Lapeyriere O., Goldfarb M., Birnbaum D., Jordan B. R. The FGF-related oncogenes hst and int.2, and the bcl.1 locus are contained within one megabase in band q13 of chromosome 11, while the fgf.5 oncogene maps to 4q21. Oncogene. 1988 Dec;3(6):703–708. [PubMed] [Google Scholar]
- Peters G., Brookes S., Placzek M., Schuermann M., Michalides R., Dickson C. A putative int domain for mouse mammary tumor virus on mouse chromosome 7 is a 5' extension of int-2. J Virol. 1989 Mar;63(3):1448–1450. doi: 10.1128/jvi.63.3.1448-1450.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters G., Brookes S., Smith R., Dickson C. Tumorigenesis by mouse mammary tumor virus: evidence for a common region for provirus integration in mammary tumors. Cell. 1983 Jun;33(2):369–377. doi: 10.1016/0092-8674(83)90418-x. [DOI] [PubMed] [Google Scholar]
- Peters G., Kozak C., Dickson C. Mouse mammary tumor virus integration regions int-1 and int-2 map on different mouse chromosomes. Mol Cell Biol. 1984 Feb;4(2):375–378. doi: 10.1128/mcb.4.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters G., Lee A. E., Dickson C. Concerted activation of two potential proto-oncogenes in carcinomas induced by mouse mammary tumour virus. Nature. 1986 Apr 17;320(6063):628–631. doi: 10.1038/320628a0. [DOI] [PubMed] [Google Scholar]
- Sakamoto H., Mori M., Taira M., Yoshida T., Matsukawa S., Shimizu K., Sekiguchi M., Terada M., Sugimura T. Transforming gene from human stomach cancers and a noncancerous portion of stomach mucosa. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3997–4001. doi: 10.1073/pnas.83.11.3997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R., Peters G., Dickson C. Multiple RNAs expressed from the int-2 gene in mouse embryonal carcinoma cell lines encode a protein with homology to fibroblast growth factors. EMBO J. 1988 Apr;7(4):1013–1022. doi: 10.1002/j.1460-2075.1988.tb02908.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenberg A., van Balen P., Hilgers J., Schuuring E., Nusse R. Oncogene expression during progression of mouse mammary tumor cells; activity of a proviral enhancer and the resulting expression of int-2 is influenced by the state of differentiation. EMBO J. 1987 Jan;6(1):121–125. doi: 10.1002/j.1460-2075.1987.tb04728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taira M., Yoshida T., Miyagawa K., Sakamoto H., Terada M., Sugimura T. cDNA sequence of human transforming gene hst and identification of the coding sequence required for transforming activity. Proc Natl Acad Sci U S A. 1987 May;84(9):2980–2984. doi: 10.1073/pnas.84.9.2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada A., Sakamoto H., Katoh O., Yoshida T., Yokota J., Little P. F., Sugimura T., Terada M. Two homologous oncogenes, HST1 and INT2, are closely located in human genome. Biochem Biophys Res Commun. 1988 Dec 15;157(2):828–835. doi: 10.1016/s0006-291x(88)80324-3. [DOI] [PubMed] [Google Scholar]
- Wilkinson D. G., Bhatt S., McMahon A. P. Expression pattern of the FGF-related proto-oncogene int-2 suggests multiple roles in fetal development. Development. 1989 Jan;105(1):131–136. doi: 10.1242/dev.105.1.131. [DOI] [PubMed] [Google Scholar]
- Wilkinson D. G., Peters G., Dickson C., McMahon A. P. Expression of the FGF-related proto-oncogene int-2 during gastrulation and neurulation in the mouse. EMBO J. 1988 Mar;7(3):691–695. doi: 10.1002/j.1460-2075.1988.tb02864.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M. C., Wada M., Satoh H., Yoshida T., Sakamoto H., Miyagawa K., Yokota J., Koda T., Kakinuma M., Sugimura T. Human HST1 (HSTF1) gene maps to chromosome band 11q13 and coamplifies with the INT2 gene in human cancer. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4861–4864. doi: 10.1073/pnas.85.13.4861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T., Miyagawa K., Odagiri H., Sakamoto H., Little P. F., Terada M., Sugimura T. Genomic sequence of hst, a transforming gene encoding a protein homologous to fibroblast growth factors and the int-2-encoded protein. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7305–7309. doi: 10.1073/pnas.84.20.7305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T., Muramatsu H., Muramatsu T., Sakamoto H., Katoh O., Sugimura T., Terada M. Differential expression of two homologous and clustered oncogenes, Hst1 and Int-2, during differentiation of F9 cells. Biochem Biophys Res Commun. 1988 Dec 15;157(2):618–625. doi: 10.1016/s0006-291x(88)80295-x. [DOI] [PubMed] [Google Scholar]
- Zhan X., Bates B., Hu X. G., Goldfarb M. The human FGF-5 oncogene encodes a novel protein related to fibroblast growth factors. Mol Cell Biol. 1988 Aug;8(8):3487–3495. doi: 10.1128/mcb.8.8.3487. [DOI] [PMC free article] [PubMed] [Google Scholar]