Abstract
Physical properties (roughness, gloss, mechanical, surface topography and adhesive) of a bioadhesive film for the transdermal delivery of drugs and its interactions with a skin model surface were studied. Roughness is a measurement of the small-scale variations in the height of a physical surface. No significant differences in Ra between the “x” and “y” dimensions for both the skin model and patch were detected, due to uniformity in their production. Scanning electron microscope pictures showed small particles projected from the film. Those particles resulted in increasing roughness and surface area. For the patch, gloss values measured at 20° were 6.0 ± 0.9 and at 60°, 32.2 ± 2.2 gloss units, respectively, indicating a semi-gloss material. Concerning the mechanical properties, the tensile strength of the film resulted four- to sevenfold greater than the peel force from the model skin used, indicating the suitability of the film for skin application. The adhesion to skin model depended on the amount of water used for film application and on the elapsed time between film application and removal. Finally, the model skin that was invented by Charkoudian can be used as an alternative to costly and highly variable human skin substrates since it possesses human topography.
Key words: adhesion, gloss, patch, peeling, roughness, transdermal
INTRODUCTION
Topical and transdermal drug delivery may exhibit some advantages over common drug delivery routes, such as oral or intravenous drug delivery. The main advantages of topical and transdermal drug delivery systems are: good compliance mainly because no invasive procedure is required, prevention of first pass effect by liver and gut enzymes, while usually production of such preparations is simple and cheap (1,2). There are different types of topical and transdermal preparations: emulsions, creams, gels and patches. Patches can be classified into two basic sub-types: reservoir systems—drug is dissolved or dispersed in a reservoir and the drug release rate is controlled by a membrane and matrix systems—drug is dissolved or dispersed in an adhesive layer which is in contact with the skin (3,4). Recently, a new and special single-layer matrix system has been developed (5,6). This system is a film, almost not adhesive in the dry state, which becomes adhesive when applied on wet skin. It is water vapor permeable so the risk of skin irritation is reduced. Successful delivery of different drugs was obtained while testing such patches in ex vivo and in vivo experiments (7–11). However, a comprehensive investigation of the physical and adhesive properties of such films has not yet performed.
Patches are designed to deliver a therapeutically effective amount of drug across the skin (12). In order to achieve a continuous and effective release of the drug to the skin, the patch must exhibit good adhesion properties. Patches can suffer from reduction in surface area of contact, falling off or lack of adhesion. By all means adhesion is a very important property for patches and the necessity for its assessment on a regular basis is increasing. Also, the patches should exhibit desirable mechanical properties so tearing or any other kind of damage will not occur during their utilization. In addition, physical properties are important for patch appearance, especially if patches are used for cosmetic purposes and topical skin treatment.
The development and evaluation of pressure-sensitive tapes for the medical industry involve complex issues of skin adhesion and related testing procedures. Procedures using human skin and animal models are highly variable, tedious, preparation-intensive, costly and difficult. Other test substances, such as steel and glass, offer ease and precision, but the results are not easily related to actual skin surfaces. Several studies have proposed the use of smooth polyamide surfaces of collagen and Nylon 66 (13); however, these materials do not include lipid components, nor do they account for the topological aspects of human skin. To avoid these difficulties, a model skin surface for testing adhesion to skin was developed by Charkoudian (14), who emphasized its potential as a substrate, although its further use was limited (15,16).
The aims of this study were to investigate different physical properties of the skin bioadhesive film including roughness, gloss, strength and fragility (brittleness). Adhesion properties of the patch were also evaluated; in particular the dependence of adhesion (peeling force) on water content and elapsed time from skin application until testing was performed. A model skin surface developed for testing adhesion of medical adhesives was adapted for the peel tests, because the film is not self-adhesive on standard surfaces such as stainless steel.
MATERIALS AND METHODS
Film Preparation
Films were prepared as previously described (6), except that no drug was included. Polyvinyl alcohol (PVA) of molecular weight of 83,400 (degree of hydrolysis 87%) was obtained from Nippon Gohsei (Osaka, J) while Eudragit E100 from Rofarma (Gaggiano, Milan, Italy). Plastoid-E35H was prepared according to the protocol of Rofarma: Eudragit E100 (15.9% w/w), lauric acid (9.2% w/w), and adipic acid (1.8% w/w) were added to hot water (72.1% w/w, temperature ∼80°C). The mixture of PVA and Plastoid was stirred, maintaining the temperature at 80°C, until a clear solution was formed. The solution was cooled to 60°C and sorbitol (4.0% w/w) was added. The mixture was then gradually cooled to room temperature while stirring. The composition of the resulting mixture (% w/w on wet basis) was: PVA 83,400—12.4%, Plastoid E35H—27%, Sorbitol—4.0% and Water—56.6%. The mixture was laminated on siliconized paper using a film casting knife (BYK Gardner, Silverspring, MD, USA; gap 200 lm) and oven-dried at 80°C for 30 min. After drying the final water content of the film was approx. 10% w/w (as determined by Karl Fisher titration). The films (10 × 20 cm) were covered with a second siliconized paper and individually sealed in aluminum pouches. The average thickness of the patch was ∼100 ± 5 μm.
Skin Model
The skin model, which mimics the physical and chemical properties of human skin, used in the peel tests, was prepared in accordance with American Patent Number 4,877,454 (17). Briefly, 10 g of granular porcine skin gelatin, 225 bloom, were dissolved in 83 g of water at 50°C while stirring. To prevent bacterial contamination, 0.05 g of propylparben (Sigma) was then dissolved in the resulting warm gelatin, followed by 4.5 ml sodium hydroxide and 3 g of 2-[(alkoyloxy)methyl]methyl-ethyl-7-(4-heptyl-5,6-dicarboxy-2-cycloheaxane-1-yl)-hepatanone (Sigma). The addition of the alkali and the triglyceride resulted in a stable, white emulsion. To the emulsion 3.95 g of formaldehyde were added before it was poured on a negative mold and allowed to set and dry under ambient conditions. After ∼24 h, it was carefully removed from the mold (17). The average thickness of the skin model was ∼200 ± 10 μm.
Roughness Measurement
In order to characterize the surface of the films and the skin model, roughness measurements were carried out using a portable surface roughness tester (18; Surftest-301, Mitutoyo Corp., Tokyo, Japan). For films and skin model, ten readings of Ra value (the arithmetical mean value of the amounts of the ordinate value within an individual measuring distance) and ten readings of Rz value (sum of the height of the highest profile peak and the depth of the deepest profile valley within an individual measuring distance) were taken in both “x” and “y” dimensions of the plane surface of the patch or skin model on a randomly selected part of each sample. Results are given as the arithmetic mean ± SD for an evaluation length of 12.5 mm at a speed of 0.5 mm/s (18).
Gloss Measurement
Films and skin model were positioned in the gloss meter (Triple Angle Novo-Gloss, Rhopoint Instrumentation Ltd., Germany) and illuminated by a light beam from a helium-neon laser at angles of 20° and 60° to a plane perpendicular to the surface. The results were obtained in gloss units. Five readings were taken at different randomly selected points on the film and skin model surfaces. Results are given as the arithmetic mean ± SD (19).
Scanning Electron Microscopy (SEM)
SEM micrographs of the film surface were taken using a JEOL JSM 35C SEM (Tokyo, Japan). Film slices (0.5 × 0.5 cm) were attached to metal stubs and gold-coated (150–200 Å) in a Polaron 5150 sputter coater (Polaron Equipment Ltd., Holywall Industrial Estate Watford, Hertfordshire, England).
Tensile Test
After their production, patches were cut to samples with dimensions of 7 × 1.1 cm. Then each sample was connected to the upper and the lower grips of a Universal Testing Machine (UTM) (Model 5544), of the Instron production. Tensile test was carried out in three replicates. The UTM was connected to an IBM-compatible personal computer using a card. Data acquisition and conversion of the Instron’s continuous voltage versus time output into digitized force versus time relationships was performed by software (‘‘Merlin’’) from Instron Corporation (Canton, MA). Finally, the force versus time data was converted to stress versus Hencky’s strain relationships using the following equations:
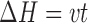 |
1 |
 |
2 |
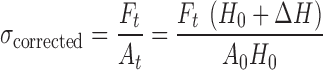 |
3 |
Where ΔH is the absolute deformation (cm), v is the deformation rate (cm/s), t is time (s), ɛH is the Hencky’s strain (−), H0 is the initial length of the sample (cm), σcorrected is the corrected stress (Pa = N cm−2), Ft is the force at a given time (g force) and A0 is the initial cross-sectional area of the film.
Peel Test
The patches were peeled from skin model samples in accordance with a previously described method (20,21). Before the peel tests were conducted, the skin model was wetted with a known amount of water and the patch was attached and left on the skin model surface. During the test, a graph showing the peel force (g force/cm) as a function of peel length (cm) was obtained. Peel force was examined as a function of water content (4.5 or 9.0 μL/cm2) and time (15, 30 or 45 min, at 9 μL/cm2). For peel tests, samples with dimensions of 10 × 2 cm were used. At least 5 replicates were carried out in each case.
Statistical Analysis
Statistical analyses were conducted using JMP software (SAS Institute, 1995), including ANOVA and the Tukey–Kramer Honestly Significant Difference test for comparisons of means. p ≤ 0.05 was considered significant.
RESULTS AND DISCUSSION
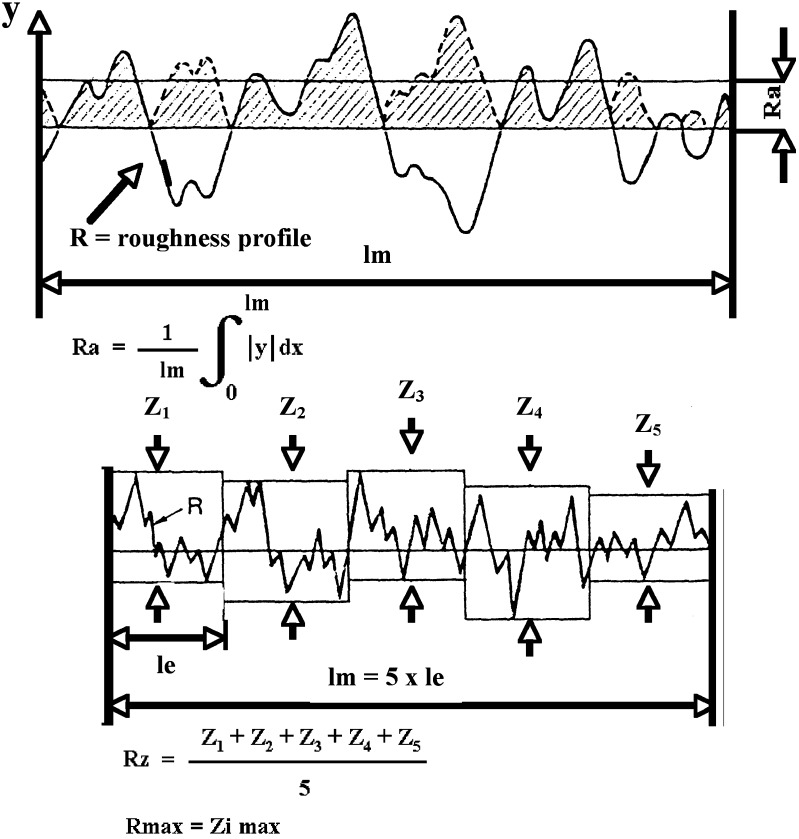
Roughness is a measure of the small-scale variations in the height of a physical surface. This is in contrast to large-scale variations, which may be either part of the geometry of the surface or unwanted ‘waviness’. The surface roughness comprises surface irregularities with relatively small distances that usually include any irregularities caused by the applied manufacturing process and/or other influences. The most commonly measured and indicated parameters of the roughness profile (R-parameters) are Ra and Rz. Since the introduction of ISO 4287 in 1997 (22), these parameters have only been distinguished by their symbol and no longer by their designation. The Ra measure is one of the most effective surface roughness measures commonly adopted in general engineering practice. Ra is defined as the arithmetical mean value of the amounts of the ordinate value within an individual measuring distance (Fig. 1). It gives a good general description of the height variations in the surface.
Fig. 1.
Representation of the calculation of roughness parameters, R a and R z (adapted from the guide of Mitutoyo Surface Roughness Tester 211)
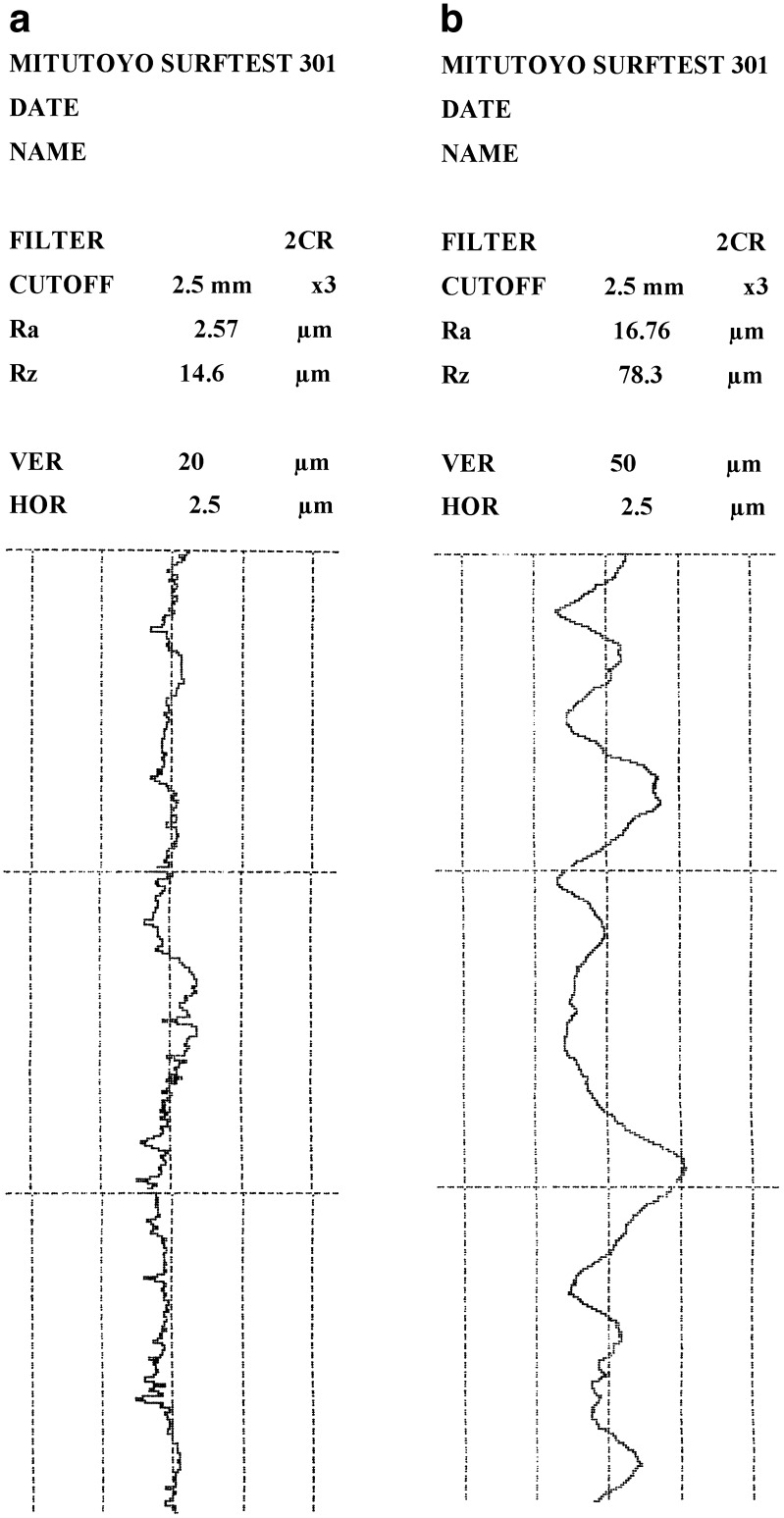
The Ra of both the patch and the skin model were measured for the plane surface of the film in the “x” and “y” dimensions. For the “x” dimension Ra amounted to 2.65 ± 0.11 and 16.85 ± 0.65 μm, respectively (Fig. 2). For the “y” dimension (perpendicular to the “x”) similar results were obtained: 2.69 ± 0.12 and 16.95 ± 0.57 μm for the patch and for the skin model, respectively. No significant differences in Ra between the “x” and “y” dimensions for both the skin model and patch were detected, due to uniformity in their production. The Ra values of the films are close to the ones obtained for films made of different hydrocolloids. For example, for films based on agar and gelatin, detected Ra values were between 1.5 and 3.0 μm (19,23).
Fig. 2.
Typical roughness profiles of patch (a) and skin model (b)
The Rz is defined as the sum of the height of the highest profile peak and the depth of the deepest profile valley within an individual measuring distance (Fig. 1). The Rz measured in the “x” dimension for the planar surfaces of both the patch and the skin model were 14.65 ± 0.55 and 74.62 ± 1.83 μm, respectively. In the “y” dimension similar results were obtained: 14.39 ± 0.51 and 75.02 ± 1.57 μm for the patch and the skin model (Fig. 2). In other words no significant differences between the “x” and “y” dimensions were detected. It was observed that the skin model has Ra and Rz values of 6.5 and 5.3 times greater than was measured for the patch, in other words the surface of the skin model is rougher than the patch. Upon wetting and attaching the patch to the simulated skin, the patch (film) adjusts itself to the rough surface and adheres to it. Other reports mention that Ra values for human and pig skin are 20 ± 3 μm (24,25). Thus, the skin model not only mimics the physical and chemical properties of human skin, but also has similar roughness values. Roughness is sometimes an undesirable property, as it may cause friction, wear, drag and fatigue, but in this specific case it is beneficial, as its texture allows surfaces to absorb the water quickly (upon wetting) and the differences between the roughnesses do not prevent them from “welding” together. The roughness of the patch is attributed to small particles of insoluble dried material (such as sorbitol added as plasiticizer or less likely the polymers) which are projected from the smooth surface. This enlarges the surface area of the patch and may interfere with its adhesion.
Gloss is an optical property which is based on the interaction of light with the physical characteristics of a surface. It is actually the ability of a surface to reflect light in the specular direction. The factors that affect gloss are the refractive index of the material, the angle of incident light and the surface topography. Gloss can be defined as a view of a material’s appearance. Materials with smooth surfaces appear glossy, while very rough surfaces do not reflect any specular light and therefore appear matte. Gloss is also expressed as luster in mineralogy, or sheen in certain specialized fields of application. Gloss is an important issue in the fields of paints and cosmetics (26). Patches that are meant to be used not only for drug delivery but also for cosmetic purposes, and that may be attached to exposed skin areas, must have pleasant colors and should perhaps be glossy.
The surface characteristics of the patch are also presented in Fig. 3. Another measured parameter that characterizes the surface of both the skin model and the patch is gloss. Gloss values measured for non-sweaty human skin (forearm) in our lab were five to eight gloss units at all angles. For the skin model gloss values measured at 20° were 2.8 ± 0.2 and at 60°: 10.8 ± 0.5 gloss units, respectively. For the patch, gloss values measured at 20° were 6.0 ± 0.9 and at 60°, 32.2 ± 2.2 gloss units, respectively. From these results three conclusions can be derived. The patch is glossier than the skin model. The patch is a semi-glossy material since its gloss value at 60° is between 20 and 70 and it seems that the lesser the roughness the greater is the gloss (26).
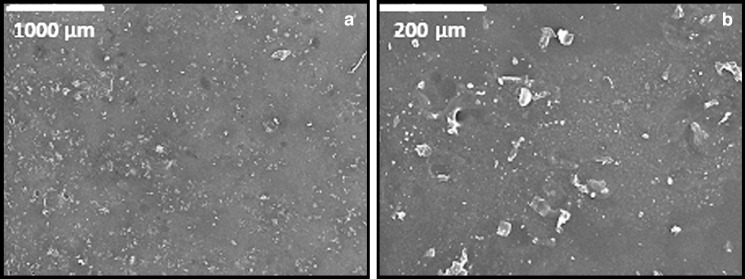
Fig. 3.
Scanning electron micrographs of the surface of the polyvinyl alcohol-based patch. Magnifications: ×35 (a) and ×200 (b)
A very limited number of studies on the gloss properties of films and their dependence on different factors can be located in the literature. From those studies, it can be concluded that gloss measurements of films should be tested at 60° (as was performed in this study), that specular reflectance of a surface is a sensitive function of its roughness, and that the gloss is also dependent on hydrocolloid (gum) concentration within the patch (19,26). Note, however, that this latter property was not tested here.
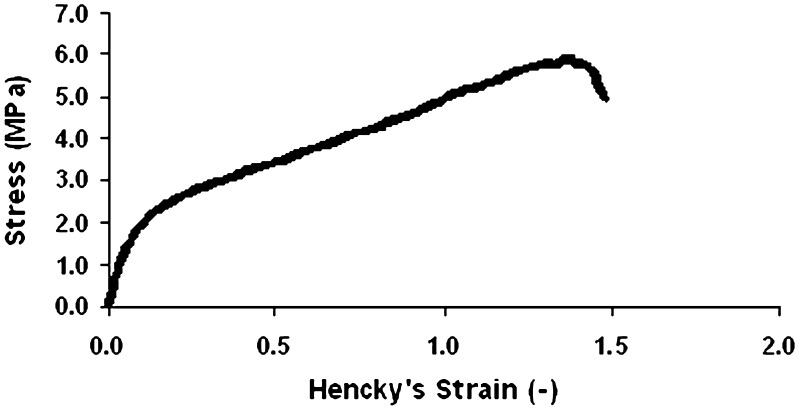
Tensile tests were carried out in order to investigate the mechanical properties of the films. The patches should exhibit desirable mechanical properties so tearing or any other kind of damage will not occur during storage or use of the patch. In other words, the integrity of the patch and its mechanical properties are most important for a successful application. A typical stress-strain relationship in a tensile mode is demonstrated in Fig. 4. The average strength (stress at failure) and failure strain were 5.91 ± 0.21 MPa and 1.37 ± 0.04 (−), respectively. It is also important to note that while peeling the patch from the skin, it is pulled and must resist tensile stresses (12). All the peeled patches (with a higher or lesser water inclusion, or different durations of time before peeling) showed no sign of tearing during peeling. As will be demonstrated next, the forces at failure obtained in tensile tests are about four- to sevenfold greater than those obtained in peel tests. Thus, it can be concluded that such tensile stress at failure is suitable for such purposes.
Fig. 4.
Typical stress–strain relationships of a patch strip in tensile mode
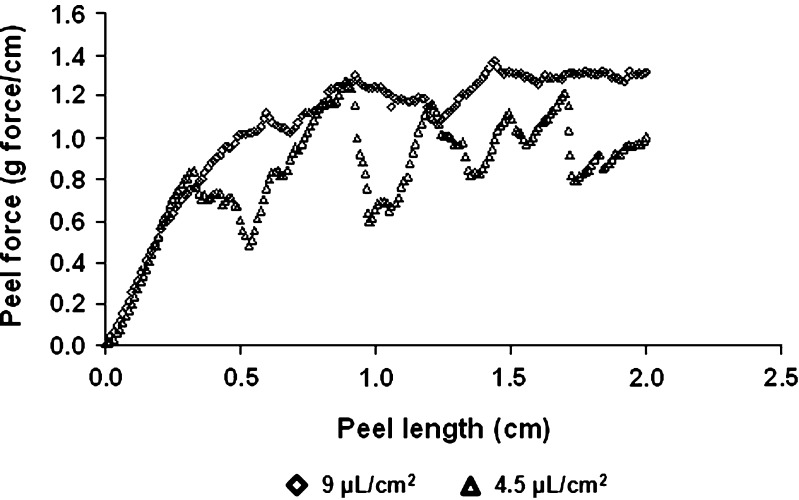
In order to have a system suitable for the purposes of topical and transdermal delivery of drugs, it should exhibit desirable adhesion properties (27). During patch wearing, the drug delivery system may not stay very well in contact with the skin or even fall off the skin (12). Still, when the treatment is over, the procedure of patch removal should not be accompanied by pain or irritation. Thus, adhesion strength needs to be, on the one hand, high enough so good adhesion will be obtained and on the other hand, not too high, so the patch can be easily removed at the end of the treatment. Therefore, an investigation of the adhesion properties of the patch is crucial. In this study, peel tests were carried out (Fig. 5) to learn about the adhesion properties of the patch. When peeling experiments were conducted, the influence of time (gluing time) and added water were considered. The water was added to the surface of the patch in two quantities 4.5 and 9.0 μL/cm2. The wetted patch was pressed on the surface of the skin model and peeling experiments were conducted after 15 min (Fig. 6). From Fig. 6 it is clear that the average peeling force for the higher content of added water was 1.2 ± 0.04 g force/cm in comparison to 0.8 ± 0.03 g force/cm for the lower content of water. These results are not surprising. The fluid (water) wets the upper surface of the patch and the water molecules are taken up by the volume, not only by the surface. In other words sorption (which covers adsorption and absorption) took place. It is also possible that either the dry skin model or the patch draw the water by capillary action as such a phenomenon is seen readily with porous paper.
Fig. 5.

Peel test configuration
Fig. 6.
Peel forces as a function of added water content: empty diamonds—9 μL/cm2 water, empty triangles—4.5 μL/cm2 water
From Fig. 6 it seems that a water quantity of 4.5 μL/cm2 is not sufficient for good wetting, since the “peaks and valleys” of the lower curve are much greater than what is observed for the 9.0 μL/cm2. The upper “peaks” in the lower curve, maybe demonstrate “places” where the patch was better glued to the skin model in comparison to “valleys” where it is weakly glued, or that for such rough skin model more added fluid is required to “soften” the patch, thus it can be better adjusted to the surface. This result is in agreement with the permeation data obtained using lidocaine films placed on skin in the absence of water (7): the amount of drug retained in the stratum corneum was approx. one fourth of that obtained by applying 15 μL/cm2 of water, indicating that skin wetting is essential to achieve skin adhesion and consequently drug permeation. Our previous data on sumatriptan succinate (11), in which no difference in permeation was observed using 7.5, 15 or 30 μL/cm2 of water, indicate that the adhesion obtained with 7.5 μL/cm2 of water was enough to guarantee the contact between the formulation and the skin surface.
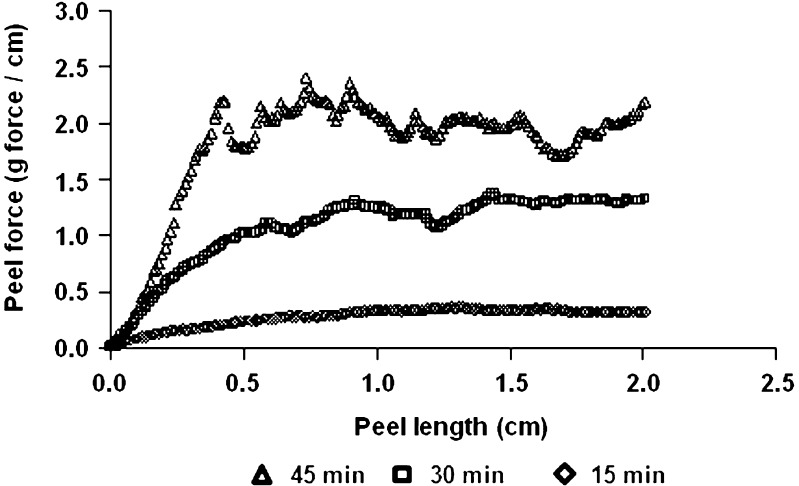
Figure 7 demonstrates that time has a crucial influence on the peeling force. The longer the time interval between the application of the film and the peeling test, the higher the measured peeling forces. The average peeling forces for 15, 30 and 45 min were 0.32 ± 0.01, 1.25 ± 0.05 and 2.00 ± 0.07 g force/cm, respectively. Those results are statistically different at p < 0.05. It is assumed that after longer periods (10 min instead of immediate test) the system reached a better equilibrium and that distribution of water molecules both within the patch and the skin model was more uniform. Another hypothesis is that wetting accelerates start of ion diffusion and permits electrostatic forces, that are responsible in part for the adhesion. A final possibility is that a certain time has to pass so that a certain quantity of water can evaporate thus strengthening film/patch adhesion. The data obtained by measuring the amount of water evaporating from the skin surface after film application clearly demonstrate that water evaporation takes place during the first hour of patch application (28).
Fig. 7.
Peel forces as a function of time elapsed from film application on skin model: empty diamonds—15 min, empty squares—30 min, empty triangles—45 min
Conclusions
When evaluating the adhesive properties of patches, a great advantage comes to mind when considering a skin-like substitute, consisting of the same physical, chemical and topographical properties as human skin, since it would enable to overcome the ethical difficulties that arise given their current use.
Acknowledgments
Lisapharma S.p.A. (Como, I) is gratefully acknowledged for supporting this work
References
- 1.Aulton M. E. Pharmaceutics: the science of dosage form design. New York: Churchill Livingstone; 2002. [Google Scholar]
- 2.Washington N., Washington C., Wilson C. G. Transdermal drug delivery. Phisiological pharmaceutics: barrier to drug absorption. New York: Taylor & Francis; 2003. [Google Scholar]
- 3.Dittgen M. Transdermal therapeutic systems. Medizinische Monatsschrift für Pharmazeuten. 1998;21(12):366–377. [PubMed] [Google Scholar]
- 4.Venkatraman S., Gale R. Skin adhesives and skin adhesion transdermal drug delivery systems. Biomaterials. 1998;19(11):119–1136. doi: 10.1016/s0142-9612(98)00020-9. [DOI] [PubMed] [Google Scholar]
- 5.P. Colombo, P. L. Catellani, C. Padula and P. Santi. Film for dermal and transdermal administration of drugs. International publication number WO 02/30402 A3, 18 April, 2002.
- 6.Padula C., Nicoli S., Colombo P., Santi P. Single-layer transdermal film containing lidocaine: modulation of drug release. Eur. J. Pharm. Biopharm. 2007;66(3):422–428. doi: 10.1016/j.ejpb.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 7.Padula C., Colombo G., Nicoli S., Catellani P.L., Massimo G., Santi P. Bioadhesive film for the transdermal delivery of lidocaine: in vitro and in vivo behavior. J. Control. Release. 2003;88(2):277–285. doi: 10.1016/S0168-3659(03)00015-4. [DOI] [PubMed] [Google Scholar]
- 8.Nicoli S., Amoretti V., Colombo P., Santi P. Bioadhesive transdermal film containing caffeine. Skin Pharmacol. Physiol. 2004;17(3):119–123. doi: 10.1159/000077237. [DOI] [PubMed] [Google Scholar]
- 9.Nicoli S., Penna E., Padula C., Colombo P., Santi P. New transdermal bioadhesive film containing oxybutynin: In vitro permeation across rabbit ear skin. Int. J. Pharm. 2006;325(1–2):2–7. doi: 10.1016/j.ijpharm.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 10.A. Pappani, C. Padula, F. Marra, and P. Santi. Thyroxine skin accumulation from bioadhesive film. Paper presented at: 5th World Meeting on Pharmaceutics, Biopharmaceutics ad Pharmaceutical Technology, Geneva (CH) (2006).
- 11.Femenia-Font A., Padula C., Marra F., et al. Bioadhesive monolayer film for the in vitro transdermal delivery of sumatriptan. J. Pharm. Sci. 2006;95(7):1561–1569. doi: 10.1002/jps.20638. [DOI] [PubMed] [Google Scholar]
- 12.Wokovich A. M., Prodduturi S., Doub W. H., Hussain A. S., Buhse L. F. Transdermal drug delivery system (TDDS) adhesion as a critical safety, efficacy and quality attribute. Eur. J. Pharm. Biopharm. 2006;64(1):1–8. doi: 10.1016/j.ejpb.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 13.J. F. Komerska, and N. Moffett. Collagen films as test surfaces for skin-contact pressure sensitive adhesives. Proceedings of Pressure Sensitive Tape Council. 1985, pp. 108–111.
- 14.Charkoudian J. C. A model skin surface for testing adhesion to skin. J. Soc. Cosmet. Chem. 1988;39(4):225–234. [Google Scholar]
- 15.Schott H. Contact angles and wettability of human skin. J. Pharm. Sci. 1971;60(12):1893–1895. doi: 10.1002/jps.2600601233. [DOI] [PubMed] [Google Scholar]
- 16.Deniau N., Ponchel G., Bonte F., Meybeck A., Duchene D. Immobilization of particulate systems on the skin by the mean of emulsions. Drug Dev. Ind. Pharm. 1993;19(13):1521–1540. doi: 10.3109/03639049309069324. [DOI] [Google Scholar]
- 17.J. C. Charkoudian. Model human skin. US Patent 4877454, 1989.
- 18.Mitutoyo. Catalog No. E4071 Brochure for Mitutoyo surface roughness Tester 211.
- 19.Nussinovitch A., Ward G. Characterizing the gloss properties of hydrocolloid films. Food Hydrocolloids. 1997;11(4):357–365. [Google Scholar]
- 20.Portelli G. B. In: Structural Adhesives Chemistry and Technology. Hartshorn S. R., editor. New York and London: Plenum Press; 1986. pp. 407–449. [Google Scholar]
- 21.Ben-Zion O., Nussinovitch A. Physical properties of hydrocolloid wet glues. Food Hydrocolloids. 1997;11(4):429–442. doi: 10.1016/S0268-005X(97)80041-0. [DOI] [Google Scholar]
- 22.ISO 4287. Geometrical product specifications (GPS)—Surface texture: profile method—Terms definition and surface texture parameters. 1997.
- 23.Nussinovitch A. Hydrocolloid applications: gum technology in the food and other industries. London: Blackie Academic & Professional; 1997. [Google Scholar]
- 24.De Pape K., Lagarde J. M., Roseeuw D., Rogiers V. Microrelief of the skin using a light transmission method. Arch. Dermatol. Res. 2000;292(10):500–510. doi: 10.1007/s004030000166. [DOI] [PubMed] [Google Scholar]
- 25.Ben-Yaakov A. Properties of tree gum exudate patches for transdermal and topical drug delivery [M. Sc.] Jerusalem: Faculty of Agricultural, Food and Enviromental Quality Sciences, The Hebrew University of Jerusalem; 2007. [Google Scholar]
- 26.Ward G., Nussinovitch A. Gloss properties and surface morphology relationships of fruits. J. Food Sci. 1996;61(5):973–977. doi: 10.1111/j.1365-2621.1996.tb10914.x. [DOI] [Google Scholar]
- 27.Nussinovitch A. Hydrocolloid adhesives. Water soluble polymer applications in foods. Oxford: Blackwell Science Ltd; 2003. pp. 1–26. [Google Scholar]
- 28.Padula C., Nicoli S., Aversa V., et al. Bioadhesive film for dermal and transdermal drug delivery. Eur. J. Dermatol. 2007;17(4):309–312. doi: 10.1684/ejd.2007.0205. [DOI] [PubMed] [Google Scholar]