Abstract
Mucociliary clearance (MC) is an important defense mechanism of the respiratory system to eliminate inhaled and possibly noxious particles from the lung. Although the principal mechanics of MC seem to be relatively clear there are still open questions regarding the long-term clearance of particles. Therefore, we have developed a new set-up based on embryonic chicken trachea (ECT) to investigate mucociliary particle clearance in more detail. ECT was placed in an incubation chamber after carbon particles were applied and tracked using optical microscopy. The aim of the study was to validate this model by investigating the impact of temperature, humidity and drugs on particle transport rates. Particles were transported reproducibly along the trachea and clearance velocity (2.39 ± 0.25) mm/min was found to be in accordance to data reported in literature. Variation in temperature resulted in significantly reduced MC: (0.40 ± 0.12) mm/min (20 °C); (0.42 ± 0.10) mm/min (45 °C). Decreasing humidity (99–60%) had no significant effect on MC, whereas reduction to 20% humidity showed a significant influence on particle clearance. The use of different cilio- and muco-active drugs (Propranolol, Terbutalin, N-acetylcysteine) resulted in altered MC according to the pharmacological effect of the substances: a concentration dependent decrease of MC was found for Propranolol. From our results we conclude that this model can be employed to investigate MC of particles in more detail. Hence, the model may help to understand and identify decisive physico-chemical parameters for MC and to answer open questions regarding the long-term clearance phenomenon.
Electronic supplementary material
The online version of this article (doi:10.1208/s12249-008-9072-6) contains supplementary material, which is available to authorized users.
Key words: chicken, in vitro, mucociliary clearance, nanoparticle, trachea
INTRODUCTION
The human lung as application site for active substances is of increasing importance for local (e.g. asthma, COPD) as well as systemic (e.g. diabetes mellitus) therapy. The attractiveness of pulmonary drug delivery is attributed to the good absorption due to a huge alveolar surface area (70–140 m2), unique barrier properties and avoidance of the first-pass effect (1). Beside these therapeutic aspects there is a constant input of exogenous material into the lung triggered by breathing. Mucociliary clearance (MC) is a most important mechanism to eliminate inhaled and possibly noxious particles, bacteria and toxins from the central airways. In the field of inhalation toxicology the aerodynamic diameter is termed to be a main decisive parameter. Particle classification is based upon a precise nomenclature which is inevitable for avoiding misunderstanding and confusion. Thus, ultra fine particles (UFP) are defined to be smaller than 100 nm in diameter, whereas particulate matter (PM) can be divided into inhalable PM10 (d < 25 μm) and respirable PM2.5 (d < 3.5 μm). Depending on the aerodynamic diameter and the inhalation manoeuvre particles will deposit in different regions within the respiratory system (2). Discrimination between deposition site of the particles is realized due to three different principal mechanisms: impaction, sedimentation and Brownian motion (3). Particles sized >5 μm will mainly be deposited in the oro-pharyngal region due to impaction, whereas particles sized 1–5 μm are suitable to enter the tracheo-bronchial region. When particle size is reduced to ≤1 μm Brownian motion will be the decisive mechanism for deposition in mainly peripheral regions of the lung. To date exclusive deposition in either the bronchial or the alveolar region is not feasible and only can be optimized using special inhalation techniques (e.g. the shallow bolus technique for targeting the airways) (4,5).
Depending on the site of particle deposition, clearance is realized by different mechanisms: I. Mucociliary clearance (MC) — the airways are covered by a mucus layer that is transported by ciliary beating resulting in a fast removal of deposited particles. II. Alveolar macrophages — in the lung periphery no ciliated cells are present and alveolar macrophages are the defence mechanism in this region (6). The present work describes the set-up of an in vitro test system to investigate the mucociliary clearance. Therefore, a closer look at the detailed morphological architecture, conditions and prerequisites in this area is appreciated.
The mucus layer in healthy individuals consist of the upper gel layer and the lower periciliary layer. The periciliary layer is a low viscosity fluid with a thickness of 5–7 μm which is slightly less than an extended cilium (7). The mucus layer on top is a gel composed of a 3-dimensional polymer network of mucus glycoproteins or mucins. These mucin macromolecules are 70–80% carbohydrate, 20% protein and 1–2% sulphate bound to oligosaccharide side chains (8,9).
About 3% of the mucus layer consists of mucins, while 90–95% consist of water, with electrolytes, serum proteins, immunoglobulins and lipids (10,11). Although optimum concentration for all components seems likely to exist, MUC5-AC and MUC5-B have been identified to be predominantly responsible for gel-forming and adhesive properties of airway mucus (12,13). Alterations in secretion rate and MUC5-AC to MUC5-B ratio correlated with impaired mucociliary clearance can be found in several pathophysiological conditions of the respiratory system e.g. asthma, cystic fibrosis and COPD (14–16).
Within the human airways 30–65% of the epithelium is covered with ciliated cells whereat each cell houses about 200 cilia (17). Cilia are motile hair-like appendages extending 5–7 μm from the surface of epithelial cells. They contain a central axoneme i.e. a bundle of microtubules arranged as nine outer doublets and one central pair (9*2 + 2 arrangement) (18).
Movement of the cilium is generated by sliding movements of the microtubules under ATP depletion (19). Cilia beat in close coordination and adjust their frequency and phase of beating in response to neighbouring cilia (20). Throughout the ciliary beat cycle, consisting of forward and backward stroke, cilia transfer kinetic energy to the on top located mucus layer during the forward stroke. Metachronal coordination and beat mechanics result in transport of the mucus layer towards the oesopharyngal region, where finally the mucus and entrapped material is swallowed.
Scheuch and Stahlhofen could show that mucociliary clearance removes all particles >6 μm in diameter within 24 h from the human airways in vivo. For particles ≤6 μm a certain fraction was retained for more than 24 h. Further reduction in particle size correlated with an increasing fraction of long-term cleared particles (21–23). Despite the phenomenon of long-term cleared particles the effects of inhaled ultra fine particles and particulate matter on human health have been for years and still are under debate (24). However, the number of epidemiological studies indicating a correlation between exposure to particulate matter and adverse health effects increases constantly (25,26).
By today the decisive parameters and mechanisms of particle long-term clearance are not understood and different hypotheses are under discussion. Depending on particle size and physico-chemistry a certain fraction of particles might penetrate into and through the mucus layer and thus escape from mucociliary clearance (27–31). In case that the mucus layer is not a fully closed blanket covering the surface of all airways, particles might penetrate after deposition directly into the periciliary layer and thereby undergo fast elimination (9,32). Another reason for delayed clearance is possibly the deposition of a certain particle fraction in the peripheral lung although the airways are the primary target.
The aim of our study was to set up an in vitro model based on chicken trachea to investigate mucociliary particle clearance under more complex constraints such as various temperatures, humidities and drugs. This model will allow for exploring mucociliary clearance and mucus-particle interactions based on functional interaction of cilia and mucus. It is well suited for this approach since former experiments showed that embryonic chicken trachea is a valid substitute for human material in studying ciliary beat frequency and ciliary toxicity (33–36). Furthermore, we examined the models’ histology in order to check for functional development of ciliated cells, goblet cells and differences compared to human tissue morphology.
MATERIAL AND METHODS
Materials
Inorganic salts (E. Merck, Darmstadt, GE); Activated carbon Ph.Eur. (E. Merck, Darmstadt, GE); Fertilized chicken eggs (Lohmann Tierzucht GmbH, Cuxhaven, GE); Propranolol hydrochloride (Sigma-Aldrich Chemie GmbH, Steinheim, GE); Terbutalin hemisulfate (Sigma-Aldrich Chemie GmbH, Steinheim, GE); N-acetylcysteine (Sigma-Aldrich Chemie GmbH, Steinheim, GE); Uranyl acetate (Fluka AG, Zürich, CH); Lead citrate (Leica AG, Heerbrugg; CH); Eppon resin (Fluka AG, Zürich, CH). All chemicals used in the experiments were of highest available quality.
Trachea Preparation
Chicken eggs of SPF quality (specific pathogen free) were incubated in a breeding chamber (Hemel Breeding Instruments GmbH, Verl, GE) at 37.8 °C/60% relative humidity for 19–20 days. All clearance experiments were performed using freshly harvested embryo chicken trachea. After dissection the trachea was placed on gauze soaked with Locke-Ringer solution (LR). LR is an isotonic solution of NaCl 7.72 g (132 mmol), KCl 0.42 g (5.63 mmol), CaCl2 × H2O (0.16 g/1.24 mmol), NaHCO3 (0.15 g/1.79 mmol) and glucose anhydrous (1.00 g/5.55 mmol) in 1 l of water. Prior to particle deposition the tracheal tube was cut oblong resulting in two tracheal half-pipes. Particle deposition on the half-pipes was realized utilizing a Dry Powder Insufflator™ Model DP-4 (PennCentury Inc., Philadelphia, USA) adopted from instillation experiments (37).
Clearance Experiments
Following particle deposition the tracheal tissue was transferred to a temperature and humidity controlled incubation chamber and subsequently placed under the microscope (AxioImager-M3, ZEISS, GE). Carbon particles were applied to establish the set-up due to easy visualization using transmission light microscopy. Particle size was 0.8–260 μm as determined by static light scattering (MasterSizer-2000, Malvern Inst., GB). Particle transport on the trachea was recorded for 10–20 s (AxioCam HSm, ZEISS, GE) at various positions on the tracheal half-pipes using a LD Plan-Neofluar 10x/0.30 objective (ZEISS, GE). Clearance velocity was calculated from three to eight videos, and three to six single particles were calculated per video. Numbers beside the bars in the graphs e.g. “100/5” account for 100 particles tracked on five tracheas.
The effects of three drugs, i.e. Propranolol, Terbutalin and N-acetylcysteine, already known to influence either ciliary beat frequency (CBF) or mucus rheology, have been investigated. Tracheal tubes were incubated for 60 s in LR/drug solution or drug free LR solution (control) prior to particle deposition. A significant effect for Propranolol 1% (m/v) on CBF was already reported by Boek (34). To investigate the model’s sensitivity for a concentration dependent effect on MC we tested Propranolol at 1% (3.4 mM), 0.1% (0.34 mM) and 0.01% (3.4 μM). Terbutalin and N-acetylcysteine were both tested at 1% (m/v). The influence of temperature (20–45 °C) and humidity (20–99%) in the incubation chamber was investigated to test the models robustness for various experimental conditions. Temperature and humidity were actively controlled and monitored online using a system provided by LIS (Life Imaging Services, Reinach, CH). Results were examined for statistical differences by ANOVA using routine statistical software (SigmaStat 3.0).
Histochemistry
Histology on Formalin-Fixed Lung Specimen
Tracheal tissue was fixed in 10% neutral-buffered formalin and embedded in paraffin at 58 °C. Sections (5 μm) were cut from paraffin-embedded tissue and mounted on glass slides. Routine histology was then carried out as described previously using Alcian blue staining (38).
SEM/TEM Imaging
For SEM imaging tracheal tissue was cut into slices of about 5 mm in width, dehydrated in ethanol, critical point dried and sputter-coated with gold. Samples were examined using a Philips XL 30-FEG scanning electron microscope (Philips AG, Zuerich, CH) operating at 10 kV. For TEM imaging tissue was fixed for 24 h in 0.03 M potassium phosphate buffer containing 2.5% glutar aldehyde. After fixation tissue was embedded in Eppon resin and cut to ultra-thin sections (60–80 nm) as described earlier (39). The ultra-thin sections were transferred to uncoated 200-mesh copper grids and stained with uranyl acetate and lead citrate before examination in a Philips 300 transmission electron microscope (Philips AG, Zuerich, CH) operating at 60 kV.
RESULTS
Clearance Experiments
Proof of Principle
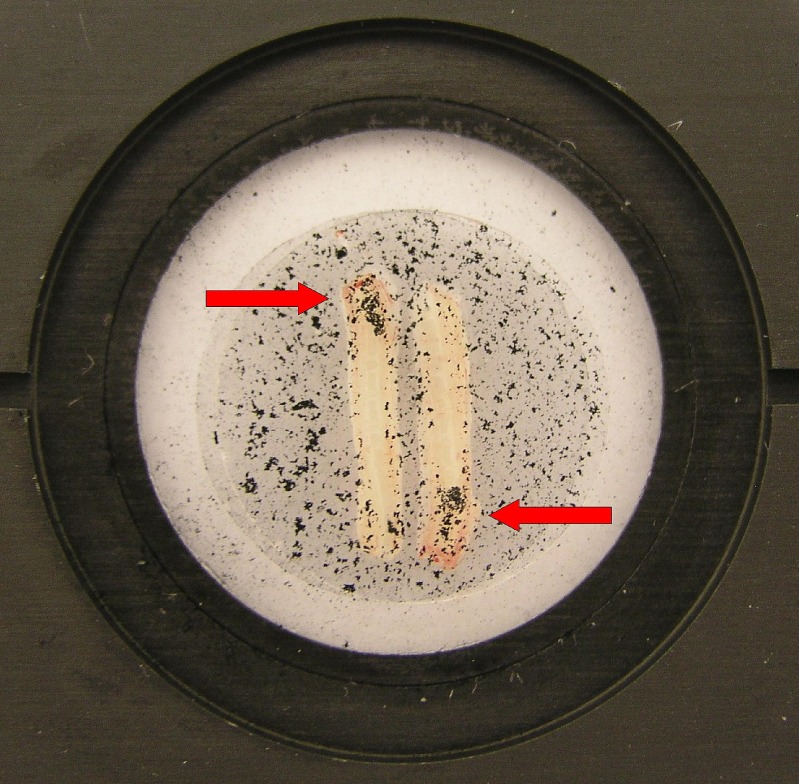
Mucus and particles were clearly and reproducibly transported along the tracheal tissue (see Video 1). Clearance due to mucociliary interaction could be successfully demonstrated by turning one of two tracheal half-pipes by 180°: particles were transported into different directions but always to the proximal end of the trachea (Fig. 1). Mucus exhibited characteristic appearance, i.e. mucus streams and mucus flakes. Mucus transparency was according to the mucus grades (MG) classified by Gerber et al. (40). Completely transparent mucus (MG-1), transparent but slightly opaque mucus (MG-2), opaque mucus with surface relief (MG-3) and non-transparent, completely opaque mucus with marked surface relief (MG-4) was present on the tracheal epithelium.
Fig. 1.
Tracheal half-pipes in the incubation chamber. Proof of principle experiment: Particles are transported into different but uniquely proximal direction after turning one of the tracheal half-pipes by 180°
Temperature and Humidity
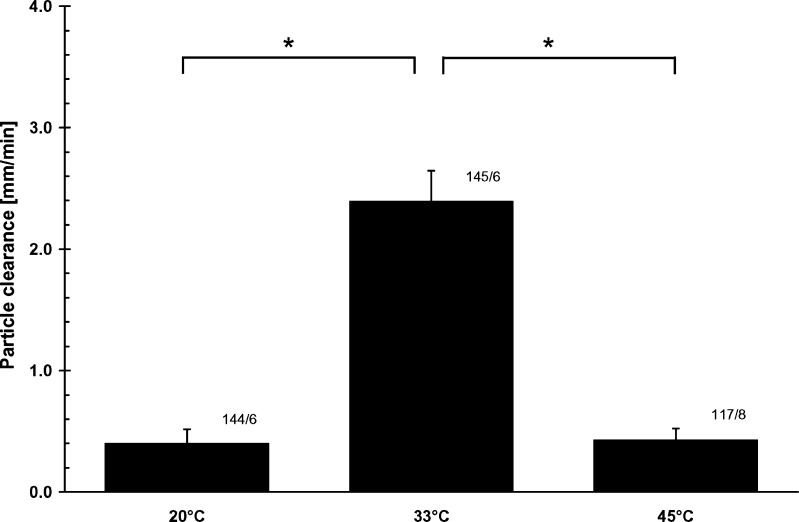
Decrease (20 °C) as well as increase (45 °C) in temperature resulted in significant reduction (P ≤ 0.001) of particle transport rates (TR). TR determined for 20, 33 and 45 °C was (0.40 ± 0.12) mm/min, (2.39 ± 0.25) mm/min and (0.42 ± 0.10) mm/min, respectively (Fig. 2).
Fig. 2.
Influence of temperature on mucociliary particle transport rates. Decreased (20 °C) as well as increased (45 °C) temperature resulted in significant reduction of particle transport rates. Numbers beside the bars e.g. “100/6” represent 100 particles tracked on six tracheas
Variation of relative humidity between 60% and 99% had no significant effect (P ≤ 0.001) on particle transport velocity. TR was (2.29 ± 0.96) mm/min, (2.32 ± 0.70) mm/min and (2.39 ± 0.25) mm/min for 60%, 75% and 99% relative humidity experiments, respectively. Further reduction to 20% relative humidity resulted in significantly (P ≤ 0.001) decreased clearance rates (0.54 ± 0.37) mm/min.
Influence of Drugs
Particle clearance under influence of three drugs known to affect mucociliary clearance was investigated. Propranolol (Pp) was capable of reducing or fully eliminating particle transport in a concentration dependent manner. No transport of particles could be observed after incubation in Pp-1% (3.4 mM) and Pp-0.1% (0.34 mM) LR/drug solution.
Incubation in Pp-0.01% (0.034 mM) LR/drug solution resulted in significantly (P ≤ 0.001) reduced transport (0.44 ± 0.21) mm/min compared to control experiments (C; 3.14 ± 0.41) mm/min, using only LR solution (Fig. 3). Clearance rate under influence of Terbutalin (T) was significantly increased (P ≤ 0.001) to (4.90 ± 0.62) mm/min, whereas N-acetylcysteine (NAC) decreased transport velocity significantly (P ≤ 0.001) to (0.66 ± 0.20) mm/min (Fig. 3).
Fig. 3.
Influence of drugs on mucociliary particle transport rates. Clearance velocity under influence of drugs was significantly changed. Terbutalin (1%) increased transport rates, whereas N-acetylcysteine (1%) and Propranolol (0.01%) reduced mucociliary particle clearance. Influence of Propranolol (0.1%) and Propranolol (1%) totally inhibited particle transport. Numbers beside the bars e.g. “100/6” represent 100 particles tracked on six tracheas
Histochemistry
TEM/SEM Images
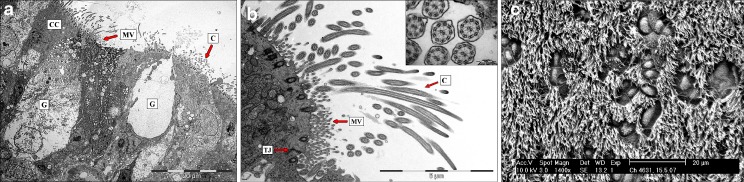
Cilia were found to be comparable to human respiratory cilia regarding cilia length (5–7 μm) and ultrastructure of the microtubules (9*2 + 2 arrangement). Basal bodies exhibited normal topology and orientation (Fig. 4).
Fig. 4.
a TEM image (overview) of tracheal epithelium cross sections. CC Ciliated cells, C cilia, MV microvilli, G goblet cells. ECT morphology was found to be comparable to human tracheal tissue—all structures essentially required for mucociliary clearance are present. b TEM image of tracheal epithelium and cilia cross sections. C Cilia, MV microvilli, TJ tight junction complexes. ECT morphology was found to be comparable to human tracheal tissue—all structures essentially required for mucociliary clearance are present. Cilia ultrastructure exhibits normal (9*2 + 2) arrangement of the central axoneme—a bundle of microtubules arranged as nine outer doublets and one central pair. c SEM image of ciliated epithelium. Cilia length (5–7 μm) and cilia density was found to be comparable to the human airway epithelium. Mucus producing goblet cells are present and exhibit characteristic morphology
Paraffin Sections
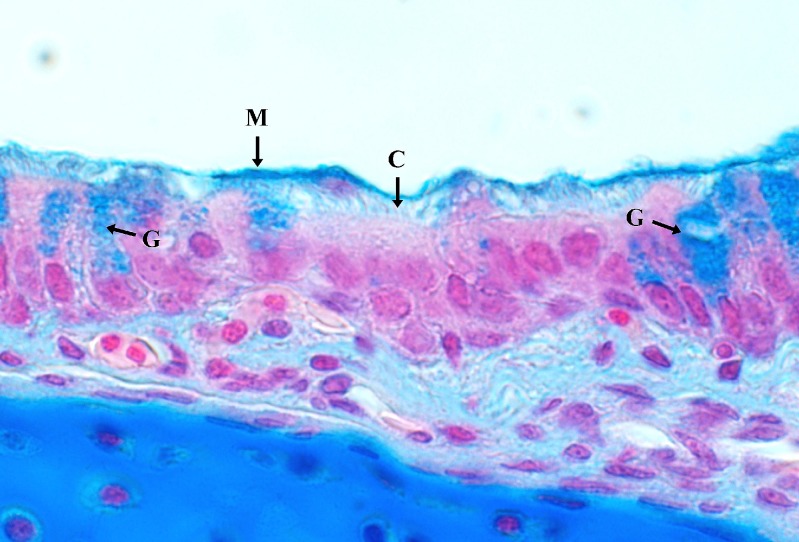
Cross sections of the tracheal tissue showed normal morphology regarding cilia length and cilia density. Presence of goblet cells and localisation of the upper mucus layer was successfully determined by characteristic staining using Alcian blue (Fig. 5).
Fig. 5.
Alcian blue staining of paraffin embedded tracheal tissue. A mucus layer (M) and goblet cells (G) are present and exhibit characteristic staining. Cilia (C) on ciliated cells. (×100, cross section)
DISCUSSION
Mucociliary clearance is a complex cleaning mechanism of the airways. Decisive parameters for mucociliary transport are ciliary beating, height of the periciliary layer (PCL) and viscoelastic properties of the mucus. Metachronal coordination of the beat pattern results in directional mucus transport only when transfer of kinetic energy from the cilia to the mucus layer is efficient. Thus, ciliary beat frequency (CBF) defines the maximum amount of kinetic energy to be transferred from the cilia to the upper mucus. As known from literature CBF is correlated to temperature. Clairy-Meinesz reported that human cilia are almost immotile at 5 °C. From 9 to 20 °C CBF increased constantly and values approximately doubled as the temperature increased by 10 °C. Between 20 and 45 °C CBF was found to be constant at around 8–11 Hz. At 50 °C cilia rapidly became immotile and cooling did not restore ciliary motility (41). Being aware of the CBF plateau between 20 to 45 °C and the comparability of human and chicken tissue at 33 °C reported by Boek (34), all control experiments were realized at 33 °C and 99% relative humidity. Rheological properties of the mucus are important parameters regarding coupling efficiency, and correlation of mucus transport rates and rheological properties have already been reported (42). If the elastic and the viscous modulus are out of range, kinetic energy will be lost and the mucus will not be propelled at normal velocity.
The experimental results in this study demonstrate efficient interaction of mucus and beating cilia. Mucus exhibited characteristic transparency and structure (streams, flakes) and entrapped particles were reproducibly and uni-directionally transported along the tracheal tissue. The results from the experiments at various temperatures clearly show a significant influence of temperature (P ≤ 0.001) on mucociliary transport (Fig. 1). Reduced clearance rates can be explained by a combinatory effect of reduced or less coordinated CBF and changes in mucus rheology (43–45). Clearance velocity (2.39 ± 0.25) mm/min for the control experiments (33 °C/99%) were found to be comparable to data reported in literature.
Tracheal mucociliary clearance rates reported for rats, guinea pigs and rabbits are (1.9 ± 0.7) mm/min, (2.7 ± 1.4) mm/min and (3.2 ± 1.1) mm/min, respectively (46). In comparison, average tracheal mucus velocities in healthy non-smokers, as measured by non-invasive radiological techniques, were found to range from 4–6 mm/min (47). The only reported value for human mucus velocity in the main bronchi is about 2.4 mm/min (48) which is in good accordance with our data.
Decreasing relative humidity from 99% to 60% had no significant effect on transport rates and can be explained by a certain stability of MC mechanistics even under suboptimal environmental conditions. Thus, duration of a single transport experiment (~10 min) might not be sufficient to change mucus rheology, PCL height or CBF to a significant extent. Further reduction in relative humidity to 20% significantly influenced transport rates, suggesting that the model is sensitive for changes in humidity, moreover reflecting the in vivo situation where small changes should not effect MC dramatically.
The choice of different active substances was made with respect to their impact on the mucociliary transport rate respectively the CBF. All drugs tested had a significant influence on mucociliary clearance (Fig. 3). Terbutalin and Propranolol are both influencing CBF via β-receptors. Terbutalin, acting as a β-adrenoceptor agonist, increases CBF by increasing intracellular levels of cyclic adenosine monophosphate (cAMP). On the contrary Propranolol, acting as β-blocker, reduces CBF via decrease of intracellular cAMP (49). Mucociliary clearance for both drugs changes according to their respective pharmacological effect. For the experiments using Propranolol a concentration dependent effect could be determined, suggesting that the model exhibits sensitivity for drugs in a concentration dependent manner. The influence of N-acetylcysteine (NAC) resulted in a significant reduction of mucociliary transport rates and can be explained by a significant change of mucus rheology. NAC is known to sever disulfide bonds, thus diminishing crosslinking of the mucin network. As a consequence the viscosity as well as the elasticity of the mucus gel is reduced and the energy transfer from the cilia to the upper mucus layer is less efficient (8).
Histochemistry was performed to investigate functional development of ciliated cells, goblet cells and differences compared to human tissue morphology. ECT morphology was found to be comparable to human tracheal tissue. Cilia length, cilia density and orientation of the basal bodies exhibit characteristic structure and orientation. Presence of goblet cells and a mucus layer was successfully determined by Alcian blue staining (Fig. 5). Hence, all structures essentially required for mucociliary clearance are present on embryo chicken trachea.
Several model systems based on frog palate, horse or bovine trachea are already available (50). Compared to those in vitro models the ECT model is favourable for several reasons. (1) Breeding eggs are commonly available in SPF quality which reduces costs and the risk for biological contamination of the experimentalist to a minimum. (2) Eggs are easy to handle and can be stored for up to 1 week prior to breeding. Thus, (3) high standardization for the ECT model is possible. This argument gains importance considering the fact that the ECT is an already validated model for investigating ciliary beat frequency (34,35). These two crucial aspects allow for generating a profound data basis. For experiments utilizing porcine or bovine tracheal tissue such standardization can be achieved hardly. Tissue from the local abattoir often undergoes critical but inevitable treatment with hot water steam. Transportation to the laboratory takes valuable time and experimental results may include artefacts. Moreover, housing conditions for the animals are not standardized and bacterial contamination or lung pneumonia can not be excluded. Raising animals for experimental use under best possible standardized conditions would be an option but suffers from increasing costs and controversial discussion on ethical and regulatory aspects.
CONCLUSIONS AND OUTLOOK
The pulmonary route is considered as a promising target for drug and protein delivery. However, there are still open question regarding long-term clearance of particles from the airways and mucus-particles interactions. Hitherto, ECT has been utilized and validated for CBF measurements under influence of various chemical substances. The aim of our study was to set up an in vitro model based on chicken trachea in order to investigate MC of particles under more complex constraints such as temperature, humidity and various drugs. Thus, MC in the experiments is a result of ciliary beating and effective energy transfer from cilia to the mucus. From our results we conclude that embryo chicken trachea can be employed to investigate the influence of cilio- and mucus-active drugs or particles on MC. The model shows a stability reflecting the in vivo situation where small changes are not supposed to impact dramatically on MC. Nevertheless, the ECT model is sensitive to changes in environmental conditions regarding temperature and humidity. Furthermore, the model allows for investigating the impact of drugs as could be shown for substances already known to influence clearance rates. As well this holds for the tissue response of the cilio-toxic substance Propanolol, which shows a concentration dependent decrease of MC as a result of the reduced ciliary function, as for Terbutalin and its clearance enhancing effect. Changes in mucus structure and the correlated rheological properties as achieved with N-acetylcysteine can be monitored as well. Therefore, the ECT is a powerful tool to investigate particulate impact on the clearance functionality of the trachea. Future experiments utilizing different sized and/or modified (nano) particles as well as further characterization of mucus structure and components (surfactant) shall help to understand and identify decisive physico-chemical parameters for MC and to answer open questions on the long-term clearance phenomenon.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Exemplary video for mucociliary clearance of carbon particles. Particles are clearly and reproducibly transported on the tracheal tissue. Particle clearance is unidirectional and always to the proximal end of the trachea. Visualization and tracking of the carbon particles was realized by transition light microscopy. (×100, top view; MPG 2.78 MB)
Acknowledgements
Leon Muijs and Beat Haenni are thanked for technical support and introduction to SEM/TEM imaging. Gregor Jung and Babette Hinkeldey are thanked for cover slip modification for the incubation chamber.
Financial support from the Federal German Ministry of Education and Research is gratefully acknowledged (Nano-Inhale-13N8890).
References
- 1.Corkery K. Inhalable drugs for systemic therapy. Resp. Care. 2000;45(7):831–835. [PubMed] [Google Scholar]
- 2.Oberdörster G., Oberdörster E., Oberdörster J. Nanotoxicology: an emerging discipline evolving from studies of ultrafine particles. Environ. Health Persp. 2005;113(7):823–839. doi: 10.1289/ehp.7339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Groneberg D. A., Witt C., Wagner U., Chung K. F., Fischer A. Fundamentals of pulmonary drug delivery. Resp. Med. 2003;97(4):382–387. doi: 10.1053/rmed.2002.1457. [DOI] [PubMed] [Google Scholar]
- 4.Brand P., Rieger C., Beinert T., Heyder J. Aerosol derived airway morphometry in healthy subjects. Eur. Resp. J. 1995;8(10):1639–1646. doi: 10.1183/09031936.95.08101639. [DOI] [PubMed] [Google Scholar]
- 5.Scheuch G., Gebhart J., Heigwer G., Stahlhofen W. New device for human inhalation studies with small aerosol boluses. J. Aerosol Sci. 1989;20(8):1293–1296. doi: 10.1016/0021-8502(89)90820-3. [DOI] [Google Scholar]
- 6.Möller W., Häu K., inger, Winkler-Heil R., et al. Mucociliary and long-term particle clearance in the airways of healthy nonsmoker subjects. J. Appl. Physiol. 2004;97(6):2200–2206. doi: 10.1152/japplphysiol.00970.2003. [DOI] [PubMed] [Google Scholar]
- 7.Marttin E., Schipper N. G. M., Coos Verhoef J., Merkus F. W. H. M. Nasal mucociliary clearance as a factor in nasal drug delivery. Adv. Drug Deliv. Rev. 1998;29(1–2):13–38. doi: 10.1016/S0169-409X(97)00059-8. [DOI] [PubMed] [Google Scholar]
- 8.Fuloria M., Rubin B. K. Evaluating the efficacy of mucoactive aerosol therapy. Resp. Care. 2000;45(7):868–873. [PubMed] [Google Scholar]
- 9.Matsui H., Randell S. H., Peretti S. W., Davis C. W., Boucher R. C. Coordinated clearance of periciliary liquid and mucus from airway surfaces. J. Clin. Invest. 1998;102(6):1125–1131. doi: 10.1172/JCI2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Verdugo P. Goblet cells secretion and mucogenesis. Ann. Rev. Physiol. 1990;52:157–176. doi: 10.1146/annurev.ph.52.030190.001105. [DOI] [PubMed] [Google Scholar]
- 11.Lethem M. I. The role of tracheobronchial mucus in drug administration to the airways. Adv. Drug Deliv. Rev. 1993;11(3):271–298. doi: 10.1016/0169-409X(93)90013-T. [DOI] [Google Scholar]
- 12.Lillehoj E. P., Kim K. C. Airway mucus: its components and function. Arch. Pharm. Res. 2002;25(6):770–780. doi: 10.1007/BF02976990. [DOI] [PubMed] [Google Scholar]
- 13.Gray T., Koo J. S., Nettesheim P. Regulation of mucous differentiation and mucin gene expression in the tracheobronchial epithelium. Toxicol. 2001;160(1–3):35–46. doi: 10.1016/S0300-483X(00)00455-8. [DOI] [PubMed] [Google Scholar]
- 14.Kirkham S., Sheehan J. K., Knight D., Richardson P. S., Thornton D. J. Heterogeneity of airways mucus: variations in the amounts and glycoforms of the major oligomeric mucins MUC5AC and MUC5B. Biochem. J. 2002;361(3):537–546. doi: 10.1042/0264-6021:3610537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Groneberg D. A., Eynott P. R., Lim S., et al. Expression of respiratory mucins in fatal status asthmaticus and mild asthma. Histopathology. 2002;40(4):367–373. doi: 10.1046/j.1365-2559.2002.01378.x. [DOI] [PubMed] [Google Scholar]
- 16.Groneberg D. A., Eynott P. R., Oates T., et al. Expression of MUC5AC and MUC5B mucins in normal and cystic fibrosis lung. Resp. Med. 2002;96(2):81–86. doi: 10.1053/rmed.2001.1221. [DOI] [PubMed] [Google Scholar]
- 17.Blake J. R., Sleigh M. A. Mechanics of ciliary locomotion. Biol. Rev. Cambr. Phil. Soc. 1974;49(1):85–125. doi: 10.1111/j.1469-185X.1974.tb01299.x. [DOI] [PubMed] [Google Scholar]
- 18.Satir P., Christensen S. T. Overview of structure and function of mammalian cilia. Ann. Rev. Physiol. 2007;69:377–400. doi: 10.1146/annurev.physiol.69.040705.141236. [DOI] [PubMed] [Google Scholar]
- 19.Afzelius B. A. Ciliary structure in health and disease. Acta Oto-Rhino-Laryngol. Bel. 2000;54(3):287–291. [PubMed] [Google Scholar]
- 20.Sleigh M. A., Blake J. R., Liron N. The propulsion of mucus by cilia. Am. Rev. Resp. Dis. 1988;137(3):726–741. doi: 10.1164/ajrccm/137.3.726. [DOI] [PubMed] [Google Scholar]
- 21.Scheuch G., Stahlhofen W. Particle deposition of inhaled aerosol boluses in the upper human airways. J. Aerosol. Sci. 1987;18(6):725–727. doi: 10.1016/0021-8502(87)90107-8. [DOI] [Google Scholar]
- 22.W. Stahlhofen, J. Gebhart, G. Rudolf, G. Scheuch, and K. Philipson. Clearance from the human airways of particles of different sizes deposited from inhaled aerosol boli. In Aerosols: Formation and Reactivity, Pergamon Press, Oxford, UK 1986.
- 23.Stahlhofen W., Koebrich R., Rudolf G., Scheuch G. Short-term and long-term clearance of particles from the upper human respiratory tract as function of particle size. J. Aerosol. Sci. 1990;21(Supp 1):407–410. doi: 10.1016/0021-8502(90)90267-2. [DOI] [Google Scholar]
- 24.Kreyling W. G., Semmler-Behnke M., Möller W. Ultrafine particle–lung interactions: does size matter? J. Aerosol. Med. 2006;19(1):74–83. doi: 10.1089/jam.2006.19.74. [DOI] [PubMed] [Google Scholar]
- 25.Dominici F., Peng R. D., Bell M. L., et al. Fine particulate air pollution and hospital admission for cardiovascular and respiratory diseases. J. Am. Med. Assoc. 2006;295(10):1127–1134. doi: 10.1001/jama.295.10.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Annesi-Maesano I., Forastiere F., Kunzli N., Brunekref B. Particulate matter, science and EU policy. Eur. Resp. J. 2007;29(3):428–431. doi: 10.1183/09031936.00129506. [DOI] [PubMed] [Google Scholar]
- 27.Gehr P., Geiser M., Hof V. I., Schurch S., Waber U., Baumann M. Surfactant and inhaled particles in the conducting airways: structural, stereological, and biophysical aspects. Micros. Res. Techniq. 1993;26(5):423–436. doi: 10.1002/jemt.1070260510. [DOI] [PubMed] [Google Scholar]
- 28.Gehr P., Green F. H. Y., Geiser M., Im Hof V., Lee M. M., Schürch S. Airway surfactant, a primary defense barrier: mechanical and immunological aspects. J. Aerosol. Med. 1996;9(2):163–181. doi: 10.1089/jam.1996.9.163. [DOI] [PubMed] [Google Scholar]
- 29.Geiser M., Gerber P., Maye I., Im Hof V., Gehr P. Retention of Teflon particles in hamster lungs: a stereological study. J. Aerosol. Med. 2000;13(1):43–55. doi: 10.1089/jam.2000.13.43. [DOI] [PubMed] [Google Scholar]
- 30.Schurch S., Gehr P., Im Hof V., Geiser M., Green F. Surfactant displaces particles toward the epithelium in airways and alveoli. Resp. Physiol. 1990;80(1):17–32. doi: 10.1016/0034-5687(90)90003-H. [DOI] [PubMed] [Google Scholar]
- 31.Peters A., Veronesi B., Calderon-Garciduenas L., et al. Translocation and potential neurological effects of fine and ultrafine particles a critical update. Part Fibre Toxicol. 2006;3:13. doi: 10.1186/1743-8977-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Iravani J., van As A. Mucus transport in the tracheobronchial tree of normal and bronchitic rats. J. Pathol. 1972;106(2):81–93. doi: 10.1002/path.1711060204. [DOI] [PubMed] [Google Scholar]
- 33.Van De Donk H. J. M., Zuidema J., Merkus F. W. H. M. Correlation between the sensitivity of the ciliary beat frequency of human adenoid tissue and chicken embryo tracheas for some drugs. Rhinology. 1982;20(2):81–87. [PubMed] [Google Scholar]
- 34.Boek W. M., Romeijn S. G., Graamans K., Verhoef J. C., Merkus F. W. H. M., Huizing E. H. Validation of animal experiments on ciliary function in vitro. I. The influence of substances used clinically. Acta Oto-Laryngol. 1999;119(1):93–97. doi: 10.1080/00016489950182016. [DOI] [PubMed] [Google Scholar]
- 35.Boek W. M., Romeijn S. G., Graamans K., Verhoef J. C., Merkus F. W. H. M., Huizing E. H. Validation of animal experiments on ciliary function in vitro. II. The influence of absorption enhancers, preservatives and physiologic saline. Acta Oto-Laryngol. 1999;119(1):98–101. doi: 10.1080/00016489950182025. [DOI] [PubMed] [Google Scholar]
- 36.Merkus P., Romeijn S. G., Coos Verhoef J., Merkus F. W. H. M., Schouwenburg P. F. Classification of cilio-inhibiting effects of nasal drugs. Laryngoscope. 2001;111(4I):595–602. doi: 10.1097/00005537-200104000-00008. [DOI] [PubMed] [Google Scholar]
- 37.Sakagami M. In vivo, in vitro and ex vivo models to assess pulmonary absorption and disposition of inhaled therapeutics for systemic delivery. Adv. Drug Deliv. Rev. 2006;58(9–10):1030–1060. doi: 10.1016/j.addr.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 38.E. B. M. B. Prophet, J. B. Arrington, and L. H. Sobin (eds.). Laboratory Methods in Histotechnology, American Registry of Pathology: Armed Forces Insitute of Pathology, Washington (DC), 1994.
- 39.Gehr P., Bachofen M., Weibel E. R. The normal human lung: ultrastructure and morphometric estimation of diffusion capacity. Resp. Physiol. 1978;32(2):121–140. doi: 10.1016/0034-5687(78)90104-4. [DOI] [PubMed] [Google Scholar]
- 40.Gerber V., Gehr P., Straub R., Frenz M., King M., Im Hof V. Mucus quality on horse tracheal epithelium: microscopic grading based on transparency. Resp. Physiol. 1997;107(1):67–74. doi: 10.1016/S0034-5687(96)02503-0. [DOI] [PubMed] [Google Scholar]
- 41.Clary-Meinesz C. F., Cosson J., Huitorel P., Blaive B. Temperature effect on the ciliary beat frequency of human nasal and tracheal ciliated cells. Biol. Cell. 1992;76(3):335–338. doi: 10.1016/0248-4900(92)90436-5. [DOI] [PubMed] [Google Scholar]
- 42.A. J. Shah, and M. D. Donovan. Formulating gels for decreased mucociliary transport using rheologic properties: polyacrylic acids. AAPS PharmSciTech.8(2) (2007). [DOI] [PMC free article] [PubMed]
- 43.Rubin B. K. Immotile cilia syndrome (primary ciliary dyskinesia) and inflammatory lung disease. Clin. Chest Med. 1988;9(4):657–668. [PubMed] [Google Scholar]
- 44.King M. Relationship between mucus viscoelasticity and ciliary transport in guaran gel/frog palate model system. Biorheology. 1980;17(3):249–254. [PubMed] [Google Scholar]
- 45.King M. Interrelation between mechanical properties of mucus and mucociliary transport: effect of pharmacologic interventions. Biorheology. 1979;16(1–2):57–68. doi: 10.3233/bir-1979-161-210. [DOI] [PubMed] [Google Scholar]
- 46.Felicetti S. A., Wolff R. K., Muggenburg B. A. Comparison of tracheal mucous transport in rats, guinea pigs, rabbits, and dogs. J. Appl. Physiol. Resp. Environ. Exercise Physiol. 1981;51(6):1612–1617. doi: 10.1152/jappl.1981.51.6.1612. [DOI] [PubMed] [Google Scholar]
- 47.Hofmann W., Asgharian B. The effect of lung structure on mucociliary clearance and particle retention in human and rat lungs. Toxicol. Sci. 2003;73(2):448–456. doi: 10.1093/toxsci/kfg075. [DOI] [PubMed] [Google Scholar]
- 48.Foster W. M., Langenback E., Bergofsky E. H. Measurement of tracheal and bronchial mucus velocities in man: relation to lung clearance. J. Appl. Physiol. Resp. Environ. Exercise Physiol. 1980;48(6):965–971. doi: 10.1152/jappl.1980.48.6.965. [DOI] [PubMed] [Google Scholar]
- 49.Salathe M. Effects of b-agonists on airway epithelial cells. J. Allergy Clin. Immun. 2002;110(6):S275–S281. doi: 10.1067/mai.2002.129412. [DOI] [PubMed] [Google Scholar]
- 50.King M. Experimental models for studying mucociliary clearance. Eur. Resp. J. 1998;11(1):222–228. doi: 10.1183/09031936.98.11010222. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Exemplary video for mucociliary clearance of carbon particles. Particles are clearly and reproducibly transported on the tracheal tissue. Particle clearance is unidirectional and always to the proximal end of the trachea. Visualization and tracking of the carbon particles was realized by transition light microscopy. (×100, top view; MPG 2.78 MB)