INTRODUCTION
Poor aqueous solubility is usually a major obstacle in the development of therapeutic agents. Some of the approaches commonly used to enhance the solubility of poorly soluble drugs include use of co-solvents (1–2), selection of salt form (3–4), increase of specific surface area by reduction of particle size (5), complex formation with excipients such as hydrophilic polymers and cyclodextrins (6–8), change of crystal form (polymorphism/amorphism) (9) and preparation of solid dispersions (10–11). Micellar solubilization is a widely used alternative for the dissolution of poorly soluble drugs (12–14). Depending upon the drug hydrophobicity, it can be solubilized in the inner core of the micelle, on the surface of the micelle or at an intermediate location in the palisade layer. Solubilization of drugs by surfactant systems has been reviewed and discussed by many investigators (15–17).
The antidiabetic drugs used in the present work belong to class II (poor solubility and high permeability) of the biopharmaceutical classification system (BCS). Improvement in the solubility of glyburide by solid dispersion technique has been reported (18). Ammar et al. (19) have shown that the association of water soluble polymers with glimepiride–cyclodextrin systems leads to great enhancement in the dissolution rate of the drug. Complexation with cyclodextrin has been reported to increase the solubility of gliclazide (20–21). Surfactants have also been used to enhance the solubility of antidiabetic drugs (22–23). Alkhamis et al. (22) have studied the solubilization efficiency and locus of solubilization of gliclazide for various surfactants. A hydroalcoholic surfactant solution with a relatively low alcohol and Tween-80 content buffered at pH 7.4 has been used as dissolution medium for glyburide (23). However, detailed studies on surfactant solubilization of a wide range of antidiabetic drugs have not been reported. In addition to improving solubility and bioavailability of drugs, the use of micelles as drug carriers also presents other advantages such as reduced toxicity, enhanced permeability and longer residence time in the system (16). In the present paper an attempt has been made to enhance the solubility of seven antidiabetic drugs, gliclazide, glyburide, glimepiride, glipizide, repaglinide, pioglitazone and rosiglitazone using cationic, anionic and non-ionic surfactants and their mixtures.
MATERIALS AND METHODS
Pure samples of sulfonylureas and glitazones were obtained as gift from M/s USV Pharmaceuticals Ltd., Solan, H.P., India. Pure repaglinide was a generous gift from Torrent Pharmaceuticals Ltd., Gujarat, India. All other reagents were of analytical grade. Water used was double distilled in an all glass apparatus.
Drug Analysis
Drug estimation was done using ultraviolet absorption spectroscopic technique. Due to the poor solubility of drugs in water/aqueous buffer, 0.1 M NaOH and 0.1 M HCl were used as solvents for drug analysis in the case of sulfonylureas (gliclazide, glyburide, glimepiride, glipizide) and glitazones (pioglitazone, rosiglitazone), respectively. Phosphate buffer (0.1 M), pH 7.4 was used as solvent for repaglinide since the drug had sufficient solubility in aqueous buffer. Standard drug solutions in the appropriate solvent were prepared in the concentration range 10–100 μM and the ultraviolet absorption spectra were measured against solvent blank. Extinction coefficients in the relevant solvent, determined from the absorbance at wavelength corresponding to absorption maxima (λmax) versus drug concentration plots, were used to calculate unknown drug concentrations using Beer Lambert law. λmax values used for drug analysis were 226, 228, 275, 280, 269, 317 nm in the case of giclazide and glyburide, glimepiride, glipizide, repaglinide, pioglitazone and rosiglitazone, respectively.
Solubility Determination
For the determination of solubility, excess of drug was placed in contact with 5 mL of solvent in sealed conical flasks. The flasks were maintained at 25°C and the contents were stirred on a magnetic stirrer for 24 h. The solution was centrifuged and the supernatant was filtered through 0.45 μm filter. The absorbance of the clear solution was determined at λmax of the drug after suitable dilution with the appropriate solvent. The concentration of drug was determined from Beer Lambert law using extinction coefficients, determined in the relevant solvent. The solubility was calculated by multiplying the drug concentration, so obtained, by the appropriate dilution factor. The reported data are an average of three determinations. The standard error of mean (SEM), calculated using statistical software SPSS for Windows, was less than ±0.01 mg/mL in most cases.
Five surfactant systems; a cationic (CTAB), anionic (SDS) and a non-ionic (Tween-80) surfactant as well as equimolar mixtures of cationic + non-ionic (CTAB + Tween-80) and anionic + non-ionic (SDS + Tween-80) surfactants were studied. Fifty millimolar micellar concentration of surfactant (Cmicellar = Csurf − CMC, where Cmicellar, Csurf and CMC are the micellar, total and critical micellar concentrations of surfactant, respectively) was employed in each case. The medium used for the preparation of surfactant solutions was water, 0.15 M NaCl and 0.1 M phosphate buffer, pH 7.4 (PB).
RESULTS AND DISCUSSION
Solubilities of the antidiabetic drugs used in this work in water, 0.15 M NaCl and 0.1 M phosphate buffer, pH 7.4 are given in Tables I, II. Solubility, less than 0.1 mg/mL in most cases, indicates poor solubility of drugs in these media. Sulfonylureas and repaglinide, being acidic drugs, solubility in phosphate buffer (pH 7.4) was higher than that in water. The presence of salt also increased solubility in most cases. Alkali and halide ions are known to be water structure breakers (24). A layer of water molecules beyond the primary hydration shell which is less ordered than the bulk water, may be available for drug dissolution resulting in small increase in drug solubility in the presence of salt.
Table I.
Solubilities of Sulfonylureas in Various Surfactants at 25°C
| Drug/surfactant | Solubility (μg/mL) in various surfactantsa | ||
|---|---|---|---|
| Medium | |||
| Water | 0.15 M NaCl | Buffer | |
| Gliclazide | 37.32b | 119.21b | 175.06b |
| SDS | 583.47 | 798.20 | – |
| CTAB | 825.27 | 780.97 | 14,733 |
| Tween-80 | 2,465.4 | 4,177.1 | 3,139.3 |
| SDS + Tween-80 | 8,991.9 (1,524.4) | 4,908.3 (2,487.7) | – |
| CTAB + Tween-80 | 14,483 (1,645.3) | 4,621.1 (2,479.0) | 13,731 (8,936.0) |
| Glyburide | 5.96b | 9.78b | 8.22b |
| SDS | 12.12 | 22.53 | – |
| CTAB | 24.72 | 29.41 | 223.31 |
| Tween-80 | 545.13 | 174.33 | 224.46 |
| SDS + Tween-80 | 425.29 (278.62) | 5,236.4 (98.43) | – |
| CTAB + Tween-80 | 476.02 (284.92) | 4,913.2 (101.87) | 1,028.3 (223.88) |
| Glimepiride | 6.40b | 13.44b | 8.63b |
| SDS | 257.31 | 499.99 | – |
| CTAB | 315.81 | 300.95 | 8,534.3 |
| Tween-80 | 407.31 | 227.70 | 703.70 |
| SDS + Tween-80 | 2,591.5 (332.31) | 3,771.2 (363.84) | – |
| CTAB + Tween-80 | 2,759.4 (361.56) | 4,267.8 (264.32) | 4,039.8 (4,619.0) |
| Glipizide | 10.23b | 14.26b | 66.48b |
| SDS | 238.27 | 242.44 | – |
| CTAB | 120.10 | 145.95 | 2676.0 |
| Tween-80 | 173.97 | 71.05 | 196.04 |
| SDS + Tween-80 | 533.60 (206.12) | 231.73 (156.74) | – |
| CTAB + Tween-80 | 648.11 (147.03) | 224.26 (108.50) | 1,929.6 (1,436.0) |
aConcentration of surfactants: SDS = 58.00 mM, CTAB = 51.00 mM, Tween-80 = 50.01 mM
bSolubilities in the absence of surfactant
Table II.
Solubilities of Repaglinide and Glitazones in Various Surfactants at 25°C
| Drug/surfactant | Solubility(μg/mL) in various surfactantsa | ||
|---|---|---|---|
| Medium | |||
| Water | 0.15 M NaCl | Buffer | |
| Repaglinide | 39.82b | 24.47b | 140.86b |
| SDS | 872.05 | 1,027.5 | – |
| CTAB | 835.98 | 819.47 | 14,596 |
| Tween-80 | 980.81 | 937.08 | 6,077.6 |
| SDS + Tween-80 | 1,221.7 (926.43) | 919.44 (982.31) | – |
| CTAB + Tween-80 | 486.45 (908.39) | 1,045.0 (878.27) | 12,779 (10,337) |
| Pioglitazone | 14.05b | 25.70b | 10.61b |
| SDS | 417.32 | 465.11 | – |
| CTAB | 89.73 | 148.13 | 1,028.2 |
| Tween-80 | 82.78 | 112.45 | 26.40 |
| SDS + Tween-80 | 565.99 (250.05) | 7,065.2 (288.78) | – |
| CTAB + Tween-80 | 177.34 (86.25) | 2,431.3 (130.29) | 194.01 (527.29) |
| Rosiglitazone | 30.67b | 35.09b | 3.79b |
| SDS | 481.19 | 574.99 | – |
| CTAB | 511.94 | 627.16 | 3124.8 |
| Tween-80 | 465.85 | 416.02 | 365.22 |
| SDS + Tween-80 | 168.36 (473.52) | 8,253.2 (495.50) | – |
| CTAB + Tween-80 | 421.66 (488.89) | 7,337.8 (521.59) | 1,048.7 (1,745.0) |
aConcentration of surfactants: SDS = 58.00 mM, CTAB = 51.00 mM, Tween-80 = 50.01 mM
bValues in the absence of surfactant
Due to a large difference in the CMC of cationic, anionic and non-ionic surfactants, for the same total surfactant concentration, the fraction of surfactant in micellar form can be quite different. Since concentration at CMC is approximately equal to the monomer surfactant concentration, total surfactant concentration minus CMC can be taken as a measure of the micellar concentration of surfactant. Solubility of various drugs in the presence of 50 mM micellar concentration of an anionic (SDS), cationic (CTAB) and non-ionic (Tween-80) surfactant, determined at 25°C in water, is given in Tables I, II. Large solubility enhancement was observed in each case. Since the solubility of a drug molecule in water is likely to depend on various diverse structural factors and physico-chemical properties such as the size, shape, hydrophobicity of substituent groups and their effect on the water structure, degree of ionization and other solute–solute and solute–solvent interactions, the relative solubility increase by various surfactants was found to vary with the nature of drug. Non-ionic surfactant (Tween-80) was found to be a better solvent as compared to ionic surfactants in the case of most of the sulfonylureas (gliclazide, glyburide, glimepiride) and repaglinide. The low CMC of non-ionic surfactants, combined with the low toxicity, makes this class of surfactants particularly important for solubilization and delivery of drugs. Solubility of glipizide, pioglitazone and rosiglitazone, on the other hand, was found to be higher in ionic surfactants as compared to the non-ionic surfactant. Amongst ionic surfactants, gliclazide, glyburide, glimepiride and rosiglitazone had higher solubilization efficiency in cationic surfactant (CTAB) as compared to the anionic surfactant (SDS). Glipizide, pioglitazone and repaglinide were more soluble in anionic surfactant (SDS). The diverse solubilization behaviour of drugs could not be correlated to the structure and commonly used physico-chemical properties.
Effect of the Presence of Salt on Solubility Enhancement
The CMC of ionic surfactants decreases in the presence of electrolytes due mainly to decrease in the electrical repulsion between ionic head groups. The screening of charges would also lead to micellar growth, resulting in increased volume in the inner core of the micelle, where drug would be located. If a drug is solubilized in the inner core of the micelle, the solubility should increase in the presence of salt whereas solubilization in the outer palisade layer should decrease solubility. Thus solubility data in the presence of salt gives an estimate of the possible locus of solubilization of a drug in the micelle.
Since even in the absence of surfactant, salt causes a small change in the aqueous solubility of drugs (Tables I, II), the effect of salt on surfactant solubilization of drugs has been discussed with reference to the increase/decrease in the aqueous solubility in control solutions (without surfactant). The data is given in Tables I, II. In the case of anionic surfactant SDS, the presence of salt resulted in increase in solubilization in all cases except glipizide. The small increase in the case of glipizide is due to the presence of salt only. Thus in SDS, all the drugs except glipizide are solubilized in the inner core of the micelle. In the case of cationic surfactant (CTAB), the presence of salt resulted in increase in solubility in the case of glipizide, pioglitazone and rosiglitazone, indicating thereby that in CTAB these drugs are solubilized in the inner core of the micelle. Gliclazide and glimepiride, on the other hand, are solubilized in the outer layer of cationic surfactant (CTAB). Small increase in the case of glyuburide and small decrease in the case of repaglinide are due to the presence of salt only. Alkhamis et al. (22) while studying the solubilization of gliclazide by aqueous micellar solutions, have reported that gliclazide is solubilized mainly in the inner core of the cationic surfactant micelles and in the outer regions of the anionic surfactant micelles. Our results, however, show a different behaviour.
In the case of non-ionic surfactant (Tween-80), in all cases except gliclazide and pioglitazone, the presence of salt decreased solubility indicating thereby that these drugs are solubilized in the outer palisade layer in most cases. Since the antidiabetic drugs used in this work contain a number of hydrogen bond donors and acceptors, it appears that hydrogen bonding interactions between drugs and the polyethylene oxide (PEO) head groups in Tween-80 are responsible for the high solubilization capacity of this surfactant. Similar results have also been reported (16) for the solubilization of non-steroidal anti-inflammatory drug, ibuprofen.
Combined Effect of Surfactant and Buffer
Although surfactants produced significant solubility enhancement, the maximum solubility was found to be less than 1 mg/mL in most cases. It was, therefore, thought of interest to study the combined effect of surfactant and buffer. For this purpose phosphate buffer (pH 7.4) was used as solvent for the preparation of surfactant solutions and solubility was determined as before. The data is given in Tables I, II. The combined effect of surfactant and buffer produced enormous increase in solubility in the case of ionic surfactants. Higher pH of buffer may be responsible for the increased solubilization. However, such large increase cannot be only due to increase in pH of the medium since in the absence of surfactant, the ratio of solubility in buffer and water (Tables I, II) is very small. Apparently the presence of both buffer and surfactant has synergistic effect. It appears that the presence of buffer affects the micellization process thereby increasing solubilization of the drugs. Rangel-Yagui et al. (16) have reported significant reduction in the CMC of ionic surfactants in phosphate buffer. Since buffer components are electrolytes, they decrease CMC due to decreased electrical repulsion between ionic head groups, thereby decreasing the CMC and increasing the aggregation number and volume of micelles (24). Since most of the drugs are solubilized in the inner micellar core, increased solubilization was observed.
In the case of non-ionic surfactant (Tween-80) also the solubility was higher in phosphate buffer in the case of all acidic drugs except glyburide. The CMC of non-ionic surfactants is not much affected by the presence of buffer (16,24) and therefore, in this case reduction in CMC is not the cause for increased solubilization. Since the polyethylene oxide hydrophilic head groups of non-ionic surfactants interact with water through hydrogen bonds, this hydrogen bonding ability should decrease in the presence of buffer, resulting in increased solubilization of drugs in the palisade layer. In the case of basic drugs, pioglitazone and rosiglitazone, non-ionic surfactant (Tween-80) was not found to be a good solvent in aqueous medium; the presence of buffer had negative effect on the solubilization. The solubility in relatively alkaline buffered Tween-80 solutions was lower than the corresponding values in the absence of buffer.
Solubilization in Surfactant Mixtures
It is known that mixture of dissimilar surfactants behave quite differently as compared to single surfactants. The mixed surfactant systems (ionic + non-ionic) show synergistic behaviour and therefore the total quantity of surfactant required is smaller and physical properties such as CMC and interfacial tensions are much lower than would be expected based on the properties of pure components (25,26). Moreover, since non-ionic surfactants have relatively less toxicity as compared to the ionic surfactants, combination with the same total surfactant concentration will have lower toxicity. It was therefore, thought of interest to study equimolar mixtures of a cationic + non-ionic and anionic + non-ionic surfactants at the same total micellar concentration (50 mM) for solubility enhancement. The results are given in Tables I, II. In general, surfactant mixtures were found to be better solvents than single surfactants. The observed solubility was found to be much larger than the value calculated for an equimolar mixture of the two surfactants, in most cases. In aqueous medium, the solubility increase was found to be 6–9, 7–8, 2–5 times as compared to the calculated value in the case of gliclazide, glimepiride, glipizide/pioglitazone, respectively. In the presence of 0.15 M NaCl, surfactant mixtures were found to be especially good solvents; the solubility was larger by 48–53, 10–16,19–25, 14–17 times in the case of glyburide, glimepiride, pioglitazone and rosiglitazone, respectively. In pH 7.4 buffer medium, the use of surfactant mixtures produced noticeable increase (about five times) only in the case of glyburide.
Surfactant mixtures were found to be particularly good solvents for very poorly soluble antidiabetic drugs such as glyburide, glimepiride, pioglitazone and rosiglitazone which could be dissolved up to 5.24, 4.27, 7.06 and 8.25 mg/mL at 25°C. A very high solubility (>10 mg/mL) could also be attained for gliclazide. The mixed surfactant systems are known (25,26) to show nonideal synergistic behaviour resulting in substantial reduction in CMC and interfacial tensions, higher aggregation number and larger micellar size compared to the pure components. Large increase in the drug solubilization efficiency of mixed micelles shows that the drugs are solubilized in the inner core of mixed micelles. The increase in solubility in the presence of salt in most cases of surfactant mixtures also indicates solubilization into the inner core of mixed micelles.
Surfactant Solubilization Parameters
Usually, the solubility of a drug molecule by a surfactant can be evaluated based on two descriptors: molar solubilization capacity, χ, and micelle–water partition coefficient, 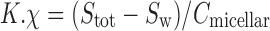 (where Stot is the total drug solubility, Sw is the water drug solubility, Cmicellar is the micellar concentration of surfactant), is defined as the number of moles of drug that can be solubilized by one mole of micellar surfactant. It characterizes the ability of the surfactant to solubilize drug. The micelle–water partition coefficient,
(where Stot is the total drug solubility, Sw is the water drug solubility, Cmicellar is the micellar concentration of surfactant), is defined as the number of moles of drug that can be solubilized by one mole of micellar surfactant. It characterizes the ability of the surfactant to solubilize drug. The micelle–water partition coefficient, 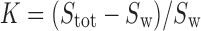 , is the ratio of the drug concentration in the micelle to the drug concentration in water for a particular surfactant concentration. From the thermodynamic point of view, the solubilization can be considered as a normal partitioning of the drug between micelle and aqueous phase and the standard free energy of solubilization,
, is the ratio of the drug concentration in the micelle to the drug concentration in water for a particular surfactant concentration. From the thermodynamic point of view, the solubilization can be considered as a normal partitioning of the drug between micelle and aqueous phase and the standard free energy of solubilization, 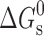 can be represented as
can be represented as 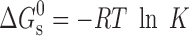 , where R, T and K are the universal gas constant, temperature and molar partition coefficient between micelle and aqueous phase, respectively. χ, K and
, where R, T and K are the universal gas constant, temperature and molar partition coefficient between micelle and aqueous phase, respectively. χ, K and 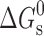 values for the solubilization of different drugs in various surfactants are given in Tables III, IV. In general, χ and K values, which give quantitative estimate of the solubilization efficiency of surfactant, were found to be higher for non-ionic surfactant (Tween-80) as compared to the ionic surfactants and equimolar ionic + nonionic surfactant mixtures as compared to the individual surfactants. The sign and magnitude of
values for the solubilization of different drugs in various surfactants are given in Tables III, IV. In general, χ and K values, which give quantitative estimate of the solubilization efficiency of surfactant, were found to be higher for non-ionic surfactant (Tween-80) as compared to the ionic surfactants and equimolar ionic + nonionic surfactant mixtures as compared to the individual surfactants. The sign and magnitude of 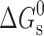 values indicated the effect of the nature of drug, nature of surfactant system and the medium (water/sodium chloride/buffer) on the spontaneity of the solubilization process.
values indicated the effect of the nature of drug, nature of surfactant system and the medium (water/sodium chloride/buffer) on the spontaneity of the solubilization process.
Table III.
Surfactant Solubilization Parameters for Various Sulfonylureas
| Drug/surfactant | Solubilization parameters | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Water | 0.15 M NaCl | Buffer | |||||||
| χ a | K |
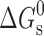 a
a
|
χ a | K |
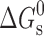 a
a
|
χ a | K |
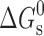 a
a
|
|
| Gliclazide | |||||||||
| SDS | 33.77 | 14.63 | −6.65 | 47.05 | 20.39 | −7.47 | – | – | – |
| CTAB | 48.73 | 21.11 | −7.56 | 45.99 | 19.93 | −7.41 | 908.79 | 393.76 | −14.81 |
| T-80 | 150.15 | 65.06 | −10.35 | 256.01 | 110.92 | −11.67 | 191.83 | 83.12 | −10.95 |
| SDS + T-80 | 553.77 | 239.93 | −13.58 | 301.24 | 130.52 | −12.07 | – | – | – |
| CTAB + T-80 | 893.36 | 387.07 | −14.77 | 283.47 | 122.82 | −11.92 | 846.82 | 366.91 | −14.63 |
| Glyburide | |||||||||
| SDS | 0.25 | 1.03 | −0.08 | 0.67 | 2.78 | −2.54 | – | – | – |
| CTAB | 0.76 | 3.15 | −2.84 | 0.95 | 3.94 | −3.40 | 8.80 | 36.49 | −8.91 |
| T-80 | 21.83 | 90.53 | −11.16 | 6.82 | 28.27 | −8.23 | 8.85 | 36.69 | −8.93 |
| SDS + T-80 | 16.98 | 70.41 | −10.54 | 211.76 | 878.22 | −16.80 | – | – | – |
| CTAB + T-80 | 19.03 | 78.93 | −10.82 | 198.67 | 823.96 | −16.64 | 41.39 | 171.65 | −12.75 |
| Glimepiride | |||||||||
| SDS | 10.23 | 39.18 | −9.09 | 20.12 | 77.07 | −10.77 | – | – | – |
| CTAB | 12.61 | 48.31 | −9.61 | 12.01 | 45.99 | −9.49 | 347.63 | 1,331.55 | −17.83 |
| T-80 | 16.34 | 62.60 | −10.25 | 9.02 | 34.55 | −8.78 | 28.42 | 108.88 | −11.62 |
| SDS + T-80 | 105.38 | 403.64 | −14.87 | 153.47 | 587.83 | −15.80 | – | – | – |
| CTAB + T-80 | 112.22 | 429.86 | −15.02 | 173.72 | 665.38 | −16.11 | 164.42 | 629.78 | −15.97 |
| Glipizide | |||||||||
| SDS | 10.24 | 22.30 | −7.69 | 10.42 | 22.70 | −7.74 | – | – | – |
| CTAB | 4.93 | 10.74 | −5.88 | 6.09 | 13.27 | −6.41 | 119.66 | 260.63 | −13.78 |
| T-80 | 7.35 | 16.01 | −6.87 | 2.73 | 5.95 | −4.42 | 8.34 | 18.17 | −7.18 |
| SDS + T-80 | 23.49 | 51.17 | −9.75 | 9.94 | 21.66 | −7.62 | – | – | – |
| CTAB + T-80 | 28.63 | 62.37 | −10.24 | 9.61 | 20.93 | −7.53 | 86.16 | 187.66 | −12.97 |
a
χ has been expressed as millimoles of drug solubilized per mol surfactant and 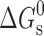 values are in kilojoules per mole at 298.15 K
values are in kilojoules per mole at 298.15 K
Table IV.
Surfactant Solubilization Parameters for Repaglinide and Glitazones
| Drug/surfactant | Solubilization parameters | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Water | 0.15 M NaCl | Buffer | |||||||
| χ | K |
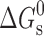 a
a
|
χ | K |
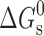 a
a
|
χ | K |
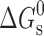 a
a
|
|
| Repaglinide | |||||||||
| SDS | 36.77 | 20.90 | −7.53 | 43.64 | 24.80 | −7.96 | – | – | – |
| CTAB | 35.18 | 19.99 | −7.42 | 34.45 | 19.58 | −7.37 | 643.23 | 365.53 | −14.62 |
| T-80 | 41.58 | 23.63 | −7.84 | 39.65 | 22.53 | −7.72 | 266.80 | 151.61 | −12.44 |
| SDS + T-80 | 52.23 | 29.68 | −8.40 | 38.87 | 22.09 | −7.67 | – | – | – |
| CTAB + T-80 | 19.74 | 11.22 | −5.99 | 44.42 | 25.24 | −8.00 | 562.91 | 319.88 | −14.29 |
| Pioglitazone | |||||||||
| SDS | 20.53 | 28.71 | −8.32 | 22.96 | 32.11 | −8.60 | – | – | – |
| CTAB | 3.85 | 5.39 | −4.17 | 6.83 | 9.55 | −5.59 | 51.62 | 72.19 | −10.60 |
| T-80 | 3.50 | 4.89 | −3.93 | 5.01 | 7.01 | −4.82 | 0.63 | 0.88 | +0.32 |
| SDS + T-80 | 28.10 | 39.29 | −9.10 | 358.93 | 501.94 | −15.41 | – | – | – |
| CTAB + T-80 | 8.31 | 11.62 | −6.08 | 123.05 | 172.07 | −12.76 | 9.16 | 12.81 | −6.32 |
| Rosiglitazone | |||||||||
| SDS | 19.03 | 14.69 | −7.53 | 22.99 | 17.75 | −7.96 | – | – | – |
| CTAB | 20.33 | 15.69 | −7.42 | 25.19 | 19.45 | −7.37 | 130.68 | 100.87 | −14.62 |
| T-80 | 18.38 | 14.19 | −7.84 | 16.28 | 12.56 | −7.72 | 14.13 | 10.91 | −12.44 |
| SDS + T-80 | 5.82 | 4.49 | −8.40 | 347.29 | 268.07 | −7.67 | – | – | – |
| CTAB + T-80 | 16.51 | 12.75 | −5.99 | 308.63 | 238.22 | −8.00 | 43.00 | 33.19 | −14.29 |
a
χ has been expressed as millimoles of drug solubilized per mole surfactant and 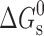 values are in kilojoules per mole at 298.15 K
values are in kilojoules per mole at 298.15 K
SUMMARY AND CONCLUSIONS
Micellar solubilization of seven poorly soluble antidiabetic drugs, gliclazide, glyburide, glimepiride, glipizide, repaglinide, pioglitazone and rosiglitazone has been studied using cationic (CTAB), anionic (SDS) and non-ionic (Tween-80) surfactants and cationic + non-ionic and anionic + non-ionic surfactant mixtures in the absence and presence of salt (0.15 M NaCl)/phosphate buffer (0.1 M, pH 7.4). In general, non-ionic surfactant was found to be a better solvent as compared to ionic surfactants. The combined effect of surfactant and buffer as well as solubilization in ionic–non-ionic mixed surfactant systems was synergistic and large solubility enhancement could be attained. The presence of salt resulted in increased solubilization in single and mixed surfactant systems in most cases. In general, drugs are solubilized in the inner core of ionic surfactants as well as mixed surfactant systems and the outer core of non-ionic surfactant. Very poorly soluble antidiabetic drugs such as glyburide, glimepiride, pioglitazone and rosiglitazone could be dissolved up to 5.24, 4.27, 7.06 and 8.25 mg/mL and for gliclazide, a very high solubility up to about 15 mg/mL could be attained. Surfactant solubilization parameters; molar solubilization capacity, χ, micelle–water partition coefficient, K and standard free energy of solubilization, 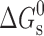 , gave quantitative estimate of the solubilization efficiency of the surfactant system.
, gave quantitative estimate of the solubilization efficiency of the surfactant system.
References
- 1.Etman M. A., Salama R. O., Shamsedeen M. A., El-Kamel A. Solubilization of etodolac for parenteral administration. Indian J. Pharm. Sci. 2001;63:459–467. [Google Scholar]
- 2.Li P., Zhao L., Yalkowsky S. H. Combined effect of cosolvent and cyclodextrin on solubilization of nonpolar drugs. J. Pharm. Sci. 1999;88:967–969. doi: 10.1021/js9901413. [DOI] [PubMed] [Google Scholar]
- 3.Bhatt P. M., Ravindra N. V., Banerjee R., Desiraju G. R. Saccharin as a salt former. Enhanced solubilities of saccharinates of active pharmaceutical ingredients. Chem. Commun. 2005;8:1073–1075. doi: 10.1039/b416137h. [DOI] [PubMed] [Google Scholar]
- 4.Nielsen A. B., Frydenvang K., Liljefors T., Buur A., Larsen C. Assessment of the combined approach of N-alkylation and salt formation to enhance aqueous solubility of tertiary amines using bupivacaine as a model drug. Eur. J. Pharm Sci. 2005;24:85–93. doi: 10.1016/j.ejps.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 5.Otsuka M., Matsuda Y. Effect of cogrinding with various kinds of surfactants on the dissolution behaviour of phenytoin. J. Pharm. Sci. 1995;84:1434–1437. doi: 10.1002/jps.2600841209. [DOI] [PubMed] [Google Scholar]
- 6.Kumar N. K., Murali M. B. G. V., Prasad C. D. S., Himasankar K., Seshasayana H. A., Murthy V. R. Comparative studies on the effect of some hydrophilic polymers on the dissolution rate of a poorly water-soluble drug meloxicam. Indian Drugs. 2002;39(6):323–329. [Google Scholar]
- 7.Corvi M. P., Cirri M., Allolio B. Enhancement of solubility and bioavailability by ternary complexation with β-cyclodextrin and glycine. J. Pharm. Sci. 2003;92:2177–2184. doi: 10.1002/jps.10485. [DOI] [PubMed] [Google Scholar]
- 8.Uekama K., Fujinaga T., Hirayama F., Otagiri M., Yamasaki M., Seo H., Hashimoto T., Tsuruoka M. Improvement of the oral bioavailability of digitalis glycosides by cyclodextrin complexation. J. Pharm. Sci. 1983;72:1338–1341. doi: 10.1002/jps.2600721125. [DOI] [PubMed] [Google Scholar]
- 9.Gupta P., Kakamanu V. K., Bansal A. K. Stability and solubility of Celecoxib–PVP Amorphous Dispersions: A Molecular Perspective. Pharm. Res. 2004;21:1762–1769. doi: 10.1023/B:PHAM.0000045226.42859.b8. [DOI] [PubMed] [Google Scholar]
- 10.Chauchan C., Shimpi S., Paradkar A. Preparation and characterization of etoricoxib solid dispersions using lipid carriers by spray drying technique. AAPS PharmaSciTech. 2005;6:50. doi: 10.1208/pt060350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saha R. N., Sajeev C., Padma P. K., Sreekhar C., Shashikanth G. Solubility enhancement of Nimesulide and ibuprofen by solid dispersion technique. Indian J. Pharm. Sci. 2002;64:529–534. [Google Scholar]
- 12.Desai K. G. H., Kulkarni A. R., Aminabhavi T. M. Solubility of rofecoxib in the presence of methanol, ethanol and sodium lauryl sulfate at (298.15, 303.15 and 308.15 K) J. Chem. Eng. Data. 2003;48:942–945. doi: 10.1021/je020213z. [DOI] [Google Scholar]
- 13.Liu C., Desai K. G. H., Liu C. Solubility of valdecoxib in the presence of Ethanol and Sodium lauryl sulfate at (298.15, 303.15 and 308.15) K. J. Chem. Eng. Data. 2004;49:1847–1850. doi: 10.1021/je0496948. [DOI] [Google Scholar]
- 14.Desai K. G. H., Park H. J. Solubility studies of valdecoxib in the presence of carriers, cosolvents and surfactants. Drug Dev. Res. 2004;62:41–48. doi: 10.1002/ddr.10365. [DOI] [Google Scholar]
- 15.Rangel-Yagui C. O., Pessoa Jr A., Tavares L. C. Micellar solubilization of drugs. J Pharm Pharmaceu Sci. 2005;8:147–163. [PubMed] [Google Scholar]
- 16.Rangel-Yagui C. O., Hsu H. W. L., Pessoa Jr A., Tavares L. C. Micellar solubilization of ibuprofen—influence of surfactant head groups on the extent of solubilization. Brazilian J Pharm Sci. 2005;41:237–246. [Google Scholar]
- 17.Attwood D., Florence A. T. Surfactant Systems: The Chemistry, Pharmacy and Biology. New York: Chapman and Hall; 1983. [Google Scholar]
- 18.Valleri M., Mura P., Maestrelli F., Cirri M., Ballerini R. Development and evaluation of glyburide fast developing tablets using solid dispersion technique. Drug Dev. Ind. Pharm. 2004;30:525–534. doi: 10.1081/DDC-120037483. [DOI] [PubMed] [Google Scholar]
- 19.Ammar H. O., Salama H. A., Ghorab M., Mahmoud A. A. Implication of inclusion complexation of glimepiride in cyclodextrin–polymer systems on its dissolution, stability and therapeutic efficacy. Int. J. Pharm. 2006;320:53–57. doi: 10.1016/j.ijpharm.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 20.Arias-Blanco M. J., Moyano J. R., Perez-Martinez J. I., Gines J. M. Study of the inclusion of gliclazide in a-cyclodextrin. J. Pharm. Biomed. Anal. 1998;18:275–279. doi: 10.1016/S0731-7085(98)00179-4. [DOI] [PubMed] [Google Scholar]
- 21.Moyano J. R., Arias-Blanco M. J., Gines J. M., Giordano F. Study of the complexation behaviour of gliclazide with partially methylated β-cyclodextrin in solution and solid state. Int. J Pharm. 1997;239:243. doi: 10.1016/s0378-5173(97)00233-0. [DOI] [PubMed] [Google Scholar]
- 22.Alkhamis K. A., Allaboun H., AL-Momani W. Y. Study of the solubilization of gliclazide by aqueous micellar solutions. J. Pharm. Sci. 2003;92:839–846. doi: 10.1002/jps.10350. [DOI] [PubMed] [Google Scholar]
- 23.El-massik M. A., Darwish I. A., Hassan E. E., El-Khordagui L. K. Development of a dissolution medium for glibenclamide. Int. J. Pharm. 1996;140:69–76. doi: 10.1016/0378-5173(96)04580-2. [DOI] [Google Scholar]
- 24.Florence A. T., Attwood D. Physicochemical Principles of Pharmacy. 3. London, UK: Macmillan; 1998. pp. 159–160, 230–232. [Google Scholar]
- 25.Paria S. The mixing behaviour of n-alkylpyridinium bromide-NP-9 mixed surfactant systems. Colloid Surf. A. 2006;281:113–118. doi: 10.1016/j.colsurfa.2006.02.023. [DOI] [Google Scholar]
- 26.Zhu L. Z., Zhu L., Shaoliang F., Feng S. L. Synergistic solubilization of polycyclic aromatic hydrocarbons by mixed ionic–non-ionic surfactants. Chemosphere. 2003;53:459–467. doi: 10.1016/S0045-6535(03)00541-1. [DOI] [PubMed] [Google Scholar]


