Abstract
The purpose of this study was to develop a lyotropic liquid crystalline formulation using the emulsifier vitamin E TPGS and evaluate its behavior after incorporation of a flavonoid, quercetin. The physical (macro and microscopic), chemical (determination of quercetin content by the HPLC method) and functional (determination of quercetin antioxidant activity by DPPH• assay) stability of the lamellar liquid crystalline formulation containing flavonoid was evaluated when stored at 4 ± 2 °C; 30 ± 2 °C/70 ± 5% RH (relative humidity) and 40 ± 2 °C/70 ± 5% RH during 12 months. The lamellar liquid crystalline structure of the formulation was maintained during the experiment, however chemical and functional stability results showed a great influence of the storage period in all conditions tested. A significant decrease in quercetin content (approximately 40%) was detected during the first month of storage and a similar significant loss in antioxidant activity was detected after 6 months. The remaining flavonoid content was unchanged during the final 6 months of the experimental period. The results suggest possible interactions between quercetin and the liquid crystalline formulation, which could inhibit or reduce the quercetin activity incorporated in the system. In conclusion, the present study demonstrated that incorporation of quercetin (1%) did not affect the liquid crystalline structure composed of vitamin E TPGS/IPM/PG–H2O (1:1) at 63.75/21.25/15 (w/w/w). Nevertheless, of the total quercetin incorporated in the system only 60% was free to act as an antioxidant.
Key words: antioxidant, DPPH•, HPLC, liquid crystals, quercetin, stability
INTRODUCTION
Human skin is frequently exposed to oxidative injury by a variety of environmental stressors, including solar radiation, nitrogen ozone oxides, and transition metals ions(1). The deleterious effects of sunlight and particularly UV radiation on the skin can lead to diverse damage as inflammation, skin aging, tumour promotion, cutaneous auto-immune disease, and phototoxicity/photosensitivity (2).
Topical administration of antioxidants provides an efficient way to enrich the endogeneous cutaneous protection system and thus may be a successful strategy for diminishing ultraviolet radiation-mediated oxidative damage to the skin (3,4). Therefore, systems to deliver an antioxidant active agent to cutaneous or subcutaneous levels may be of great interest as a therapeutic or a cosmetic approach for selective treatment and prevention of skin disorders (5,6).
Quercetin is one of the most abundant natural flavonoids present in various common vegetables and fruits. Numerous in vitro studies have revealed diverse biological effects of quercetin including antioxidant activity, which can be explained by its metal ion chelations, inhibition of lipid peroxidation and scavenging of oxygen radicals (7,8). Recently, it was demonstrated that topical formulations containing quercetin inhibit UVB-induced cutaneous oxidative stress and inflammation (9,10).
The design of new administration forms that increase the effectiveness of existing drugs is a new and recent trend observed in pharmaceutical technology. In this context, the use of vehicles having a liquid crystalline structure to carry drugs for topical use has been employed. It allows an easier diffusion of biological active substances through the skin besides having a considerable solubilizing capacity for both oil and water soluble compounds (11–13). Furthermore, liquid crystals are thermodynamically stable and can be stored for long periods of time without phase separation (13).
The stability evaluation of formulations containing quercetin that could be useful in the treatment of UVB-induced oxidative skin damage is one of the important issues in the study of new pharmaceutical products (14,15).
Chemical stability studies evaluate drug capacity to remain in concentrations necessary to guarantee its efficacy and safety (16). However, considering the fact that an antioxidant formulation could become pro-oxidant or loose its activity without apparently altering the drug content, chemical stability studies should always be accompanied by functional stability evaluations.
Functional stability guarantees the efficacy of a product with a specific function and has been proposed as a different approach to evaluate the stability of quercetin as an active pharmaceutical ingredient and also in different topical formulations (17,18).
The aim of this study was to investigate the physical, chemical and functional stability of a lamellar liquid crystalline formulation containing quercetin, stored for one year under different conditions. In the course of the experimental work different lyotropic liquid crystal systems were developed and the effect of quercetin on their phase behavior evaluated.
MATERIALS AND METHODS
Materials
Quercetin dihydrate 99% (C15H10O7.2H2O, Mw = 338.26) was purchased from Acros Organics (New Jersey, USA), vitamin E TPGS (d-alpha-tocopheryl polyethylene glycol 1000 succinate) from Eastman (Kingsport, Tennessee, USA), isopropyl myristate from Vetec (Rio de Janeiro, Brazil) and 2,2-diphenyl-1-picryl-hydrazyl (DPPH•) from Sigma Chemical Co. (St. Louis, MO, USA). Methanol (MeOH) and glacial acid acetic, both high-performance liquid chromatography (HPLC) grade were from J.T. Baker (USA) and Merck (Darmstadt, Germany), respectively. Solutions or mobile phase mixtures were prepared with water purified in a Milli-Q-plus System (Millipore, Bedforte, MA, USA).
Preparation of the Formulations
To determine the optimum ratio of isopropyl myristate (IPM), vitamin E TPGS and propylene glycol (PG)-H2O to obtain liquid crystalline phases, formulations containing different amounts of these compounds (2.5–67.5% IPM, 2.5–67.5% vitamin E TPGS, and 5–67.5%/2.5–45% PG–H2O, w/w/w) were prepared. Vitamin E TPGS was melted (40°C) and IPM was added under vortex stirring. Immediately thereafter, PG–H2O mixture pre-warmed to 40 °C was added, and the resulting formulations were allowed to rest in closed vials for 1 week at room temperature to reach equilibrium. Sample homogeneity and liquid crystalline phase formation were, respectively, examined by visual inspection and through a polarized light microscope (Carl Zeiss, Oberkichen, Germany).
Quercetin (final concentration of 1% w/w) was incorporated into the systems in which lamellar or cubic phases were previously obtained. For this, the flavonoid was firstly added under vortex stirring to melted (40 °C) vitamin E TPGS and immediately followed by the incorporation of IPM and PG–H2O mixture pre-warmed to 40 °C as described above.
Stability Studies
Lamellar liquid crystalline formulations with or without quercetin were stored at 4 ± 2 °C; 30 ± 2 °C/70 ± 5% RH (relative humidity) and 40 ± 2 °C/70 ± 5% RH for 12 months into BOD MA 415 UR incubators (Marconi®) with controlled temperature and humidity. At pre-determined times (immediately after preparation, 1, 2, 3, 6, 9 and 12 months) samples were collected for the evaluation of physical, chemical and functional stability as described below (19,20).
Physical Stability
Formulations were macroscopically characterized by visual analysis and microscopically through a polarized light microscope (Carl Zeiss, Oberkichen, Germany) to detect changes in consistence and liquid crystalline structure.
Chemical Stability
Lamellar liquid crystalline formulations were diluted in methanol to a final quercetin concentration equivalent to 50 μg/ml and samples were evaluated with regards to quercetin content by HPLC method.
Analyses were performed using a Shimadzu (Kyoto, Japan) liquid chromatograph, equipped with an LC-10 AT VP solvent pump unit and an SPD-10A VP UV-Visible detector. Samples were injected manually through a 20 μl loop with a Rheodyne injector. The separation was performed in a C18 Hypersyl BDS-CPS ciano (5 μm), 250 × 4.6 mm column with a mobile phase of methanol: water (60:40 v/v) containing 2% acetic acid (flow rate of 1 ml/min) and the drug detected at 254 nm. Data was collected using a Chromatopac CR8A integrator (Shimadzu, Kyoto, Japan). Values obtained for methanolic quercetin showed linearity over the concentration range of 0.1 to 200 μg/ml with a correlation coefficient (r) of 0.999. The quantification limit in the HPLC assay was 0.03 μg/ml and the average for relative standard variation and error was no more than 4.68% in all concentrations tested, which is considered adequate for analytical assays (21). No unidentified peaks were seen in the HPLC chromatograms.
Functional Stability
DPPH• assay was used to evaluate the antioxidant activity of quercetin incorporated in the formulations, which were submitted to different storage conditions.
Formulations added with 1% of quercetin were diluted to a final quercetin concentration of 50 μg/ml and the H-donor ability evaluated using an ethanolic solution of DPPH•, a stable nitrogen-centered free radical. Briefly, for radical scavenging measurements, 1 ml of 0.1 M acetate buffer (pH 5.5), 1 ml of ethanol and 0.5 ml of 250 μM ethanolic solution of DPPH• were mixed with 50 μl of the test sample and the light absorbance measured after 10 min at 517 nm (22).
The positive control was prepared by adding quercetin-free formulations submitted to the same storage conditions, and indicates the maximum odd electrons of DPPH•, which were considered as 100% free radicals in the solution and used to calculate the hydrogen-donating ability (%) of quercetin. The blank was prepared from the reaction mixture without DPPH• solution and all measurements were performed in triplicate. Values obtained for methanolic quercetin showed linearity over the concentration range of 0.1 to 2.0 μg/ml with a correlation coefficient (r) of 0.996. The average for relative standard variation and error was no more than 6.43% in all concentrations tested, in agreement with literature recommendations (21).
Statistical Analysis
Data were statistically analyzed by one way ANOVA, followed by Bonferroni’s multiple comparisons t-test to evaluate the influence of temperature and time of storage in the quercetin content and antioxidant activity of the liquid crystalline formulations. Results were considered significantly different when P < 0.05 was obtained.
RESULTS AND DISCUSSION
The present report describes the preparation and characterization of a liquid crystalline system containing the flavonoid quercetin. The liquid crystalline systems, as carriers for topical delivery of quercetin have not been explored to date.
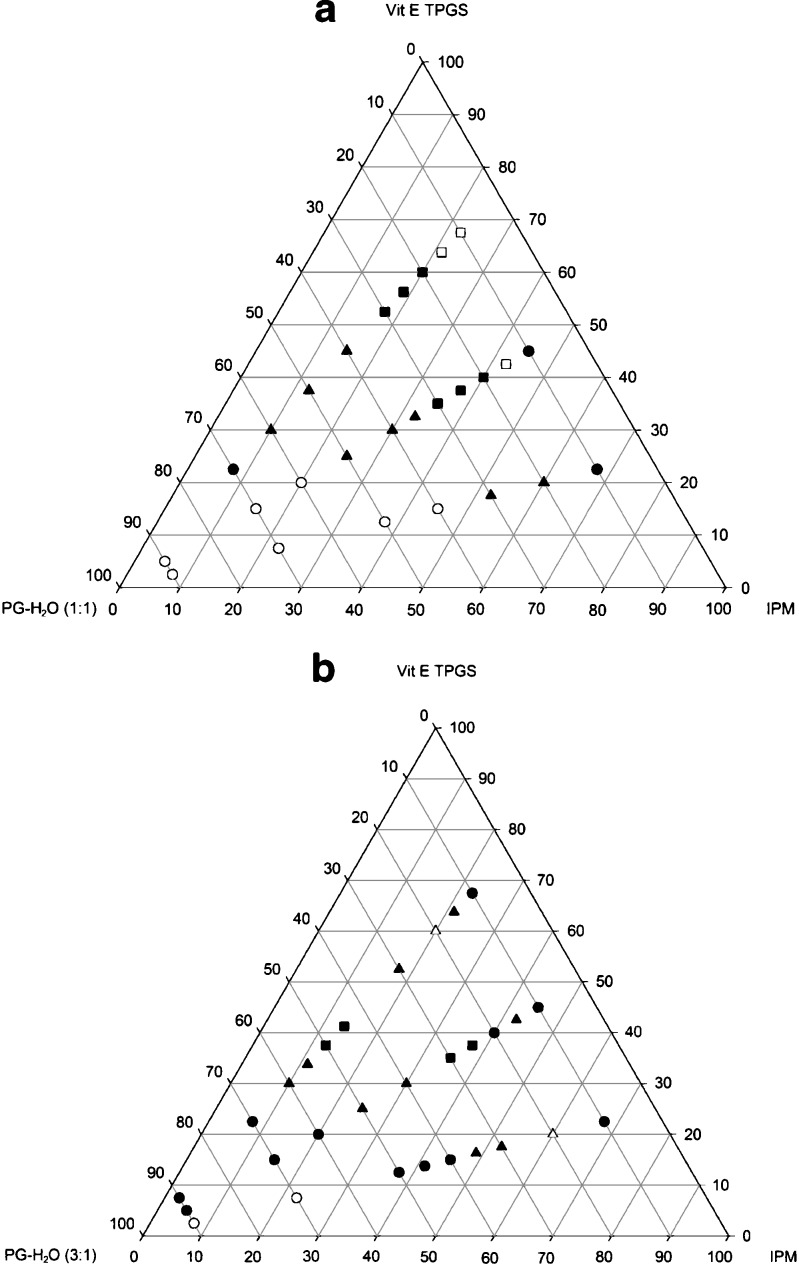
As a first step, different ratios of vitamin E TPGS/IPM/PG–H2O were tested to prepare the liquid crystalline systems. The phase diagrams of systems containing the PG–H2O components in 1:1 and 3:1 ratios are shown in Fig. 1a and b, respectively. The first ternary phase diagram shows that a lamellar phase was formed when the aqueous phase (PG–H2O 1:1) content was less than 15% and birefringence points when it was between 20% and 30%. Higher percentage increases led to a change to cubic phase. This could be explained by increased lamellar phase instability as the concentration of propylene glycol increases (23).
Fig. 1.
Phase diagrams of vitamin E TPGS/IPM/PG–H2O systems at a 1:1 and b 3:1 ratios. The phase regions are: filled circle isotropic liquid, filled triangle cubic phase, filled square birrefringence, empty circle unstable emulsion, open triangle cubic + anisotropic phases and empty square lamellar phase
Vitamin E TPGS/IPM/PG–H2O (1:1) systems, which resulted in the lamellar phase, were macroscopically more fluid while the cubic phase ones were transparent and very viscous. Polarized light microscopy of the liquid crystalline phases showed characteristic textures: the cubic phase is identified as a black field and the lamellar phase as oily streaks with inserted maltese crosses.
In the ternary phase diagram of vitamin E TPGS/IPM/PG–H2O (3:1) only cubic phases were formed when propylene glycol concentration varied from 11.25% to 45% and the water content from 3.75% to 15%.
A wide region containing liquid, homogeneous and isotropic systems when analyzed in the microscopic were also observed in the diagram and characterized as an isotropic liquid system. This system was observed when great (90%) or little (10%) amounts of PG–H2O (3:1) were used. An instable emulsion, which has two distinct phases, was formed when it was mixture 90 and 70% of PG–H2O (3:1), 7.5% and 2.5% of IPM and 22.5% and 7.5% of vitamin E TPGS, respectively.
The results corroborate with other studies (24–28), demonstrating the effectiveness of using vitamin E TPGS as a surfactant. Adequate systems (cubic and lamellar phases) are produced when equal amounts of propylene glycol and water are used in combination with the other components.
Alterations in the liquid crystalline structure and system properties by drug loading might have implications on drug release, system stability, and interactions of the system with the site of action. Since parameters such as pH, temperature, and the presence of other compounds in the system can influence the packing parameter of the lipid and consequently the liquid crystalline phase formed (29), we studied whether quercetin affects the liquid crystalline structure (cubic and lamellar phases) of some of the systems composed of vitamin E TPGS/IPM/PG–H2O.
The systems selected to incorporate quercetin are described in Table I. Addition of 1% quercetin to formulations containing PG–H2O (1:1) and previously showing cubic phases (F1 and F2), induced a phase transformation from cubic phase to (cubic phase + water), which may suggest that the system cannot accommodate the total amount of water when the drug was included. It suggests that the incorporated drug participates in the microstructure of the system and may even influence it due to molecular interactions (30).
Table I.
Percent Composition of Formulations in which Quercetin was Incorporated in the Final Concentration of 1% (w/w)
| Components | F1 (%) | F2 (%) | F3 (%) | F4 (%) | F5 (%) | F6 (%) |
|---|---|---|---|---|---|---|
| Vitamin E TPGS | 17.5 | 20.0 | 63.8 | 42.5 | 63.8 | 67.5 |
| IPM | 52.5 | 60.0 | 21.2 | 42.5 | 21.2 | 22.5 |
| PG–H2O (1:1) | 30.0 | 20.0 | 15.0 | 10.0 | ||
| PG–H2O (3:1) | 15.0 | 15.0 |
Drug incorporation into two other cubic phase systems containing less water (F3 and F4) similarly did not retain the cubic phase. Although cubic phase liquid crystalline systems are able to dissolve or disperse drugs of varies polarities, in some cases the active substance might interact with curved bicontinuous lipid bilayer of this phase making it unstable and resulting in a phase transformation (31).
F5 was the only system retaining the characteristic structure (lamellar phase) of liquid crystalline systems (Fig. 2) after addition of quercetin, demonstrating that it was the proper one for incorporating poorly water-soluble drugs. In addition, considering that lamellar lyotropic liquid crystalline systems are thermodynamically stable (32), F5 was selected for stability studies.
Fig. 2.
Polarized light microscopy of the lamellar liquid crystalline formulation composed of vitamin E TPGS/IPM/PG–H2O (1:1)/quercetin at 63.11:21.04:14.85:1.0 w/w/w/w at magnification of ×200. The lamellar phase is identified as oily streaks with inserted maltese crosses
Stability evaluation of active principles in formulations stored for various periods of time at different climatic conditions constitutes an important step in the development of new products. It provides information about the shelf-life of pharmaceutical products, as well as storage conditions (15,19). In the present study, the physical, chemical and functional stability of a lamellar liquid crystalline formulation containing quercetin was evaluated under different conditions of storage during 12 months.
Macro and microscopic analysis carried out in parallel with chemical and functional studies represent a necessary approach to predict physical sample behavior during stability tests.
The physical stability studies demonstrated that although it is known that temperature is one of the important factors influencing liquid crystalline structures, microscopic analysis indicated that the distinct conditions of storage did not compromise its structure.
Liquid crystalline system components interference in the HPLC and DPPH• methods were investigated in advance of the chemical and functional stability studies of the system containing quercetin. No interfering peaks were detected in the chromatographic patterns (data not shown) and no significant statistical difference was detected in the hydrogen-donating ability of quercetin compared to formulation only (33). These results further confirm the adequacy of these assays to evaluate the chemical and functional stabilities of quercetin in the liquid crystalline formulation, respectively. Furthermore, the quercetin content in the liquid crystalline formulation was determined by both methods, HPLC and DPPH•, showing very similar values which indicates the adequacy of DPPH• assay to determine drug concentration in the sample (Table II). A variation coefficient of approximately 5% in both methodologies could be attributed to intrinsic errors associated to them (21).
Table II.
Quercetin Content in the Lamellar Liquid Crystalline Formulation, Composed of Vitamin E TPGS/IPM/PG–H2O (1:1) at 63.75:21.25:15 (w/w/w), Determined by HPLC and by its Antioxidant Activity
| Samples | % inhibition in the DPPH• assay | Corresponding quercetin content (µg/ml)a | Quercetin content determined by HPLC (µg/ml) |
|---|---|---|---|
| 1 | 50.1 ± 0.4 | 50.0 ± 0.5 | 49.8 ± 0.3 |
| 2 | 50.0 ± 0.3 | 50.2 ± 0.3 | 49.8 ± 1.0 |
| 3 | 50.1 ± 0.6 | 50.2 ± 0.8 | 50.7 ± 0.6 |
Results are presented as mean ± SD (standard deviation) of three experiments run in parallel.
aQuercetin content in the DPPH• assay was estimated by the regression equation obtained by plotting the concentration of quercetin against the inhibition (%) in each concentration.
At the beginning of the study the choice of the concentration of flavonoid added to the liquid crystalline system to be used in chemical and functional stability studies was a matter of concern since the decrease in drug activity during the stability studies, if any, was not to known. It was necessary to select a concentration, which corresponded to a significant inhibition but it could not be one leading to the higher percentage of radical inhibition due to the plateau effect in the quercetin dose-response curve where different concentrations of the flavonoid showed the same inhibition against the radical (33). Quercetin concentrations in the plateau range might hinder stability results, since a decrease in content is not followed by the same decrease in antioxidant activity. For this reason, the concentration selected, to be used in chemical and functional stability studies, inhibits 50% of DPPH• and corresponds to 50 µg/ml of the flavonoid as determined by both DPPH• and HPLC methods.
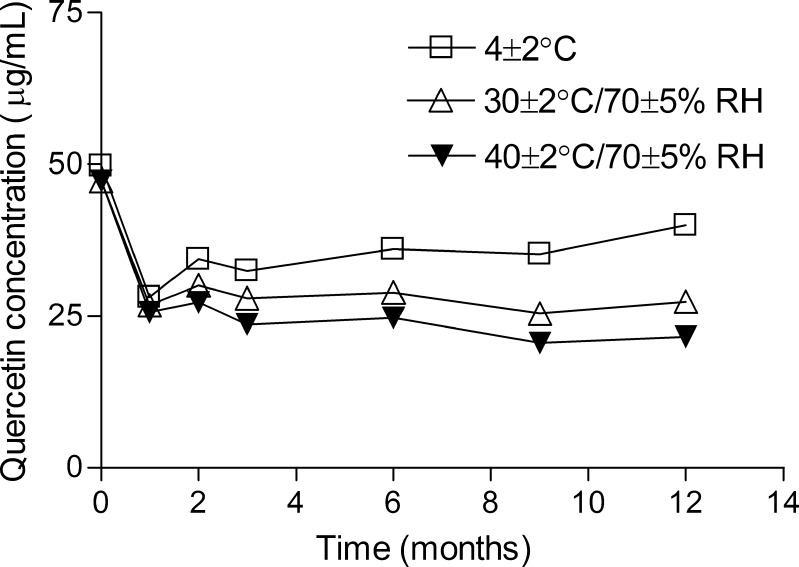
The chemical stability study demonstrated a significant decrease in quercetin content (approximately 40%) in the first month for samples under all storage conditions employed but it was maintained stable during the rest of the study (Fig. 3).
Fig. 3.
Quercetin concentration in the lamellar liquid crystalline formulation under storage at 4 ± 2 °C; 30 ± 2 °C/70 ± 5% RH and 40 ± 2 °C/70 ± 5% RH for 12 months determined by HPLC. Results are represented by mean ± SEM of three experiments run in parallel
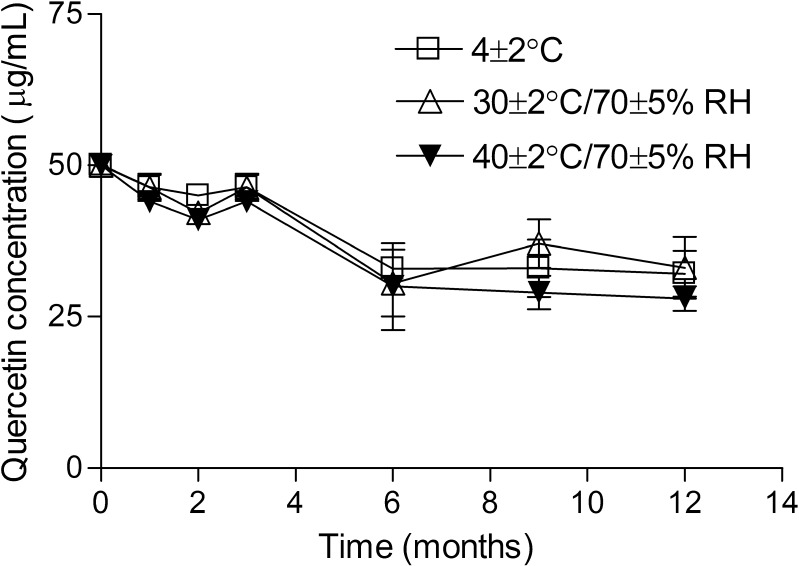
The functional stability study, however, showed that quercetin antioxidant activity did not decrease during the first months, corresponding to approximately 100% of the quercetin concentration incorporated in the formulation up to the sixth month of storage when the DPPH• assay showed a significant loss in antioxidant activity of approximately 40%, the remaining quercetin content being maintained thereafter (Fig. 4).
Fig. 4.
Quercetin concentration in the lamellar liquid crystalline formulation under storage at 4 ± 2 °C; 30 ± 2 °C/70 ± 5% RH and 40 ± 2 °C/70 ± 5% RH for 12 months determined by DPPH• assay. Results are represented by mean ± SEM of three experiments run in parallel
Statistical analysis of the results for chemical and functional stability assays detected significant differences between quercetin concentrations determined by the HPLC and DPPH• methods at different storage conditions at the first, second and third months (p < 0.001), but there was no difference in the concentrations determined at the other periods of time. Furthermore, in both stability studies (chemical and functional), the period of storage plays a greater influence than the conditions of storage in the stability of the liquid crystalline formulations added with quercetin.
Considering that both methodologies are equally precise and exact in the determination of quercetin concentration, the difference observed for chemical and functional stability studies could be possible due to interactions between quercetin and the liquid crystalline formulation. Although these interactions might prevent quercetin behavior at the chromatographic column they are not able to abolish the flavonoid capacity to donate H+ to the DPPH• radical, suggesting that it occurred with quercetin groups not responsible for its antioxidant activity. However, interactions established in the first month of storage could be modified further on during the time of storage (until the sixth month), and lead to the loss of quercetin antioxidant capacity. Future studies are planned in order to best elucidate the possible interactions suggested in this report.
CONCLUSION
The present report demonstrates that liquid crystalline formulations composed of vitamin E TPGS/ IPM/PG–H2O (1:1) at 63.75:21.25:15 (w/w/w), respectively, containing 1% quercetin maintain its lamellar phase. Nevertheless, only 60% of the total quercetin incorporated in the system is free to act as an antioxidant.
Acknowledgments
The authors thank Lívia N. Borgheti for technical support in the initial experiments. This study was supported by “Coordenação de Aperfeiçoamento de Pessoal de Nível Superior” (CAPES, Brazil) and “Fundação de Amparo à Pesquisa do Estado de São Paulo” (FAPESP, Brazil). F.T.M.C.Vicentini was the recipient of a CAPES fellowship.
References
- 1.Fuchs J., Weber S., Podda M., Groth N., Herrling T., Packer L., Kaufmann R. HPLC analysis of vitamin E isoforms in human epidermis: correlation with minimal erythema dose and free radical scavenging activity. Free Radic. Biol. Med. 2003;34:330–336. doi: 10.1016/S0891-5849(02)01293-5. [DOI] [PubMed] [Google Scholar]
- 2.Fuchs J., Packer L. Photooxidative stress in the skin. In: Sies H., editor. Oxidative Stress: Oxidants and Antioxidants. London, UK: Academic; 1991. pp. 559–583. [Google Scholar]
- 3.Montenegro L., Bonina F., Rigano L., Giogilli S., Sirigu S. Protective effect evaluation of free radical scavengers on UVB induced human cutaneous erythema by skin reflectance spectrophotometry. Int. J. Cosmet. Sci. 1995;17:91–103. doi: 10.1111/j.1467-2494.1995.tb00113.x. [DOI] [PubMed] [Google Scholar]
- 4.Saija A., Tomaino A., Trombetta D., Giacchi M., De Pasquale A., Bonina F. Influence of different penetration enhancers on in vitro skin permeation and in vivo photoprotective effect of flavonoids. Int. J. Pharm. 1998;175:85–94. doi: 10.1016/S0378-5173(98)00259-2. [DOI] [Google Scholar]
- 5.Bonina F. P., Montenegro L., Scrofani N., Esposito E., Cortesi R., Menegatti E., Nastruzzi C. Effects of phospholipid based formulations on in vitro and in vivo percutaneous absorption of methyl nicotinate. J. Control. Release. 1995;34:53–63. doi: 10.1016/0168-3659(94)00125-E. [DOI] [Google Scholar]
- 6.Saija A., Tomaino A., Trombetta D., De Pasquale A., Uccella N., Barbuzzi T., Paolino D., Bonina F. In vitro and in vivo evaluation of caffeic and ferulic acids as topical photoprotective agents. Int. J. Pharm. 2000;199:39–47. doi: 10.1016/S0378-5173(00)00358-6. [DOI] [PubMed] [Google Scholar]
- 7.Inal M. E., Kahraman A., Koken T. Beneficial effects of quercetin on oxidative stress induced by ultraviolet A. Clin. Exp. Dermatol. 2001;26:536–539. doi: 10.1046/j.1365-2230.2001.00884.x. [DOI] [PubMed] [Google Scholar]
- 8.Maiti K., Mukherjee K., Gantait A., Ahamed H. N., Saha B. P., Mukherjee P. K. Enhanced therapeutic benefit of quercetin-phospholipid complex in carbon tetrachloride-induced acute liver injury in rats: a comparative study. Iranian J. Pharmacol. Ther. 2005;4:84–90. [Google Scholar]
- 9.Casagrande R., Georgetti S. R., Verri Jr W. A., Dorta D. J., dos Santos A. C., Fonseca M. J. V. Protective effect of topical formulations containing quercetin against UVB-induced oxidative stress in hairless mice. J. Photochem. Photobiol. B: Biol. 2006;84:21–27. doi: 10.1016/j.jphotobiol.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 10.F. T. M. C. Vicentini, T. R. M. Simi, J. O. Del Ciampo, N. O. Wolga, D. L. Pitol, M. M. Iyomasa, M. V. L. B. Bentley, and M. J. V. Fonseca. Quercetin in w/o microemulsion: In vitro and in vivo skin penetration and efficacy against UVB-induced skin damages evaluated in vivo. Eur. J. Pharm. Biopharm. (in press) 2008, DOI 10.1016/j.ejpb.2008.01.012. [DOI] [PubMed]
- 11.Farkas E., Zelkó R., Németh Z. s., Pálinkás J., Marton S., Rácz I. The effect of liquid crystalline structure on chlorhexidine diacetate release. Int. J. Pharm. 2000;193:239–245. doi: 10.1016/S0378-5173(99)00346-4. [DOI] [PubMed] [Google Scholar]
- 12.Nesseem D. I. Formulation and evaluation of itraconazole via liquid crystal for topical delivery system. J. Pharmaceut. Biomed. 2001;26:387–399. doi: 10.1016/S0731-7085(01)00414-9. [DOI] [PubMed] [Google Scholar]
- 13.Makai M., Csányi E., Németh Z. s., Pálinkás J., Erós I. Structure and drug release of lamellar liquid crystals containing glycerol. Int. J. Pharm. 2003;256:95–107. doi: 10.1016/S0378-5173(03)00066-8. [DOI] [PubMed] [Google Scholar]
- 14.Bonina F., Lanza M., Montenegro L., Puglisi C., Tomaino D., Francesco C., Saija A. Flavonoids as potential protective agents against photo-oxidative skin damage. Int. J. Pharm. 1996;145:87–94. doi: 10.1016/S0378-5173(96)04728-X. [DOI] [Google Scholar]
- 15.Wessels P., Holz M., Erni F., Krummen K., Ogorka J. Statistical evaluation of stability data of pharmaceutical products for specification setting. Drug Dev. Ind. Pharm. 1997;23:427–439. doi: 10.3109/03639049709148492. [DOI] [Google Scholar]
- 16.Mendez A. S. L., Steppe M., Schapoval E. E. S. Validation of HPLC and UV spectrophotometric methods for the determination of meropenem in pharmaceutical dosage form. J. Pharm. Biom. Anal. 2003;33:947–954. doi: 10.1016/S0731-7085(03)00366-2. [DOI] [PubMed] [Google Scholar]
- 17.Casagrande R., Georgetti S. R., Verri Jr W. A., Jabor J. R., dos Santos A. C., Fonseca M. J. V. Evaluation of functional stability of quercetin as a raw material and in different topical formulations by its antilipoperoxidative activity. AAPS PharmSciTech. 2006;7:E1. doi: 10.1208/pt070110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guaratini T., Gianeti M. D., Campos P. M. B. G. M. Stability of cosmetic formulations containing esters of Vitamins E and A: Chemical and physicals aspects. Int. J. Pharm. 2006;327:12–16. doi: 10.1016/j.ijpharm.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 19.Singh S. Drug stability testing and shelf-life determination according to international guidelines. Pharmac. Technol. 1999;23:68–88. [Google Scholar]
- 20.Singh S. Stability test storage conditions for zones III and IV—some unresolved issues. Pharmac. Technol. 1999;23:131–141. [Google Scholar]
- 21.Vicentini F. T. M. C., Georgetti S. R., Jabor J. R., Caris J. A., Bentley M. V. L. B., Fonseca M. J. V. Photostability of quercetin under exposure to UV irradiation. Lat. Am. J. Pharm. 2007;26(1):119–124. [Google Scholar]
- 22.Dinis T. C. P., Madeira V. M. C., Almeida L. M. Action of phenolic derivates (acetaminophen, salicylate, and 5-aminosalicylate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. Arch. Biochem. Biophys. 1994;315:161–169. doi: 10.1006/abbi.1994.1485. [DOI] [PubMed] [Google Scholar]
- 23.Martino A., Kaler E. W. The stability of lamellar phases in water, propylene glycol, and surfactant mixtures. Colloids Surf. A. 1995;99:91–99. doi: 10.1016/0927-7757(95)03149-8. [DOI] [Google Scholar]
- 24.Mu L., Feng S. S. A novel controlled release formulation for the anticancer drug paclitaxel (Taxol®): PLGA nanoparticles containing vitamin E TPGS. J. Control. Release. 2003;86:33–48. doi: 10.1016/S0168-3659(02)00320-6. [DOI] [PubMed] [Google Scholar]
- 25.Mu L., Seow P. H. Application of TPGS in polymeric nanoparticulate drug delivery system. Colloids Surf. B Biointerfaces. 2006;47:90–7. doi: 10.1016/j.colsurfb.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 26.Sheu M. T., Wu A. B., Lin K. P., Shen C. H., Ho H. O. Effect of tocopheryl polyethylene glycol succinate on the percutaneous penetration of minoxidil from water/ethanol/polyethylene glycol 400 solutions. Drug Dev. Ind. Pharm. 2006;32:595–607. doi: 10.1080/03639040600599848. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Z., Feng S. S. Self-assembled nanoparticles of poly(lactide)-vitamin E TPGS copolymers for oral chemotherapy. Int. J. Pharm. 2006;324:191–198. doi: 10.1016/j.ijpharm.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 28.Suppasansatorn P., Nimmannit U., Conway B. R., Du L., Wang Y. Microemulsions as topical delivery vehicles for the anti-melanoma prodrug, temozolomide hexyl ester (TMZA-HE) J. Pharm. Pharmacol. 2007;59:787–94. doi: 10.1211/jpp.59.6.0005. [DOI] [PubMed] [Google Scholar]
- 29.Lopes L. B., Lopes J. L. C., Oliveira D. C. R., Thomazini J. A., Garcia M. T. J., Fantini M. C. A., Collett J. H., Bentley M. V. L. B. Liquid crystalline phases of monoolein and water for topical delivery of cyclosporine A: characterization and study of in vitro and in vivo delivery. Eur. J. Pharm. Biopharm. 2006;63:146–155. doi: 10.1016/j.ejpb.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 30.Müller-Goymann C. C. Physicochemical characterization of colloidal drug delivery systems such as reverse micelles, vesicles, liquid crystals and nanoparticles for topical administration. Eur. J. Pharm. Biopharm. 2004;58:343–356. doi: 10.1016/j.ejpb.2004.03.028. [DOI] [PubMed] [Google Scholar]
- 31.Shah J. C., Sadhale Y., Chilukuri D. M. Cubic phase gels as drug delivery systems. Adv. Drug Deliv. Rev. 2001;47:229–250. doi: 10.1016/S0169-409X(01)00108-9. [DOI] [PubMed] [Google Scholar]
- 32.Makai M., Csanyi E., Eros I., Dekany I. Preparation and structural determination of lyotropic lamellar liquid crystalline systems of pharmaceutical importance. Acta Pharm. Hung. 2003;73:71–76. [PubMed] [Google Scholar]
- 33.Vicentini F. T. M. C., Casagrande R., Georgetti S. R., Bentley M. V. L. B., Fonseca M. J. V. Influence of vehicle on antioxidant activity of quercetin: a liquid crystalline formulation. Lat. Am. J. Pharm. 2007;26(6):805–10. [Google Scholar]