Abstract
The purpose of this research work was to formulate and characterize self-micro emulsifying drug delivery system containing exemestane. The solubility of exemestane was determined in various vehicles. Pseudo ternary phase diagram was used to evaluate the micro-emulsification existence area. SMEDDS formulations were tested for micro-emulsifying properties, and the resultant formulations loaded with exemestane (ME1, ME2, ME3, ME4 and ME5) were investigated for clarity, phase separation, globule size and shape, zeta potential, effect of various diluents and dilutions, thermodynamic and thermal stability. From the results it is concluded that increase in droplet size is proportional to the concentration of oil in SMEDDS formulation. Minor difference in the droplet size and zeta potential was observed by varying the diluents (deionized water and 0.1 N HCl) and dilutions (1:10, 1:50 and 1:100). Formulations, which were found to be thermodynamically stable (ME1, ME2, ME3 and ME4), were subjected to stability studies as per International Conference on Harmonization (ICH) guidelines. No significant variations were observed in the formulations over a period of 3 months at accelerated and long-term conditions. TEM photographs of microemulsions formulations further conformed the spherical shape of globules. Among the various SMEDDS formulations, ME4 offer the advantages of good clarity systems at high oil content and thus offer good solubilization of exemestane. Thus this study indicates that the SMEDDS can be used as a potential drug carrier for dissolution enhancement of exemestane and other lipophilic drug(s).
KEY WORDS: aromatase inhibitors, exemestane, microemulsion, SMEDDS
INTRODUCTION
It is generally accepted that many of today’s new chemical entities (NCEs) are poorly water-soluble and pose a challenge in developing an optimum solid oral dosage form. Oral route has been the major route of drug delivery for the treatment of various chronic diseases like cancer. However, oral delivery of approximately 40% of the drug compounds is limited because of low aqueous solubility, which leads to limited oral bioavailability, high intra and inter subject variability and lack of dose proportionality (1).
To overcome the above discussed drawbacks, various other formulation strategies have been adopted including the use of cyclodextrins, nanoparticles, solid dispersions and permeation enhancers (1,2). In recent years, much attention has focused on lipid-based formulations to improve the oral bioavailability of poorly water-soluble drug compounds (3). In fact, the most popular approach is the incorporation of the drug compound into inert lipid vehicles such as oils and surfactant dispersions (4), self-emulsifying formulations (5–7), emulsions (8) and liposomes (9) with particular emphasis on self-microemulsifying drug delivery systems (SMEDDS) (10,11).
Self micro-emulsifying drug delivery systems (SEDDS) or self-emulsifying oil formulations (SEOF) are defined as isotropic mixtures of natural or synthetic oils, solid or liquid surfactants, or alternatively, one or more hydrophilic solvents and co solvents/surfactants. Upon mild agitation followed by dilution in aqueous media, such as GI fluids, form the droplets of emulsion (5–100 nm). Because of their unique solubilization properties SMEDDS offer the following advantages (12,13)
Bio-availability enhancement of poorly aqueous soluble drugs: SMEDDS offer the opportunity to present lipophilic drugs to the gastrointestinal tract in a dissolved state, avoiding the dissolution step (which can limit absorption rate of BCS Class 2 and 4 drugs).
Reduction in inter-subject and intra-subject variability.
Reduction of food effect.
Ease of manufacturing and scale up.
Ability to deliver peptides that are prone to enzymatic hydrolysis in GIT.
No influence of lipid digestion process.
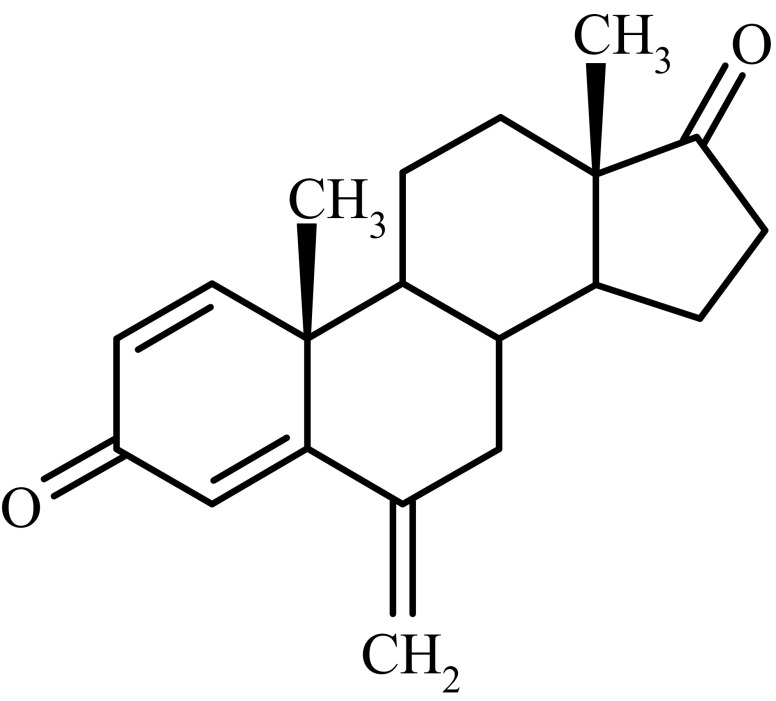
Breast cancer cell growth is often estrogen-dependent and antitumor activity is expected following effective and continuous estrogen suppression in patients with hormone-sensitive breast cancer. Aromatase is the key enzyme that converts androgens to estrogens both in pre- and postmenopausal women (14,15). Exemestane (androsta-1,4 diene-3,17-dione-6-methylene) (Fig. 1) is a potent irreversible Type I aromatase inhibitor, causing estrogen suppression and inhibition of peripheral aromatisation. It acts as a false substrate for the aromatase enzyme, and is processed to an intermediate that binds irreversibly to the active site of the enzyme causing its inactivation, an effect also known as suicide inhibition1 (16,17).
Fig. 1.
Structure of exemestane
Exemestane is practically insoluble in water (0.08 mg/ml) and have high hydrophobicity (log P 4.222). Exemestane exhibits low bioavailability in various animal models at a single dose of 25 mg. Food was shown to enhance absorption, resulting in plasma levels 30–40% higher than those observed in subjects under fasting conditions (17). Hence, exemestane was selected as a model drug for this study.
The aim of this study was to evaluate and characterize a system known to produce self-microemulsifying drug delivery system (SMEDDS) containing poorly water soluble drug (exemestane) with special emphasis on:
The solubility in SMEDDS and solubilization capacity after dispersion;
The influence of exemestane on dispersion properties and particle size of the identified SMEDDS; and
Investigate whether dilution would have any effect on the particle size of the identified SMEDDS and if this was dependent on drug load.
In this study optimized SMEDDS formulation was characterized for various physicochemical parameters (like droplets size and size distribution, zeta potential, dilution studies, thermodynamic stability studies morphology and thermal stability studies).
MATERIALS AND METHODS
Materials
Exemestane was obtained from Dabur Research Foundation (Ghaziabad, India). Cremophore ELP (Polyoxyl 35 castor oil) obtained from Dabur Pharma Ltd. (Kalyani, India), Labrafil M1944, Labrafil M2125, Transcutol HP (Diethylene glycol monoethyl ether) and Capryol 90 (Propylene glycol monocaprylate) obtained from Gattefosse (Saint Priest, France). Olive oil, Castor oil, Iso-propyl Myristate (IPM), oleic acid obtained from Loba Chem. All other chemicals and solvents were of analytical grade.
HPLC Analysis of Exemestane
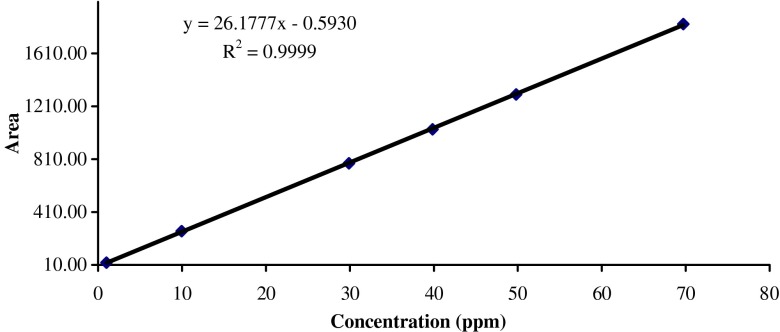
The concentration of exemestane was determined by HPLC method. The system consists of Agilent 1100 series with a UV detector. The chromatographic column was Inertsil ODS-3 (150 cm and 4.6 mm i.d.) with 5 μm particle size. The mobile phase (55:45) was acetonitrile and Milli-Q water at a flow rate of 1.0 ml/min and run time was 10 min. A 10-μl volume was injected into the system and the eluent was monitored at 248 nm. The retention time of exemestane was 5.8 ± 0.05 min at ambient room temperature. The mean calibration curve was given by the equation
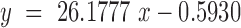 |
with a correlation coefficient, r2 = 0.9999, where y represents area under the curve and x the concentration in microgram per milliliter. The method was validated for accuracy, precision, specificity and solution stability. Linearity curve of exemestane was demonstrated in Fig. 2.
Fig. 2.
Linearity plot of exemestane by HPLC method
Solubility Studies
The solubility of exemestane in various oils was determined by HPLC method. An excess amount of exemestane was introduced into 2 ml of each excipients and mixture was kept in a sealed vials. Vortex mixer (Heidolph Multi Reax) was used to facilitate the solubilization (18). Sealed vials were stirred in a water bath (Julabo SW 23) at 40°C for 72 h. After standing for 72 h and reaching equilibrium at 30°C, each vials was centrifuged at 15,000 rpm for 10 min using a centrifuge (Eppendorf Centrifuge 5810). Undissolved exemestane was removed by filtering with a membrane filter (0.45 μm). The concentration of dissolved exemestane was determined. Results of solubility studies were reported in Table I (mean±SD; n = 3).
Table I.
Solubility Results of Exemestane in Various Oils
| S. No. | Oils | Solubility (mg/ml) |
|---|---|---|
| 01 | Corn oil | 9.6 ± 0.3 |
| 02 | Castor oil | 30.4 ± 0.7 |
| 03 | Cotton seed oil | 11.7 ± 1.2 |
| 04 | Olive oil | 10.0 ± 1.4 |
| 05 | Soyabean oil | 11.4 ± 0.4 |
| 06 | Oleic acid | 23.9 ± 1.5 |
| 07 | Iso propyl myristate (IPM) | 10.3 ± 0.8 |
| 10 | Labrafil M 2125 | 22.7 ± 1.1 |
| 11 | Labrafil M 1944 | 19.7 ± 0.2 |
| 12 | Capryol 90 | 88.7 ± 0.4 |
Construction of Phase Diagram
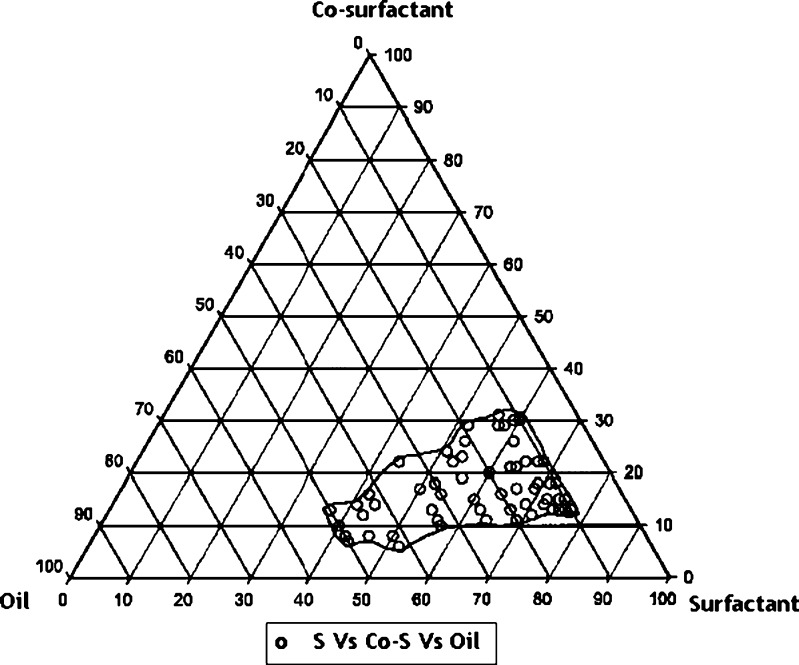
On the basis of solubility study data presented in Table I, Capryol 90 was selected as a lipid phase. Cremophore ELP and Transcutol HP were used as a surfactant and co-surfactant, respectively. To determine the concentration of components for the existing range of SMEDDS, pseudo-ternary phase diagram was constructed using water titration method at ambient temperature (25°C). Surfactant and co-surfactant were mixed in different volume ratios (1:1, 1:2, 1:3, 1:4, 1:4, 1:5, 1:6 and 2:1). Oil and surfactant/co-surfactant mixture (S/Co-S) were mixed thoroughly in different volume ratios (1:9, 1:8.5, 1:8, 1:7.5, 1:7, 1:6.5, 1:6, 1:5.5, 1:5, 1:4.5, 1:4, 1:3.5, 1:3, 1:2.5, 1:2, 1:1.5, 1:1, 1.5:1 and 2:1). The mixtures of oil, surfactant and co-surfactant at certain weight ratios were titrated with water by drop wise addition under gentle addition. Deionized water was used as diluting medium and added into the formulation. The proper ratio of one excipient to another in the SMEDDS formulation was analysed. The pseudo-ternary phase diagrams of the formulation composed of Capryol 90, Cremophore ELP and Transcutol HP is described in Fig. 3. Pseudo-ternary plot was constructed using Sigma Plot 10 software.
Fig. 3.
Ternary plot
After being equilibrated, the efficiency of self-emulsification, dispersibility, and appearance and flow ability was observed according to the five grading systems shown in Table II. Above observations were recorded in Table III. By the investigation of pseudo ternary phase diagram, some optimal placebo formulations, containing various ratios of oil, surfactant and co-surfactant, were selected to develop exemestane loaded SMEDDS formulations.
Table II.
Classification of the SMEDDS Formulation in Accordance to Comparative Grades
| Grade | Dispersibility and appearance | Time of self-microemulsification (min) |
|---|---|---|
| I | Rapid forming microemulsion, which is clear or slightly bluish in appearance | <1 |
| II | Rapid forming, slight less clear emulsion, which has a bluish white appearance | <2 |
| III | Bright white emulsion (similar to mill in appearance) | <2 |
| IV | Dull, grayish white emulsion with a slight oily appearance that is slow to emulsify | >3 |
| V | Exhibit poor or minimal emulsification with large oils droplets present on the surface | >3 |
Table III.
Visual Observation of SMEDDS Formulations
| Surfactant (S) | Cremophore ELP | |||||
|---|---|---|---|---|---|---|
| Co-surfactant (Co-S) | Transcutol HP | |||||
| Lipophilic phase (oil) | Caproyl 90 | |||||
| Smix to oil ratio | Surfactant/co-surfactant ratio (Smix) | |||||
| 6:1 | 5:1 | 4:1 | 3:1 | 2:1 | 1:1 | |
| 9:1 | I | I | I | I | II | III |
| 8.5:1 | I | I | I | I | II | III |
| 8:1 | I | I | I | I | II | III |
| 7.5:1 | I | I | I | I | II | III |
| 7:1 | I | I | II | I | II | III |
| 6.5:1 | I | I | I/II | I | II | III |
| 6:1 | I | I | I | I | II | III/IV |
| 5.5:1 | I | I | I | I | II/III | III/IV |
| 5:1 | I | I/II | I/II | II | II/III | III/IV |
| 4.5:1 | I/II | I/II | II | II | II/III | III |
| 4:1 | I | II | II | II/III | III | III |
| 3.5:1 | II | II | III | II/III | III | III/IV |
| 3:1 | II | II | III | II/III | III | III/IV |
| 2.5:1 | II | II/III | III | II/III | III | III/IV |
| 2:1 | II/III | II/III | IV | II | III | IV |
| 1.5:1 | II/III | II/III | III | I/II | III/IV | IV |
| 1:1 | I/II | II/III | III | I/II | III | V |
| 1:1.5 | III | III | IV | II | IV | V |
| 1:2 | III | IV | V | IV | V | V |
Preparation of Exemestane SMEDDS
Exemestane was added in the oily phase in small increment with continues stirring. The surfactant system was prepared by mixing separately the chosen surfactant and co-surfactant in their determined ratios. Exemestane containing oil solution was added in the surfactant system solution with continuous stirring and vortex mixing. Continued the stirring till the homogenous mixture formed. Finally, the mixture was kept at 25°C. Exemestane loaded SMEDDS formulations (ME1, ME2, ME3, ME4 and ME5) were subjected to further characterization. Detailed compositions of SMEDDS formulations were summarized in Table IV.
Table IV.
Comparative Grades for Assessment of Efficiency of Self-microemulsification Based in the Dispersibility, Appearance and Time of Microemulsification
| Composition | Formulation (g) | ||||
|---|---|---|---|---|---|
| ME1 | ME2 | ME3 | ME4 | ME5 | |
| Exemestane | 25 mg | 25 mg | 25 mg | 25 mg | 25 mg |
| Cremophore ELP | 755 mg | 640 mg | 690 mg | 430 mg | 370 mg |
| Transcutol HP | 125 mg | 220 mg | 110 mg | 70 mg | 130 mg |
| Caproyl 90 | 120 mg | 140 mg | 200 mg | 500 mg | 500 mg |
| Assessment of SMEDDS diluted with deionized water | |||||
| Visual observation grade | I | I | I | I/II | I/II |
| Droplet size (after 0.5 h) nm | 12.3 | 14.1 | 25.6 | 28.5 | 31.0 |
| Polydispersity index (after 0.5 h) | 0.11 | 0.23 | 0.08 | 0.06 | 0.02 |
| Zeta potential (after 0.5 h) mv | −2.2 | −7.3 | −0.7 | −9.7 | −5.4 |
| Droplet size (after 24 h) nm | 12.8 | 14.3 | 27 | 29.6 | 32.3 |
| Polydispersity index (After 24 h) | 0.04 | 0.05 | 0.37 | 0.09 | 0.08 |
| Zeta potential (after 24 h) mv | −1.8 | −7.1 | −0.9 | −10.8 | −4.2 |
| Assessment of SMEDDS diluted with 0.1 N HCl | |||||
| Visual observation grade | I | I | I | I/II | II |
| Droplet size (after 0.5 h) nm | 14.1 | 13.7 | 22.9 | 28.1 | 30.1 |
| Polydispersity index (after 0.5 h) | 0.07 | 0.01 | 0.08 | 0.23 | 0.18 |
| Zeta potential (after 0.5 h) mv | −2.7 | −6.9 | −1.0 | −10.6 | −5.9 |
| Droplet size (after 24 h) nm | 15.3 | 16.3 | 28.1 | 29.2 | 32.8 |
| Polydispersity index (after 24 h) | 0.11 | 0.15 | 0.08 | 0.03 | 0.34 |
| Zeta potential (after 24 h) mv | −2.9 | −7.8 | −1.6 | −11.8 | −4.1 |
Determination of Droplets Size Distribution and Zeta Potential
The droplet size, size distribution and zeta potential were analysed by dynamic light scattering with particle size apparatus (Malvern Zetasizer 3000 HS). Exemestane SMEDDS were diluted with deionized water and 0.1 N HCl in a drop-wise manner at 25°C under gentle shaking. After equilibrium droplet size and zeta potential were recorded in Table IV.
Dilution Studies
Dilution may better mimic conditions in the stomach following oral administration of SMEDDS pre-concentrate. Dilution study was done to access the effect of dilution on SMEDDS pre-concentrates. In this study selected formulations were subjected to various dilutions (i.e.1: 10, 1:50 and 1:100) with various diluents (i.e. deionized water, 0.1 N HCl) and the visual observation were recorded in Table V.
Table V.
Observation of Dilution Studies
| S. No. | Formulation code | Cremophore (%) | Transcutol HP (%) | Capryol 90 (%) | Dilution with deionized water | Dilution with 0.1 N HCl | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1:10 | 1:50 | 1:100 | 1:10 | 1:50 | 1:100 | |||||
| 01 | ME1 | 75.5 | 12.5 | 12.0 | I | I | I | I | I | I |
| 02 | ME2 | 64.0 | 22.0 | 14.0 | I | I | I | I | I/II | I/II |
| 03 | ME3 | 69.0 | 11.0 | 20.0 | I/II | I/II | II | I/II | II | II |
| 04 | ME4 | 43.0 | 7.0 | 50.0 | I | I | I | I | I/II | I/II |
| 05 | ME5 | 37.0 | 13.0 | 50.0 | I/II | I/II | II | II | I/II | I/II |
Thermodynamic Stability Studies of Exemestane SMEDDS
The objective of thermodynamic stability is to evaluate the phase separation and effect of temperature variation on SMEDDS formulations. Exemestane SMEDDS were diluted with aqueous medium and centrifuged at 15,000 rpm for 15 minutes and formulation were observed visually for phase separation. Phase separation was observed in ME5 sample.
Formulations were subjected to freeze thaw cycles (−20°C for 2 days followed by +40°C for 2 days) (19). No change in the visual description of samples after freeze-thaw cycles. Formulations, which are thermodynamically stable, were selected for further characterization.
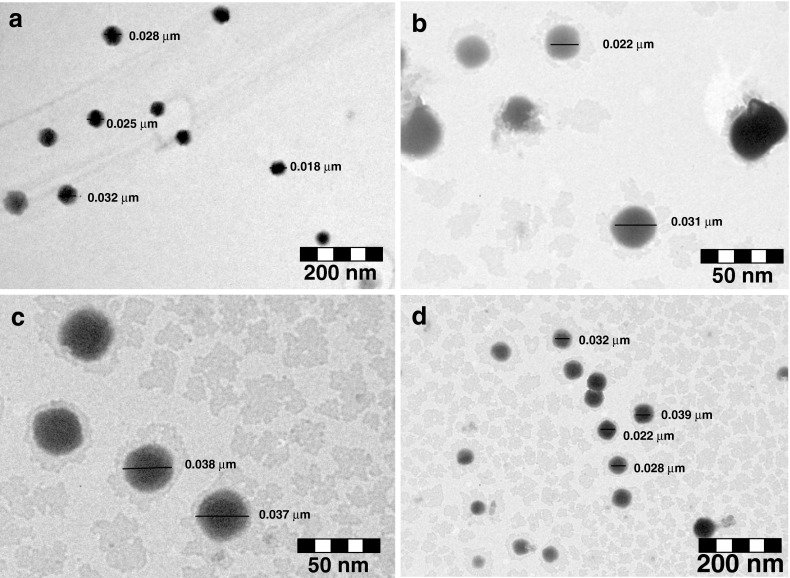
Transmission Electron Microscopy
From the results of thermodynamic stability studies four formulations (i.e. ME1, ME2, ME3 and ME4) were selected for morphological characterization using transmission electron microscopy (TEM). Transmission electron microscope (TEM; Philips CM12 Electron Microscope, Eindhoven, The Netherlands) was used as a visualizing aid. SMEDDS formulations were diluted with water (1:100). A drop of the diluted microemulsion was directly deposited on the holey film grid and observed the morphology of formulations Fig. 4a, b, c, d.
Fig. 4.
TEM photograph a ME1 formulation; b ME2 formulation; c ME3 formulation; d ME4 formulation
Stability Studies
Formulations, which were found to be thermodynamically stable, were subjected to stability studies. Samples of stability studies were charged on 25°C ± 2°C/60 ± 5% RH (Newtronics stability chamber) and 40°C ± 2°C/75 ± 5% RH (Newtronics stability chamber Samples were subjected to stability studies for 3 months period. Observations of stability studies were recorded in the Table VI.
Table VI.
Stability Assessment of SMEDDS Formulations
| Formulations | Drug content (%) | |||
|---|---|---|---|---|
| ME1 | ME2 | ME3 | ME4 | |
| 40°C ± 2°C/75% ± 5% RH-1 month | ||||
| Drug content (%) | 99.6 | 99.2 | 99.5 | 99.5 |
| Assessment of SMEDDS diluted with deionized water | ||||
| Visual observation grade | I | I | I/II | I/II |
| Droplet size (nm) | 13.2 | 15.1 | 29.8 | 30.5 |
| Polydispersity Index | 0.16 | 0.18 | 0.09 | 0.08 |
| Assessment of SMEDDS diluted with 0.1 N HCl | ||||
| Droplet size (nm) | 14.6 | 19.3 | 24.5 | 34.1 |
| Polydispersity Index | 0.08 | 0.19 | 0.06 | 0.12 |
| 40°C ± 2°C/75% ± 5% RH-3 month | ||||
| Drug content (%) | 99.5 | 99.3 | 99.1 | 99.6 |
| Assessment of SMEDDS diluted with deionized water | ||||
| Visual observation grade | I | I | I/II | I/II |
| Droplet size (nm) | 15.1 | 17.9 | 26.9 | 24.8 |
| Polydispersity Index | 0.11 | 0.24 | 0.13 | 0.14 |
| Assessment of SMEDDS diluted with 0.1 N HCl | ||||
| Droplet size (nm) | 14.4 | 18.0 | 25.4 | 25.1 |
| Polydispersity Index | 0.13 | 0.21 | 0.07 | 0.11 |
| 25°C ± 2°C/60% ± 5% RH-3 month | ||||
| Drug content (%) | 99.7 | 99.6 | 99.3 | 99.4 |
| Assessment of SMEDDS diluted with deionized water | ||||
| Visual observation grade | I | I | I/II | I/II |
| Droplet size (nm) | 16.9 | 18.8 | 31.4 | 28.8 |
| Polydispersity Index | 0.04 | 0.21 | 0.07 | 0.11 |
| Assessment of SMEDDS diluted with 0.1 N HCl | ||||
| Droplet size (nm) | 21.0 | 24.4 | 33.8 | 29.4 |
| Polydispersity Index | 0.22 | 0.09 | 0.13 | 0.14 |
RESULT AND DISCUSSION
SMEDDS is a homogenous mixture of lipids, surfactants and co-surfactants, which get emulsified on contact with aqueous phase under gentle agitation. It is considered that the excipients in the SMEDDS could enhance the dissolution and permeability of drug by significantly decreasing the droplet size. To develop an optimum self-emulsifying formulation (SMEDDS), it is very important to evaluate (a) the drug solubility in various components; (b) area of self-emulsifying region in the phase diagram; (c) and distribution of droplet size (20).
The components used for developing a SMEDDS formulation should have high solubilization capacity for the drug, ensuring maximum solubilization of drug in the resultant dispersion. Solubility of exemestane in various oils was determined by HPLC method. Since the exemestane exhibit maximum solubility in Capryol 90 than other oils, Capryol 90 was selected as an oil phase for exemestane SMEDDS formulation.
Self-microemulsifying systems form fine oil–water emulsions with only gentle agitations, upon their introduction into aqueous media. Surfactant and co-surfactant get preferentially absorbed at the interface, reducing the interfacial energy as well as providing a mechanical barrier to coalescence. The decrease in the free energy required for the emulsion formation consequently improve the thermodynamic stability of the microemulsion formulations. The efficiency of self-emulsification of surfactant and co-surfactant is much related to their hydrophilic–lipophilic balance (HLB) value. Generally surfactants with HLB 12–15 are regarded as being of good efficiency for self emulsification (21). Considering the safety and biocompatibility of the excipients, the selected system, known to produce SMEDDS consist of a nonionic surfactant (Cremophor ELP), propylene glycol monocaprylate (Capryol 90) and Transcutol HP (Diethylene glycol monoethyl ether) was selected for the development of exemestane SMEDDS.
The construction of Pseudo-ternary phase diagram makes it easy to find out the concentration range of components for the existence range of SMEDDS. Pseudo-ternary plot was constructed by using Capryol 90, Cremophore ELP and Transcutol HP as presented in the Fig. 3. Formation of microemulsion systems was observed at room temperature. Phase behavior investigation of this system demonstrated the suitable approach to determining an optimum oil, surfactant and co-surfactant ratio with which transparent microemulsion system was formed.
Microemulsion region that contains the oil component approximately 10–50% resulting in an extensive microemulsion region of SMEDDS. From this region five different ratio of Oil/S/Co-S were selected. In the selected pre-concentrate mixture exemestane was incorporated and the formulations (ME1, ME2, ME3, ME4 and ME5) were subjected to further characterization.
The effect of concentration of oil on the droplet size was investigated after SMEDDS formulations were dispersed with deionized water at 25°C. The droplet increased from 12.3 nm to 31.0 nm, when the concentration of oil added increased from 12.0% to 50.0%.
An increase in the ratio of the oil phase (Capryol 90) resulted in a proportional increase in particle size, because of the simultaneous decrease in the S/CoS proportion. Increasing the S/CoS ratio led to a decrease in mean droplet size. ME1, with the highest proportion of surfactant (75.5% wt/wt), had the lowest mean particle diameter. This could be attributed to an increased surfactant proportion relative to co-surfactant. It is well known that the addition of surfactants to the microemulsion systems causes the interfacial film to stabilize and condense, while the addition of co-surfactant causes the film to expand; thus, the relative proportion of surfactant to co-surfactant has varied effects on the droplet size.
To investigate the effect of the dispersing medium on zeta potential, SMEDDS formulations were dispersed with deionized water and 0.1 N HCl, respectively. Minor difference in zeta potential was observed between the two dispersing media at the same dilution. Composition and detailed assessment of optimized formulations are summarized in Table IV.
The influence of increasing the dilution factor from (1:10, 1:50 and 1:100) was evaluated; larger dilutions may better mimic conditions in the stomach following oral administration of SMEDDS (pre-concentrate). In all cases, increased dilution resulted in the microemulsion remaining with the same clarity.
Thermodynamic stability study was designed to identify and avoid the metastable SMEDDS formulations. In thermodynamic stability studies, formulations selected were subjected to different stress tests like centrifugation and freeze–thaw test. If the SMEDDS formulations are stable in this condition, metastable formulations thus avoided and frequent tests need not to be performed during storage. Thermodynamic stability of formulations is directly proportional to content of surfactant (Cremophore ELP) in the formulation. ME5 formulation of exemestane, which contains 37% of Cremophore ELP, found to be thermodynamically unstable. Formulations that found to be thermodynamically stable were considered for further characterization.
Samples of exemestane SMEDDS were charged on accelerated and long term stability conditions. Chemical and visual observations of samples were shown in Table VI. No significant change in the drug content in the formulations was observed over the period of 3 months at accelerated and long-term stability conditions. However exemestane SMEDDS demonstrate insignificant difference in the particle size and polydispersity results when diluted with deionized water and 0.1 N HCl.
The morphology of microemulsion was examined with a transmission electron microscope. The droplet on the microemulsion appears dark with the bright surroundings. TEM photographs [Fig. 4 (a, b, c, d)] further conformed that the globules are spherical in shape.
CONCLUSION
An optimized exemestane loaded formulation consisting of Capryol 90 (50% w/w), Cremophore ELP (43%), Transcutol HP (7%) offers the advantage of good clarity systems at high oil content and thus should offer good solubilization of exemestane. Thus our studies conformed that SMEDDS can be used as a possible alternative to conventional oral formulation of exemestane. Results further conclude that SMEDDS can be explored as a potential drug carrier for dissolution enhancement of exemestane and other lipophilic drug.
Acknowledgement
The authors would like to thank Gattefosse (Saint Priest, France) and GPS Pharma (Delhi, India) for providing the excipients for this study.
References
- 1.Robinson J. R. Introduction: semi-solid formulations for oral drug delivery. Bull. Tech.-Gattefosse. 1996;89:11–13. [Google Scholar]
- 2.Aungst B. J. Novel formulation strategies for improving oral bioavailability of drugs with poor membrane permeation or presystemic metabolism. J. Pharm. Sci. 1993;82:979–987. doi: 10.1002/jps.2600821002. [DOI] [PubMed] [Google Scholar]
- 3.Humberstone A. J., Charman W. N. Lipid-based vehicles for the oral delivery of poorly water-soluble drugs. Adv. Drug Del. Rev. 1997;25:103–128. doi: 10.1016/S0169-409X(96)00494-2. [DOI] [Google Scholar]
- 4.Chiou W. L., Chen S. J., Athanikar N. Enhancement of dissolution rates of poorly water-soluble drugs by crystallization in aqueous surface solutions. I. Sulfathiazole, prednisone, and chloramphenicol. J. Pharm. Sci. 1976;65:1702–1704. doi: 10.1002/jps.2600651137. [DOI] [PubMed] [Google Scholar]
- 5.Pouton C. W. Self-emulsifying drug delivery systems: assessment of the efficiency of emulsification. Int. J. Pharm. 1985;27:335–348. doi: 10.1016/0378-5173(85)90081-X. [DOI] [Google Scholar]
- 6.Pouton C. W. Effects of the inclusion of a model drug on the performance self-emulsifying formulations. J. Pharm. Pharmacol. 1985;37:1P. [Google Scholar]
- 7.Pouton C. W. Formulation of self-emulsifying drug delivery systems. Adv. Drug Deliv. Rev. 1997;25:47–58. doi: 10.1016/S0169-409X(96)00490-5. [DOI] [Google Scholar]
- 8.Kararli T. T., Needham T. E., Grifaen M., Schoenhard G., Ferro L. J., Alcorn L. Oral delivery of a rennin inhibitor compound using emulsion formulation. Pharm. Res. 1992;9:888–893. doi: 10.1023/A:1015896731545. [DOI] [PubMed] [Google Scholar]
- 9.Schwendener R. A., Schott H. Lipophilic 1-beta-d-arabinofuranosyl cytosine derivatives in liposomal formulations for oral and parenteral antileukemic therapy in the murine L1210 leukemia model. J. Cancer Res. Clin. Oncol. 1996;122:723–726. doi: 10.1007/BF01209119. [DOI] [PubMed] [Google Scholar]
- 10.Shen H., Zhong M. Prepration and evaluation of self-microemulsifying drug delivery systems (SMEDDS) containing atorvastatin. J. Pharm. Pharmacol. 2006;58:1183–1191. doi: 10.1211/jpp.58.9.0004. [DOI] [PubMed] [Google Scholar]
- 11.Wang D. K., Shi Z. H., Liu L., Wang X. Y., Zhang C. X., Zhao P. Development of Self-microemulsifying drug delivery systems for oral bioavailability enhancement of a-Asarone in beagle dogs. PDA J. Pharm. Sci. Tech. 2006;60(6):343–349. [PubMed] [Google Scholar]
- 12.Constantinides P. P. Lipid microemulsion for improving drug dissolution and oral absorption: physical and biopharmaceutical aspects. Pharm. Res. 1995;12(11):1561–1572. doi: 10.1023/A:1016268311867. [DOI] [PubMed] [Google Scholar]
- 13.Ghosh P. K., Murthy R. S. R. Microemulsions: a potential drug delivery system. Curr. Drug Deliv. 2006;3:167–180. doi: 10.2174/156720106776359168. [DOI] [PubMed] [Google Scholar]
- 14.Lonning P. E. Pharmacological profiles of exemestane and formestane, steroidal aromatase inhibitors used for the treatment of post-menopausal breast cancer. Breast Can. Res. Treat. 1998;49:S45–S52. doi: 10.1023/A:1006048722559. [DOI] [PubMed] [Google Scholar]
- 15.Weippl K. S., Goss P. E. Prevention of breast cancer using SERMs and aromatase inhibitors. J. Mammary Gland Biol. Neoplasia. 2003;8:5–18. doi: 10.1023/A:1025727103811. [DOI] [PubMed] [Google Scholar]
- 16.Dowsett M. Theoretical considerations for the ideal aromatase inhibitors. Breast Can. Res. Treat. 1998;49:S39–S44. doi: 10.1023/A:1006088405721. [DOI] [PubMed] [Google Scholar]
- 17.Physician Desk Reference. Thomson Healthcare, Montvale, NJ. 60th edition, 2006, pp. 2600–2602.
- 18.Kang B. K., Lee J. S., Chon S. K., Jeong S. Y., Yuk S. H., Khang G., Lee H. B., Cho S. H. Development of self-microemulsifying drug delivery systems (SMEDDS) for oral bioavailability enhancement of simvastatin in beagle dogs. Int. J. Pharm. 2004;274:65–73. doi: 10.1016/j.ijpharm.2003.12.028. [DOI] [PubMed] [Google Scholar]
- 19.T. Lucas, R. Bishara, and R. Seevers. A stability program for the distribution of drug products. Pharma. Tech. 68–71 (2004).
- 20.Kommuru T. R., Gurley B., Khan M. A., Reddy I. K. Self-emulsifying drug delivery systems (SEDDS) of co-enzyme Q10: formulation development and bioavailability assessment. Int. J. Pharm. 2001;212:233–246. doi: 10.1016/S0378-5173(00)00614-1. [DOI] [PubMed] [Google Scholar]
- 21.Constantinides P. P., Lancaster C. M., Marcello J. Enhanced intestinal absorption of an RGD peptide from water-in-oil microemulsions of different composition and particle size. J. Control. Release. 1995;34:109–116. doi: 10.1016/0168-3659(94)00129-I. [DOI] [Google Scholar]