Abstract
The aim of this study was to investigate the influence of polymer level and type of some hydrophobic polymers, including hydrogenated castor oil (HCO); Eudragit RS100 (E-RS100); Eudragit L100 (E-L100), and some fillers namely mannitol [soluble filler], Dibasic calcium phosphate dihydrate (Emcompress) and anhydrous dibasic calcium phosphate [insoluble fillers] on the release rate and mechanism of baclofen from matrix tablets prepared by a hot-melt granulation process (wax tablets) and wet granulation process (E-RS100 and E-L100 tablets). Statistically significant differences were found among the drug release profile from different classes of polymeric matrices. Higher polymeric content (40%) in the matrix decreased the release rate of drug because of increased tortuosity and decreased porosity. At lower polymeric level (20%), the rate and extent of drug release was elevated. HCO was found to cause the strongest retardation of drug. On the other hand, replacement of Emcompress or anhydrous dibasic calcium phosphate for mannitol significantly retarded the release rate of baclofen, except for E-L100 (pH-dependent polymer). Emcompress surface alkalinity and in-situ increase in pH of the matrix microenvironment enhanced the dissolution and erosion of these matrix tablets. The release kinetics was found to be governed by the type and content of the excipients (polymer or filler). The prepared tablets showed no significant change in drug release rate when stored at ambient room conditions for 6 months.
Key words: baclofen, Emcompress, Eudragit, hydrogenated castor oil, mean dissolution time
INTRODUCTION
A previous study carried out on baclofen, a water soluble widely used centrally acting skeletal muscle relaxant drug, showed that baclofen release showed pH dependency owing to pH dependent solubility of the drug. Also, the effect of aging on the drug release showed that methylcellulose and sodium alginate were good candidate excipients for retarding baclofen release rate, compared with standard commercial tablets and the major release mechanism was non-Fickian (Anomalous), coupled diffusion and polymer matrix relaxation (1). Therefore, this work aims at preparation and evaluation of controlled release tablet formulations of baclofen. In an attempt to obtain more sustained drug release rates and different release mechanisms for the water soluble agent and endow the formulator the flexibility in tailoring the proper controlled drug release with a convenient oral bioavailability during eventual in vivo studies.
Baclofen with a dose of 25 mg and a solubility of 26 mg/ml in pH 1.2 at 37 °C, has a dose: solubility ratio of just 1 ml. In other words, baclofen is thought to be freely soluble in simulated gastric fluid (1). For such drugs with high water solubility, hydrophobic polymers are suitable as matrixing agents for developing sustained-release dosage forms. Hydrophobic polymers provide several advantages, ranging from good stability at varying pH values and moisture levels to well-established safe application. Hydrophobic polymers include inert insoluble polymers and wax polymers. An example of the former is Eudragit, which is a group commercially available in anionic, cationic, and zwitterionic forms (2,3). Eudragit Ll00 (E-L100) is an anionic co-polymer of methacrylic acid and methyl methacrylate. The ratio of free carboxyl group to the ester is approximately 1:1. It has a pH-dependent solubility and is readily soluble in neutral to weakly alkaline conditions and forms salts with alkalis (4). Eudragit RS100 (E-RS100) is an acrylic resin, copolymer, synthesized from acrylic and methacrylic acid esters with a low content of quaternary ammonium groups. These groups are responsible for the permeability of the coating film of E-RS100. It is pH-independent polymer, inert to the digestive tract, impermeable to water, as well as capable of swelling and release active ingredients by diffusion (4). The latter, wax matrix systems, are a simple concept. They are easy to manufacture using standard direct compression, roller compaction or melt granulation. An example of wax polymers is hydrogenated castor oil (HCO) (5). HCO is a white to slightly yellow fine powder obtained by hydrogenating castor oil using a catalyst. HCO has been used in pharmaceutical formulation or technology as a sustained-release coating material and hardening agent (6). In this study, also, the effect of type of filler, mannitol (water soluble); dibasic calcium phosphate dihydrate, and dibasic calcium phosphate anhydrous (water insoluble), on drug release has been investigated.
MATERIALS AND METHODS
Materials
Eudragit RS100 and Eudragit L100 were purchased from Rohm GmbH & Co. KG, Darmstadt, Germany. Hydrogenated castor oil was purchased from Kawaken Fine Chemical Co., Japan. Dibasic calcium phosphate dihydrate was purchased from E.Mendell Co., Inc., Carmel, New York, USA. Dibasic calcium phosphate anhydrous was purchased from Edward Mendell Co., Inc., New York, USA. Magnesium stearate was purchased from Witco Chemical Co., Chicago, USA.
Thermal Analysis
Thaw–Melt Method
Drug-wax physical mixtures were mixed using a mortar and pestle for 2 min to prepare 0%, 10%, 30%, 50%, 70%, 90%, and 100% (w/w) drug concentrations, in an attempt to construct a melting point phase diagram. This accomplished using a digital melting point apparatus (Stuart Scientific SMP10, UK) which enabled accurate heating rates, better visualization, and improved temperature hold capabilities. Sample (drug-HCO physical mixtures) were placed in glass capillary tubes and individually heated at a rate 2 °C/min. The sample was heated at a constant rate by means of a heating stage interfaced with a digital temperature display. Visualization was possible using a high-powered magnifying glass fixed on a mount over the sample and heating stage. The apparatus thus resembled a hot-stage microscope but lacked the polarizing lenses of the latter. All determinations were made in triplicate. For constructing phase diagram, observation was made during heating to note the temperature at which melting started (thaw point) and the temperature at which complete melting was affected (melting point). These two temperatures were used to define the melting point range.
Differential Scanning Calorimetry (DSC)
Differential Scanning Calorimetry (DSC) was utilized to investigate the physicochemical compatibilities and solid interaction of the drug and the used excipients. DSC thermograms of baclofen and its physical mixtures (1:1 w/w) with Emcompress, Eudragit RS100, and Eudragit L100 were tested. Confirmatory to the above-mentioned visual data, baclofen-wax physical mixtures at 0%, 10%, 50%, 70%, and 100% (w/w) drug concentration were tested using a DSC (Perkin-Elmer, 2-C,New York, USA) with a thermal analysis data station TADS system, computer, and plotter interface. The instrument was calibrated with an indium standard. The samples (2–4 mg) were heated (50 to 300 °C) at a constant scanning speed (10 °C/min) in sealed aluminum pans, using nitrogen as purging gas.
Preparation of Wet Granulated Tablets
Sixteen tablet formulations were prepared by wet granulation technique. Composition of a 25 mg-baclofen tablet is given in Tables I and II. Stock ethanolic solutions of E-RS100 (50% w/v) and E-L100 (50% w/v) were prepared, separately. A calculated amount, required to prepare a 20-tablet batch, of the drug, and filler (mannitol or Emcompress) was mixed thoroughly. The powder mix was sieved through a no. 60 sieve, thereafter, it was kneaded using suitable volume of E-RS100 or E-L100 stock solutions, pure ethanol might be added if the blend needed. After enough cohesiveness was obtained, the mass was screened portionwise through a 2-mm sieve. The granules were dried at 50 °C for 2 h thereafter kept in a desiccator for 24 h at room temperature. The granules were rescreened through a 1-mm sieve. Prior to the compression, the prepared granules were evaluated for several tests as mentioned below. Finally, magnesium stearate (lubricant) was added and mixed using mortar and a spatula for 1 min. A 100 mg sample of the granules was weighed and then compressed using a hydraulic press (Shimadzu laboratory, Japan) equipped with a 6 mm flat-faced punch and dies set. The force of compression was 2 tons. All compressed tablets were stored in airtight container at room temperature for further study.
Table I.
Composition of Eudragit RS100-Based Baclofen Matrix Tablet
| Formulations | RS-1 | RS-2 | RS-3 | RS-4 | RS-5 | RS-6 |
|---|---|---|---|---|---|---|
| Baclofen | 25 | 25 | 25 | 25 | 25 | 25 |
| E-RS100 | 20 | 30 | 40 | 20 | 30 | 40 |
| Mannitol | 54 | 44 | 34 | – | – | – |
| Emcompress | – | – | – | 54 | 44 | 34 |
| Mg stearate | 1 | 1 | 1 | 1 | 1 | 1 |
| Total weight | 100 | 100 | 100 | 100 | 100 | 100 |
Table II.
Composition of Eudragit L100-Based Baclofen Matrix Tablet
| Formulations | L-1 | L-2 | L-3 | L-4 | L-5 | L-6 |
|---|---|---|---|---|---|---|
| Baclofen | 25 | 25 | 25 | 25 | 25 | 25 |
| E-L100 | 20 | 30 | 40 | 20 | 30 | 40 |
| Mannitol | 54 | 44 | 34 | – | – | – |
| Emcompress | – | – | – | 54 | 44 | 34 |
| Mg stearate | 1 | 1 | 1 | 1 | 1 | 1 |
| Total weight | 100 | 100 | 100 | 100 | 100 | 100 |
Preparation of Hot-Melt Granulated Tablets
Eight tablet formulations were prepared by melt-granulation technique. Composition of a 25 mg-baclofen tablet is given in Table III. HCO and filler (mannitol or dibasic calcium phosphate anhydrous) were mixed thoroughly. The blend was then heated to 80–90 °C in a heating mantel (Super aparatos cientificos 307, Barcelona, Spain). The drug was dispersed in the melted wax with continuous stirring then allowed to cool at room temperature. The congealed solid mass was pulverized, screened through a 425 μm-sieve. The prepared granules were evaluated for the aforementioned tests. All granules lubricated, and compressed at 0.5 tons using a Shimadzu laboratory hydraulic press equipped with 6 mm flat-faced punch and die set. A compression force higher than 0.5 tons produced sticky and deformed tablets. This could be ascribed to softening and/or even melting of the wax at higher compression forces. It is worthy to note that anhydrous dibasic calcium phosphate should replace the dihydrate form (Emcompress) in conditions require heat treatment such the present case of hot-melt granulation (7).
Table III.
Composition of HCO-Based Baclofen Matrix Tablet
| Formulations | H-1 | H-2 | H-3 | H-4 | H-5 | H-6 |
|---|---|---|---|---|---|---|
| Baclofen | 25 | 25 | 25 | 25 | 25 | 25 |
| HCO | 20 | 30 | 40 | 20 | 30 | 40 |
| Mannitol | 54 | 44 | 34 | – | – | – |
| CaHPO4 | – | – | – | 54 | 44 | 34 |
| Mg stearate | 1 | 1 | 1 | 1 | 1 | 1 |
| Total weight | 100 | 100 | 100 | 100 | 100 | 100 |
Evaluation of Starting Material and Granules
Angle of Repose
Static angle of repose was determined according to the fixed funnel and freestanding cone method, according to the method reported by Raghuram et al. (8), whereby accurately weighed granules (3 g) were carefully poured through the funnel with its tip 2 cm height, H; until the apex of the conical heap so formed just reach the tip of the funnel. The mean diameter, 2R, of the base for the powder cone was measured and the angle of repose (θ) was calculated using the following equation:
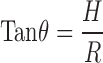 |
1 |
Bulk Density
Both poured (or fluff) bulk (Do) and tapped bulk densities (DF) were determined, according to the method reported by Raghuram et al. (8), whereby a quantity (3 g) of granules from each formula, previously lightly shaken to break any agglomerates formed, was introduced into a 10-ml measuring cylinder. After the initial volume was observed, the cylinder allowed to fall under its own weight onto a hard surface from the height of 2.5 cm at 2-s intervals. The tapping was continued until no further change in the volume was noted.
Compressibility Percent
The compressibility index of the granules was determined by Carr’s compressibility percent: (9)
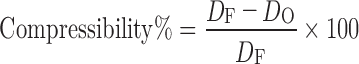 |
2 |
EVALUATION OF TABLETS
Thickness
The thickness of the tablets was determined using a thickness gauge (Mitutoyo, New Delhi, India). Ten tablets from each batch were used. Thickness values were reported in millimeter. Mean and standard deviation (SD) were calculated.
Average Weight of the Dosage Unit
To study weight variation, ten tablets of each formulation were weighed using an electronic balance (Mettler Toledo, Switzerland). Weight values were reported in mg. Mean and SD were calculated.
Drug Content
Five tablets were weighed individually, then placed in a mortar and powdered with a pestle. An amount equivalent to 25 mg drug (100 mg) was extracted with 100 ml of 0.1 M hydrochloric acid, sonication for 15 min. The solution was filtered through a filter (0.22 μm pore size), properly diluted with 0.1 M hydrochloric acid and the drug content was measured as previously mentioned.
Hardness Test
For each formulation, the hardness of six tablets was determined using a hardness tester (VK 200, Vankel, Varian Inc,Palo Alto, CA). Hardness values were reported in kilogram (kg). Mean and SD were calculated.
Friability Test
For each formulation, six tablets were weighed. The tablets were placed in a friabilator (Campbell electronics, Mumbai, India) and subjected to 100 rotations in 4 min. The tablets were then dedusted and reweighed. The friability was calculated as the percent weight loss.
In Vitro Release Studies
In-vitro release studies of standard tablets, control tablets, and baclofen matrix tablets were monitored. The release experiments were carried out in a 900-ml dissolution medium of hydrochloric acid pH 1.2 for the first 2 h, then replaced with the same volume of a phosphate buffer solution pH 6.8 kept at 37 ± 0.5 °C, and stirred at 50 rpm, using USP dissolution apparatus 2 (perfect sink conditions). A 5-ml sample was withdrawn through a 0.45 μm filter, and replaced with another 5-ml of a suitable fresh dissolution medium at preselected intervals up to 8 h. The amount of the drug was determined first-derivative (D1) spectrophotometrically at 226.5 nm adopting the peak height method (Shimadzu-UV 160A Spectrophotometer). Each test was performed in triplicate (correlation of variance, CV < 1.5%).
Release Kinetics
Different kinetic equations (zero-order, first-order and Higuchi’s equation) were applied to interpret the release rate of the drug from matrix systems. The best fit with higher correlation (r2 > 0.98) was found with the Higuchi’s equation for all the formulations. Two factors, however, diminish the applicability of Higuchi’s equation to matrix systems. This model fails to allow for the influence of swelling of the matrix (upon hydration) and gradual erosion of the matrix. Therefore, the dissolution data were also fitted according to the well-known exponential Korsmeyer-Peppas equation (10), which is often used to describe the drug release behavior from polymeric systems:
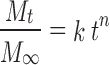 |
3 |
Mt/M∞ is the fraction of drug release at time t, and k is the kinetic constant, n is the release exponent (indicating the general operating release mechanism). In addition, for determination of the exponent n, one must use only the initial portion of the release curve (Mt/M∞ < 0.6) (11,12). Ritger and Peppas (13) have defined the exponent n as a function of the aspect ratio for 1-dimensional to 3-dimensional systems (slabs, cylinders, and discs). The aspect ratio (2a/l) is defined as the ratio of diameter (2a) to thickness (l). For tablets, depending on the aspect ratios, n value between 0.43 and 0.5 indicating Fickian (case I) diffusion-mediated release, non-Fickian (Anomalous) release, coupled diffusion and polymer matrix relaxation, occurs if 0.5 < n < 0.89, purely matrix relaxation or erosion-mediated release occurs for n = 1 (zero-order kinetics), and super case II type of release for n > 0.89. The release exponent, n, is the slope of log fraction of drug release vs. log time curve, using GraphPad Software, Inc., 3.05. from the previous study, baclofen showed marked pH-dependent release profile (1). Thus, significant curvatures are predicted in release-time plots resulting from abrupt decrease in release rate in simulated intestinal medium (SIF). Furthermore, to avoid any curvatures predicted in the plots due to changes in the effective area of drug release of a whole tablet since these occur greater than 60% is released (13). Therefore, the acid portion of the plots will be involved in the kinetic studies unless 60% of drug release is not reached.
This equation was successfully applied to evaluate the drug release mechanism from hydrophilic (14), wax (15), and plastic matrix tablets (15).
Due to the difference in drug release kinetics, the constant k, though is one of the measures of release rate, should not be used for comparison. Therefore, to characterize the drug release rates in different experimental conditions, mean dissolution time (MDT) was calculated from dissolution, according to Mockel and Lippold (16) using the following equation:
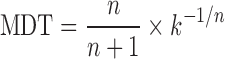 |
4 |
Where n is the release exponent and k is the kinetic constant calculated from Eq. 3.
Effect of Aging on Release of Baclofen from Prepared Tablets
The effect of aging on the drug release from the prepared tablets was conducted by storing the tablets in amber bottles at ambient room conditions for 6 months. The dissolution studies of the drug from selected matrix tablets were followed the same procedure as previously described.
Statistics
To compare the means of all release data and to assess statistical significance between them, either one-way analysis of variance (ANOVA) or an unpaired two-tailed t-test was carried out at 5% significance level, using GraphPad Software, Inc., 3.05.
RESULTS AND DISCUSSION
Eutectic transformation, interactions resulting in the formation of monotectic or peritectic compounds, formation of solid solution, and possibly, stiochiometric compounds might be associated with a matrix solid dispersion system such as the drug-wax combination. However, the properties of a eutectic mixture would be expected to differ from those of a simple mixture, if particle size and solubility of the drug were altered, then the dissolution characteristics and the in vivo availability could be influenced. Therefore, an investigation of the matrix-type sustained-release dosage form should commence with some basic phase studies to determine whether a drug-wax interaction occurs and a t what composition a eutectic mixture exists (17).
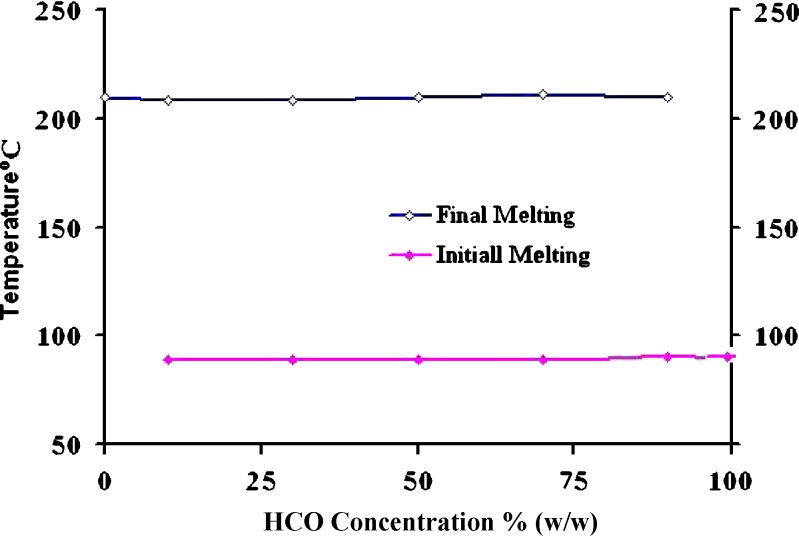
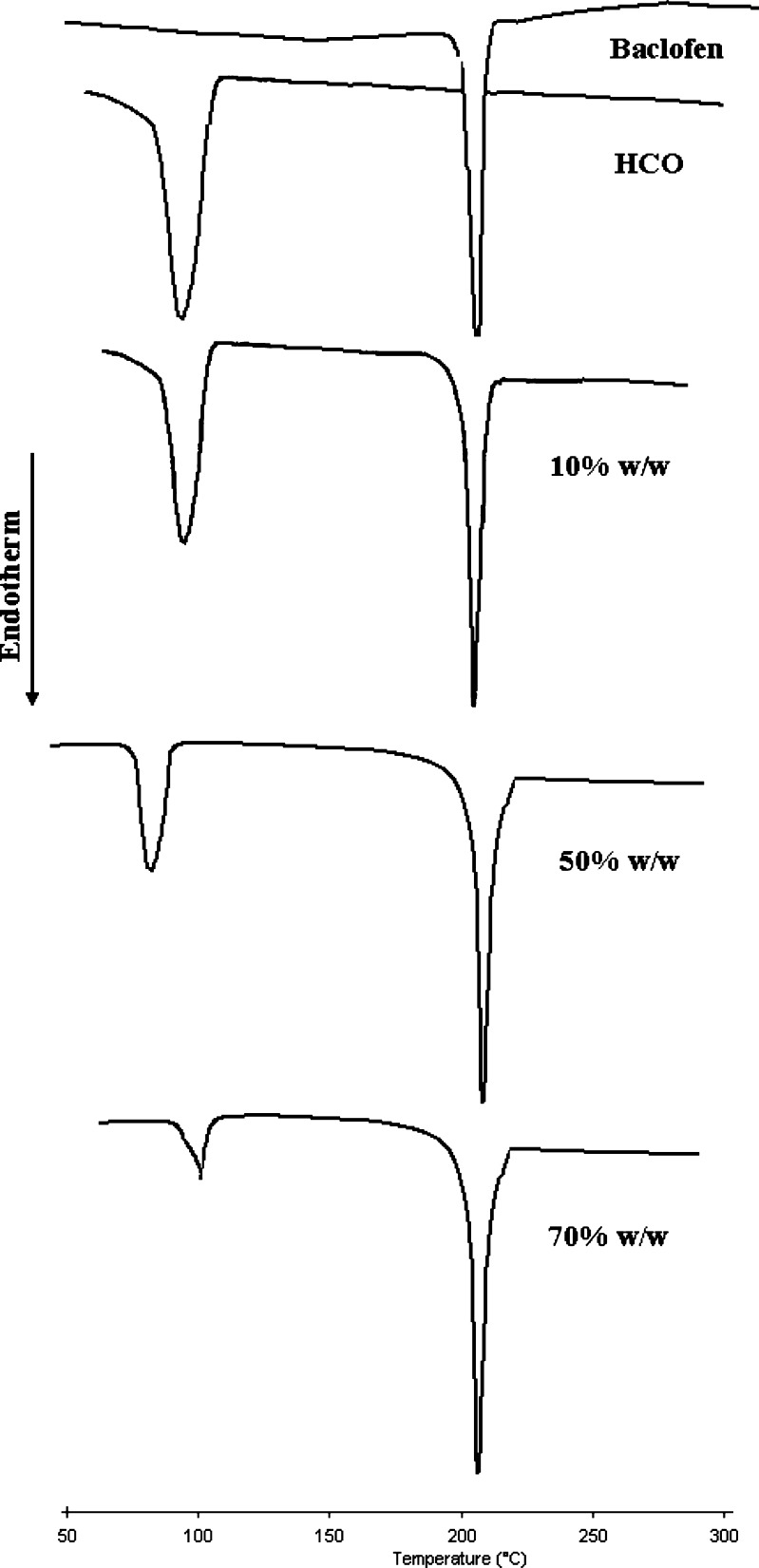
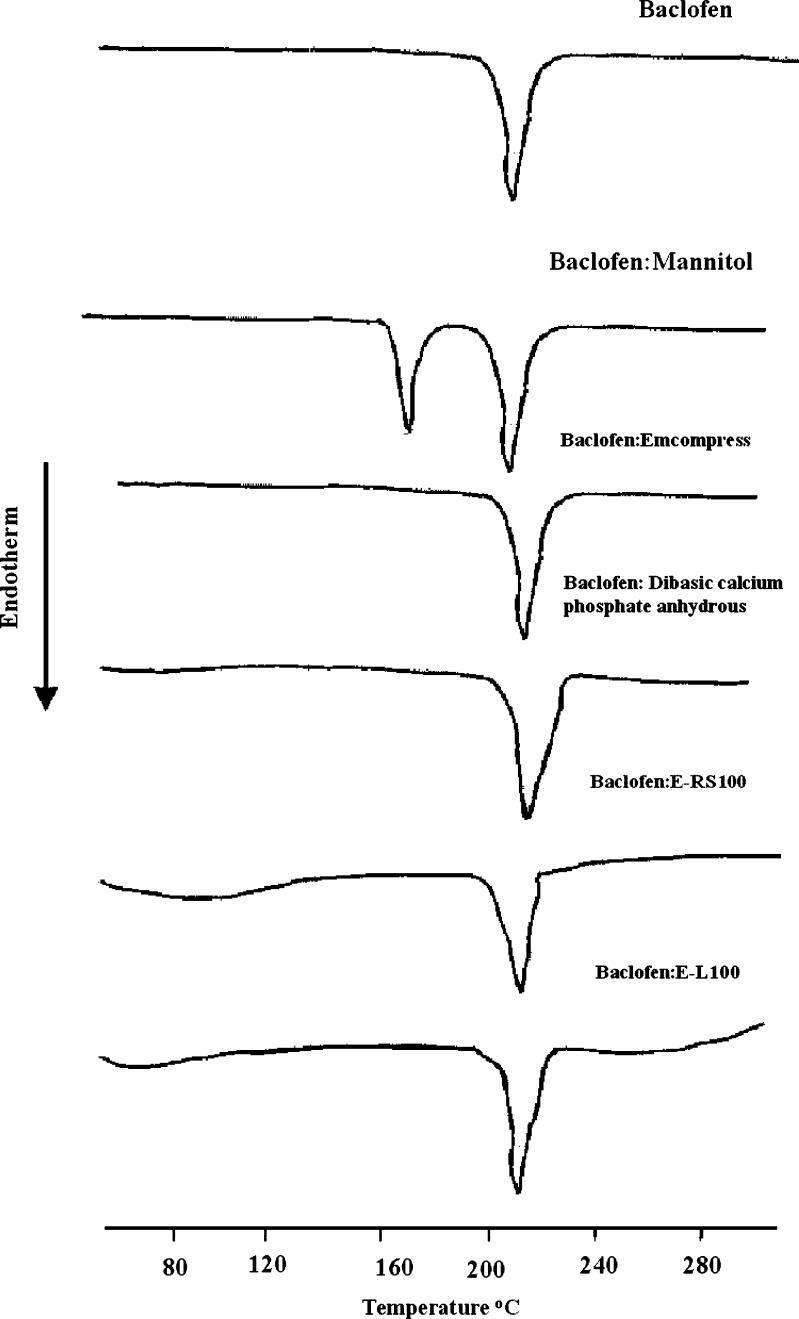
The baclofen-HCO phase diagram, Fig. 1, was constructed to investigate the possibility of alteration of drug particle size and the matrix type as a consequence of heat treatment, during hot-melt granulation. Figure 1 shows the phase diagram for the baclofen-HCO system. There was no indication of interaction or eutectic formation at above 10% drug concentration since the melting characteristics of the two components remained substantially unchanged. These findings were in a good agreement with that of DSC studies, Fig. 2. Also, DSC thermograms of the drug alone and the drug with the retardant excipients are shown in Fig. 3. The DSC analysis of the drug alone elicited an endothermic peak at 210 °C, very close to the reported value of baclofen melting point, which is 208 °C (18), whereas pure mannitol exhibited an endothermic peak at 170 °C. Furhter, it was found that the endothermic peaks of physical mixtures of the drug with mannitol, Emcompress, dibasic calcium phosphate anhydrous, E-RS100, or E-L100 reflected the characteristic features of baclofen alone. Thus, it was thought to indicate that there was no evidence of solid interactions between baclofen and the used excipients, which might eventually affect the rate of drug release or drug release mechanism from the matrix system.
Fig. 1.
Phase diagram of HCO-baclofen binary system
Fig. 2.
DSC thermograms of various baclofen-HCO physical mixtures
Fig. 3.
DSC thermograms of baclofen various physical mixtures of baclofen with the used excipients
Granulation is the key process in the production of many dosage forms involving the controlled release of a drug from coated or matrix-type particles. A granule is an aggregation of component particles that is held together by the presence of bonds of finite strength. Physical properties of granules such as specific surface area, shape, hardness, surface characteristics, and size can significantly affect the rate of dissolution of drugs contained in a heterogeneous formulation (8). The granules of different formulations and drug powder were evaluated for angle of repose, bulk densities, Hausner factor, and compressibility index, Table IV. The angle of repose could not be measured by the above-mentioned method for baclofen powder. The powder was too cohesive to flow through the funnel whereas the results of angle of repose (<30) indicate good flow properties of the prepared granules (9,19). This was further supported by hausner and lower compressibility index values, Table IV. Hausner showed that powders with low interparticle friction had ratios of approximately 1.2 (19). Further, compressibility index values up to 20% result in good to excellent flowability and compressibility (19).
Table IV.
Physical Properties of the Prepared Granules, Using Eudragit RS100 and Eudragit L100, as Matrix Forming Polymers
| Formulations | Angle of repose(Ө) | Poured density (g/cm3) | Tapped density (g/cm3) | Hausner factor | Compressibility (%) |
|---|---|---|---|---|---|
| Pure Drug | – | 0.241 | 0.611 | 2.535 | 60.55 |
| RS-1 | 19 | 0.460 | 0.511 | 1.110 | 9.98 |
| RS-2 | 21 | 0.461 | 0.512 | 1.110 | 11.06 |
| RS-3 | 18 | 0.425 | 0.495 | 1.164 | 7.00 |
| RS-4 | 18 | 0.561 | 0.600 | 1.069 | 6.50 |
| RS-5 | 22 | 0.501 | 0.550 | 1.097 | 8.90 |
| RS-6 | 20 | 0.510 | 0.545 | 1.068 | 6.42 |
| L-1 | 14 | 0.510 | 0.544 | 1.066 | 6.25 |
| L-2 | 16 | 0.490 | 0.523 | 1.067 | 6.30 |
| L-3 | 12 | 0.427 | 0.448 | 1.049 | 4.68 |
| L-4 | 15 | 0.550 | 0.594 | 1.080 | 7.40 |
| L-5 | 18 | 0.551 | 0.601 | 1.091 | 8.31 |
| L-6 | 17 | 0.590 | 0.631 | 1.069 | 6.49 |
| H-1 | 14.5 | 0.627 | 0.752 | 1.199 | 12.50 |
| H-2 | 14 | 0.601 | 0.680 | 1.131 | 11.61 |
| H-3 | 10 | 0.521 | 0.592 | 1.136 | 11.99 |
| H-4 | 15 | 0.702 | 0.800 | 1.139 | 12.25 |
| H-5 | 12 | 0.703 | 0.771 | 1.096 | 8.81 |
| H-6 | 12 | 0.660 | 0.770 | 1.166 | 14.28 |
The thickness of the tablets ranged from 1.66 ± 0.01 to 2.52 ± 0.02 mm. The average percentage deviation of ten tablets of each formula was less than ±5%. It is worthy to note that tablets containing Emcompress showed significantly higher thickness values than those containing mannitol, for the same polymer type and level. These could be ascribed to an obvious difference in the densities of the two fillers. The density of Emcompress is 2.4 g/cm3 whereas mannitol density is 1.48 g/cm3 (7,20). Drug content was found to be uniform among different batches of the tablets and ranged from 98.86 ± 0.55 to 102.25 ± 0.45. The hardness and percentage friability of the tablets of all batches ranged from 3.22 ± 0.44 to 14.50 ± 1.08 kg and 0.204 to1.65, respectively, Table V. Tablet hardness is not an absolute indicator of strength (9). Another measure of a tablet’s strength is friability. Conventional compressed tablets that lose less than 1% of their weight are generally considered acceptable. In the present study, the percentage friability for all the formulations, except for HCO-based tablets, was below 1%, indicating that the friability is within the prescribed limits, according to European and USA pharmacopoeia. These findings might be ascribed to the low compression force applied and/or poor compression properties of HCO. Any attempt to increase the applied compression forces over than 0.5 tonne resulted in deformation of the tablet, as previously mentioned in tablet preparation. Consequently, data generated from such systems require sufficient scaling up development and should not be directly extrapolated to commercially prepared controlled-release tablets.
Table V.
Thickness, Diameter, Weight, Drug Content, Hardness, and Friability of the Prepared Tablets, Expressed as Mean ± SD
| Formulations | Thickness (mm) | Weight (mg) | Drug Content (%) | Hardness (kg) | Friability (%) |
|---|---|---|---|---|---|
| RS-1 | 2.11 ± 0.02 | 100.05 ± 0.98 | 99.55 ± 0.45 | 7.55 ± 0.45 | 0.25 |
| RS-2 | 2.24 ± 0.01 | 102.45 ± 0.88 | 101.11 ± 0.48 | 8.22 ± 0.82 | 0.24 |
| RS-3 | 2.42 ± 0.02 | 99.88 ± 1.00 | 100.05 ± 0.8 | 9.00 ± 0.77 | 0.22 |
| RS-4 | 2.50 ± 0.01 | 100.43 ± 1.06 | 102.25 ± 0.45 | 10.25 ± 0.16 | 0.18 |
| RS-5 | 1.79 ± 0.05 | 100.23 ± 1.23 | 100.52 ± 0.79 | 10.45 ± 0.81 | 0.28 |
| RS-6 | 1.90 ± 0.08 | 101.03 ± 1.80 | 100.00 ± 0.65 | 12.00 ± 1.05 | 0.25 |
| RS-7 | 2.05 ± 0.07 | 99.45 ± 1.01 | 99.00 ± 0.65 | 13.25 ± 1.30 | 0.22 |
| RS-8 | 2.21 ± 0.04 | 101.53 ± 0.63 | 99.25 ± 0.55 | 14.50 ± 1.08 | 0.204 |
| L-1 | 2.08 ± 0.04 | 98.34 ± 0.53 | 100.44 ± .97 | 8.25 ± 0.85 | 0.5 |
| L-2 | 2.21 ± 0.15 | 99.52 ± 0.98 | 100.55 ± 1.05 | 8.4 ± 0.66 | 0.5 |
| L-3 | 2.31 ± 0.02 | 100.54 ± 1.52 | 101.22 ± 1.05 | 11.00 ± 0.88 | 0.2 |
| L-4 | 2.38 ± 0.01 | 99.37 ± 1.58 | 100.82 ± 0.88 | 15.55 ± 0.85 | 0.15 |
| L-5 | 1.80 ± 0.02 | 101.03 ± 1.87 | 99.88 ± 0.97 | 5.25 ± 0.66 | 0.5 |
| L-6 | 1.85 ± 0.01 | 99.45 ± 1.31 | 99.44 ± 0.52 | 5.00 ± 0.75 | 0.5 |
| L-7 | 1.92 ± 0.01 | 101.53 ± 1.43 | 100.47 ± 0.75 | 5.8 ± 0.22 | 0.38 |
| L-8 | 2.00 ± 0.02 | 98.34 ± 1.13 | 99.57 ± 1.00 | 6.00 ± 0.28 | 0.32 |
| H-1 | 2.30 ± 0.05 | 97.52 ± 0.98 | 100.00 ± 1.00 | 3.97 ± 0.33 | 0.85 |
| H-2 | 2.40 ± 0.07 | 100.54 ± 1.02 | 99.44 ± 0.45 | 4.21 ± 1.00 | 1 |
| H-3 | 2.45 ± 0.05 | 100.54 ± 0.52 | 98.86 ± 0.55 | 3.45 ± 0.45 | 1.5 |
| H-4 | 2.52 ± 0.02 | 99.37 ± 0.58 | 100.54 ± 1.00 | 3.22 ± 0.44 | 1.65 |
| H-5 | 1.66 ± 0.01 | 102.45 ± 1.08 | 99.00 ± 0.22 | 4.11 ± 0.32 | 1 |
| H-6 | 1.81 ± 0.07 | 99.88 ± 0.75 | 99.05 ± 0.55 | 4.00 ± 0.67 | 1.25 |
| H-7 | 2.02 ± 0.05 | 100.43 ± 0.66 | 100.05 ± 0.33 | 3.88 ± 0.15 | 1.2 |
| H-8 | 2.17 ± 0.01 | 100.23 ± 0.83 | 100.65 ± 0.55 | 3.77 ± 0.58 | 1.3 |
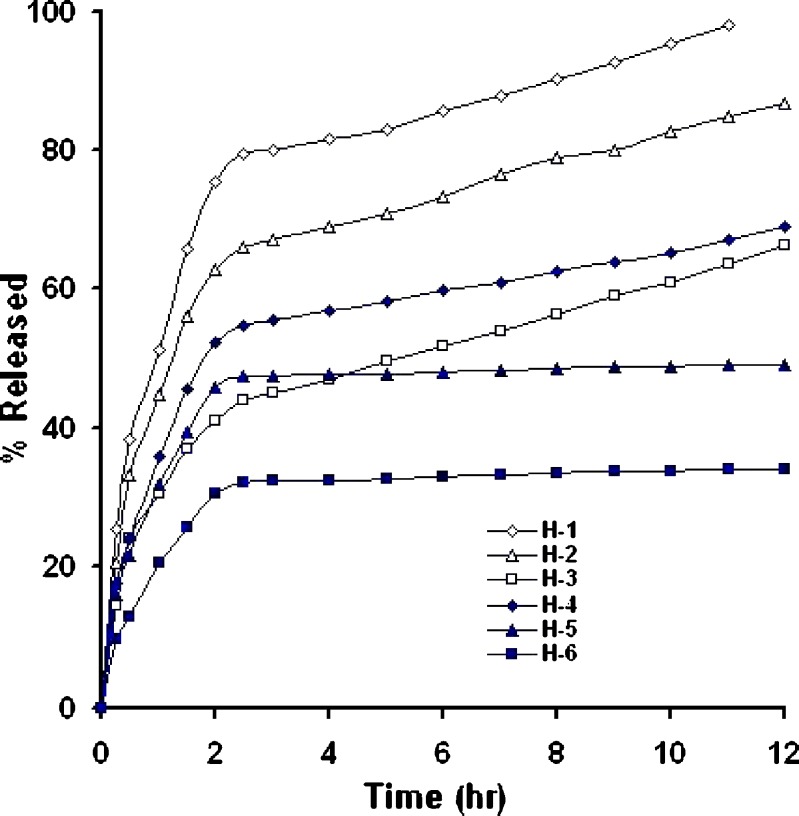
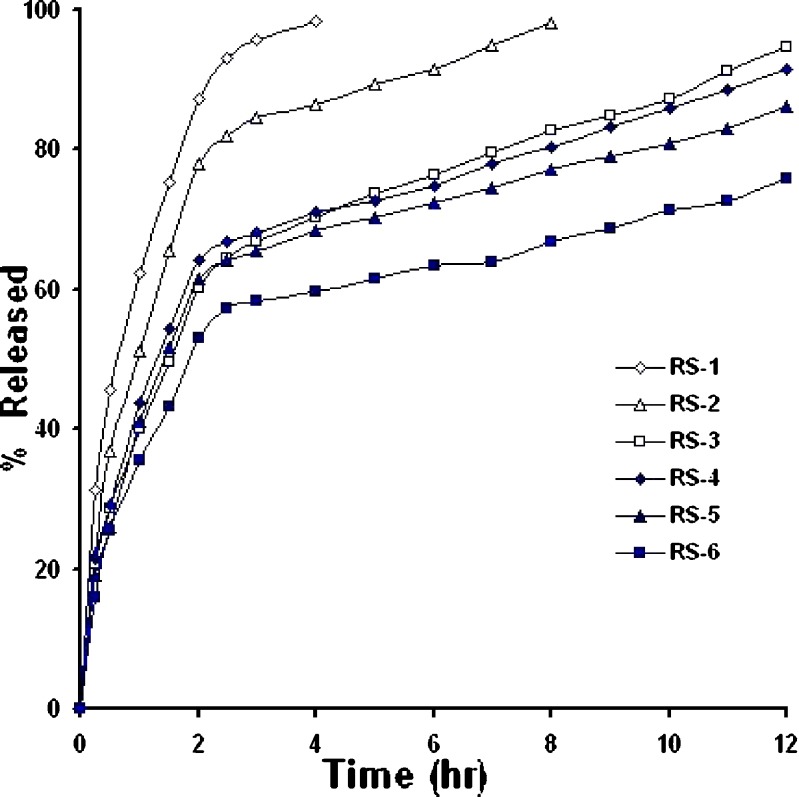
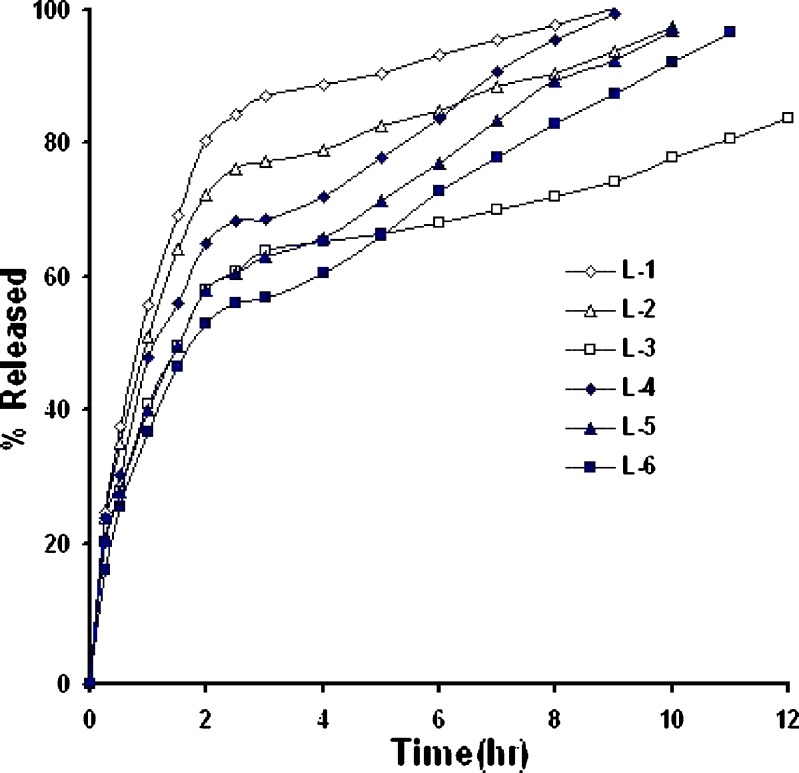
In-vitro drug release from matrix systems depends on several factors, such as the manufacturing process, the type of excipient, drug solubility and concentration, polymer concentration and pH of the dissolution medium (8,21). Figures 4,5,6 show in-vitro release profile of baclofen from the prepared controlled release tablets, using hydrophobic polymers, namely HCO; E-RS-100; E-L100.Also, the effect of filler type on drug release was investigated whereby Emcompress or dibasic calcium phosphate anhydrous (water insoluble fillers) replaced mannitol (a water soluble filler) in the prepared formulations.
Fig. 4.
Percent of baclofen released from HCO-based matrix tablets, carried out in dissolution media of pH 1.2 and 6.8 (n = 3)
Fig. 5.
Percent of baclofen released from E-RS100-based matrix tablets, carried out in dissolution media of pH 1.2 and 6.8 (n = 3)
Fig. 6.
Percent of baclofen released from E-RS100-based matrix tablets, carried out in dissolution media of pH 1.2 and 6.8 (n = 3)
Drug particles present in the surface of the matrix were initially released into surrounding media generating many pores and cracks which facilitates further release of the drug. Irrespective of polymer nature, as the polymer content increased, the release rate significantly decreased, (P < 0.001, one-way ANOVA), suggesting that the matrix with higher polymer content provides a more tortuous pathway, and/or a less porous tablet was formed, Figs. 4,5,6. For instance, at a 6-h release period formulations H-1, H-2, and H-3 loaded with 20%, 30%, and 40% (w/w) of wax polymer released about 70% of the drug within 1.5, 4, and 12 h, respectively, Fig. 4. A similar trend was obtained when Emcompress found replaced mannitol. For example, formulations RS-4, and RS-6 loaded with 20%, and 40% (w/w) of E-RS100 (pH-independent polymer) released about 70% of the drug within 5 and 12 h, respectively, Fig. 5. Also, formulations L-4, and L-6 loaded with 20%, and 40% (w/w) of E-L100 (pH-dependent polymer) released about 70% of the drug within 3 and 6 h, respectively, Fig. 6. These results might be explained on the basis that As the polymer content decreased the drug release rate increased due to decreasing the total porosity of the matrices (initial porosity plus porosity due to the dissolution of the drug) and increasing tortuosity (the length of the diffusion path of the solute) (15,22).
Irrespective of polymer level, Replacement of Emcompress to mannitol in E-L100-based formulations significantly enhanced drug release rate, when compared with that from E-L100-basd tablets, Fig. 6. For example, formulations L-3 and L-6 released about 80% of the drug within 7 and 11 h, respectively. These results could be ascribed to in-situ increase in pH in the diffusion layer where the surface of dibasic calcium phosphate dihydrate (Emcompress) is alkaline (23). Consequently, dissolution and erosion rates of E-L 100, pH-dependent solubility polymer, was markedly higher.
The effect of some fillers on the release of baclofen was investigated, Figs. 4,5,6. Emcompress (insoluble filler) replaced mannitol (soluble filler) in all formulations prepared by wet granulation technique. Also, anhydrous dibasic calcium phosphate (thermostable insoluble filler) replaced mannitol in HCO-based formulations prepared by hot-melt granulation technique. It is worthy to note that the presence of soluble filler (mannitol) in tablet formulation gave higher drug release rate than that containing either Emcompress or anhydrous dibasic calcium phosphate at the same polymer level. The dissolution of mannitol, however, may create void spaces in hydrophobic matrix tablets, increasing matrix porosity, besides; mannitol caused decrease in the tortuosity of the diffusion path of the drug that resulted in increasing of the drug release. These results were in accordance with that obtained by Amaral et al. (21), when lactose replaced Emcompress in naproxen sustained release matrix tablet, using HCO as matrixing agent, naproxen release rate was significantly enhanced. Furthermore, manufacturing process displayed apparent effect on the drug release rate. Wax matrices (H-1 to 6), prepared by melt granulation, imparted significantly higher retarding effect and extensively delayed drug release than that from inert insoluble matrices (RS-1 to 6,and L-1 to 6), wet granulated tablets. Heat treatment of the tablets made by melt granulation further retarded drug release. Heat treatment redistributed the wax, forming a new matrix system with higher tortuosity. These results were in accordance with results obtained by many authors (15,21,24,25). Total release of drug from formulations H-5 and 6, containing 30% and 40% of HCO, respectively, was not possible since a certain fraction of the dose may be coated with an impermeable wax film, where HCO is extremely hydrophobic in nature with lower wettability, Fig. 4. These findings were in a good agreement with the results obtained by Reza et al. (21). Significant curvature in the release plots was observed on media replacement. These results could be attributed to the marked difference in the drug solubility in the two dissolution media, the solubility in pH 1.2 (26 mg/ml) is five-fold greater than that in pH 6.8 (5.2 mg/ml) (1). Consequently, in vitro release of the drug from the hydrophobic matrix tablets was a direct function of its solubility in the dissolution medium.
The values of release exponent (n), kinetic constant (k), mean dissolution time (MDT), as calculated from Eqs. 3 and 4, are presented in Table VI. As observed from the table, the values of correlation coefficient (r2) for all formulations were high enough to evaluate the drug dissolution behaviour by Eq. 3. In case of drug release from a cylindrical device such as the prepared tablets, n values ranged from 0.49 to 0.5 for E-RS100-based matrix tablets suggested Fickian (Case I) transport. However, a little contribution of coupled diffusion and polymer relaxation (non-Fickian) was observed from n values, 0.5 < n < 0.89, Table VI, when Emcompress replaced mannitol in formulations RS-5, and RS-6. Further, release data analysis for acid portion of the drug release profile from E-L100-based tablet formulations (containing pH-dependent polymer) showed dominating Fickian release, Table VI. These results could be ascribed to absence of polymer relaxation or dissolution. However, formulations L-1 and L-2 showed coupled diffusion and erosion, as indicated from the values of release exponent (0.5 < n < 0.89), Table VI. These results might be attributed to the following two reasons: firstly, high level (54% and 44% w/w, respectively) of the soluble filler (mannitol) and insufficient polymer to coat drug particles allowing the matrices to be rapidly eroded, and secondly, the poor swellability and permeability of E-L100 (4).
Table VI.
Values of Release Exponent (n), Kinetic Constant (k), Mean Dissolution Time (MDT), and Correlation Coefficient (r 2) of Baclofen Tablet Formulations
| Formulations | Release exponent (n) | Kinetic constant (k) | MDT (h) | Correlation coefficient (r 2) |
|---|---|---|---|---|
| RS-1 | 0.49 | 0.63 | 0.84 | 0.996 |
| RS-2 | 0.51 | 0.52 | 1.2 | 0.99 |
| RS-3 | 0.5 | 0.41 | 2 | 0.997 |
| RS-4 | 0.5 | 0.43 | 1.75 | 0.99 |
| RS-5 | 0.55 | 0.4 | 1.86 | 0.99 |
| RS-6 | 0.55 | 0.35 | 2.35 | 0.997 |
| L-1 | 0.58 | 0.55 | 1 | 0.999 |
| L-2 | 0.55 | 0.51 | 1.2 | 0.9999 |
| L-3 | 0.5 | 0.4 | 2 | 0.999 |
| L-4 | 0.5 | 0.46 | 1.57 | 0.997 |
| L-5 | 0.47 | 0.409 | 2 | 0.995 |
| L-6 | 0.57 | 0.35 | 2.3 | 0.999 |
| H-1 | 0.52 | 0.52 | 1.17 | 0.999 |
| H-2 | 0.5 | 0.45 | 1.6 | 0.99 |
| H-3 | 0.47 | 0.297 | 4.2 | 0.97 |
| H-4 | 0.5 | 0.356 | 2.63 | 0.998 |
| H-5 | 0.47 | 0.3 | 3.3 | 0.998 |
| H-6 | 0.5 | 0.202 | 6 | 0.99 |
The mechanism of drug release from wax matrices, however, has been a matter of controversy since wax-systems tend to be crude and more heterogeneous than other classes of polymeric system (26). In some cases, it has been reported that the mechanism of release from wax matrices involves the leaching of drug by the eluting medium. Fluid enters through the cracks and pores of the matrix with diffusion of the drug through the matrix being insignificant (27). Others have reported that release from a typical wax matrix is diffusion-controlled and is best described by Higuchi model (15,28,29). In the present study, the release exponent (n) of the prepared wax formulations H-1 to H-6 ranged from 0.45 to 0.5 suggested Fickian transport. MDT value is used to characterize the drug release rate from the dosage form and the retarding efficacy of the polymer. A higher value of MDT indicates a higher drug retarding ability of the polymer and vice-versa. Table VI shows that MDT value increased with the polymer loading, irrespective of filler and polymer type. Supportive to drug release data, it was found that, MDT values of wax matrices were significantly higher than those of inert insoluble ones irrespective of filler type (P < 0.0001, one way ANOVA). This can be attributed to the higher drug-retarding ability of HCO due to their extremely hydrophobic and water repelling nature.
Effect of Aging on Release of Baclofen from Prepared Tablets
The effect of 6-month shelf storage at ambient conditions on the drug release was studied. Under these storage conditions, no significant change in baclofen profiles from the prepared tablets (data not shown). The result of stability tests suggested that baclofen release properties from the prepared tablets were stable under the above storage conditions.
CONCLUSION
In vitro release studies demonstrated that the release of baclofen from the prepared matrix tablet formulations was dependent on polymeric level and type, and filler, as well as release kinetics was found to be governed by the type and content of the excipients. However, the dominant release mechanism was Fickian transport. Anomalous release, however, was exhibited with some E-L100-besed tablets. In addition, the prepared tablets showed no significant change in drug release rate when the tablets stored at ambient conditions for 6 months. Therefore, the hydrophobic polymers were good candidate for preparation of controlled release tablets of baclofen.
Acknowledgements
The authors wish to thank Pharo Pharmaceutical Company, Alexandria; Egypt, for baclofen gift.
Reference
- 1.Abdelkader H., Abdalla O. Y., Salem H. Formulation of controlled-release baclofen matrix tablets: influence of some hydrophilic polymers on the release rate and in vitro evaluation. AAPS Pharm. Sc. iTech. 2007;8:E100. doi: 10.1208/pt0801014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ammar H. O., Khalil R. M. Preparation and evaluation of sustained-release solid dispersions of drugs with Eudragit polymers. Drug Dev. Ind. Pharm. 1997;23:1043–1054. doi: 10.3109/03639049709150492. [DOI] [Google Scholar]
- 3.Kislalioglu M. S., Khan M. A., Blount C., Goettch R. W., Bolton S. Physical characterization and dissolution properties of ibuprofen: Eudragit coprecipitates. J. Pharm. Sci. 1991;80:799–804. doi: 10.1002/jps.2600800820. [DOI] [PubMed] [Google Scholar]
- 4.A. H. Kibbe, ed. Handbook of Pharmaceutical Excipients. London, UK: American Association and The Pharmaceutical Society of Great Britain; 2000:401–406.
- 5.E. A. Michael, ed. Pharmaceutics: The Science of Dosage Form Design. London, UK: Churchill Livingstone; 2002:289–303.
- 6.A. H. Kibbe, ed. Handbook of Pharmaceutical Excipients. London, UK: American Association and The Pharmaceutical Society of Great Britain; 2000:94–95.
- 7.A. H. Kibbe, ed. Handbook of Pharmaceutical Excipients. London, UK: American Association and The Pharmaceutical Society of Great Britain; 2000:63–67.
- 8.R. K. Raghuram, M. Srinivas, R. Srinivas. Once-daily sustained-release matrix tablets of nicorandil: formulation and in vitro evaluation. AAPS PharmSciTech. 2003; 4 (4) Article 61. Available at: http://www.pharmscitech.org. [DOI] [PMC free article] [PubMed]
- 9.L. Lachman, H. A. Lieberman, eds. The Theory and Practice of Industrial Pharmacy. Philadelphia, PA: Leas and Febiger; 1987:317–318.
- 10.Korsmeyer R. W., Gurny R., Doelker E., Buri P., Peppas N. A. Mechanisms of solute release from porous hydrophilic polymers. Int. J. Pharm. 1983;15:25–35. doi: 10.1016/0378-5173(83)90064-9. [DOI] [PubMed] [Google Scholar]
- 11.Peppas N. A. Analysis of Fickian and non-Fickian drug release from Polymers. Pharm. Acta. Helv. 1985;60:110–112. [PubMed] [Google Scholar]
- 12.Peppas N. A., Sahlin J. J. A simple equation for the description of solute release III. coupling of diffusion and relaxation. Int. J. Pharm. 1989;57:169–17. doi: 10.1016/0378-5173(89)90306-2. [DOI] [Google Scholar]
- 13.Riteger P. L., Peppas N. A. A simple equation for description of solute release. I. Fickian and non-Fickian release from non-swellable devices in the form of slabs, spheres, cylinders or discs. J. Control Rel. 1987;5:23–35. doi: 10.1016/0168-3659(87)90034-4. [DOI] [PubMed] [Google Scholar]
- 14.Talukdar M. M., Rommbaut P., Kinget R. Comparative study on xanthan gum and hydroxypropyl methylcellulose as matrices for controlled-release drug delivery. I. Int. J. Pharm. 1996;129:231–241. doi: 10.1016/0378-5173(95)04355-1. [DOI] [Google Scholar]
- 15.Reza M. S., Abdul Quadir M., Haider S. S. Comparative evaluation of plastic, hydrophobic and hydrophilic polymers as matrices for controlled-release drug delivery. J. Pharm. Pharmaceut Sci. 2003;6:282–291. [PubMed] [Google Scholar]
- 16.Mockel J. E., Lippold B. C. Zero-order release from hydrocolloid matrices. Pharm. Res. 1993;90:1066–1070. doi: 10.1023/A:1018931210396. [DOI] [PubMed] [Google Scholar]
- 17.Schroeder H. G., Dakkuri A., Deluca P. P. Sustained release from inert wax matrices I: Drug-wax combinations. J. Pharm. Sci. 1978;67:350–353. doi: 10.1002/jps.2600670320. [DOI] [PubMed] [Google Scholar]
- 18.M. J. O’Neil ed. The Merck Index: An Encyclopedia of Chemicals, Drugs and Biologicals. 13th ed. USA: Merck research Laboratories; 2001:164–165.
- 19.E. A. Michael, ed. Pharmaceutics: The Science of Dosage Form Design. London, UK: Churchill Livingstone; 2002: 197–210.
- 20.A. H. Kibbe, ed. Handbook of Pharmaceutical Excipients. London, UK: American Association and The Pharmaceutical Society of Great Britain; 2000:294–297.
- 21.M. H. Amaral, J. M. Sousa, D. C. Ferreira. Effect of hydroxypropyl methylcellulose and hydrogenated castor oil on naproxen release from sustained-release tablets. AAPS PharmSciTech., 2001; 2(2) Article 6. Available at: http://www.pharmscitech.org. [DOI] [PMC free article] [PubMed]
- 22.Quadir M. A., Reza M. S., Haider S. S. Effect of PEGs on release of diclofenac sodium from directly compressed carnauba wax matrix tablets. J. Bang. Acad. Sci. 2002;26:1–12. [Google Scholar]
- 23.Schmidt P. C., Herzog R. Calcium phosphates in Pharmaceutical tableting I: Physico-phatmaceutical properties. Pharm. Wld. Sci. 1993;15:105–110. doi: 10.1007/BF02113938. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Y. E., Schwartz J. B. Melting granulation and heat treatment for wax matrix-controlled release. Drug Dev. Ind. Pharm. 2003;29:131–138. doi: 10.1081/DDC-120016720. [DOI] [PubMed] [Google Scholar]
- 25.B. Sandip, T. Tiwari, K. Murthy, P. M. Raveendra, P. R. Mehta, and P. B. Chowdary. Controlled release formulation of tramadol hydrochloride using hydrophilic and hydrophobic matrix system. AAPS PharmSciTech. 2003; 4(3) Article 31. Available at: http://www.pharmscitech.org. [DOI] [PMC free article] [PubMed]
- 26.Dakkuri A., Schroeder H. G., Deluca P. P. Sustained release from inert wax matrices II: Effects of surfactants on tripellenamine hydrochloride release. J. Pharm. Sci. 1978;67:354–358. doi: 10.1002/jps.2600670321. [DOI] [PubMed] [Google Scholar]
- 27.Higuchi T. Mechanism of sustained-action medication. Theoretical analysis of rate of release of solid drugs dispersed in solid matrices. Ibid. 1963;52:1145–1153. doi: 10.1002/jps.2600521210. [DOI] [PubMed] [Google Scholar]
- 28.Goodhart F. W., McCoy R. H., Niger F. C. Release of a water soluble drug from a wax matrix times release tablet. ibid. 1974;63:1748–1755. doi: 10.1002/jps.2600631117. [DOI] [PubMed] [Google Scholar]
- 29.Reza M. S., Quadir M. A., Haider S. S. Development of theophylline sustained release dosage form based on kollidon SR. Pak J Pharm Sci. 2002;15:63–73. [PubMed] [Google Scholar]