Abstract
The release of propranolol hydrochloride from matrix tablets with hydroxy propyl methyl cellulose (HPMC K15M) or Kollidon®SR at different concentrations was investigated with a view to developing twice daily sustained release dosage form. A hydrophilic matrix-based tablet using different concentrations of HPMC K15M or Kollidon®SR was developed using direct compression technique to contain 80 mg of propranolol hydrochloride. The resulting matrix tablets prepared with HPMC K15M or Kollidon®SR fulfilled all the official requirements of tablet dosage forms. Formulations were evaluated for the release of propranolol hydrochloride over a period of 12 h in pH 6.8 phosphate buffer using USP type II dissolution apparatus. Propranolol hydrochloride and pure Kollidon®SR or HPMC K15M compatibility interactions was investigated by using Fourier-transform infrared (FTIR) spectroscopy and differential scanning calorimetry (DSC). FTIR spectroscopic and DSC studies revealed that there was no well defined chemical interaction between propranolol hydrochloride with Kollidon®SR or HPMC K15M. Tablets were exposed to 40 °C/75% of RH in open disc for stability. The in vitro drug release study revealed that HPMC K15 at a concentration of 40% of the dosage form weight was able to control the release of propranolol hydrochloride for 12 h, exhibit non-Fickian diffusion with first-order release kinetics where as at 40% Kollidon®SR same dosage forms show zero-order release kinetics. In conclusion, the in vitro release profile and the mathematical models indicate that release of propranolol hydrochloride can be effectively controlled from a single tablet using HPMC K15M or Kollidon®SR matrix system.
Key words: hydroxypropylmethylcellulose, Kollidon®SR, matrix tablets, propranolol HCL
INTRODUCTION
For many drugs, the optimal therapeutic response is only observed when adequate blood levels are achieved and maintained with minimal fluctuations. Sustained release or controlled release products have became popular for the oral administration of such drugs because they give more consistent blood levels. Sustained release or controlled release with zero order release kinetics maintains plasma concentration of such types of drugs at constant level for better effect. Propranolol hydrochloride is a nonselective β-adrenergic blocking agent used in divided daily doses every 6 to 8 h for the treatment of heart diseases which require long term treatment, hence selected as model drug (1,2). The increased need for patient compliance and demand for improved therapeutic efficacy of propranolol Hydrochloride necessitates controlled release drug delivery system with zero order release kinetics.
Literature survey indicated a few methods published describing approaches for sustained release formulations of propranolol hydrochloride. Sanghavi et al. prepared matrix tablets of propranolol hydrochloride using hydroxypropyl methylcellulose (HPMC) which exhibited first order release kinetics (3). Velasco-De-Paola et al. described dissolution kinetics of controlled release tablets containing propranolol hydrochloride prepared using eudragit (4). Mohammadi-Samani et al. described controlled-release dosage forms of propranolol hydrochloride using coating of ethyl cellulose (5).
Kollidon®SR is a directly compressible polymeric blend composed primarily of polyvinyl acetate (PVAc) and povidone (6,7). The amorphous nature of PVAc coupled with its unusually low glass transition temperature of 28–31 °C (8) imparts certain unique characteristics to this binary matrix. Polyvinyl acetate is a very plastic material that produces a coherent matrix; the water soluble povidone is leached out to form pores through which the active ingredient slowly diffuses outwards.
HPMC is the dominant hydrophilic polymer carrier used for the preparation of oral controlled drug delivery systems (9). It has been used widely for development of sustained release dosage forms such as matrix tablets of high water soluble drugs (1).
A comparative study of controlled release matrix tablets of diclofenac sodium, ciprofloxacin hydrochloride and theophylline using HPMC has been reported (10). Also controlled release matrix tablets of verapamil hydrochloride using HPMC K15 as release control polymer has been reported (11). This paper describes a comparative study of propranolol hydrochloride release from matrix tablets with Kollidon®SR or HPMC K15M aiming to get zero order release kinetics. The effect of pH of the dissolution media and hardness on the release profile from the formulations and the effect of storage on the stability and release profiles were also investigated.
MATERIALS AND METHODS
Materials
A gift sample of Propranolol hydrochloride was received from Aristo Pharmaceuticals Ltd., (Mumbai, India). Kollidon®SR was received from BASF (Germany). HPMC was received from Colorcon Asia Ltd (Goa). Double distilled water was used throughout the study and all the other chemicals used were of analytical grade.
Compatibility Studies
The interaction between the drug and polymer was investigated by using Fourier-transform infrared (FTIR) spectroscopy and differential scanning calorimetry (DSC). The drug was characterized by various official tests of identification.
Fourier-Transform Infrared Spectroscopy
FTIR spectra were obtained by using an FTIR spectrometer-430 (Jasco, Japan). The samples (Propranolol hydrochloride or formulations PrH3 and Pk3) were previously ground and mixed thoroughly with potassium bromide, an infrared transparent matrix, at 1:5 (Sample:KBr) ratio, respectively. The KBr discs were prepared by compressing the powders at a pressure of 5 tons for 5 min in a hydraulic press. Forty scans were obtained at a resolution of 4 cm−1, from 4,000 to 300 cm−1.
Differential Scanning Calorimetry
The DSC measurements were performed on a DSC-6100 (Seiko Instruments, Japan) differential scanning calorimeter with a thermal analyzer. All accurately weighed samples (about 1.675 mg of propranolol hydrochloride or its equivalent) were placed in sealed aluminum pans, before heating under nitrogen flow (20 ml/min) at a scanning rate of 10 °C min−1 from 25 °C to 200 °C. An empty aluminum pan was used as reference.
Preparation of Matrix Tablets
Controlled release matrix-embedded tablets were prepared separately by direct compression process using different proportions of HPMC K15M (10%, 20%, 40%, and 60%) and denoted as PrH1, PrH2, PrH3 and PrH4 respectively or of Kollidon®SR(10%, 20%, 40%, and 60%) and denoted as PrK1, PrK2, PrK3 and PrK4 respectively. The composition of various formulations is given in Table I. Propranolol hydrochloride, and HPMC K15M or Kollidon®SR were mixed in a polybag, and the mixture was passed through mesh no. 40 and mixed with 1% aerosil (Aerosil-200, Degussa Corp, Dusseldorf, Germany) and 1% of magnesium stearate. Tablets were compressed at 250 mg weight on a 10-station Mini Press-I rotary tablet compression machine (Karnavati engineering Ltd, India) fitted with 6-mm oval-shaped punches with compression pressure 10 kN and dwell time 2 s.
Table I.
Composition of Various Tablet Formulations
| Formulation code | Drug (mg) | Kollidon® SR(mg) | HPMC-K15M (mg) | Microcrystalline cellulose (mg) | Mag. Stearate (mg) | Aerosil (mg) | Total wt. (mg) |
|---|---|---|---|---|---|---|---|
| PrH1 | 80 | – | 25 | 140 | 2.5 | 2.5 | 250 |
| PrH2 | 80 | – | 75 | 90 | 2.5 | 2.5 | 250 |
| PrH3 | 80 | – | 100 | 65 | 2.5 | 2.5 | 250 |
| PrH4 | 80 | – | 150 | 15 | 2.5 | 2.5 | 250 |
| PrK1 | 80 | 25 | – | 140 | 2.5 | 2.5 | 250 |
| PrK2 | 80 | 75 | – | 90 | 2.5 | 2.5 | 250 |
| PrK3 | 80 | 100 | – | 65 | 2.5 | 2.5 | 250 |
| PrK4 | 80 | 150 | – | 15 | 2.5 | 2.5 | 250 |
Evaluation of Tablets
Physical Properties of Tablets
The drug content of the manufactured tablets was determined. From each batch 20 tablets were taken, weighed, and finely ground. An adequate amount of this powder equivalent to 10 mg of drug was accurately weighed, suitably dissolved and diluted in distilled water and analyzed by ultraviolet (UV) spectrophotometric method at 290 nm.
The tablets were also evaluated as per IP1996 for weight variation (n = 20), hardness (n = 6), thickness (n = 20), and friability. Hardness was determined by using a Monsanto tablet hardness tester (Campbell Electronics, Mumbai, India).
Friability test was conducted using Roche friabilator (F. Hoffmann-La Roche Ltd, Basel, Switzerland). Thickness of the tablets was measured by digital Vernier calipers (Mitutoyo Corp, Kawasaki, Japan).
In vitro Drug Release
Drug release was assessed by dissolution test under the following conditions: n = 6 (in triplicate), USP type II dissolution apparatus (Lab India, DISSO 2000) at 50 rpm in 900 mL of phosphate buffer at pH 6.8 maintained at 37 ± 0.5 °C. Five milliliters of the sample was withdrawn by using a syringe filter (0.45 μm; Sepyrane, Mumbai) at regular intervals and replaced with the same volume of prewarmed (37 ± 0.5 °C) fresh dissolution medium. The drug content in each sample was analyzed after suitable dilution using UV spectrophotometer method at 290 nm. The effect of hardness and pH of the dissolution medium on release characteristics of the best formulations were compared.
In-vitro Release Kinetics
To compare dissolution profiles, several approaches can be followed such as analysis of variance (ANOVA)-based, model-independent and model dependent approaches (12). In this work, model-independent and model dependent approaches were used for comparison of dissolution profiles. ANOVA based is commonly used to detect significant differences between groups and thereby can be used to detect statistically significant differences between dissolution profiles. Model-independent approaches are based on the ratio of area under the dissolution curve (dissolution efficiency) or on mean dissolution time (13,14). Percent dissolution efficiency and mean dissolution time were also computed to compare the relative performance of various concentrations of HPMC K15 or Kollidon®SR in formulations.
In the model-dependent approaches, release data were fitted to five kinetic models including the zero order (Eq. 1), first order (Eq. 2), Higuchi matrix (Eq. 3), Peppas-Korsmeyer (Eq. 4) and Hixson-Crowell (Eq. 5) release equations to find the equation with the best fit using PCP Disso v3 software (Pune, India).
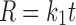 |
1 |
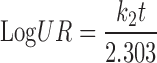 |
2 |
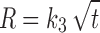 |
3 |
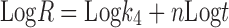 |
4 |
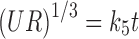 |
5 |
Where R and UR are the released and unreleased percentages, respectively, at time (t); k1, k2, k3, k4, and k5 are the rate constants of zero-order, first-order, Higuchi matrix, Peppas-Korsmeyer, and Hixon-Crowell model, respectively.
Quantification of Water Uptake and Erosion Determinations
Tablets were placed in flat dissolution vessels. At time intervals of 60, 120, 180, 240, 300 and 360 min, the tablets were withdrawn from the medium and weighed after excess of water at the surface had been removed with filter paper. The wetted samples were then dried in an oven at 40 °C up to constant weight. The increases in weight of the tablets reflect the weight of the liquid uptake. It was estimated according to Eq. 6
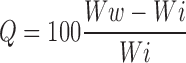 |
6 |
Where Q is the percentage of liquid uptake,
Ww and Wi are the masses of the hydrated samples before drying and the initial starting dry weight, respectively.
The degree of erosion (expressed as % erosion of the polymer content, E) was determined using Eq. 7
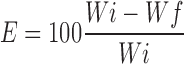 |
7 |
Where Wf is the final mass of the same dried and partially eroded sample.
Effect of Change of Dissolution Media
Propranolol hydrochloride release from PrH3 and PrK3 was studied using 0.1N HCl (pH 1.2) and phosphate buffer (pH 7.4).
Batch Reproducibility Study
All the formulations were prepared in six different batches. The dissolution study was carried out for all the batches and standard deviation was calculated. The standard deviations indicated that the variations were minimum. It was thus, observed that batch to batch variation was insignificant.
Stability Studies
Tablets were put into open disc and exposed inside humidity chambers (Tabai Espec Corp., Osaka, Japan) preequiliberated to 40 °C/75% RH for 4 weeks. After 4 weeks, disc was pulled from the chamber and tested for hardness by Monsanto tablet hardness tester (Campbell Electronics, Mumbai, India). Dissolution testing and hardness measurements were performed after the tablets were allowed to cool down to room temperature for overnight or longer. The in-vitro release profile was studied and compared with initial release profile.
RESULTS AND DISCUSSION
Compatibility Studies
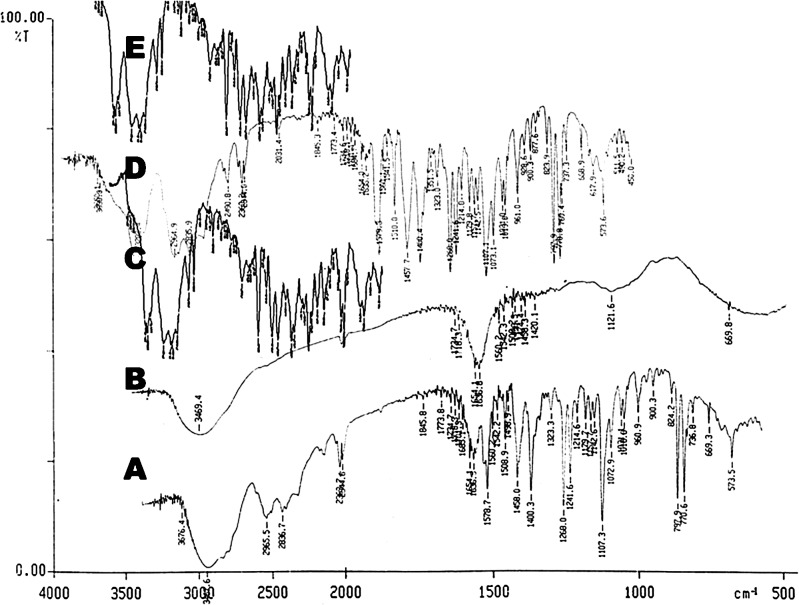
The IR spectra of propranolol hydrochloride with HPMC K15 and propranolol hydrochloride with Kollidon® SR were compared with the standard spectrum of propranolol hydrochloride (Fig. 1). IR spectrum of propranolol hydrochloride is characterized by the absorption of NH group at 3467 cm−1(Fig. 1A). In spectra of propranolol hydrochloride with HPMC K15 and propranolol hydrochloride with Kollidon® SR, this band was shifted towards lower frequencies at 3,282 and 3,280 cm−1 respectively. Mentioned evidences thus lead to the conclusion that changes seen are as a result of physical interaction between the drug and polymer.
Fig. 1.
FTIR Spectrograms of pure Propranolol hydrochloride (A), Pure HPMC K 15 (B), Pure Kollidon®SR (C), Propranolol hydrochloride + Pure HPMC K 15 (D), Propranolol hydrochloride + with Kollidon®SR (E)
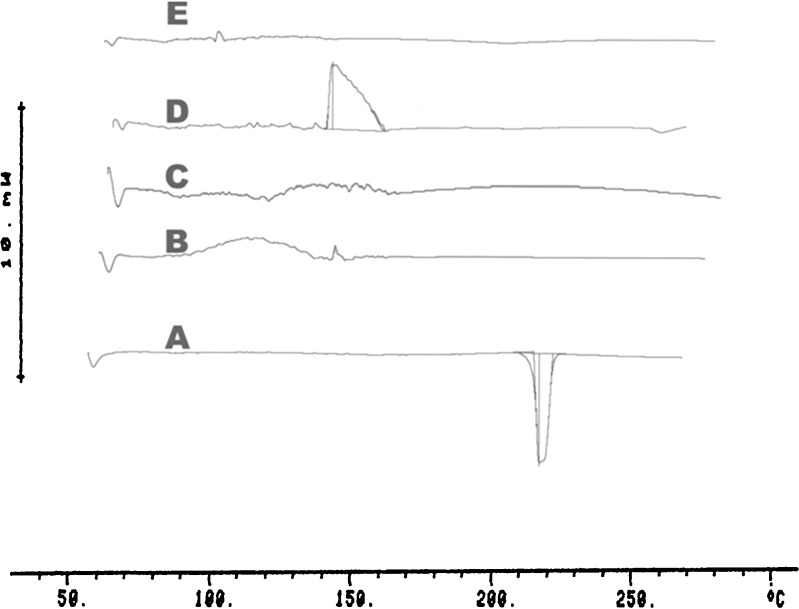
The DSC curve of pure propranolol hydrochloride exhibited a single endothermic response corresponding to the melting of drug. Onset of melting was observed at 162.19 °C, the corresponding heat of fusion (ΔHF) was −90.02.8 J/g (Fig. 2A), where as in DSC thermogram of the drug with polymer (HPMC K15 or Kollidon® SR) the melting endothermic peak of the drug disappeared.
Fig. 2.
DSC Thermograms of pure Propranolol hydrochloride (A), Pure HPMC K 15 (B), Pure Kollidon®SR (C), Propranolol hydrochloride + Pure HPMC K 15 (D) Propranolol hydrochloride + with Kollidon®SR (E)
Evaluation of Tablets
Physical Properties of Tablets
The results of the uniformity of weight, hardness, drug content and friability of the tablets are given in Table II. All the samples of the test product complied with the official requirements of uniformity of weight. The drug content was found to be close to 100% of the label claim for propranolol in all formulations. The low friability indicates that the matrix tablets are compact and hard. The results were reproducible, even on tablets that had been stored for about 6 months at 25 °C and 60% relative humidity.
Table II.
Physical Properties of Compressed Tablets
| Formulation code | PrH1 | PrH2 | PrH3 | PrH4 | PrK1 | PrK2 | PrK3 | PrK4 |
|---|---|---|---|---|---|---|---|---|
| Drug content (mg/tab) | 81.2 ± 1.6 | 80.3 ± 1.7 | 80.8 ± 1.2 | 80.2 ± 0.5 | 80.1 ± 0.5 | 80.3 ± 0.9 | 80.9 ± 0.8 | 81.6 ± 0.9 |
| Weight variation | ±4.9 | ±4.6 | ±5.2 | ±4.6 | ±4.4 | ±4.8 | ±4.3 | ±4.5 |
| Hardness (kg/cm2) | 5.2 ± 0.5 | 5.1 ± 0.3 | 5.4 ± 0.6 | 5.3 ± 0.8 | 5.4 ± 0.3 | 5.2 ± 0.6 | 5.1 ± 0.5 | 5.3 ± 0.3 |
| Friability (%) | 0.91 | 0.81 | 0.83 | 0.75 | 0.85 | 0.76 | 0.72 | 0.89 |
Release Rate Studies
In-vitro dissolution studies were conducted on three tablets of each of the formulations such as PrH1, PrH2, PrH3, PrH4, PrK1, PrK2, PrK3, and PrK4. The mean cumulative percent of propranolol hydrochloride released at different time intervals for each formulation is shown in Table III. The rate of release of the drug decreased as the concentration of polymer increased. The release of propranolol hydrochloride was prolonged upto 12 h by increasing the proportion of HPMC K15 from 10% to 40% w/w and in case of Kollidon® SR the release rate was prolonged upto 12 h by increasing the proportion from 10% to 40% w/w. It was observed that the initial rate of release for PrH1 and PrK1 was high and around 98% of the drug was released within 4 h. In case of formulation, PrH2 and PrK2 about 99% of the drug was released within 10 h. However, formulations containing 40% of HPMC K15 (PrK3) and Kollidon®SR (PrK3) controlled the release of drug up to 12 h. The formulations containing about 60% of HPMC K15 (PrH4) and Kollidon®SR (PrK4) released 86% and 80% of drug within 12 h. So the formulations PrH3 and PrK3 containing the optimum quantity of polymer to sustain the drug release for 12 h were chosen for the controlled drug delivery.
Table III.
In-vitro Dissolution Profile of Propranolol HCl from the Formulations Containing HPMC K15 or Kollidon® SR in pH 6.8 Phosphate Buffer
| Sr. No. | Mean cumulative percent ± S.D drug released | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Time (h) | PrH 1 | PrH 2 | PrH 3 | PrH 4 | PrK1 | PrK2 | PrK3 | PrK4 | |
| 1. | 1 | 33.31 ± 0.98 | 13.36 ± 1.17 | 13.788 ± 1.22 | 11.56 ± 0.93 | 32.23 ± 1.21 | 13.23 ± 1.34 | 9.22 ± 1.23 | 8.22 ± 1.1 |
| 2. | 2 | 62.06 ± 0.91 | 25.87 ± 0.39 | 27.894 ± 1.54 | 18.74 ± 1.61 | 58.76 ± 0.76 | 24.26 ± 1.56 | 18.24 ± 1.65 | 16.65 ± 2.1 |
| 3. | 3 | 87.32 ± 1.44 | 36.06 ± 1.30 | 42.373 ± 0.98 | 28.24 ± 0.69 | 81.34 ± 1.22 | 35.34 ± 1.67 | 27.31 ± 1.87 | 23.34 ± 1.43 |
| 4. | 4 | 98.18 ± 0.52 | 53.39 ± 0.57 | 52.755 ± 0.78 | 39.36 ± 0.76 | 97.98 ± 1.21 | 51.25 ± 1.98 | 35.32 ± 1.90 | 30.56 ± 1.34 |
| 5. | 5 | 67.14 ± 0.77 | 62.260 ± 0.87 | 50.99 ± 0.69 | 65.28 ± 2.10 | 44.31 ± 1.98 | 36.29 ± 1.87 | ||
| 6. | 6 | 73.86 ± 0.62 | 71.189 ± 1.34 | 59.18 ± 0.85 | 76.23 ± 1.67 | 52.38 ± 1.76 | 42.09 ± 1.98 | ||
| 7. | 7 | 78.48 ± 0.09 | 75.973 ± 1.23 | 67.49 ± 0.72 | 87.45 ± 1.98 | 60.11 ± 0.89 | 47.59 ± 1.65 | ||
| 8. | 8 | 84.60 ± 0.66 | 80.469 ± 1.43 | 73.43 ± 0.43 | 93.56 ± 1.87 | 68.22 ± 0.87 | 52.34 ± 1.56 | ||
| 9. | 9 | 91.30 ± 0.64 | 83.311 ± 1.32 | 75.18 ± 0.96 | 97.45 ± 0.95 | 77.31 ± 0.89 | 57.98 ± 0.98 | ||
| 10. | 10 | 95.84 ± 0.53 | 86.682 ± 1.12 | 79.45 ± 0.64 | 85.22 ± 0.67 | 62.6 ± 1.89 | |||
| 11 | 11 | 99.35 ± 0.31 | 89.669 ± 1.94 | 82.31 ± 0.68 | 91.22 ± 1.76 | 66.4 ± 1.95 | |||
| 12 | 12 | 91.215 ± 1.21 | 86.93 ± 0.81 | 97.23 ± 1.13 | 80.9 ± 1.76 | ||||
Table IV shows the regression parameters obtained after fitting various release kinetic models to the in vitro dissolution data. The goodness of fit for various models investigated for PrH3 ranked in order of First order > Hixson-Crowell cube root law Higuchi > Korsemeyer-Peppas > Zero order. In vitro release data of PrH3 best fitted to first-order model, however for Korsemeyer-Peppas n value is 0.5, hence exhibits Fickian diffusion. Where as for PrK3 kinetics model ranked in order of Zero order > Korsemeyer-Peppas > Hixson-Crowell cube root law > First order > Higuchi. In vitro release data of PrK3 best fitted to Zero-order model, however for Korsemeyer-Peppas n value is 1, hence exhibits Zero order release.
Table IV.
Statistical Parameters of Various Formulations after Fitting Drug Release Data into Various Release Kinetics Models
| Formulations | Zero-order Model | First-order Model | H-M Model | P-K Model | H-C Model | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| R | K 1 | R | K 2 | R | K 3 | R | K 4 | R | K 5 | |
| PrH3 | 0.9143 | 9.260 | 0.9987 | −0.202 | 0.9827 | 27.074 | 0.9812 | 16.701 | 0.9918 | −0.050 |
| PrK3 | 0.9982 | 6.971 | 0.9742 | −0.117 | 0.9353 | 19.787 | 0.9976 | 6.338 | 0.9906 | −0.032 |
H-M Highuchi Matrix, P-K Peppas-Korsmeyer, H-C Hixon-Crowell, R correlation coefficient, K 1–K 5 constants of release kinetics
Effect of Change of Dissolution Media
Propranolol hydrochloride release from PrH3 and PrK3 was studied using 0.1N HCl (pH 1.2) and phosphate buffer (pH 7.4). With increase in pH the release rate was increased in case of tablets containing HPMC. However, the release rate was unchanged in case of tablets containing Kollidon® SR.
Effect of Change of Hardness
Since the tablets were non disintegrating, low tablet hardness (2–3 kg/cm2) caused disintegration of the tablet containing HPMC K15. For this reason, the hardness in the tablet containing HPMC K15 was kept in between 5–6 kg/cm2. The tablets containing Kollidon® SR were not disintegrating at low tablet hardness (3–4 kg/cm2) and the release rate was unchanged as compared to the tablets having hardness between 5–6 kg/cm2.
Water Uptake and Erosion by the Matrix Tablets
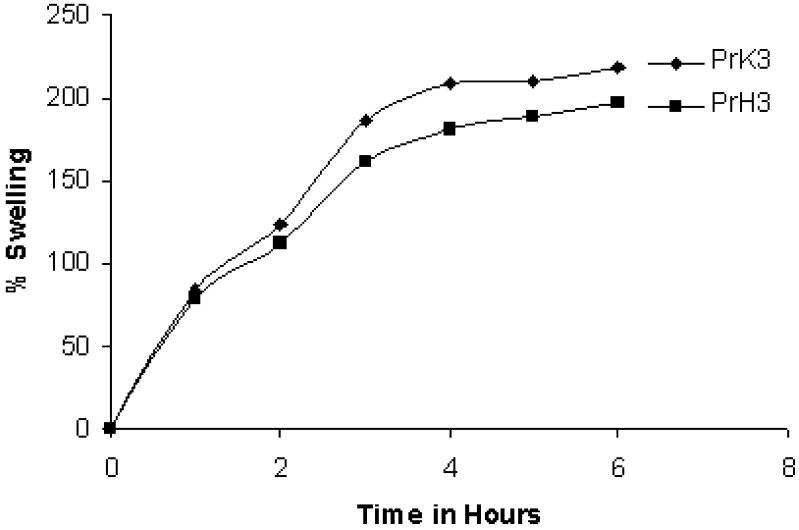
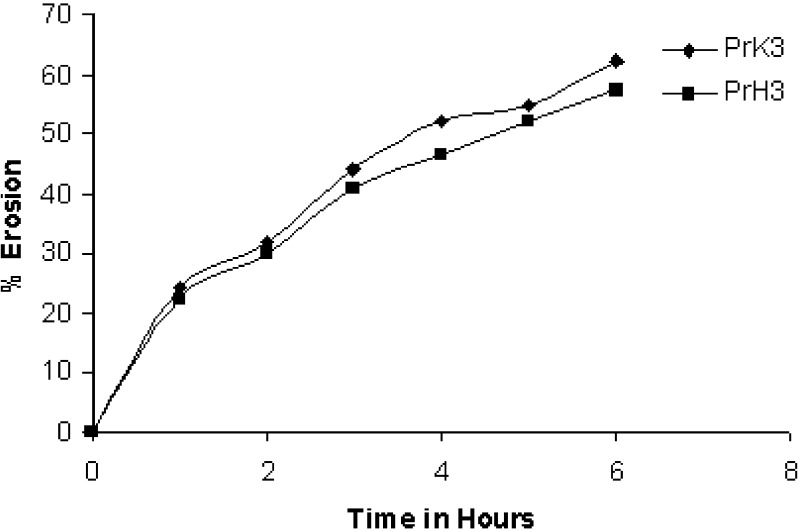
Since the rate of swelling and erosion is related and may affect the mechanism and kinetics of drug release, the penetration of the dissolution medium and the erosion of the hydrated tablets were determined. The percentage increase in weight of the hydrated tablets containing HPMC K15 at various time intervals upto 8 h are shown in (Fig. 3). Simultaneously with the penetration liquid study, the degree of polymer erosion was measured (Fig. 4). The degree of swelling is dependent on the concentration and type of polymer present in the matrix. The order of swelling observed in these polymers could indicate the rates at which the preparations were able to absorb water and swell. The percentage of erosion and swelling were higher in case of tablets containing Kollidon®SR compared to formulation containing HPMC K15M.
Fig. 3.
Percentage of swelling
Fig. 4.
Percentage of erosion
Batch Reproducibility Study
The standard deviations indicated that the variations are minimum and batch to batch variation was insignificant.
Stability Studies
There was no significant change in drug release and hardness of tablets of PrH3 and PrK3 batch observed after 4 weeks.
CONCLUSIONS
The matrix embedding technique using HPMC K15 or Kollidon® SR has successfully extended the release of propranolol hydrochloride. It is particularly suitable for obtaining directly compressed sustained-release matrix tablets with appropriate standards and well reproducible drug release profiles. In contrast to thrice daily prescriptions of the conventional formulation, the designed formulations can be prescribed twice daily leading to a reduction in dose frequency and per-day drug dose. It is concluded that Kollidon® SR in appropriate proportions is suitable for formulating controlled release tablets which exhibit zero-order release kinetics for propranolol hydrochloride.
References
- 1.Martindale W. H. The Extra Pharmacopeia. 30. London: Pharmaceutical; 1993. p. 1783. [Google Scholar]
- 2.Korolkovas A. Essentials of Medicinal Chemistry. 2. New York: Wiley; 1988. p. 435. [Google Scholar]
- 3.Sanghavi N. M., Sarawade V. B., Kamath P. R., Bijilani C. P. Matrix tablets of propranolol hydrochloride. Indian Drugs. 1998;26(8):404–407. [Google Scholar]
- 4.Velasco-De-Paola M. V. R., Santoro M. I. R. M., Gai M. N. Dissolution kinetics evaluation of controlled-release tablets containing propranolol hydrochloride. Drug Dev. and Ind. Pharm. 1999;25(4):535–541. doi: 10.1081/DDC-100102205. [DOI] [PubMed] [Google Scholar]
- 5.Mohammadi-Samani S., Adrangui M., Siahi-Shadbad M. R., Nokhodchi A. An approach to controlled-release dosage form of propranolol hydrochloride. Drug Dev. and Ind. Pharm. 2000;26(1):91–94. doi: 10.1081/DDC-100100332. [DOI] [PubMed] [Google Scholar]
- 6.Ò. SR. Kollidon . Technical Information. Germany: BASF Aktiengesellschaft; 1999. pp. 1–10. [Google Scholar]
- 7.Ruchatz F., Kolter K., Wittemer S., Frauenhofer W. KollidonÒSR a new excipient for sustained release matrice. Proc. Int. Symp.Control release bioactive Materials. 1999;26:6312. [Google Scholar]
- 8.Daniels W. Encyclopedia of Polymer Science and Engineering. 2. New York: Wiley; 1989. pp. 402–425. [Google Scholar]
- 9.Colombo P. Swelling-controlled release in hydrogel matrixes for oral route. Advance Drug Delivery Review. 1993;11:37–57. doi: 10.1016/0169-409X(93)90026-Z. [DOI] [Google Scholar]
- 10.Saha R. N., Sanjeev C., Sahoo J. A comparative study of controlled release matrix tablets of Diclofenac Sodium, Ciprofloxacin Hydrochloride, and Theophylline. Drug Delivery. 2001;8:149–154. doi: 10.1080/107175401316906919. [DOI] [PubMed] [Google Scholar]
- 11.J. Sahoo, P. N. Murthy, S. Biswal, K. A. Mahapatra, and K. S. Sahoo. Preparation and release rate study of controlled release matrix tablets of verapamil hydrochloride using hydroxy propyl methyl cellulose. Int. J. Pharm. Excipients. (2008) In press. [DOI] [PMC free article] [PubMed]
- 12.Polli J. E., Rekhi G. S., Augsburger L. L., Shah V. P. Methods to compare dissolution profiles and a rationale for wide dissolution specification for metoprolol tartrate tablets. J. Pharm. Sci. 1997;8:690–700. doi: 10.1021/js960473x. [DOI] [PubMed] [Google Scholar]
- 13.Khan C. A., Rhodes C. T. The concept of dissolution efficiency. J. Pharm. Pharmacol. 1975;27:48–49. doi: 10.1111/j.2042-7158.1975.tb09378.x. [DOI] [PubMed] [Google Scholar]
- 14.Arias M. J., Gines J. M., Moyano J. R., Rabasco A. M. Dissolution properties and in vivo behaviour of triamterene in solid dispersions with polyethylene glycols. Pharm. Acta Helv. 1996;71:229–235. doi: 10.1016/S0031-6865(96)00017-9. [DOI] [PubMed] [Google Scholar]