Abstract
The objective of the present investigation was to reduce the bitterness with improved dissolution, in acidic medium (pH 1.2), of mefloquine hydrochloride (MFL). Microparticles were prepared by coacervation method using Eudragit E (EE) as polymer and sodium hydroxide as precipitant. A 32 full factorial design was used for optimization wherein the drug concentration (A) and polymer concentration (B) were selected as independent variables and the bitterness score, particle size and dissolution at various pH were selected as the dependent variables. The desirability function approach has been employed in order to find the best compromise between the different experimental responses. The model is further cross validated for bias. The optimized microparticles were characterized by FT-IR, DSC, XRPD and SEM. Bitterness score was evaluated by human gustatory sensation test. Multiple linear regression analysis revealed that the reduced bitterness of MFL can be obtained by controlling the dissolution of microparticles at pH 6.8 and increasing the EE concentration. The increase in polymer concentration leads to reduction in dissolution of microparticles at pH > 5 due to its insolubility. However the dissolution studies at pH 1.2 demonstrated enhanced dissolution of MFL from microparticles might be due to the high porosity of the microparticles, hydrophilic nature of the EE, and improved wettability, provided by the dissolved EE. The bitterness score of microparticles was decreased to zero compared to 3+ of pure ARM. In conclusion the bitterness of MFL was reduced with improved dissolution at acidic pH.
Key words: bitterness, eudragit, full factorial design, mefloquine HCl, microparticles
INTRODUCTION
In recent years, the importance of patient compliance, not only in drug efficacy per se, but also in overall economics of healthcare, has been increasingly recognized. Efforts to improve patient compliance have included attempts to improve the palatability of orally administered pharmaceutical agents especially for children and elderly (1,2). In particular, a bitter taste is known to decrease patient compliance, and thus reduce effective pharmacotherapy.
In the present study, the possibility of masking the bitterness of mefloquine hydrochloride (MFL), a drug used as a treatment for malaria, was investigated. MFL has an extremely unpleasant bitter taste due to presence of quinine moiety (3). As this is likely to give rise to non-compliance when administered orally, it would be a considerable advantage to be able to mask the bitterness of oral formulations containing MFL.
In order to achieve an acceptable palatability, the addition of flavors or sweeteners is limited and may not be efficient enough to mask the bitter taste of drugs, requiring the use of technological processes (4–6). A number of taste-masking approaches have been described in the literature, including the use of cyclodextrin (7), ion exchange resin (8,9), film coating (10), viscosity modification (11) and melt granulation (12). Among the various techniques, microencapsulation has often proved to be the most successful in reducing the bitterness of bitter active pharmaceutical ingredients because it is simple, economic and advantageous (13).
The objective of present investigation was to completely mask the bitter taste of MFL by encapsulation in microparticles, while allowing the complete release of MFL under the acidic conditions of stomach (pH 1.2). The pH inside the oral cavity has been reported to be about 6.8 (14). A 32 full factorial design was used for optimization wherein the drug concentration (A) and polymer concentration (B) were selected as independent variables and the bitterness, particle size and dissolution at various pH were selected as the dependent variable.
Eudragit E, an acid-soluble polymer, was selected for the encapsulation of MFL. It has been reported that this polymer is a cationic copolymer based on dimethyl aminoethyl methacrylate and neutral methacrylic esters soluble up to pH 5; however it is swellable and permeable above pH 5 (15,16). Sodium hydroxide was used as precipitating agent. This alternative microparticles preparation method can be applied, replacing the complicated and sophisticated quasi emulsion solvent diffusion (17), spray drying (16), solvent evaporation (18), solid dispersion by supercritical fluids (19), co evaporates (20) and non-aq. granulation (15) used in prior formulations. Further the present method avoids use of special instrument, hazardous organic solvent and is easy to scale up.
MATERIALS AND METHODS
Materials
Mefloquine hydrochloride (Batch no. 031209) was a gift from Ajanta Pharma Ltd, (Mumbai, India). Eudragit E (EE) (Batch no. G041131159) was a gift from Degussa India Pvt. Ltd., (Mumbai, India). Methanol was purchased from Qualigens Fine Chemicals (Mumbai, India) and was used as received. Sodium hydroxide, hydrochloric acid, potassium dihydrogen phosphate, and acetic acid were purchased from S. D. Fine-Chem Ltd., (Mumbai, India) and were used as received.
Preparation of Microparticles
Microparticles were prepared by coacervation phase separation method. A concentrated solution of EE (∼1% w/v) was prepared in 1% v/v acetic acid. The required quantity of the MFL (0.6 g in 50 mL of final EE solution) was mixed for 5 min. Ten milliliters of 10% w/v sodium hydroxide solution was introduced into a 10-ml of glass syringe with a 18G × 1/2” flat-cut hypodermic needle. The droplets were amputated at a flow rate of 3 ml/min into EE solution. The resulting microparticles were allowed to harden for 60 min under gentle stirring at 400 rpm (Remi Equipments Pvt. Ltd., Mumbai, India) with small magnetic bar. Actual values of independent factors are listed in Table I. Different concentrations of MFL and EE were used, according to the experimental runs, mentioned in Table II. The microparticles were collected, decanted, washed with deionized water and dried to a constant weight in oven (Shree Kailash Industries, Baroda, India) at 80 °C for 24 h, and then stored in the desiccator until use. The percentage yield was calculated as:
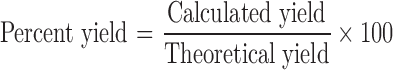 |
1 |
Table I.
Variables in 32 Full Factorial Design
| Variables | Characteristic | Actual Values | |
|---|---|---|---|
| Drug Conc. (A in g) | Polymer Conc. (B in mL)a | ||
| Independent variables | |||
| A | Drug concentration | ||
| B | Polymer concentration | ||
| Dependent variables | |||
| Y1 | Particle size | ||
| Y2 | Dissolution at pH 1.2 | ||
| Y3 | Dissolution at pH 6.8 | ||
| Y4 | Bitterness score | ||
| Coded values | |||
| −1 | 0.3 | 10 | |
| 0 | 0.5 | 30 | |
| 1 | 0.7 | 50 | |
amL of 1% w/v EE solution, prepared in 1% v/v acetic acid
Table II.
Presentation of Experiments with Coded Values for Factor Levels for 32 Full Factorial Design with their Percent Yield and Incorporation Efficacy of Microparticles
| Formulation no. | Factors and Factor Levels | Percent Yield ± SDa | Incorporation Efficiency (%) ± SDa | |
|---|---|---|---|---|
| Drug Conc. (A) | Polymer Conc. (B) | |||
| MFL 1 | −1 | 1 | 88.75 ± 1.34 | 31.17 ± 1.51 |
| MFL 2 | 0 | 0 | 99.50 ± 0.50 | 94.48 ± 1.17 |
| MFL 3 | 0 | 1 | 88.53 ± 0.42 | 59.97 ± 1.74 |
| MFL 4 | −1 | −1 | 95.00 ± 1.81 | 91.81 ± 0.48 |
| MFL 5 | 1 | −1 | 89.71 ± 0.48 | 94.52 ± 0.89 |
| MFL 6 | −1 | 0 | 85.00 ± 1.36 | 66.33 ± 0.72 |
| MFL 7 | 1 | 1 | 84.67 ± 1.17 | 57.93 ± 1.39 |
| MFL 8 | 1 | 0 | 89.00 ± 1.87 | 82.60 ± 0.94 |
| MFL 9 | 0 | −1 | 88.33 ± 1.10 | 83.28 ± 1.73 |
aValues represent the mean ± SD of three experiments
Experimental Design
A 32 full factorial design was employed to systematically study the joint influence of the effect of independent variables A and B on the dependent variables. In this design, two factors were evaluated, each at three levels, and experimental trials were performed at all nine possible combinations. A statistical model incorporating interactive and polynomial terms was used to evaluate the response (21).
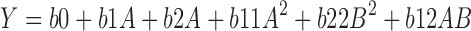 |
2 |
where, Y is the dependent variable, b0 is the arithmetic mean response of the nine runs, and bi is the estimated coefficient for the factor, drug (A) and polymer concentration (B). The main effects (A and B) represent the average result of changing one factor at a time from its low to high value. The interaction terms (AB) show how the response changes when two factors are simultaneously changed. The polynomial terms (A2 and B2) are included to investigate nonlinearity.
Optimization of Responses Using Desirability Function
This technique involves a way of overcoming the difficulty of multiple, sometimes opposing responses (22). Each response is associated with its own partial desirability function. If the value of the response is optimum, its desirability equals 1, and if it is totally unacceptable, its value is zero. Thus the desirability for each response can be calculated at a given point in the experimental domain. The optimum is the point with the highest value for the desirability.
The dissolution at pH 1.2 was targeted to maximize in the procedure, as higher values of this is desirable. Greater dissolution at pH 1.2 leads to greater availability of MFL in stomach. Moreover microparticles showed complete release within few min. Hence dissolution at pH 1.2 in 15 min (t15) was selected. So the desirability function of this parameter was calculated by using Eq. 3.
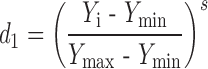 |
3 |
Where d1 is individual desirability and Yi is experimental results of dissolution at pH 1.2. The values of Ymin and Ymax of dissolution at pH 1.2 were 59.89 and 99.59 % respectively.
To avoid grittiness of microparticles after ingestion in oral cavity, minimum particles size was desired. The observed Ymin and Ymax values of particle size were 32.08 and 236.78 μ, respectively. Further the problem of bitter taste of the drug, generally encountered due to dissolution of the active component in oral cavity. In addition the microparticles remain for maximum 5 min in oral cavity. To avoid this, minimum dissolution at 5 min was desired. The values of Ymin and Ymax of dissolution at pH 6.8 in 5 min (t5) were 2.45 and 5.25 %, respectively. Similarly the lowest value of bitterness score was desired for complete taste masking. Though the observed Ymax value of bitterness score was 3, it was selected as 0.5 because no bitterness to very slightly bitterness was desired. The values of Ymax and Ymin of bitterness score were 0.5 and 0, respectively. So the desirability function of particle size, drug release at pH 6.8 and bitterness score was calculated by using following equation.
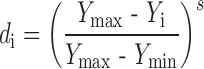 |
4 |
where di is the individual desirability while Yi is the experimental result. In all the experiments performed, all the experimental values were acceptable, however, the values far from the target, were little penalized, by choosing 0 < s < 1 (1 in this case) in Eqs. 5, 6 and 7.
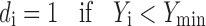 |
5 |
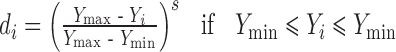 |
6 |
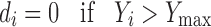 |
7 |
The combined desirability value was calculated from the individual values by using following equation:
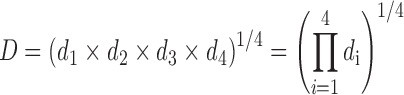 |
8 |
CHARACTERIZATION OF MICROPARTICLES
Determination of Incorporation Efficiency
Microparticles containing 10 mg MFL were weighed accurately and dissolved in methanol. Drug concentration was determined by UV spectrophotometry (Shimadzu UV visible spectrophotometer 1601) at 284 nm. A calibration curve was used, based on standard solutions in methanol. The polymer did not interfere with the analysis at this wavelength. The percent yield and incorporation efficiency for all formulations are shown in Table II. To determine the incorporation efficiency, the following practical relationship was used:
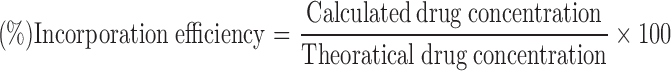 |
9 |
Particle Size Analysis
The average particle diameter and size distribution of microparticles were determined by using Malvern (Mastersizer 2000 Malvern Instruments, UK). Approximately 10 mg of microparticles were dispersed in 2–3 ml of filtered and degaussed phosphate buffer pH 6.8 containing 0.1% Tween 80 for 1 min using an ultrasonic bath. An aliquot of the microparticle suspension was then added into the small volume recirculation unit and circulated 3500 times/min. Each sample was measured in triplicate in the analysis. Particle size was expressed as the weighted mean of the volume distribution.
Drug Release
The in vitro drug release profile of microparticles was determined according to the paddle method described in the United State Pharmacopoeia (USP; XXIV). The in vitro drug release study was carried out in phosphate buffer pH 6.8 because the pH of the saliva is in the range from 6.3 to 7.2. Further the in vitro drug release study was performed in hydrochloric acid buffer pH 1.2 to demonstrate the availability of MFL in gastric pH. Both the buffers of particular pH were prepared according to Indian Pharmacopoeia. An appropriate amount of microparticles containing 250 mg of MFL were suspended in 900 mL of the buffer solution, and 3 mL sample was withdrawn at 1, 5, 10, 15, 30, 45 and 60 min and analyzed using UV spectrophotometer at 284 nm. Each sample was replaced with fresh 3 ml buffer solution having the same temperature.
Gustatory Sensation Test
Twenty volunteers participated in sensory test. Microparticles containing 500 mg of MFL were dispersed in 25 ml of water for 15 s. Immediately after preparation, each volunteer held about 1 ml of the dispersion in the mouth for 30 s. After expectoration, bitterness was evaluated using bitterness score, classified in eight grades, corresponding to increasing bitterness and comparison of bitterness among the samples was performed on the total number of persons who selected “bitter” and “slightly bitter”. The ranking scheme used is shown in Table III. The threshold of bitterness of microparticles was determined as point at which half of the volunteers described the taste as bitter or slightly bitter.
Table III.
Grading for Gustatory Sensory Test
| Parameter | Score |
|---|---|
| Tasteless | 0 |
| Very slightly bitter | 0.5 |
| Slightly bitter | 1.0 |
| Slight to moderate bitter | 1.5 |
| Moderately bitter | 2.0 |
| Moderate to strong bitter | 2.5 |
| Strongly bitter | 3.0 |
| Very strongly bitter | 3.0 + |
Fourier Transform Infra-red Spectroscopy (FTIR)
IR transmission spectra were obtained using a Fourier Transform Infrared spectrophotometer (FTIR-8300, Shimadzu, Japan). A total of 2% (w/w) of sample, with respect to the potassium bromide (KBr; S. D. Fine Chem Ltd., Mumbai, India) disc, was mixed with dry KBr. The mixture was ground into a fine powder using an agate mortar before compressing into KBr disc under a hydraulic press at 10,000 psi. Each KBr disc was scanned 16 times at 4 mm/s at a resolution of 2 cm−1 over a wave number region of 500–4,000 cm−1. The characteristic peaks were recorded.
Differential Scanning Calorimeter (DSC)
Differential scanning calorimetry study of pure MFL, EE and microparticles was performed using Differential Scanning Calorimeter (Mettler Toledo, DSC 822). All the samples were accurately weighed (5–8 mg), sealed in aluminium pan and heated at a scanning rate of 5 °C/min. Nitrogen was used as the purge gas with the flow rate set at 40 mL/min. Aluminum pans and lid were used for all samples. An empty aluminum pan was used as reference.
X-ray Powder Diffractometry (XRPD)
Vacuum grease was applied over a glass slide to stick the sample. About 100 mg of sample was sprinkled over it to make a layer having a thickness of ~0.5 mm. All the experiments were performed on an X-ray diffractometer (Philips X’Pert MPD, Eindhoven, The Netherlands) having a sensitivity of 0.1 mg. The sample slide was placed vertically at an angle of 0° in the sample chamber. An X-ray beam (Philips Cu target X-ray tube) of 2 kW was allowed to fall over the sample. As the slide moves at an angle of theta degree, a proportional detector detects diffracted X-rays at angle of 2θ°. XRD patterns were recorded using Philips JPCD software for powder diffractometry.
Scanning Electron Microscopy (SEM)
The microparticles were mounted on brass stubs using carbon paste. SEM micrographs were taken using a scanning electron microscope (JSW-5610LV, Jeol Ltd, Tokyo, Japan) at the required magnification at room temperature. a working distance of 39 mm was maintained, and the acceleration voltage used was 5 kv, using the secondary electron image (SEI) as the detector.
RESULTS AND DISCUSSION
MFL is extremely bitter due to presence of quinine moiety, suggesting a strong need to reduce the bitterness of MFL. Therefore, microencapsulation method was employed for reducing the bitterness of MFL in the present investigation.
Experimental Design
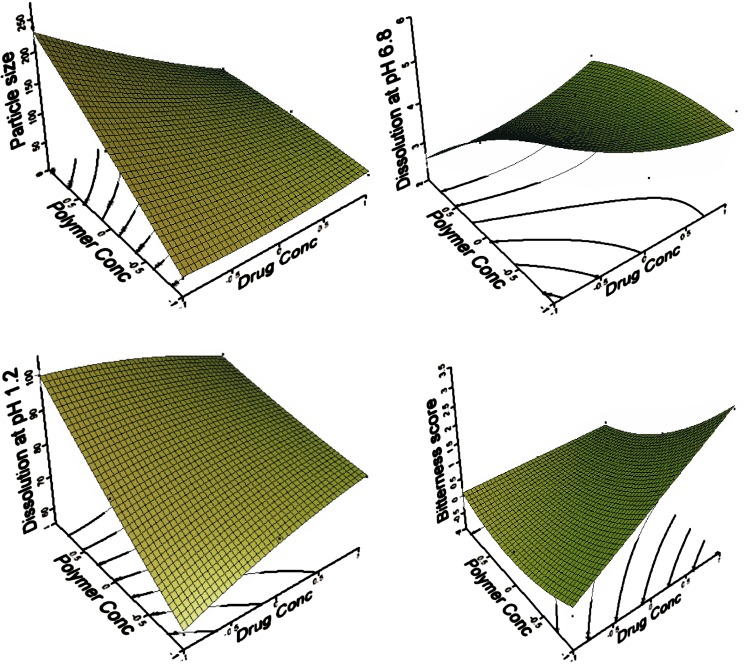
Preliminary investigations of the process parameters revealed that factors, drug (A) and polymer (B) concentration highly influenced the bitterness in human volunteers, particle size and dissolution at pH 1.2 and 6.8. Hence these responses were used for further systematic studies. The dependent and independent variables were related using mathematical relationships obtained with the statistical package, DOE v6.0.5 (Stat-Ease, Inc.). The fitted polynomial equations (full and reduced model) relating the response to the transformed factors are shown in Table IV. The polynomial equations can be used to draw conclusions after considering the magnitude of coefficient and the mathematical sign it carries, i.e., positive or negative.
Table IV.
Results of Regression Analysis
| Terms | Particle Size (μ) | Drug Release at pH 1.2 (t 15 in %) | Dissolution at pH 6.8 (t 5 in %) | Bitterness Score | ||||
|---|---|---|---|---|---|---|---|---|
| FM | RM | FM | RM | FM | RM | FM | RM | |
| Intercept | 83.39 | 83.39 | 83.04 | 81.63 | 4.48 | 4.48 | 0.22 | 0.33 |
| A | −44.81 | −44.81 | 0.46 | 0.46 | 0.39 | 0.39 | 0.50 | 0.50 |
| B | 49.16 | 49.16 | 10.46 | 10.46 | −0.61 | −0.61 | −1.00 | −1.00 |
| A 2 | 7.71 | − | −3.67 | − | 0.31 | 0.31 | 0.17 | − |
| B 2 | −3.74 | − | 1.57 | − | −1.28 | −1.28 | 0.67 | 0.67 |
| AB | −48.71 | −48.71 | −8.01 | −8.01 | 0.095 | – | −0.50 | −0.50 |
− Indicates term is omitted in reduced model, FM full model, RM reduced model, t5 and t15 percent drug released at 5 and 15 min, respectively
Table V shows the results of analysis of variance (ANOVA), which was performed to identify insignificant factors (23). High values of correlation coefficient (R2) for all dependent variables indicate a good model fit. F-value compares the variance with the residual (error) variance. If the variances are close, the ratio will be close to one and it is less likely that the term has a significant effect on the response. The model F-value implies that the model is significant for all dependent variable. Prob > F is a probability seeing the observed F value, if the null hypothesis is true (there is no factor effect). Smaller probability values call for rejection of the hypothesis i.e. if the Prob > F value is very small (less than 0.05), terms in the model have a significant effect on the model. The terms having Prob > F value more than 0.05 were omitted in reduced model (24,25).
Table V.
ANOVA Results for Measured Responses
| Responses | Sum of Square (SS) | DF | Mean Square (MS) | F Value | (Prob > F) 100 | SD | Mean | CV | PRESS | R 2 | Adj-R 2 | Pred-R 2 | Adeq Precision | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Particle size (Y1 in μ) | FM | 36,184.28 | 5 | 7,236.86 | 66.16 | 0.29 | 10.46 | 86.03 | 12.16 | 3979.0 | 0.99 | 0.97 | 0.89 | 23.36 |
| RM | 36,037.54 | 3 | 1,2012.51 | 126.47 | 0.01 | 9.75 | 86.03 | 11.33 | 1880.91 | 0.98 | 0.97 | 0.94 | 30.12 | |
| Dissolution at pH 1.2 (Y2 in %) | FM | 1,032.07 | 5 | 206.41 | 99.38 | 0.16 | 1.44 | 81.08 | 1.78 | 71.66 | 0.99 | 0.98 | 0.93 | 32.80 |
| RM | 1,022.98 | 3 | 340.99 | 111.29 | 0.01 | 1.75 | 81.08 | 2.16 | 56.84 | 0.98 | 0.97 | 0.94 | 33.07 | |
| Dissolution at pH 6.8 (Y3 in %) | FM | 6.67 | 5 | 1.33 | 274.85 | 0.03 | 0.07 | 3.83 | 1.82 | 0.17 | 0.99 | 0.99 | 0.97 | 48.59 |
| RM | 6.64 | 3 | 1.66 | 130.98 | 0.02 | 0.11 | 3.83 | 2.94 | 0.26 | 0.99 | 0.98 | 0.96 | 31.82 | |
| Bitterness Score (Y4) | FM | 9.44 | 5 | 1.89 | 51.00 | 0.42 | 0.19 | 0.78 | 24.74 | 0.83 | 0.98 | 0.96 | 0.91 | 20.15 |
| RM | 9.39 | 4 | 2.35 | 56.33 | 0.09 | 0.20 | 0.78 | 26.24 | 0.47 | 0.98 | 0.96 | 0.95 | 20.81 | |
ANOVA Indicates analysis of variance, df degrees of freedom, SS sum of squares, MS mean of squares, F Fischer’s ratio, R 2 regression coefficient, FM full model, RM reduced model
PRESS (predicted residual error sum of squares) indicates how well the model fits the data. The coefficients for the model were calculated without the first point. This new model was then used to estimate the first point and calculate the residual for point one. This was done for each data point and the squared residuals were summed. PRESS values for all formulation shows good fit of model.
Adj-R2 measures variation around the mean explained by the model, adjusted for the number of terms in model.
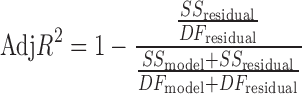 |
10 |
Pred-R2 measures amount of variation in new data explained by model. Adj-R2 and Pred-R2 values are in reasonable agreement, signifying good model fit.
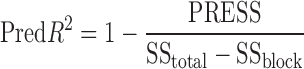 |
11 |
Adequate precision (Adeq Precision) is a signal to noise ratio. It compares the range of predicted value at the design points to the average prediction error.
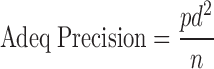 |
12 |
Where p is number of model parameters including intercept (b0), d is residual MS from ANOVA table and n is number of experiments. Both models, full model (FM) and reduced model (RM), showed Adeq precision value greater than 4, indicating adequate model discrimination.
Multiple linear regression analysis (Table IV) revealed that A2 and B2 terms were insignificant for particle size and dissolution at pH 1.2 while AB term was insignificant for dissolution at pH 6.8. A2 term was insignificant for bitterness score. The surface plots are shown in Fig. 1. The theoretical (predicted) values were obtained by substituting the values of A and B in the equation. It was found that the predicted (theoretical) and experimental (observed) values were in reasonably close agreement. Table VI shows the experimental, predicted and residual values.
Fig. 1.
Surface plots showing the effect of various formulation components
Table VI.
Observed and Predicted Values of Response for 32 Full Model
| Form No. | Particle Size (μ) ± SD* | Dissolution at pH 1.2 (t 15) ± SDa | Dissolution at pH 6.8 (t 5) ± SDa | Bitterness Score | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Experimental | Predicted | Residual | Experimental | Predicted | Residual | Experimental | Predicted | Residual | Experimental | Predicted | Residual | |
| MFL1 | 236.78 ± 2.98 | 230.03 | 6.75 | 99.59 ± 0.48 | 99.50 | 0.09 | 2.45 ± 0.52 | 2.41 | 0.04 | 0 | 0.06 | −0.06 |
| MFL2 | 81.96 ± 3.14 | 83.39 | −1.43 | 81.29 ± 0.37 | 81.92 | −0.63 | 4.46 ± 0.53 | 4.48 | −0.02 | 0 | 0.22 | −0.22 |
| MFL3 | 120.56 ± 2.72 | 128.81 | −8.25 | 93.11 ± 1.32 | 93.95 | −0.84 | 2.57 ± 0.39 | 2.58 | −0.01 | 0 | −0.11 | 0.11 |
| MFL4 | 32.08 ± 1.86 | 34.30 | −2.22 | 59.83 ± 1.41 | 60.90 | −1.07 | 3.84 ± 0.79 | 3.82 | 0.02 | 1.00 | 1.06 | −0.06 |
| MFL5 | 34.63 ± 1.33 | 42.09 | −7.46 | 77.42 ± 1.68 | 77.83 | −0.41 | 4.36 ± 0.56 | 4.41 | −0.05 | 3.00 | 3.06 | −0.06 |
| MFL6 | 131.38 ± 2.41 | 135.91 | −4.53 | 80.44 ± 1.39 | 79.46 | 0.98 | 4.35 ± 0.49 | 4.40 | −0.05 | 0 | −0.11 | 0.11 |
| MFL7 | 44.51 ± 2.51 | 43.00 | 1.51 | 85.15 ± 0.62 | 84.40 | 0.75 | 3.35 ± 1.17 | 3.37 | −0.02 | 0 | 0.06 | −0.06 |
| MFL8 | 52,24 ± 1.93 | 46.29 | 5.95 | 80.04 ± 1.82 | 80.38 | −0.34 | 5.25 ± 1.09 | 5.18 | 0.07 | 1.00 | 0.89 | 0.11 |
| MFL9 | 40.17 ± 1.78 | 30.49 | 9.68 | 77.84 ± 0.91 | 71.36 | 1.48 | 3.84 ± 0.86 | 3.81 | 0.03 | 2.00 | 1.89 | 0.11 |
t 15 Percent drug dissolved in 15 min, t 5 percent drug dissolved in 5 min
aValues represent the mean ± SD of 3 experiments
Incorporation Efficiency
Percent yield and incorporation efficiency were two important factors in the evaluation of the quality of the microparticles. The percent yield of most of the microparticles was always exceeded 80%, while the incorporation efficiency varied for all formulations, showed in Table II. Incorporation efficiency improves with increase in polymer (26). However higher quantity of EE solution prepared in 1% acetic acid, showed solubilization of MFL. This resulted in decreased incorporation efficiency (27). This finding suggests that the present method is suitable for the preparation of microparticles of a poorly water-soluble drug, such as MFL in EE solution.
Particle Size
For particle size, drug concentration (A) is negative while polymer concentration (B) is positive. This indicates that on increasing EE concentration, particle size increases. It was observed that the polymer viscosity influenced particle size (26). Increasing the EE concentration have led to an increase in its viscosity and consequently a decrease in the frequency of dissociation or separation of the particles with the addition of sodium hydroxide. This results in an increase in the overall size of the microparticles.
In Vitro Drug Release
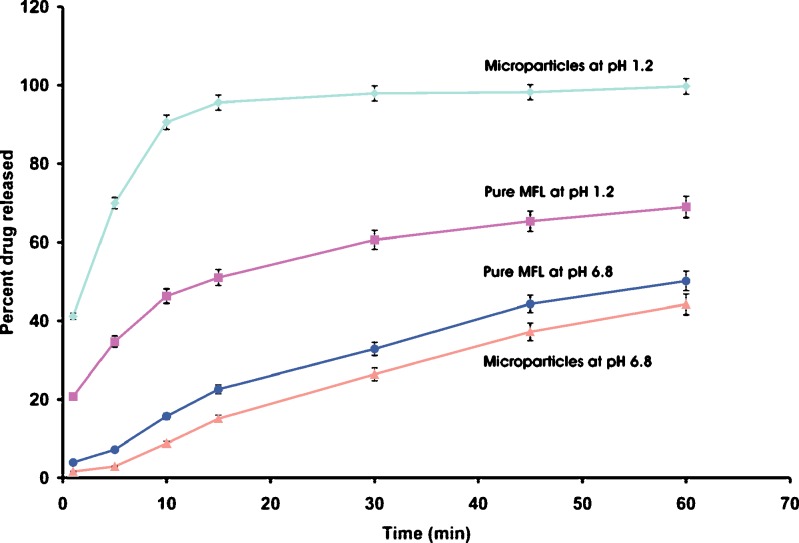
For dissolution in acidic pH, both drug (A) and polymer (B) concentrations are positive. This indicates additive effect of MFL concentration and EE concentration. This suggests that the MFL release and solubility would be improved at acidic pH. Release of MFL from the microparticles was completed within few minute at acidic pH, followed by a plateau. This may be because of the high porosity of the microparticles, the hydrophilic nature of the EE, and improved wettability, provided by the dissolved EE (17,20,28). Dissolution profile is shown in Fig. 2.
Fig. 2.
Dissolution of optimized microparticles batch and MFL
For dissolution at pH 6.8, drug concentration (A) is positive while polymer concentration (B) is negative. This indicates that on increasing EE concentration, dissolution of microparticles at pH 6.8 decreases. This finding suggests that the drug release is polymer dependent. As the concentration of EE was increased, thicker film was formed around the MFL particles, which retarded the MFL release, because of being insoluble at salivary pH (15). EE is expected to behave as insoluble and inert material at pH 6.8 and showed slightly decreased release rate. This is due to the decrease in drug diffusion and/or membrane infiltration (20,28). In a neutral or alkaline environment, the EE films swells, and slowly erodes and dissolves (29). However, after 15 min the microparticles starts swelling and releases MFL in normal way (30).
Gustatory Sensation Test
For bitterness score, drug concentration (A) is positive while polymer concentration (B) is negative. This indicates that on increasing EE concentration, bitterness score of microparticles decreases. This finding is in agreement with dissolution studies carried out at pH 6.8, because the pH of the saliva is in the range from 6.3 to 7.2. Further it has been reported that the MFL quinine moiety is responsible for the higher bitterness score. It has been reported that MFL produces bitterness by depolarizing taste cells by closing K+ channels (31). The microparticles are insoluble at salivary pH and forms physical barrier between the MFL and K+ channel present in the cell membrane of taste buds. Thus reducing the bitterness score of microparticles.
Optimization Using Desirability Function
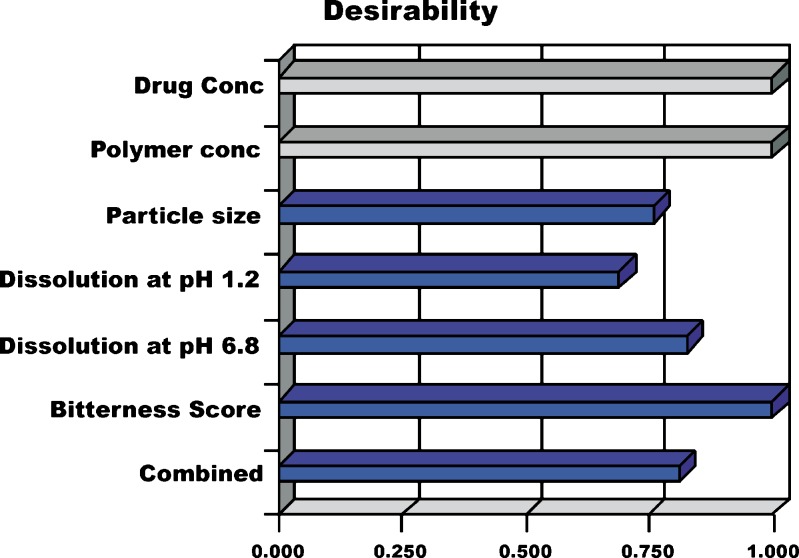
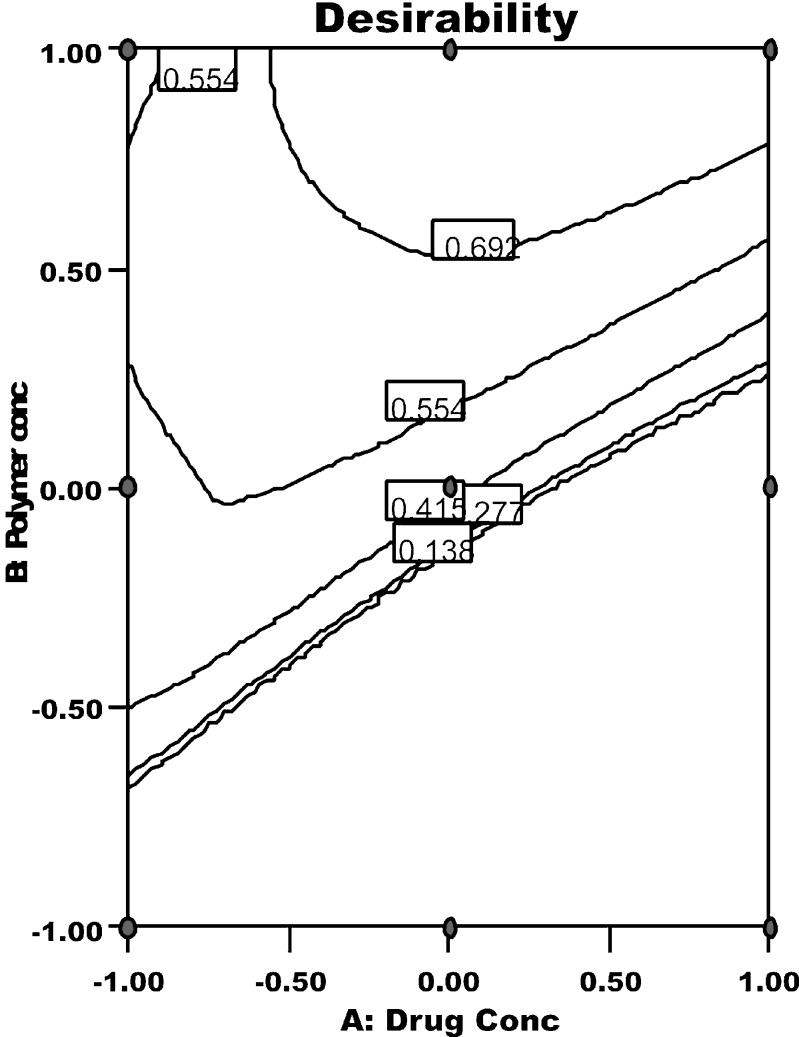
Any process can only be authenticated when optimum level of its variables (affecting the process) for microparticles of best quality characteristics is recognized. Desirability function is one excellent tool for identifying the optimum levels of variables. In this procedure, all the measured responses for independent variables which are supposed to affect the quality of the microparticles are taken into consideration. Some of these responses have to be minimized and some have to be maximized, in order to pour desired characteristics in the microparticles. Using the desirability function, all the dependant variables were combined to get one combined response i.e., the overall or combined desirability. The combined desirability response was calculated from the individual desirability of each of the responses using DOE v6.0.5 (Stat-Ease, Inc.). The individual desirability of all measured responses is reported in Fig. 3. The optimized batch was identified with a combined desirability value of 0.83 (Fig. 4). Table VII enlists the optimized values for all the independent process variables and their responses.
Fig. 3.
Individual and combined desirability for measured responses
Fig. 4.
Response surface of combined desirability for measured responses
Table VII.
Optimum Levels for the Independent Variables and their Responses
| Actual Values of Independent Variables | Particle Size (μ) | Dissolution at pH 1.2 (t 15 in %) | Dissolution at pH 6.8 (t 5 in %) | Bitterness Score | Combined Desirability | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| A in g | B in mL# | Actual ± SD* | predicted | Actual ± SD* | Predicted | Actual ± SD* | Predicted | Actual | predicted | |
| 0.6 | 50 | 81.61 ± 1.29 | 84.14 | 90.05 ± 0.78 | 88.22 | 2.92 ± 0.53 | 2.86 | 0 | 0 | 0.83 |
amL of 1% EE solution, t 15 – percent drug dissolved in 15 min, t 5 percent drug dissolved in 5 min
bValues represent the mean ± SD of three experiments
Cross Validation of the Model
The reliability of the equation that described the influence of factors on all responses was assessed by cross validation of the model. The response data for two independent check point batches was collected (32). The experimental values and predicted values of each response are shown in Table VIII. The percent relative error between predicted values and experimental values of each response was calculated using following equation.
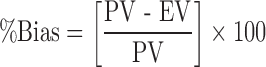 |
13 |
Table VIII.
Comparison of Responses Between Predicted and Experimental Values for the Cross-validation Set
| Responses | Test | Factors/coded Levels | Experimental Values ± SDa | Predicted Values | Bias (%) | |
|---|---|---|---|---|---|---|
| A | B | |||||
| Particle size (μ) | 1 | 0.36 | 1 | 91.79 ± 1.92 | 96.42 | 4.80 |
| 2 | 0.26 | 1 | 108.20 ± 2.11 | 104.78 | −3.26 | |
| Dissolution at pH 1.2 (t 15) | 1 | 0.36 | 1 | 93.62 ± 1.58 | 91.90 | −1.87 |
| 2 | 0.26 | 1 | 94.31 ± 1.23 | 92.82 | −1.60 | |
| Dissolution at pH 6.8 (t5) | 1 | 0.36 | 1 | 2.57 ± 0.79 | 2.79 | 7.88 |
| 2 | 0.26 | 1 | 2.69 ± 0.47 | 2.73 | 1.46 | |
| Bitterness Score | 1 | 0.36 | 1 | 0 | −0.08 | −100 |
| 2 | 0.26 | 1 | 0 | −0.10 | −100 | |
t 15 Percent drug dissolved in 15 min, t 5 percent drug dissolved in 5 minute, *Values represent the mean ± SD of 3 experiments
Where PV is predicted value and EV is experimental value. The percent bias obtained from checkpoint batches was in range of −100 to 4.80. A low value of percent bias depicts that in all cases there was a reasonable agreement in predicted and experimental values (33).
Fourier Transform Infra-red Spectroscopy (FTIR)
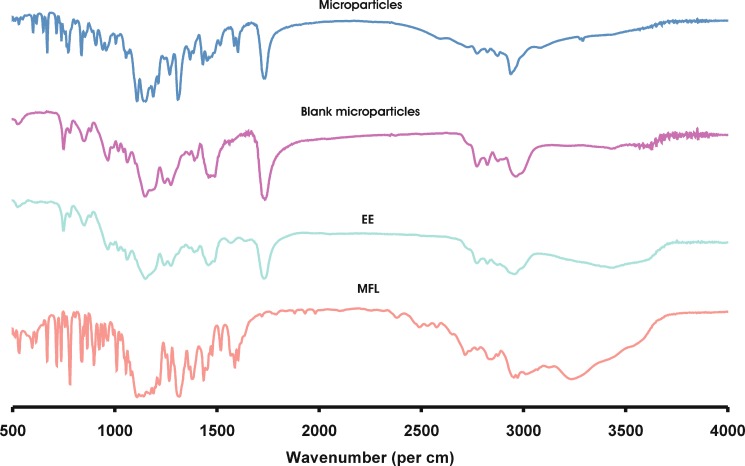
The optimization batch following the acceptable limits has been further evaluated for physical characterization viz. FT-IR, DSC and XRPD. Pure MFL and EE were also run as control. The samples used for the study were prepared 48 h before and preserved in desiccator before use. The FT-IR spectrum of pure MFL, EE, blank microparticles and optimized microparticles are shown in Fig. 5. The characteristic peaks of MFL at 3,110 cm−1 are assigned to N–H stretching vibration. In addition, the absorption peaks at 1,603, 1,363, 1,111, and 1,069 cm−1 can be assigned to quinine ring stretching vibration. The peak at 1,316 cm−1 can be assigned to CF3 stretching vibration. The peaks at 2,875 and 2,918 cm−1 are assigned to C–H bridge and CH2 respectively. The peak at 1,555 cm−1 is assigned to C=N/C=C. The peaks at 1,288 and 1,055 cm−1 are assigned to C–N and piperidine ring respectively. The peak at 1,174 cm−1 is due to the C–C/N-H stretching vibration. The spectrum of EE is dominated by the carbonyl (C=O) stretching vibration at 1,735 cm−1 and the ester C–O stretching vibrations at 1,148 and 1,188 cm−1. In addition, C–H vibrations can be discerned at 1,389, 1,450–1,490 and 2,962 cm−1. The absorptions at 2,772 and 2,822 cm−1 can be assigned to the dimethyl-amino groups. The spectrum of microparticles corresponds to the superimposition of MFL and EE with no significant shift in the major peaks. This confirms presence of MFL in microparticles.
Fig. 5.
FT-IR spectra of MFL, EE, blank microparticles and optimized microparticles
Differential Scanning Calorimeter (DSC)
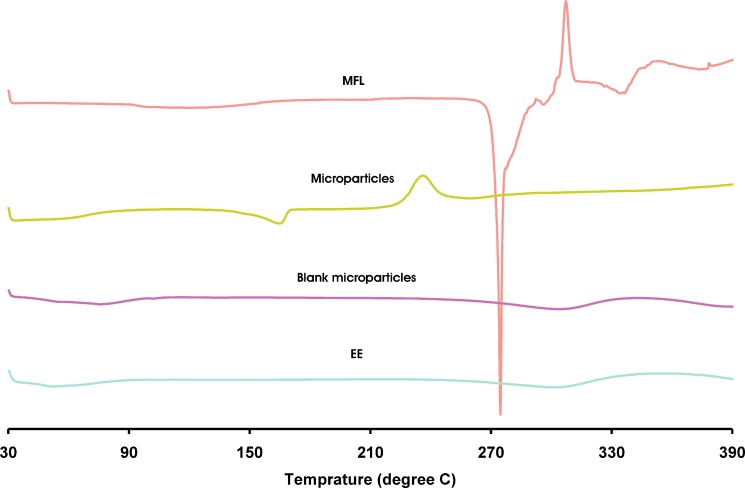
Figure 6 shows the DSC curve of pure MFL, EE, blank microparticles and optimized microparticles. The pure MFL shows an endothermic peak at 271.38 °C, followed by exothermic peak at 308.36 °C. The characteristic endothermic peak corresponding to melting peak of MFL was shifted towards lower temperature (164.53 °C), with reduced intensity in the microparticles, suggesting phase transition of MFL in EE microparticles.
Fig. 6.
DSC curve of MFL, EE, blank microparticles and optimized microparticles
X-ray Powder Diffractometry (XRPD)
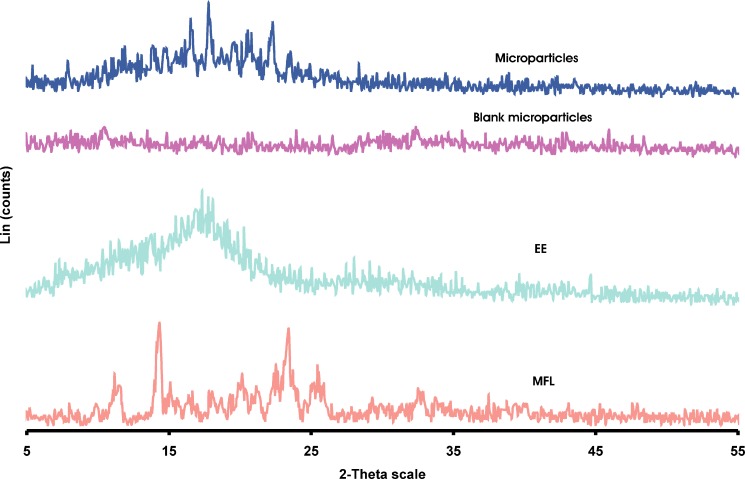
XRPD analysis was performed to confirm the results of DSC studies. XRPD patterns of MFL, EE, blank microparticles and optimized microparticles are shown in Figure 7. In X-ray diffractogram of MFL, sharp peaks at a diffraction angle (2θ) of 11.52°, 14.31°, 16.37°, 18.03°, 20.11°, 21.26°, 23.37°, 25.50°, 32.57° indicates the presence of crystalline drug, while microparticles shows sharp peaks at 7.88°, 13.84°, 14.80°, 16.56°, 17.82°, 19.57°, 20.50°, 22.23°, 23.39°. New peaks at 7.88°, 13.84°, 17.82°, 19.57° and 22.23° were observed in microparticles, indicating phase transition of MFL in EE microparticles.
Fig. 7.
XRPD patterns of MFL, EE, blank microparticles and optimized microparticles
Scanning Electron Microscopy (SEM)
Figure 8 illustrated the SEM micrographs of MFL, EE and optimized microparticles. MFL existed in needle shape whereas EE was seen as small cubes. Original morphology of both components was disappeared in microparticles. Microparticles showed encapsulation of the drug particles. Therefore the close contact between the polymer and drug might be responsible for masking the bitter taste in microparticles.
Fig. 8.
SEM photographs of MFL, EE, MFL-EE physical mixture and optimized microparticles
Conclusion
The study conclusively demonstrated complete taste masking of MFL in microparticles using EE as polymer. Present work suggests that both variables have its own significant complimentary role in enhancement of the process rather than having exclusive effect. The FTIR, DSC and XRPD studies indicated interaction of MFL, at the molecular level, in EE microparticles. The results of the experiments presented may be of value for the pharmaceutical industries dealing with bitter drugs to improve patient compliance and thus effective pharmacotherapy. Further it is possible to mask the bitterness of single high dose (250 mg) drugs like MFL with comparatively minimum concentration of polymer for oral formulations.
Acknowledgement
The authors are thankful to Degussa India Pvt. Ltd., Mumbai for providing the polymeric material. Further the support from STIC, Cochin is greatly acknowledged.
References
- 1.G. M. Roy. Taste masking in oral pharmaceuticals. Pharm. Tech. (Europe) 24–35 (1994).
- 2.Harmik S., Yasmin S., Roop K. K. Taste masking technologies in oral pharmaceuticals: recent developments and approaches. Drug Dev. Ind. Pharm. 2004;30:429–448. doi: 10.1081/DDC-120037477. [DOI] [PubMed] [Google Scholar]
- 3.William N. Anti-infective. In: Gennaro A. R., editor. Remington’s: The Science and Practice of Pharmacy, Vol. 2. Philadelphia, PA: Lippincott Williams and Wilkins; 1547. [Google Scholar]
- 4.Shimano K., Kondo O., Miwa A., Higashi Y., Goto S. Evaluation of uniform-sized microcapsules using a vibration-nozzle method. Drug Dev. Ind. Pharm. 1995;21:331–347. doi: 10.3109/03639049509048114. [DOI] [PubMed] [Google Scholar]
- 5.Cuña M., Lamosa M. L., Vila Jato J. L., Torres D., Aloso M. J. pH dependent cellulosic microspheres containing cefuroxime axetil: stability and in vitro release behaviour. Drug Dev. Ind. Pharm. 1997;23:259–265. doi: 10.3109/03639049709149802. [DOI] [Google Scholar]
- 6.Barra J., Lescure F., Doelker E. Taste masking as a consequence of the organisation of powder mixes. Pharm. Acta Helv. 1999;74:37–42. doi: 10.1016/S0031-6865(99)00013-8. [DOI] [PubMed] [Google Scholar]
- 7.Szejtli J., Szente L. Elimination of bitter, disgusting tastes of drugs and foods by cyclodextrins. Eur. J. Pharm. Biopharm. 2005;61:115–125. doi: 10.1016/j.ejpb.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 8.Agarwal R., Mittal R., Singh A. Studies of ion-exchange resin complex of chloroquine phosphate. Drug Dev. Ind. Pharm. 2000;26:773–776. doi: 10.1081/DDC-100101297. [DOI] [PubMed] [Google Scholar]
- 9.Lu M. Y. F., Borodkin S., Woodward L., Li P., Diesner C., Hernandez L., Vadnere M. A polymer carrier system for taste masking of macrolide antibiotics. Pharm. Res. 1991;8:706–712. doi: 10.1023/A:1015889631314. [DOI] [PubMed] [Google Scholar]
- 10.Chopra R., Alderborn G., Newton J. M., Podezeck F. The influence of film coating on pellet properties. Pharm. Dev. Tech. 2002;7:59–68. doi: 10.1081/PDT-120002231. [DOI] [PubMed] [Google Scholar]
- 11.C. M. Blase, and M. N. Shah. Aqueous pharmaceutical suspensions for pharmaceutical actives. EP Patent 0556057. Aug 18, 1993.
- 12.Appelgren C., Eskilson C. Novel method for the granulation and coating of pharmacologically active substance. Drug Dev. Ind. Pharm. 1990;16:2345–2351. doi: 10.3109/03639049009043804. [DOI] [Google Scholar]
- 13.Sjoqvist R., Graffeer C., Ekamn J. In vitro validation of the release rate and palatability of remoxipride-modified release suspension. Pharm. Res. 1993;10:1020–1030. doi: 10.1023/A:1018966823600. [DOI] [PubMed] [Google Scholar]
- 14.Hashimoto Y., Tanaka M., Kishimoto H., Shiozawa H., Hasegawa K., Matsuyama K., Uchida T. Preparation, characterization and taste masking properties of polyvinylacetal diethylamino acetate microsphere containing trimebutine. J. Pharm. Pharmacol. 2002;54:1323–1328. doi: 10.1211/002235702760345383. [DOI] [PubMed] [Google Scholar]
- 15.Sinha V. R., Rachna K. Binders for colon specific drug delivery: an in vitro evaluation. Int. J. Pharm. 2002;249:23–31. doi: 10.1016/S0378-5173(02)00398-8. [DOI] [PubMed] [Google Scholar]
- 16.Elisabetta E., Roberta R., Rita C., Franco C., Claudio N. Production of Eudragit microparticles by spray-drying technique: influence of experimental parameters on morphological and dimensional characteristics. Pharm. Dev. Tech. 2000;5:267–278. doi: 10.1081/PDT-100100541. [DOI] [PubMed] [Google Scholar]
- 17.Mingshi Y., Fude C., Bengang Y., Jian Y., Liang W., Liqiang Z., Yoshiaki K. A novel pH-dependent gradient-release delivery system for nitrendipine I. Manufacturing, evaluation in vitro and bioavailability in healthy dogs. J. Control Rel. 2004;98:219–229. doi: 10.1016/j.jconrel.2004.04.022. [DOI] [PubMed] [Google Scholar]
- 18.Bogataj M., Mrhar A., Kristl A., Kozjek F. Eudragit E microspheres containing bacampicillin: preparation by solvent removal methods. J. Microencapsul. 1991;8:401–406. doi: 10.3109/02652049109069567. [DOI] [PubMed] [Google Scholar]
- 19.Anne M. J., Catherine B., Cynthia K. Evaluation of solid dispersion particles prepared with SEDS. Int. J. Pharm. 2003;250:385–401. doi: 10.1016/S0378-5173(02)00577-X. [DOI] [PubMed] [Google Scholar]
- 20.Valizadeh H., Nokhodchi A., Qarakhani N., Zakeri-Milani P., Azarmi S., Hassanzadeh D., Löbenberg R. Physicochemical characterization of solid dispersions of indomethacin with PEG 6000, Myrj 52, lactose, sorbitol, dextrin and Eudragit E100. Drug Dev. Ind. Pharm. 2004;30:303–317. doi: 10.1081/DDC-120030426. [DOI] [PubMed] [Google Scholar]
- 21.Y. Rane, R. Mashru, M. Sankalia, and J. Sankalia. Effect of hydrophilic swellable polymers on dissolution enhancement of carbamazepine solid dispersion studied using response surface methodology. AAPS PharmSciTech.8:Article 27 (2007). [DOI] [PMC free article] [PubMed]
- 22.Lewis G. A., Mathieu D., Phan-Tan-Luu R. Pharmaceutical experimental design. New York: Marcel Dekker; 1999. [Google Scholar]
- 23.Bolton S., Charles S. Pharmaceutical Statistics. New York, NY: Marcel Dekker Inc; 2004. pp. 249–255. [Google Scholar]
- 24.Gohel M. C., Panchal M. K. Novel use of similarity factor f2 and Sd for the development of diltiazem HCL modified release tablets using a 32 factorial design. Drug Dev. Ind. Pharm. 2002;28:77–87. doi: 10.1081/DDC-120001488. [DOI] [PubMed] [Google Scholar]
- 25.Shah T. J., Amin A. F., Parikh J. R., Parikh R. H. Process optimisation and characterisation of poloxamer solid dispersion of a poorly water soluble drug. AAPS PharmSciTech. 2007;8:29. doi: 10.1208/pt0802029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Satturwar P. M., Mandaogade P. M., Dorle A. K. A novel method for preparation of Eudragit RL microcapsules. J. Microencapsul. 2002;19:407–413. doi: 10.1080/02652040210140454. [DOI] [PubMed] [Google Scholar]
- 27.Rama Rao K., Senapati P., Das M. K. Formulation and in vitro evaluation of ethyl cellulose microspheres containing zidovudine. J. Microencapsul. 2005;22:863–876. doi: 10.1080/02652040500273498. [DOI] [PubMed] [Google Scholar]
- 28.Rao V. M., Engha K., Qiu Y. Design of pH-independent controlled release matrix tablets for acidic drugs. Int. J. Pharm. 2003;252:81–86. doi: 10.1016/S0378-5173(02)00622-1. [DOI] [PubMed] [Google Scholar]
- 29.Claudia S. L., Dorothee E. Basic coating polymers for the colon-specific drug delivery in inflammatory bowel disease. Drug Dev. Ind. Pharm. 2000;26:1239–1246. doi: 10.1081/DDC-100102305. [DOI] [PubMed] [Google Scholar]
- 30.Jinhe L., Libo Y., Sheila M. F., Tom J. H., Shunsuke W., Masataka K., Fix J. A. In vitro evaluation of dissolution behavior for a Colon-Specific Drug Delivery System (CODESä) in multi-pH media using united states pharmacopeia apparatus II and III. AAPS PharmSciTech. 2002;3:33. doi: 10.1208/pt030433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yamamoto T., Nagai T., Shimura T., Yasoshima Y. Roles of chemical mediators in taste system. Jpn. J. Pharmacol. 1998;76:325–348. doi: 10.1254/jjp.76.325. [DOI] [PubMed] [Google Scholar]
- 32.Sankalia M. G., Mashru R. C., Sankalia J. M., Sutariya V. B. Physicochemical characterisation of papain entrapped in ionotropically cross-linked Kappa-Carrageenan gel beads for stability improvement using doehlert shell design. J. Pharm. Sci. 2006;95:1994–2013. doi: 10.1002/jps.20665. [DOI] [PubMed] [Google Scholar]
- 33.Rane Y. M., Mashru R. C., Sankalia M. G., Sutariya V. B., Shah P. P. Investigation on factors affecting chitosan for dissolution enhancement of oxcarbazapine by spray dried microcrystal formulation with an experimental design approach. Drug Dev. Ind. Pharm. 2007;33:1008–1023. doi: 10.1080/03639040601179749. [DOI] [PubMed] [Google Scholar]