Abstract
The main aim of the present study was to evaluate potential of ternary complexation (comprising of drug, cyclodextrin and polymer) as an approach for taste masking. For this purpose famotidine with property of bitter taste was selected as a model drug. Improvement in taste masking capability of cyclodextrin towards famotidine was evaluated by formulating a ternary complex including hydrophilic polymer hydroxyl propyl methyl cellulose (HPMC 5 cps) as the third component. Phase solubility analysis at 25 °C was carried out for both the binary systems (viz. drug–cyclodextrin and drug–polymer) and the ternary system (drug–cyclodextrin–polymer). Ternary complex was prepared using solution method and was further characterized using XRD, DSC, FT-IR and microscopic studies. In vitro dissolution study was carried out to see the effect of ternary complexation on drug release. Taste perception study was carried out on human volunteers to evaluate the taste masking ability of ternary complexation. Results obtained from phase solubility analysis showed that the combined use of polymer and cyclodextrin effectively increased the stability constant of the complex [from 538 M−1 for binary system to 15,096 M−1 for ternary system]. Ternary system showed effective taste masking as compared to binary complex and at the same time showed no limiting effect on the drug release (D.E15min = 90%). The effective taste masking was attributed to the enhanced complexation of famotidine in ternary system compared to binary system and the same was confirmed from the characterization studies. In conclusion, the study confirmed that ternary complexation can be utilized as an alternative approach for effective taste masking.
Key words: cyclodextrin, famotidine, hydrophillic polymer, stability constant, taste masking
INTRODUCTION
Cyclodextrins (CDs) are cyclic oligosaccharides with a hydrophilic outer surface and a lipophilic central cavity. On account of their relatively hydrophobic interiors, CDs have the ability to form inclusion complexes with a wide range of substrates (1). This complex forming ability of CDs have been widely exploited in the pharmaceutical field for various applications; taste masking of bitter drugs is one such application (2–3).
Use of cyclodextrins as taste masking agent is widely reported (4–5). However, the application of CDs (especially β-cyclodextrins) for taste masking is generally restricted to drugs that forms complex with high binding/stability constant because a low stability constant would lead to a rapid release of free drug in the oral cavity, resulting in inefficient taste masking (6).
Reports claim that the release of drug from inclusion complex is dependent on two factors: First, the stability constant of the complex (governing the association/dissociation of complex) and second, the dilution at the site of drug release. Thus, complex with higher stability constant will usually require greater dilution to affect the release of drug whereas; complex with lower stability constant will release the drug even at lower dilutions (7–9).
In case of taste masking the general aim is minimum free drug release in the oral cavity. It is therefore important to find methods of forming complex with higher stability constants which would release negligible amount of drug at the site of minimal dilution (i.e. oral cavity) and give a rapid and complete release at comparatively higher dilutions (i.e. gastric lumen). Ternary complexation in the presence of suitable auxiliary substances has proved to be an effective method of increasing stability constant (10–12). This stability constant increasing capability of ternary complexation could be utilized for effective taste masking by cyclodextrin. Thus, the present work was carried out to investigate for the first time, the possibility of using ternary complexation as an approach for taste masking.
Famotidine-[(N′-(Aminosulfonyl)-3-(((2-((diaminomethylene)amino)-4thiazolyl) methyl) thio) propanimidamide] (Mol.Wt. 337.43), an H2 histamine receptor antagonist, has extremely bitter taste and its taste masked formulations are available in doses of 20 and 40 mg as rapidly disintegrating tablets. Thus, for the present work famotidine was selected as a model drug for taste masking study.
Hydroxyl propyl methyl cellulose (HPMC) is a class of hydrophilic polymers used in pharmaceutical formulations for varied purposes (13). HPMC polymers are available in various grades and they are categorized as low, intermediate and high viscosity grades based on the viscosity of their solution (2% solution at 20 °C) (13). HPMC 5 cps is a low viscosity grade polymer and likewise used extensively in film coating for immediate release solid formulations (14). Thus, for the present study HPMC 5 cps with attributes of low viscosity and bland taste was selected as the third component for ternary complexation. The novelty of this work lies in the application of ternary complexation (comprising of drug, cyclodextrin and hydrophilic polymer) as an approach for taste masking. The formation of ternary complex was confirmed by characterization study and the taste masking ability was evaluated by taste perception study in human volunteers.
MATERIALS AND METHODS
Materials
Famotidine was supplied by Cipla Ltd. (Mumbai, India), Hydroxy propyl methyl cellulose (HPMC 5 cps) Methocel E5 PREM LV was obtained from Dow Chemicals (Mumbai, India), β-cyclodextrin was generously donated by Cerestar (Mumbai, India) All other chemicals and solvents used were of pharmaceutical and analytical grade. Double distilled water was used throughout the study for experimental work.
Methods
Phase Solubility Studies
Phase solubility equilibrium diagrams (in water at 25 °C) were obtained for both binary and ternary systems as per Higuchi and Connors method (15). Studies for binary systems were carried out by adding an excess amount of drug to 25 ml aqueous solutions containing increasing concentrations of β-cyclodextrin (from 0 to 16.29 mM, i.e., to its saturation solubility at 25 °C) or HPMC 5 cps (from 0% to 1.5% w/v). Experiment for ternary system was performed analogously to those for the binary systems, but in presence of 0.75% w/v HPMC 5 cps (value obtained from the PSA results of drug: polymer binary system). These series of suspensions were equilibrated for 48 h on a mechanical shaker followed by filtration and analysis. The samples were filtered through a 0.45 μm membrane filter (Millex-HA filter units, Millipore, Molsheim, France) and suitably diluted for analysis. The drug content was determined by UV spectrophotometry (JASCO 530 S Spectrophotometer, Tokyo, Japan) at 265 nm. The presence of β-CD and the polymer did not interfere with the spectrophotometric assay of the drug. Each experiment were performed in triplicate; the coefficient of variation associated with each measurement was never >3%.
Preparation of Famotidine–Cyclodextrin Systems
Inclusion complex of drug: β-CD was prepared by the solution method. Firstly the required amount of β-CD was dissolved in double distilled water to get a clear solution. The drug was then dispersed into this aqueous solution of β-CD in molar ratio (1:1 M) followed by continuous stirring for 24 h at room temperature. The suspension obtained was then subjected to freeze drying using LABCONCO, Freeze Dry System Freezone 4.5® (Schwabach, Germany).
Ternary complex comprising of 0.75% w/v polymer (HPMC 5 cps) was prepared by first dissolving required amounts of β-cyclodextrin and polymer in double distilled water followed by addition of drug (equivalent to its corresponding ratio in the complex i.e. 1:1 M). The suspension was stirred for 24 h and subjected to freeze drying. Physical mixture (PM) of drug/β-cyclodextrin/polymer was prepared by mixing all three components in geometric proportion followed by passing through no. 80 sieve with minimum abrasion. All samples were stored in dessicator until further use.
In-Vitro Dissolution Studies
Dissolution study of samples (equivalent to 40 mg famotidine) was performed using USP XXIV type II apparatus with 900 ml of buffer pH 4.5 as the official dissolution medium for famotidine (USP 23, 1995). The stirring speed employed was 50 rpm, and the temperature was maintained at 37 ± 0.5 °C. Five milliliter aliquots of dissolution medium was withdrawn at predetermined time intervals and replaced by 5 ml of fresh dissolution medium. The filtrates of the samples were analyzed for the content of drug by UV spectrophotometer at 265 nm. The experiments were done in triplicate. The dissolution profiles were evaluated on the basis of dissolution efficiency (DE) parameter at 15 min and dissolved percentage (DP) at 15 min. Dissolution efficiency was calculated from the area under the curve of percent cumulative release versus time (measured using the trapezoidal rule) and expressed as a percentage of the area of the rectangle described by 100% dissolution in the same time (16).
To investigate the inhibiting effect of lower dilution on drug release from ternary complex, the samples were subjected to in vitro dissolution study using smaller volume (50 ml) of dissolution medium. The study was carried out using dissolution apparatus (ELECTROLAB TDT–08L Dissolution Test Apparatus) equipped with mini jar conversion kit (250 ml). The aliquots were sampled at 15 min and analyzed for drug content as described earlier.
Differential Scanning Calorimetry (DSC) Studies
The samples were subjected to DSC studies using Perkin Elmer, pyris 4 series DSC equipment (Massachusetts, USA). Samples were sealed in 40 μl aluminium pans. An identical empty pan was used as a reference; all samples were scanned at 5 °C/min with a 20 ml/min nitrogen purge.
Fourier Transform Infrared (FT-IR) Studies
Fourier transform infrared (FT-IR) spectra of the samples were obtained in the range of 400 to 4,000 cm−1 using a Jasco-FT-IR spectrophotometer (Jasco, Essex, UK) by the KBr disc method.
X-Ray Diffraction Studies
X-ray diffraction patterns were obtained by using a Diano X-ray diffractometer (Woburn, USA) equipped with Co Kα. The tube operated at 45 kV, 9 mA and the scan range used was 10–50° of the diffraction angle 2θ. Equal amount of samples were accurately weighed and used in the study.
Photo-Microscopy
Photo microscopic analysis was carried out using Kruss MBL 3100 Inverse microscope (NJ, USA) equipped with Canon Power Shot S80 digital camera.
Scanning Electron Microscopy
SEM analysis was performed using a Philips XL-30 SEM (Basel, The Netherlands). Before examination, samples were gold-sputter coated to render them electrically conductive.
Taste Perception Study and Statistical Analysis
Taste perception was carried out on a previously consented group of 12 healthy human volunteers. The protocol for the investigation was approved by institutional animal and human ethics committee (IAHEC) and informed consent was signed by the volunteers before starting the study.
To find a suitable amount of drug for the evaluation of the bitter taste intensity during the comparative test, the perception and bitterness recognition threshold of pure drug was evaluated. For this purpose, six standard weighing of drug were selected: 2.5 mg (A), 5 mg (B), 7.5 mg (C), 10 mg (D), 12.5 mg (E), 15 mg (F) and 17.5 mg (G). The determination of the threshold was carried out as follows. The volunteers were asked to taste samples (starting from 2.5 mg) kept in the mouth for 5 s. Then, the volunteers were asked for the following perceptions:
“I feel bitter taste”.
“I feel something but I cannot identify the taste”.
“I do not feel any taste”
The volunteers who answered 2–3 were then made to taste increasing amounts of drug (A to G) and stopped at the point when the answer was 1. The results gave the bitterness recognition threshold of 6.25 mg. Thus, 6.25 mg was selected as the preferred amount for rest of the study.
For comparative analysis, volunteers were made to taste the samples (6.25 mg of plain drug and complexes and physical mixture equivalent to 6.25 mg), and rate them on a hedonic rating scale (Table I) as per the bitterness of the samples (ranging from “excellent” for no taste perception and “extremely poor” for bitter taste) The average rating score was then used to categorize the sample. Significant differences among the test scores were analyzed using the student’s unpaired t test; a value of P < 0.05 was accepted as index of a significant difference.
Table I.
Hedonic Rating Scale for Taste Perception Study
| Rating | Description |
|---|---|
| 7 | Excellent |
| 6 | Very good |
| 5 | Good |
| 4 | Fair |
| 3 | Poor |
| 2 | Very poor |
| 1 | Extremely poor |
Ratings were used to categorized the taste perception of the samples ranging from 1 = extremely poor to 7 = excellent
RESULTS AND DISCUSSION
Phase Solubility Analysis
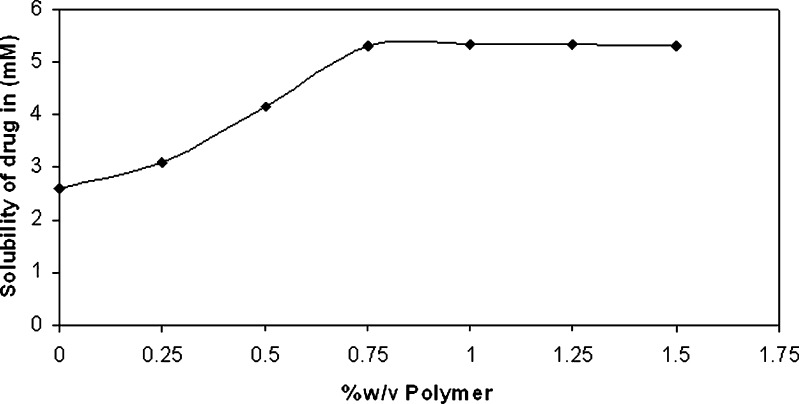
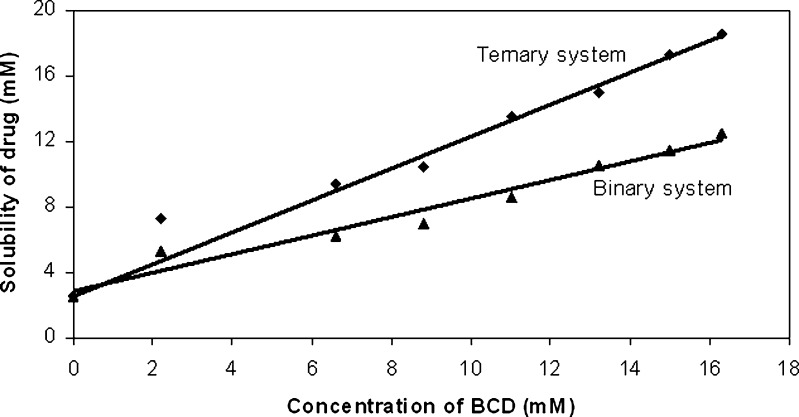
It is been reported that hydrophilic polymers tend to have solubility enhancement effect on poorly soluble drugs via formation of weak water soluble complexes (17–18). Thus, the aqueous solubility of famotidine at various concentrations of HPMC 5 cps was studied. It was found that the solubility of drug increased with the increasing concentration of HPMC 5 cps up till an optimal concentration of 0.75% w/v, attaining a plateau thereafter, suggesting no further increase in the solubility with further addition of polymer (Fig. 1). Thus, 0.75% w/v of HPMC 5 cps was used for the phase solubility studies of ternary system and it was seen that the presence of polymer resulted in a significant increase (threefold) in the stability constant. Phase solubility curve for both the binary and ternary system i.e. with or without polymer was found to be of Higuchi’s AL type: that is a linear increase of drug concentration was observed as a function of cyclodextrin concentration (Fig. 2). Values of stability constants of Drug-CD complex both when no polymer was present or in the presence of 0.75 % w/v polymer were found to be 538 M−1 and 15,096 M−1 respectively. The slopes in both cases were less than unity, thus confirming the formation of 1:1 complexes (15).
Fig. 1.
Phase solubility curve of binary system of drug–polymer
Fig. 2.
Phase solubility curve of binary system i.e. Drug–β-cyclodextrin and ternary system i.e. Drug–β-cyclodextrin–polymer
The increase in the stability constant could be attributed to the increase in the complexing ability of cyclodextrin towards the drug by establishing interactions such as hydrophobic bonds, Van der Waals dispersion forces, or hydrogen bonds and/or promoting the release of high-energy water molecules present in the cyclodextrin cavity (19). It was assumed at this point, that using polymer for preparation of ternary complex with increased stability constant could serve the purpose of effective taste masking as compared to binary complex.
In vitro Dissolution Studies
As discussed earlier, the higher stability constant could result in decreased release of drug from the complex at lower dilutions whereas at higher dilutions the release would remain unaffected. In the present study, in vitro drug release study was carried out to evaluate the effect of enhanced stability constant on dissolution rate of ternary system. Results obtained from the dissolution studies are presented in Table II. It was seen that at higher dilutions (900 ml), ternary system resulted in a complete release with D.E15min of 90% as compared to 30.0% and 70.9% in the case of plain drug and Drug-β-CD binary complex respectively. Whereas, at lower dilutions (50 ml), the release of the drug from ternary complex was found to be lower (D.E15min = 1.8%) as compared to 8.7% and 21.4% in the case of plain drug and Drug-β-CD binary complex respectively.
Table II.
Results from the In-Vitro Dissolution Studies of Various Systems
| Systems | Dissolution percentage D.P.15min D.E.15min | Dissolution efficiency (%) |
|---|---|---|
| (Dissolution medium = 900 ml) | ||
| Plain drug | 40.1 | 30.0 |
| Binary complex | 79.7 | 70.9 |
| Ternary complex | 100.0 | 90.0 |
| (Dissolution medium = 50 ml) | ||
| Plain drug | 18.2 | 8.7 |
| Binary complex | 31.3 | 21.4 |
| Ternary complex | 9.5 | 1.8 |
The improved dissolution rate at higher dilutions can be attributed to the polymer assisted enhanced complexation in ternary system and release of drug as a function of dilution. It also suggested that increase in the stability constant had limiting effect on drug release at lower dilutions, thus confirming our hypothesis.
Differential Scanning Calorimetry
DSC thermograms of famotidine, β-cyclodextrin, HPMC 5 cps, binary complex, physical mixture and the ternary complex are illustrated in Fig. 3. The thermogram of β-CD showed a broad peak at 85–100 °C, attributed to desolvation of water molecules present in β-CD cavity and a relatively sharp peak at 290–300 °C corresponding to its melting point. Famotidine exhibited a sharp peak at 161–163 °C which corresponds to its melting point.
Fig. 3.
DSC curves of Famotidine; HPMC 5 cps; β-Cyclodextrin; binary complex {Drug/β-CD}; physical mixture (PM) {Drug/β-CD/polymer} and ternary complex
The existence of an interaction between two components can be obtained by thermal analysis (DSC). When guest molecules are included in the CD cavity, their melting, boiling and sublimation points usually shift to a different temperature or disappear (20–21). The value of peak area and the DELTA H value give an estimation of the number of molecules undergoing the transition (20–21). Thus, the decrease in both peak area and DELTA H value for ternary complex (Table III) suggests that the complexation efficiency was enhanced due to the presence of polymer leading to a decrease in the amount of free drug in ternary complex as compared to both binary complex and physical mixture.
Table III.
Peak Area and DELTA H Values Obtained from DSC Curves
| Samples | Peak area (mJ) | DELTA H (J/g) |
|---|---|---|
| Famotidine | 910.7 | 182.14 |
| Binary complex [FAM: β-CD] | 387.6 | 38.76 |
| Physical mixture [FAM: β-CD:HPMC] | 607.1 | 60.72 |
| Ternary complex [FAM: β-CD:HPMC] | 89.3 | 8.93 |
Fourier Transform Infrared (FT-IR) Studies
The frequency of vibrations of band depends on the masses of atoms and bond stiffness, and any factor that influences the stiffness will also alter the frequency of vibration (22). Thus, IR spectroscopy can be used as a useful tool to characterize complex formation.
FT-IR spectra of the samples are illustrated in Fig. 4. As seen from the spectra, there were no major changes in the FT-IR spectra confirming the absence of any chemical interaction between the components in the ternary complex. However, there were some minor changes in peaks (reported in Table IV) especially, at 900–650 cm−1, which corresponds to the out plane bending vibrations of aromatic rings (22). The shift in the peaks in these regions can be attributed to change in the atmosphere of the aromatic group thus it can be assumed to be included in the cyclodextrin cavity. Further, absence of peaks at 3,502.4 cm−1 and 1,599.2 cm−1 in both binary and ternary complex suggested that hydroxyl group of cyclodextrin showed a weak interaction with the amine group of famotidine.
Fig. 4.
FT-IR spectra of Famotidine; HPMC 5 cps; β-Cyclodextrin; binary complex {Drug/β-CD}; physical mixture (PM) {Drug/β-CD/polymer} and ternary complex
Table IV.
Peak Values Obtained from the FT-IR Studies
| Peak assignment | Peaks (cm−1) | ||
|---|---|---|---|
| Famotidine | Binary complex (FAM: β-CD 1:1M) | Ternary complex (FAM: β-CD:HPMC) | |
| N–H stretching | 3,502.4 | Absent | Absent |
| Aromatic –C=C– stretching | 1,599.2 | Absent | Absent |
| –C=N– stretching | 1,492.9 | 1,491.2 | 1,458.9 |
| O=S=O stretching | 1,430.7 | 1,427.7 | 1,415.1 |
| Aromatic –C=C– stretching and bending | 891.0 | Absent | Absent |
| Aromatic –C=C– stretching and bending | 777.1 | 777.6 | Absent |
| Aromatic –C=C– stretching and bending | 542.3 | 536.8 | Absent |
X-Ray Diffraction Studies
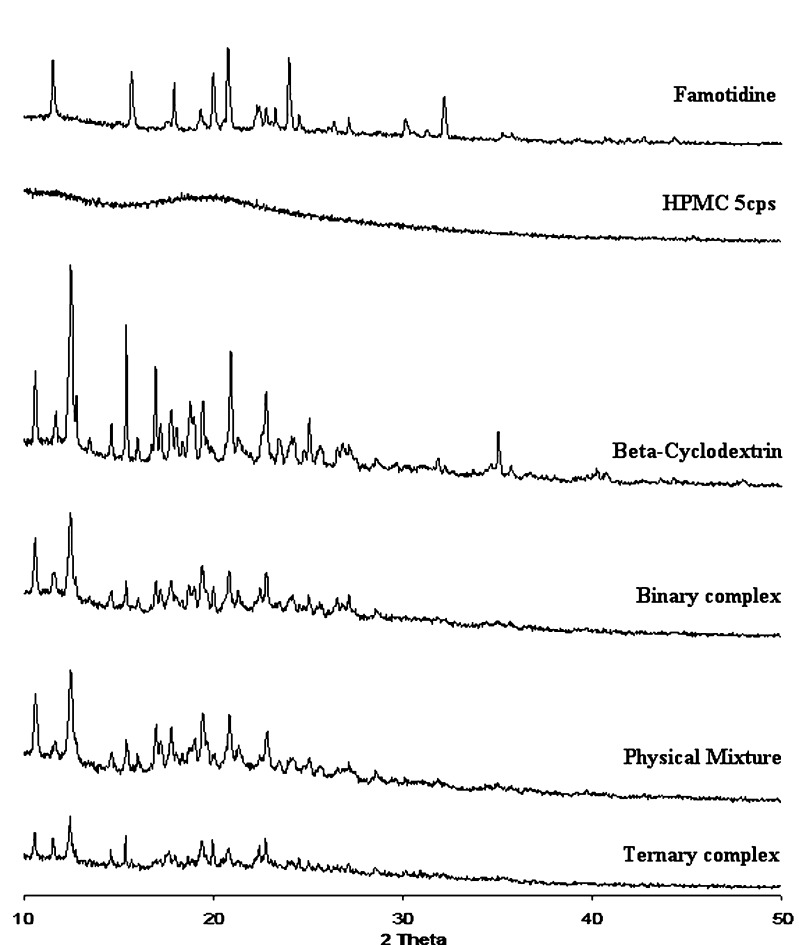
XRD analysis was carried out to confirm formation of a new solid state. XRD patterns and data of samples are represented in Fig. 5 and Table V. The diffractogram of β-CD exhibited characteristic peaks at 10.62, 12.46, 15.42, 16.98, 20.94 and 22.80 due to its crystalline nature. Famotidine exhibited a series of intense peaks at 11.56, 15.70, 17.94, 20.00, 20.80, 24.00, 32.22 and 35.10, which were indicative of crystalline nature of famotidine.
Fig. 5.
XRD spectra of Famotidine; HPMC 5 cps; β-Cyclodextrin; binary complex {Drug/β-CD}; physical mixture (PM) {Drug/β-CD/polymer} and ternary complex
Table V.
XRD Data of Various Systems
| 2θ | Famotidine | β-CD | Counts binary complex (A) | Ternary complex (B) | Difference in the intensity (A−B) |
|---|---|---|---|---|---|
| 10.62 | 1,537.2 | 1,332.4 | 686.4 | 646.0 | |
| 11.56 | 1,102.6 | 889.7 | 694.2 | 195.5 | |
| 12.46 | 2,818.6 | 1,628.4 | 871.1 | 757.3 | |
| 15.42 | 2,026.7 | 807.6 | 453.0 | 354.6 | |
| 15.70 | 957.7 | 500.9 | 430.2 | 70.7 | |
| 16.98 | 1,590.5 | 787.2 | 409.3 | 377.9 | |
| 17.94 | 840.0 | 614.7 | 465.2 | 149.5 | |
| 20.00 | 946.7 | 717.2 | 574.0 | 143.2 | |
| 20.80 | 1,244.2 | 846.6 | 562.5 | 284.1 | |
| 20.94 | 1,778.4 | 674.1 | 436.0 | 238.1 | |
| 22.80 | 1,233.4 | 883.1 | 595.0 | 288.1 | |
| 24.00 | 1,128.3 | 583.0 | 398.4 | 184.6 | |
| 32.22 | 591.4 | 331.1 | 237.9 | 93.2 | |
| 35.1 | 739.6 | 268.6 | 177.3 | 91.3 |
On comparing the diffratograms of all the samples, it can be suggested that the formation of ternary complex resulted in an amorphous solid system. The increased complexation of famotidine led to a decrease in its free concentration in the ternary complex. Moreover, the XRD pattern of ternary complex was found to be more diffuse compared to both the binary complex and the physical mixture of three components. Thus, it can be claimed that ternary complexation resulted in an increase in the complexation efficiency, resulting in formation of amorphous solid state.
Microscopic Studies
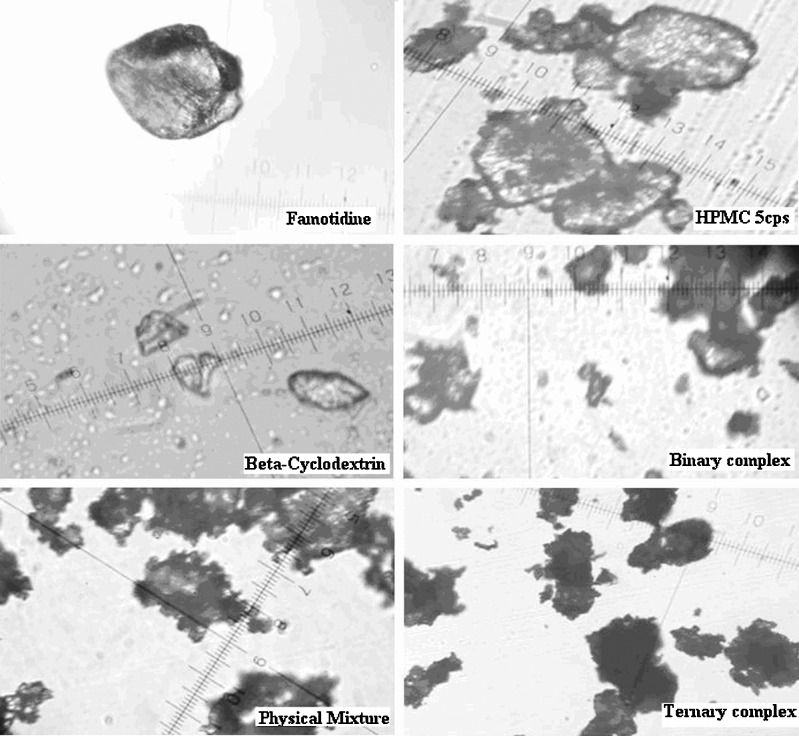
The particular nature of systems was studied using Photomicrography and Scanning Electron Microscopy (Figs. 6 and 7). Drug and β-CD and HPMC crystals were well detectable in the PM, whereas the formation of amorphous aggregates was observed in binary complex which was further seen prominently in ternary system, where it was no more possible to differentiate the two components.
Fig. 6.
Photo-micrographic images of Famotidine; HPMC 5 cps; β-Cyclodextrin; binary complex {Drug/β-CD}; physical mixture (PM) {Drug/β-CD/polymer} and ternary complex
Fig. 7.
SEM images of Famotidine; HPMC 5 cps; β-Cyclodextrin; binary complex {Drug/β-CD}; physical mixture (PM) {Drug/β-CD/polymer} and ternary complex
Taste Perception Studies
In order to evaluate the efficiency of taste masking by ternary complexation a taste perception study was designed and followed. Precisely, a hedonic rating scale (Table I) of 1 to 7 was used for the quantification of the results of taste perception study. The overall ratings for various samples were obtained and the mean rating was used to qualify the taste masking efficiency. It was found that ternary complex showed an average rating of 6.80 (SD = 0.38; P < 0.001) as compared to 1.08 (SD = 0.29; P < 0.005), 2.25 (SD = 0.45; P < 0.001) and 3.58 (SD = 0.51; P < 0.005) in case of plain drug, drug-β-CD-polymer physical mixture and drug-β-CD binary complex respectively. Based on the average ratings it can be concluded that ternary complex was better at masking the bitterness of the drug as compared to binary complex.
CONCLUSION
It has been well reported in literature that ternary complexation can be used as an approach for improving the complexation efficiency of β-cyclodextrin by increasing the stability constant of the formed complex. The investigation here confirmed that the same principal can be applied for improving the taste masking capabilities of β-cyclodextrin. As evident from the results, it can be suggested that ternary complexation can be effectively used as a novel approach for taste masking.
Acknowledgement
Authors wish to thank University Grant Commission (UGC), India for providing financial assistance through senior research fellowship.
References
- 1.Loftsson T., Brewster M. E. Pharmaceutical applications of cyclodextrins: 1. Drug solubilization and stabilization. J. Pharm. Sci. 1996;85:1017–1025. doi: 10.1021/js950534b. [DOI] [PubMed] [Google Scholar]
- 2.Sohi H., Sultana Y., Khar R. Taste masking technologies in oral pharmaceuticals: Recent developments and approaches. Drug Dev. Ind. Pharm. 2004;30:429–448. doi: 10.1081/DDC-120037477. [DOI] [PubMed] [Google Scholar]
- 3.Szejtli J. Past, present, and future of cyclodextrin research. Pure Appl. Chem. 2004;76:1825–1845. doi: 10.1351/pac200476101825. [DOI] [Google Scholar]
- 4.Giancarlo C., Arianna B., Enzo B., Paolo C., Trotta F. Cyclodextrins as food additives and in food processing. Curr. Nutri. Food Sci. 2006;2:343–350. doi: 10.2174/157340106778699485. [DOI] [Google Scholar]
- 5.Noriaki F., Ikumi U., Takashi O., Shun H., Saburo N. Masking mechanisms of bitter taste of drugs studied with ion selective electrodes. Chem. Pharm. Bull. 2006;54:1155–1161. doi: 10.1248/cpb.54.1155. [DOI] [PubMed] [Google Scholar]
- 6.Rajewski R. A., Stella V. J. Pharmaceutical applications of cyclodextrins II: In vivo drug delivery. J. Pharm. Sci. 1996;85:1142–1169. doi: 10.1021/js960075u. [DOI] [PubMed] [Google Scholar]
- 7.Okimoto K., Rajewski R. A., Jona J. A., Stella V. J. The interaction of charged and uncharged drugs with a neutral (HP-b-CD) and anionically charged (SBE7-b-CD) b-cyclodextrin. Pharm. Res. 1996;13:256–264. doi: 10.1023/A:1016047215907. [DOI] [PubMed] [Google Scholar]
- 8.Stella V. J., Rajewski R. A. Cyclodextrins: Their future in drug formulation and delivery. Pharm. Res. 1997;14:556–567. doi: 10.1023/A:1012136608249. [DOI] [PubMed] [Google Scholar]
- 9.Stella V. J., Rao V. M., Zannou E. A., Zia V. Mechanisms of drug release from cyclodextrin complexes. Adv. Drug Del. Rev. 1999;36:3–16. doi: 10.1016/S0169-409X(98)00052-0. [DOI] [PubMed] [Google Scholar]
- 10.Patel A. R., Vavia P. R. Effect of hydrophilic polymers on solubilization of fenofibrate by cyclodextrin complexation. J. Incl. Phenom. Macrocycl. Chem. 2006;56:247–251. doi: 10.1007/s10847-006-9091-4. [DOI] [Google Scholar]
- 11.Loftsson T., Frioriksdottir H. The effect of water-soluble polymers on the aqueous solubility and complexing abilities of b-cyclodextrin. Int. J. Pharm. 1998;163:115–121. doi: 10.1016/S0378-5173(97)00371-2. [DOI] [Google Scholar]
- 12.Sigurdardottir A. M., Loftsson T. The effect of polyvinylpyrrolidone on cyclodextrin complexation of hydrocortisone and its diffusion through hairless mouse skin. Int. J. Pharm. 1995;126:73–78. doi: 10.1016/0378-5173(95)04095-1. [DOI] [Google Scholar]
- 13.Rowe R. C., Paul J. S., Paul J. W. Handbook of Pharmaceutical Excipients. London: Pharmaceutical Press; 2003. [Google Scholar]
- 14.Li S. P., Martellucci S. A., Bruce R. D., Kinyon A. C., Hay M. B., Higgins J. D. Evaluation of the film-coating properties of a hydroxyethyl cellulose/hydroxypropyl methylcellulose polymer system. Drug Dev. Ind. Pharm. 2002;28:389–401. doi: 10.1081/DDC-120003000. [DOI] [PubMed] [Google Scholar]
- 15.Higuchi T., Connors K. A. Phase solubility techniques. Adv. Anal. Chem. Instrum. 1965;4:117–118. [Google Scholar]
- 16.Khan K. A. The concept of dissolution efficiency. J. Pharm. Pharmacol. 1975;27:48–49. doi: 10.1111/j.2042-7158.1975.tb09378.x. [DOI] [PubMed] [Google Scholar]
- 17.Acarturk F., Kislal O., Celebi N. The effect of some natural polymers on the solubility and dissolution characteristics of nifedipine. Int. J. Pharm. 1992;85:1–15. doi: 10.1016/0378-5173(92)90127-N. [DOI] [Google Scholar]
- 18.Loftsson T., Frikdriksdottir H., Sigurkdardottir A. M., Ueda H. Evaluation of cyclodextrin solubilization of drugs. Int. J. Pharm. 1994;110:169–177. doi: 10.1016/0378-5173(94)90155-4. [DOI] [Google Scholar]
- 19.Rekharshy M. Y., Inoue Y. Detection of paramagnetic pH dependent Inclusion complexes. Chem. Rev. 1998;98:1875–1896. doi: 10.1021/cr970015o. [DOI] [PubMed] [Google Scholar]
- 20.Jadhav G. S., Patel A. R., Vavia P. R., Malde A. K., Coutinho E. C. Interaction of valdecoxib with β-cyclodextrin: Experimental and molecular modeling studies. J. Incl. Phenom. Macrocycl. Chem. 2006;56:247–251. doi: 10.1007/s10847-006-9093-2. [DOI] [Google Scholar]
- 21.Sinha V. R., Anitha R., Ghosh S., Nanda A., Kumria R. Complexation of celecoxib with β-cyclodextrin: Characterization of the interaction in solution and in solid state. J. Pharm. Sci. 2005;94:676–687. doi: 10.1002/jps.20287. [DOI] [PubMed] [Google Scholar]
- 22.George B., Mcintyre P. Introduction to spectrum interpretation. In: Mowthorpe D. J., editor. Infrared Spectroscopy: Analytical Chemistry by Open Learning. London, UK: Wiley; 1987. pp. 161–201. [Google Scholar]