Abstract
The purpose of the present study was to develop and design pectin and polyvinyl pyrrolidone (PVP) blended hydrogel membranes (PEVP), with different pectin: PVP ratios (1:0.2, 1:0.4, 1:0.6, 1:0.8 and 1:1 w/w), which were prepared by using a conventional solution casting technique. An attempt has been made to characterize the hydrogel membranes by various instrumental techniques like, FTIR (Fourier transform infrared) spectroscopy, X-ray diffraction (XRD), Differential scanning calorimetry (DSC), tensile strength test and scanning electron microscopy (SEM). The release patterns of the drug (salicylic acid) from the hydrogel membrane were done in three different release mediums (pH 1.4, pH 7.4 and distilled water) and samples were analyzed spectrophotometrically at 294 nm wavelength on a UV Vis spectrophotometer. MTT assay was done to ensure cytocompatibility of the pectin/PVP hydrogel membranes using B16 melanoma cells. FTIR spectroscopy indicated the presence of secondary amide (I) absorption bands. The XRD study shows decrease in crystallinity of the hydrogel membranes with increase in PVP ratio. DSC study shows an increase in Tg of pectin after blending with PVP. It was found that tensile strength increases with increasing PVP ratios in the hydrogel membranes. The prepared hydrogel membranes were found to be biocompatible with B16 melanoma cells.
Key words: biocompatibility, pectin, PVP, salicylic acid
INTRODUCTION
Hydrogels are polymeric networks, which absorb and retain large amount of water. Their hydrophilic surface has a low interfacial free energy in contact with body fluids, which results in a low tendency for protein and cells to adhere to these surfaces. Moreover, the soft and rubbery nature of hydrogels minimizes irritation to surrounding tissues. As a result, in comparison to other synthetic materials, hydrogels resemble nature living tissues closely in their physical properties due to their high water contents and softness, which also contributes to biodegradability and biocompatibility. Therefore, it can find applications in different technological arias such as materials for contact lenses and protein separation, matrices for cell encapsulation and devices for controlled release of drugs and proteins (1–5). Pectin is one of the major constituents of citrus by products and has good gelling properties (6–7) chemically; pectin is poly α 1-4-galacturonic acids, with varying degree of methylation of carboxylic acid residues (8). Pectin has been used since long time by the researchers as a potential drug carrier for colon specific drug delivery (9–13). However pectin films have low thermal stability and poor mechanical properties, hence it was blended with different polymers to improve its thermal and mechanical stability (14–17). As a water-soluble polymer polyvinyl pyrrolidone (PVP) has beneficial effect on protection, viscosity, absorbency, solubilization, with its most significant features being excellent solubility and biocompatibility. Additionally, PVP has low toxicity and it is used in medical, food, cosmetics and as a film-forming agent (18). PVP is a component of hydrogels that is widely used for biomedical applications (19) its monomer N-vinyl pyrrolidone has been copolymerized with acrylic acid, methacrylates and other vinyl monomers for their application in contact lenses (20), for the controlled delivery of drugs (21), and for immobilization of the enzyme lipase (22). Recently a great deal of interest has been shown by few workers on synthesis, characterization and swelling studies of PVP based hydrogels (23–26). Considering the film forming property of PVP, it was blended with pectin to improve its mechanical properties. Attempts were made to characterize the hydrogel membranes by FTIR, XRD, DSC and tensile test. The in vitro drug release of SA (model drug) through the hydrogel membranes were evaluated by using modified Franz diffusion cell.
EXPERIMENTAL
Materials and Methods
Pectin (MW ~30,000–100,000), glutaraldehyde (GA) 25% solution and salicylic acid (SA, a model drug) were purchased from Loba-chemie Indoaustranal Co. Mumbai, India. Poly vinyl pyrrolidone (MW 40,000 and viscosity 2.4CP) was purchased from Sisco research laboratories, Mumbai, India. Hydrochloric acid 35% pure was obtained from Merck Limited, Mumbai, India. Double distilled water was used throughout the study. B16 melanoma cells, dimethyl sulphoxide (DMSO), and MTT solution were obtained from Department of Biotechnology, Indian Institute of Technology, India.
Preparation of Hydrogels
Fifty milliliters of 10% (w/v) of pectin solution in water was taken in a beaker. To the pectin solution 50 ml of PVP solution (containing 1, 2, 3, 4, 5 g of PVP so as to give pectin: PVP ratios, (1:0.2, 1:0.4, 1:0.6, 1:0.8 and 1:1) was added with continuous stirring to get a homogeneous mixture. A glutararaldehyde reagent (0.2 ml HCl + 1 ml GA) was added as a crosslinking agent to the solutions and resulting dispersions were stirred at room temperature for half an hour for proper reaction between pectin and PVP. The thick dispersions so obtained were converted in to hydrogel membranes by conventional solution casting method by casting on glass petri plates. The hydrogel membranes were allowed to dry at room temperature for 72 h. The hydrogel membranes so obtained were peeled of and thoroughly washed with distilled water to remove HCl and GA if any. The hydrogel membranes obtained were named as PEVP-1, PEVP-2, PEVP-3, PEVP-4 and PEVP-5.
Characterization
The FTIR spectrum of Pectin hydrogel membrane and pectin/PVP hydrogel membranes were taken in the range of 4,000–400 cm−1 as KBr pellet and Attenuated total reflectance (ATR) technique with the help of FTIR spectrophotometer (NEXUS-870, Thermo Nicolet Corporation) at 19 °C temperature. In the FTIR studies of the prepared membranes a uniform resolution of 4 cm−1 was maintained and the number of scan used was 32.
For the X-ray diffraction study the raw materials and the pectin/PVP hydrogel membranes were subjected to X-ray diffraction (XRD-PW 1700, Philips, USA), at 19 °C temperature, using Cu Kα radiation generated at 40 kV and 40 mA; the range of diffraction angle was 10°–70° 2θ. The % crystallinity from the XRD plots were calculated by using Fit gaussian method with the help of origin software. It was calculated by using the following formula;
 |
1 |
Differential scanning calorimetry was done in the DSC unit (Netzsch DSC-200 PC Phox, Germany). The samples were heated in a closed aluminium pan at a rate of 40 °C/min from −75 to 255 °C. Nitrogen was used as a purge gas with a flow rate of 50 mL/min.
The tensile strength of the Pectin/PVP blended hydrogel membranes were carried out using Hounsfield H10KS tensile testing machine. Cross-Head Speed: 50 mm/min, Temperature: 18 °C and Relative Humidity: 60%
The morphology of pure pectin, PVP and pectin/PVP blended hydrogel membranes have been investigated by using a Scanning electron microscope (CAMSCAN-2, JEOL, Japan).
Drug Loading
Drug release from the crosslinked pectin/PVP hydrogel membrane was studied by using salicylic acid (SA) as a model drug. SA was incorporated in the hydrogel membrane by diffusion method. The pectin/PVP hydrogel membrane was immersed for 5 h in SA solution in acetone (1 g SA in 10 ml acetone). The drug loaded hydrogel membrane was washed with distilled water to remove the drug adhered on the surface of the membrane.
Preparation of Buffer Solutions
The pH buffers were prepared according to Indian pharmacopoeia 1996. Buffer solution of pH 1.4 was prepared by taking 50 ml of 0.2 M KCl and 53.2 ml 0.2 N HCl in volumetric flask to make volume 200 ml with distilled water. 0.2 M KCl solution was prepared by dissolving 14.912 g of KCl in distilled water to make the volume 1,000 mL with distilled water. Buffer solution of pH 7.4 was prepared by taking 50 ml of 0.2 M KH2PO4 and 39.1 ml of 0.2 N NaOH in volumetric flask to make volume 200 ml with distilled water. 0.2 M KH2PO4 was prepared by dissolving 27.218 g of KH2PO4 in distilled water to make the volume 1,000 ml with distilled water.
Drug Release Study
The drug (SA) loaded Pectin/PVP hydrogel membrane (PEVP-5) was put in a 100 ml beaker containing 100 ml of distilled water and was mechanically stirred with a magnetic stirrer. Samples were periodically withdrawn from the beaker using a 1-mL pipette and the volume of the withdrawn samples was replaced with distilled water. The drug release study was also carried out at pH 1.4 and pH 7.4 by using the same method. Then samples were analyzed spectrophotometrically at 294 nm wavelength on a UV Vis spectrophotometer.
Swelling Study
The hydrogel membranes (PEVP-1 and PEVP-5) were immersed directly in buffer solutions of pH 1.4, 5.4, 7.4 and 9.4 (prepared as per Indian pharmacopoeia 1996) at room temperature for 72 h and thereafter the swollen product was dried at 37 °C under vacuum to a constant weight. The equilibrium percentage of swelling (% swelling) of the product was calculated as
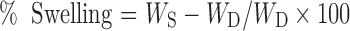 |
2 |
Where WS is the weight of the product after hydration for 72 h and WD is the weight of dried test sample (27).
MTT Assay
B16 melanoma cells were used to evaluate the biocompatibility of hydrogel membranes by MTT (3-[4, 5-dimethylthiazole-2-yl]-2, 5 diphenyltetrazolium bromide) assay (28). Cells were seeded into a 96-well microplates at 3,000 cells per well. The medium was removed after 24 h of plating and fresh media containing different concentrations of polymers (1–100 μg/ml) were added. After incubation for 1 h the medium was discarded, the cells were washed twice with phosphate-buffered saline (PBS: 150 mM NaCl; 1.9 mM NaH2PO4; 8.1 mM Na2HPO4; pH 7.4) and 50 ml of 5 mg/ml MTT solution in PBS were added to each well. The plates were incubated 37 °C for 4 h and the formazan crystals were dissolved by adding 100 ml dimethyl sulfoxide (DMSO) to each well. The absorptions were measured in triplicate at 570 nm. Results were recorded as percentage absorbance relative to untreated control cells. The cytotoxicity assay results were used to calculate cell viability after incubation with polymers as follows:
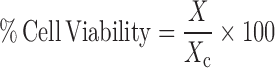 |
3 |
where X is the absorbance in a well containing a particular polymer concentration and Xc is the absorbance for untreated control cells.
RESULT AND DISCUSSION
In this preliminary study, pectin/ PVP based hydrogels has been synthesized containing varying amount of PVP ratios by crosslinking with GA. The FTIR spectrum of pectin/PVP hydrogel membranes indicates lowering in the intensity of –OH stretching vibration peaks as compared to usual –OH stretching vibration peak at 3,402 cm−1 in pectin. This can be attributed to the presence of hydrogen bonding between pectin and PVP. This indicates the proper molecular interactions between the two polymers pectin and PVP. The X-ray diffraction studies of raw materials (pectin and PVP) and pectin/PVP-blended membranes in different ratio of PVP indicate that change in crystallinity was observed from crystalline to amorphous phase. This may be attributed to the fact that increase in PVP ratio in membranes leads to a disruption in the crystalline regions of pectin due to increase in the amorphousity of the blended membranes. The DSC study shows increase in glass transition temperature (Tg) of the pectin/PVP-blended hydrogel (PEVP-5) with increase in PVP, which indicates the miscibility of pectin and PVP due to the formation of intermolecular hydrogen bonding between hydroxyl group (–OH) of pectin and carbonyl group (–C=O) of PVP (Fig. 1). This has been also confirmed by the FTIR spectra of the prepared hydrogels. The presence of single Tg in the PEVP-5 indicates the completely miscible nature of the blend with a homogeneous amorphous phase. Few workers have reported similar results during their studies on miscibility behavior and hydrogen bonding in blends of poly (vinyl phenol) and poly (vinyl pyrrolidone; 29). The mechanical properties of the blended membranes were found to be much higher than pectin and PVP. The tensile properties of blend membranes indicate that PVP significantly improves the mechanical properties of the composite membranes and the enhancement of tensile strength may be due to the formation of hydrogen bond between pectin and PVP which leads to the formation of intermolecular complex or the physical crosslinkage. The swelling behavior of pectin/PVP hydrogels (PEVP-1 and PEVP-5) indicates that maximum swelling was obtained at alkaline pH (pH 7.4) as compared to acidic pH (pH 1.4). This is attributed to the highly hydrophilic nature of PVP and complete dissociation of carboxymethyl groups (COOCH3) present in pectin at higher pH. It shows the pH dependent swelling behavior of the prepared hydrogel membranes. It has also been observed that swelling increases with increase in PVP ratio in the blended membranes. The higher swelling was observed in the membrane, which is designated as PEVP-5. It has been observed that there is a direct correlation between swelling behavior and antibiotic release properties of the hydrogels. The drug release study indicates that different pH buffer solutions (pH 1.4 and pH 7.4) have pronounced effect on salicylic acid (SA) release through the pectin/PVP hydrogel. The maximum SA release was observed at pH 7.4 as compared to pH 1.4 and distilled water. The matrix systems developed by us were studied in an environment having abundant amount of dissolution media. This is why the release of the drug was quicker and the equilibrium reached at ≈3 h. The fast SA release at higher pH is a desirable feature for controlled release systems operating in alkaline environment of small intestine i.e. colon. The pH sensitive swelling and subsequent drug release will thus assure that antibiotic can be released only at high pH in small intestine rather than at low pH in stomach. The SEM study of pectin/PVP hydrogels indicates completely different surface morphology than the raw materials pectin and PVP. The SEM micrograph of both the hydrogels (PEVP-1 and PEVP-5) indicates that the complete diffusion of drug occurs from the matrix.
Fig. 1.
Reaction mechanism for preparation of pectin/PVP hydrogel crosslinked with GA
Characterization
FTIR Characterization
The FTIR spectra of pectin and pectin-PVP blended hydrogel membranes are shown in Fig. 2 and the peaks assigned were listed in Table I. The FTIR spectra of pectin hydrogel show clear absorption peaks at 3,402, 2,932 cm−1, that was due –OH and –CH stretching vibration peaks. The peaks at 1,454 and 1,357 cm−1 could be assigned to CH2 and –OH bending vibration peaks respectively. The FTIR spectra of pectin/PVP blended hydrogel membranes (PEVP-1, PEVP-5) shows that there are common peaks were observed between 1,630–1,660 cm−1 in all the hydrogel membranes, which are corresponding to amide (I), and also corresponding to the –CO and –CN groups (30). The formation of hydrogen bonding weakened the strength of carbonyl bond of PVP in all the hydrogel membranes and shifted the carbonyl stretching to lower frequency (31–32). The FTIR spectra of pectin/PVP hydrogel membranes indicates that upon blending with PVP the characteristic –OH stretching vibration peak of pectin at 3,402 cm−1 is shifted to lower frequency, 3,357, 3,325, 3,340, 3,345, 3,352 cm−1. This lowering in frequency of the –OH groups can be accounted due to presence of hydrogen bonding in the hydrogel membranes. The peaks between 2,930–2,970 cm−1 indicate presence of –CH stretching vibration peak and peaks between 1,420–1,460 cm−1 indicate –CH bending vibration peak. The presence of peaks in the range of 1,740–1,760 cm−1 can be accounted due to the presence of carboxy methyl groups (–COOCH3) in the hydrogel membranes. The peaks at 1,030, 1,017, 1,042, 1,023, 1,17 cm− shows the presence of –CN stretching vibration peak in the hydrogel membranes.
Fig. 2.
FTIR Spectra of pectin and pectin/PVP blended hydrogel membranes (PEVP-1 to PEVP-5)
Table I.
Assignment and Peaks of the FTIR Bands of Pectin and Pectin/PVP Membranes (a–f)
| Sample Identity | –CN stretch (cm−1) | –OH Stretch (cm−1) | N–H (amide I; cm−1) | C=O (cm−1) | CH2 bending (cm−1) | –CH Stretch (cm−1) |
|---|---|---|---|---|---|---|
| Pectin | 1,023 | 3,402 | – | 1,740 | 1,460 | 2,932 |
| PEVP-1 | 1,030 | 3,357 | 1,634 | 1,750 | 1,428 | 2,952 |
| PEVP-2 | 1,017 | 3,325 | 1,647 | 1,750 | – | – |
| PEVP-3 | 1,042 | 3,340 | 1,640 | 1,750 | – | 2,958 |
| PEVP-4 | 1,023 | 3,345 | 1,634 | 1,756 | – | 2,965 |
| PEVP-5 | 1,017 | 3,352 | 1,640 | 1,750 | 1,422 | 2,958 |
XRD Characterization
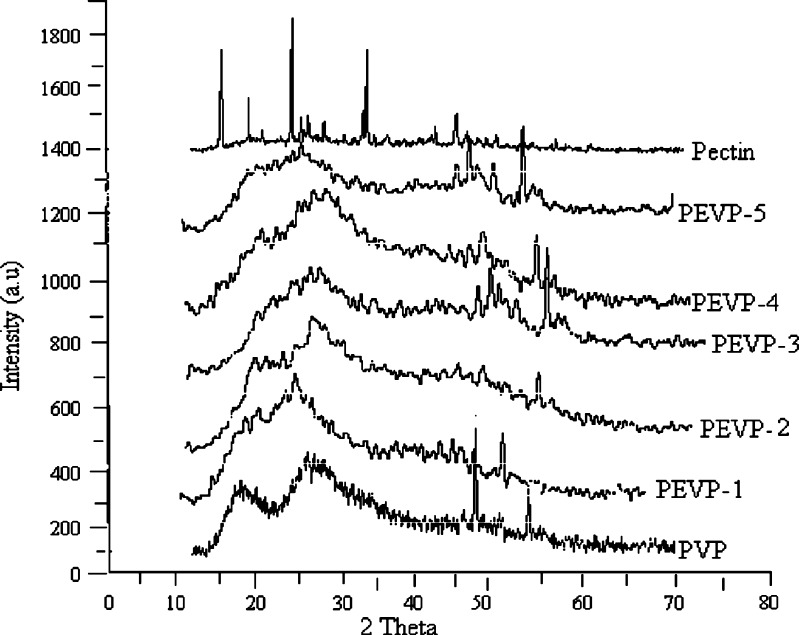
The XRD pattern of pectin, PVP and pectin/PVP blended membranes are depicted in Fig. 3. The X-ray diffractogram of PVP shows two broad peaks at 2θ equals to 11.55oθ and 21.36oθ, which shows amorphous nature of PVP. It can be observed from the X-ray diffractogram of pure pectin that it shows sharp crystalline peaks at 2θ equals to 9oθ, 12.70oθ, 18.42oθ, 28.22oθ, and 40.14oθ, which clearly indicate the crystalline behavior of pectin. Whereas the X-ray diffractograms of the blended membranes (PEVP-1 to 5) shows broad peaks between 20oθ–3oθ, it can be attributed to the amorphous nature of PVP (33). The result shows that with increasing ratio of PVP the crystallinity of the blend membranes decreases as compared to parent pectin, it might be due to disruption of the crystalline fractions of pectin with increase in PVP ratio in the blended membranes. From the XRD plots of the pectin, PVP and pectin/PVP blended membranes the percent crystallinity was calculated and it was found to be 42.89, 20.03, 27.69, 14.02, 14.42, 16.55 and 6.77% respectively (Table II). It shows a considerable decrease in crystallinity of the blended hydrogel membranes with increase in concentration of PVP. However, a slight variation in percent crystallinity of the membrane was observed which is designated as PEVP-4.
Fig. 3.
XRD pattern of pectin, PVP and pectin/PVP blended membranes (PEVP-1 to PEVP-5)
Table II.
Percent Crystallinity of Pectin, PVP and Pectin/PVP Blended Membranes
| Sample Identity | Percent Crystallinity |
|---|---|
| Pectin | 42.89 |
| PVP | 20.30 |
| PEVP-1 | 27.69 |
| PEVP-2 | 14.02 |
| PEVP-3 | 14.42 |
| PEVP-4 | 16.55 |
| PEVP-5 | 6.77 |
DSC Study
The DSC thermograms of pectin and pectin/PVP blended hydrogel membrane (PEVP-5) are presented in Fig. 4. The DSC of pectin shows a small endothermic peak at 95 °C, which is corresponding to the glass transition temperature (Tg) of pectin; it can be also associated with the elimination of bound water in the pectin sample. There is another endothermic peak was observed near 137 Superscript>/Superscript>C it can be assigned to melting temperature (Tm) of pure pectin. From the thermogram of PVP (Fig. 4) it can be observed that, it shows a sharp endothermic peak at 140 °C, it is corresponding to Tg of pure PVP, which is less than the Tg reported by few workers (34). The thermogram of pectin/PVP membrane shows an endothermic peak near 158 °C. This endothermic peak shows the glass transition (Tg) temperature of the blended membrane. These peaks were assigned due to the elimination of water and formation of complex between the pectin and PVP molecules. This shows that there is increase in Tg of the pure pectin after blending with poly vinyl pyrrolidone. This increase in Tg may be attributed to the presence of intermolecular hydrogen bonding between pectin (–OH) and PVP (–C=O).
Fig. 4.
DSC thermograms of pectin, PVP and pectin/PVP blended membrane (PEVP-5)
Tensile Study
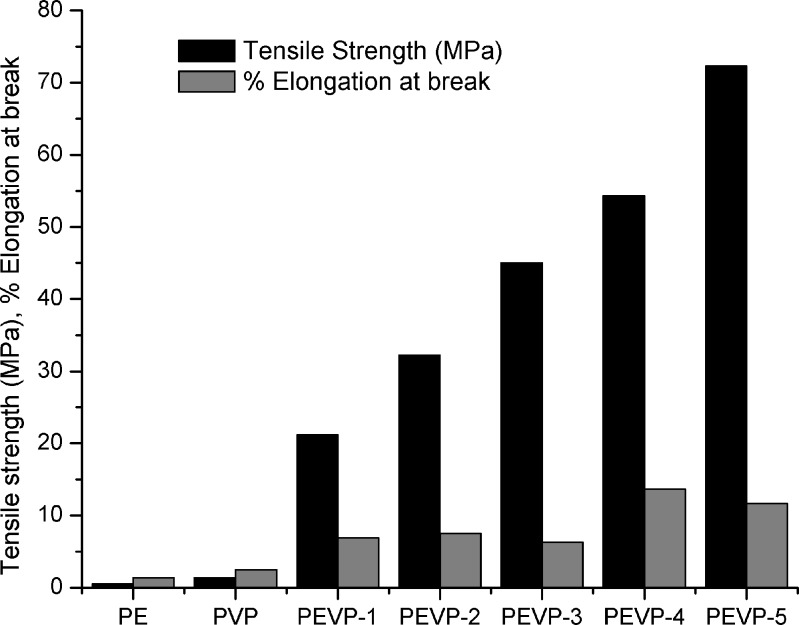
The tensile strength of the pectin and PVP membrane were found to be 0.50 and 1.4 MPa respectively. The tensile strength of the pectin/PVP blended hydrogel membranes in dry state are shown in Fig. 5. From the figure, it can be observed that the tensile strength of the Pectin/PVP hydrogel membranes increases considerably with increase in concentration of PVP. The highest tensile strength 72.29 MPa was observed in the case of the hydrogel membrane, which is designated as PEVP-5 (1:1), and was found to be higher than the tensile strength of skin (34 MPa) (35). This increase in tensile strength of the hydrogel membranes with increase in PVP ratio may be attributed to the restriction in the mobility experienced by the polymer chains in the pectin-polyvinyl pyrrolidone complex that have electrostatic attraction with another polymer chain. However, a slight variation in % elongation at break was observed for pectin and PVP blended membranes.
Fig. 5.
Tensile strength and percent elongation at break of the raw materials and pectin/PVP membranes (PEVP-1 to 5)
Swelling Study
The crosslinked pectin/PVP hydrogel membranes (PEVP-1, PEVP-5) were allowed to swell in 25 ml of buffer solutions of pH 1.4, 5.4, 7.4 and 9.4. Figure 6 shows the swelling behavior of crosslinked pectin/PVP hydrogel membranes. The result indicates that swelling was found to increase with pH for both compositions of pectin/PVP hydrogels (PEVP-1 and PEVP-2). It has been observed that maximum degree of swelling (295% and 175%) was observed at pH 7.4 as compared to pH 1.4. Since the apparent pKa value of pectin of different degree of esterification (DE) ranges from 3.55 to 4.10 (36), hence at pH value lower than the pKa value, the carboxymethyl groups (–COOCH3) of pectin are completely collapsed and which results in low degree of swelling. In addition to this hydrogen bond formation occurs between hydroxyl group (–OH) of pectin and carbonyl group (–C=O) of PVP, which further leads to low degree of swelling at the said pH. However at higher pH (pH 7.4) values than the pKa value, the swelling of the hydrogel increases because of dissociation of the hydrogen bonding between hydroxyl group of pectin and carbonyl group of PVP. Similar results have been reported by few other workers for swelling behavior of crosslinked PVA/PVP and PVP/AAc hydrogels at varying pH buffer solutions (37–38). After pH 7.4 there is a decrease in swelling because of the partial solubility of the crosslinked hydrogels (39).
Fig. 6.
Swelling studies of the pectin/PVP hydrogel membranes (PEVP-1, PEVP-2)
Drug Release Study
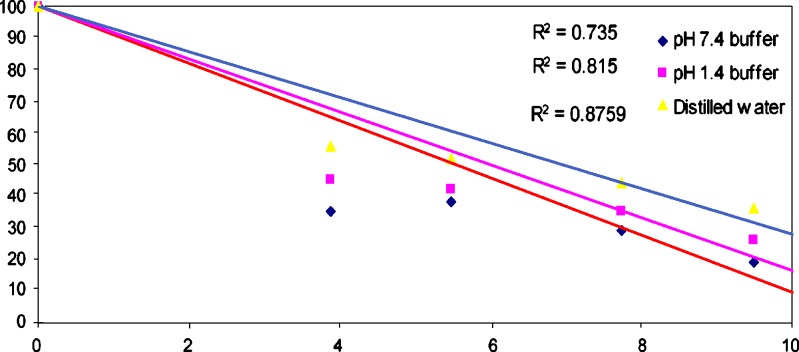
The release of water-soluble drugs, entrapped in hydrogels, occur only after water gets in to the polymer networks to swell and dissolve the drug, fallowed by that drug diffuses out through the aqueous pathways to the surface of the device (40). The SA release profile of the drug loaded pectin/PVP hydrogel membrane (PEVP-5) in different release medium, pH 1.4, pH 7.4 and distilled water has been presented in Fig. 7. From the percent release profile of the hydrogel membrane at pH 7.4 it has been observed that total 65% drug release occurred in 15 min, whereas in pH 1.4 and distilled water total 55%, 48% drug released in 15 min. After 3 h the release pattern shows that at pH 7.4 total 85% drug released fallowed by 82% and 72% drug release at pH 1.4 and distilled water respectively. It can be suggested from the results that the amount of SA release in pH 7.4 was higher than release medium of pH 1.4 and distilled water. It can be correlated with the swelling behavior of the pectin/PVP hydrogel membranes (Fig. 6), where the swelling increased when pH of the medium changed from acidic to mildly basic. At high pH the carboxymethyl group in the pectin/PVP complex gets ionized and as a result the –COO− groups repel each other, which lead to higher swelling and SA release from the prepared hydrogels.
Fig. 7.
Drug release studies from the pectin/PVP hydrogel membrane (PEVP-5), at different release medium pH 1.4, 7.4 and distilled water
Drug Release Kinetics from the Hydrogels
The relaese kinetics from the hydrogel (PEVP-5) in different release medium (pH 7.4, pH 1.4 and distilled water) indicates that it follows Fickian kinetics, which shows a diffusion controlled drug release process (Fig. 8). Diffusion system may release drug following higuchian or Fickian kinetics. The rate of release of a drug dispersed as a solid in an inert matrix has been described by Higuchi (41–43). In this model, it is assumed that solid drug dissolves from the surface layer of the device first when this layer becomes exhausted of the drug; the next layer begins to be depleted by dissolution and diffusion through the matrix to the external solution. In this fashion, the interface between the region containing dispersed drug and that containing dispersed drug moves into the interior as a front. For the purposes of data treatment the above model is depicted by the following equation;
Fig. 8.
Drug release kinetics from the pectin/PVP hydrogel at different release mediums pH 1.4, 7.4 and distilled water
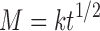 |
4 |
Where M is the mass of the drug released per unit area, k is a constant, so that a plot of amount of drug released versus the square root of time, t, should be linear if the release of the drug from the matrix is diffusion controlled.
MTT Assay
MTT assay was performed to measure change in viability of B16 melanoma cells after incubation with pectin: PVP hydrogel membranes. The relative percent cell viability with respect to concentration of pectin/PVP hydrogel membranes are presented in Fig. 9. The figure shows that the pectin/PVP hydrogel membranes did not induce significant cytotoxic effect even at higher concentration of the polymer solution used, it indicates the biocompatibility of the pectin/PVP hydrogel membranes.
Fig. 9.
Cytotoxicity test of pectin/PVP hydrogel membranes (1 = 1 μg/l, 2 = 10 μg/l ,3 = 100 μg/l, 4 = 200 μg/l, 5 = 300 μg/l)
Scanning Electron Microscopy
The SEM micrographs of pectin, PVP and pectin/PVP hydrogel membranes (PEVP-1 and PEVP-5) are shown in Fig. 10 (a–f). It can be observed from the SEM micrograph of pectin that it shows discrete elongated granular structures separated from one another. Whereas the surface topology of PVP indicates a spherical shape type morphology. The SEM micrograph of PEVP-1 shows circular pits, which confirms that drug, has diffuses out of the matrix using leaving behind the empty channels. The SEM of PEVP-5 shows capsules type morphology, which indicates that drug was adhered on the surface of the matrix, whereas after diffusion it shows clear island type morphology. This confirms that SA was incorporated in to the hydrogels by diffusion method and it also suggests that it fallows Fickian kinetics for SA release through the hydrogels.
Fig. 10.
SEM micrographs of pectin, PVP, PEVP-1 (before diffusion), PEVP-1 (after diffusion), PEVP-5 (before diffusion), PEVP-5 (after diffusion)
CONCLUSION
The present work confirms the successful development of Pectin/PVP based novel hydrogel membranes. The membranes show pH sensitive swelling and controlled drug release behavior; these properties make these hydrogels a promising candidate for different biomedical applications, such as controlled drug delivery systems etc.
Acknowledgement
The authors are thankful to Department of Biotechnology, Indian Institute of Technology, India for their kind support in carrying out the cytotoxicity tests.
References
- 1.Peppas N. A. Hydrogels in medicine and pharmacy. Florida: CRC press Boca Raton; 1986. [Google Scholar]
- 2.Peppas N. A., Bures P., Leobandung W., Ichikawa H. Hydrogels in pharmaceutical formulations. Eur. J. Pharm. Biopharm. 2000;50:27–46. doi: 10.1016/S0939-6411(00)00090-4. [DOI] [PubMed] [Google Scholar]
- 3.Peppas N. A., Huang Y., Torres-Lugo M., Ward J. H., Zhang J. Physicochemical foundations and structural design of hydrogels in medicine and biology. Annual Rev. Biomed. Eng. 2000;2:9–29. doi: 10.1146/annurev.bioeng.2.1.9. [DOI] [PubMed] [Google Scholar]
- 4.Gehrke S. H. Drugs. Pharm. Sci. 2000;102:473–546. [Google Scholar]
- 5.Park K., Shalaby W. S. W., Park H. Biodegradable hydrogels for drug delivery. Basel, Switzerland: Technomic Publishing company; 1993. [Google Scholar]
- 6.Cann M. C., Roberts K. Plant cell wall architecture: the role of pectins and pectinases. In: Visser J., Voragen A. G. J., editors. Pectins and pectinases. Amsterdam, Netherlands: Elsevier science; 1996. pp. 91–107. [Google Scholar]
- 7.May C. D. Industrial pectins, sources, production and applications. Carbohy polym. 1990;12:91–107. [Google Scholar]
- 8.Grant G. T., Morris E. R., Rees D. A., Smith P. J. C., Thom D. Biological interactions between polysaccharides and divalent cations: the egg box model. FEBS Letters. 1973;32:195–198. doi: 10.1016/0014-5793(73)80770-7. [DOI] [Google Scholar]
- 9.Ashford M., Fell J., Attwood D., Sharma H., Woodhead P. An evolution of pectin as a carrier for drug targeting to the colon. J Control Release. 1993;26:213–220. doi: 10.1016/0168-3659(93)90188-B. [DOI] [Google Scholar]
- 10.Rubenstein A., Radai R., Ezra M., Pathak S., Rokem J. S. In vitro evolution of calcium pectinate a potential colon specific drug delivery carrier. Pharm Res. 1993;10:258–263. doi: 10.1023/A:1018995029167. [DOI] [PubMed] [Google Scholar]
- 11.Radai R., Rubenstein A. In vitro and vivo analysis of colon specificity of calcium pectinate formulations. Eur. J. Pharm Biopharm. 1995;41:291–295. [Google Scholar]
- 12.Warkley Z., Fell J. T., Attwood D., Parkins D. Pectin/ethyl cellulose film coating formulations for colonic drug delivery. Pharm Res. 1996;13:1210–1212. doi: 10.1023/A:1016016404404. [DOI] [PubMed] [Google Scholar]
- 13.Warkley Z., Fell J. T., Attwood D., Parkins D. Studies on drug release from Pectin/ethyl cellulose film-coated tablets: a potential colonic delivery system. Int. J. Pharm. 1997;153:219–224. doi: 10.1016/S0378-5173(97)00110-5. [DOI] [Google Scholar]
- 14.Tharanathan R. N. Biodegradable films and composite coatings: past present and future. Trends food Science Technol. 2003;14:71–78. doi: 10.1016/S0924-2244(02)00280-7. [DOI] [Google Scholar]
- 15.Kang J. C. H., Lee N. Y., Kwon J. H., Byun M. W. Pectin and gelatin based film: effect of gamma irradiation on mechanical properties and biodegradation. Radiat. Phys. Chem. 2005;72:745–750. doi: 10.1016/j.radphyschem.2004.05.045. [DOI] [Google Scholar]
- 16.Fishman M. L., Coffin D. R. Mechanical, microstructural and solubility properties of pectin/poly vinyl alcohol blends. Carbohydr. Polym. 1998;35:195–203. doi: 10.1016/S0144-8617(97)00245-2. [DOI] [Google Scholar]
- 17.Fishman M. L., Coffin D. R., Ly T. V. Thermomechanical properties of blends of pectin and poly (vinyl alcohol) J. Appl. Poly. Sci. 1996;61:71–79. doi: 10.1002/(SICI)1097-4628(19960705)61:1<71::AID-APP8>3.0.CO;2-R. [DOI] [Google Scholar]
- 18.Yeh J. T., Chen C. L., Huang K. S., Nien Y. H., Chen J. L., Huang P. Z. Synthesis, characterization and application of PVP/chitosan blended polymers. J. Appl. Polym. Sci. 2006;101:885–891. doi: 10.1002/app.23517. [DOI] [Google Scholar]
- 19.Rostak J. M., Olejniczac J. Medical applications of radiation formed hydrogels. J. Radiat. Phys. Chem. 1993;42:903. doi: 10.1016/0969-806X(93)90398-E. [DOI] [Google Scholar]
- 20.Lai Y. C. Effect of crosslinkers on photopolymerization of N- vinyl pyrrolidone and methacrylates to give hydrogels. J. Appl. Polym. Sci. 1997;66:1475. doi: 10.1002/(SICI)1097-4628(19971121)66:8<1475::AID-APP8>3.0.CO;2-B. [DOI] [Google Scholar]
- 21.Yuang J. F., Kwei T. K. pH sensitive hydrogel based on Poly (vinyl pyrrolidone)- Poly (acrylic acid) (PVP-PAA) Semi-interpenetrating networks (semi IPN): Swelling and controlled release. J. Appl. Polym. Sci. 1998;69:921. doi: 10.1002/(SICI)1097-4628(19980801)69:5<921::AID-APP11>3.0.CO;2-R. [DOI] [Google Scholar]
- 22.Basri M., Wong C. C. M., Ahmad M. B., Razak C. N. A., Salleh A. B. Immobilization of lipase on Poly (N 2-pyrrolidone-co-2 hydroxyethyl methacrylate) hydrogel for the synthesis of Butyl oleate. J. Am. Oil Chem. Soc. 1999;76:571. doi: 10.1007/s11746-999-0006-6. [DOI] [Google Scholar]
- 23.Doria-Serrano M. C., Riva-Palacio G., Ruiz-Travino F. A., Hernandez-Esparza M. Poly (N-vinyl pyrrolidone)-calcium alginate (PVP-Ca-alg) composite hydrogels: Physical properties and activated sludge Immobilization for waste water treatment. Ind. Eng. Chem. Res. 2002;41:3163–3168. doi: 10.1021/ie0109399. [DOI] [Google Scholar]
- 24.Zhai M., Ha H., Yoshii F., Makuuchi K. Effect of kappa-carageenan on the properties of Poly (N-vinyl pyrrolidone)/ kappa-carageenan blend hydrogels synthesized by γ-radiation technology. J. Radia. Phys. Chem. 2000;57:459–464. doi: 10.1016/S0969-806X(99)00415-6. [DOI] [Google Scholar]
- 25.Park K. R., Nho Y. C. Synthesis of PVA/PVP hydrogels having two layer by radiation and their physical properties. J. Radia. Phys. Chem. 2003;67:361–365. doi: 10.1016/S0969-806X(03)00067-7. [DOI] [Google Scholar]
- 26.Bajpai S. K. Poly (N-vinyl-2-pyrrolidone)-polyacrylamide hydrogels as extraction solvents. Iran. Polym. J. 2000;9:1026–1265. [Google Scholar]
- 27.Pal K., Banthia A. K., Majumdar D. K. Preparation and characterization of polyvinyl alcohol–gelatin hydrogel membranes for biomedical applications. AAPS PharmSciTech. 2007;8:1–5. doi: 10.1208/pt080121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hansen M. B., Nielsen S. E., Berg K. Re-examination and further development of precise and rapid dye method for measuring cell growth/cell kill. J. Immunol. Methods. 1989;119:203–210. doi: 10.1016/0022-1759(89)90397-9. [DOI] [PubMed] [Google Scholar]
- 29.Kuo S. W., Chang F. C. Studies of miscibility behavior and hydrogen bonding in blends of Poly (vinyl phenol) and Poly (vinyl pyrrolidone) Macromol. 2001;34:5224–5228. doi: 10.1021/ma010517a. [DOI] [Google Scholar]
- 30.Chalapathi V. V., Ramiah K. V. Normal vibration of N, N-Dimethylpropionamide. Curr. Sci. 1968;37:453–454. [Google Scholar]
- 31.Martinez A., Iruin J. J., Ferrnandez- Berridi M. J. Macromol. 1995;39:3707. doi: 10.1021/ma00114a026. [DOI] [Google Scholar]
- 32.Guo Q., Huang J., Li X. Miscibility of poly (N-vinyl-2-pyrrolidone) with poly (hydroxyether of phenolphthalein) and polyacrylonitrile. Eur. Polym. J. 1996;32:423–426. doi: 10.1016/0014-3057(95)00166-2. [DOI] [Google Scholar]
- 33.Bhattacharya R., Phaniraz T. N., Shailaza D. Polysulfone and polyvinyl pyrrolidone blend membranes with reverse phase morphology as controlled release systems: experimental and theoretical studies. J. Membr. Sci. 2003;227:23–37. doi: 10.1016/j.memsci.2003.07.014. [DOI] [Google Scholar]
- 34.Karavas E., Georgarakis E., Bikiaris D. Application of PVP/HPMC miscible blends with enhanced mucoadhesive properties for adjusting drug release in predictable pulsatile chronotherapeutics. Eur. J. Pharm. Biopharm. 2006;64:115–126. doi: 10.1016/j.ejpb.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 35.Bhat S. V. Biomaterials. New Delhi, India: Narosa publishing house; 2002. [Google Scholar]
- 36.Plaschina I. G., Braudo E. E., Tolstoguzov V. B. Circular dichroism studies of pectin solutions. Carbohydr. Res. 1978;60:1–8. doi: 10.1016/S0008-6215(00)83459-X. [DOI] [Google Scholar]
- 37.Lakouraj M. M., Tajbakhsh M., Mokhtary M. Synthesis and swelling characterization of crosslinked PVP/PVA hydrogels. Iran. Polym. J. 2005;14:1022–1030. [Google Scholar]
- 38.El-Hag Ali A., Shawkay H. A., Abd-El Rehim H. A., Hegazy E. A. Synthesis and characterization of PVP/AAc copolymer hydrogel and its applications in the removal of heavy metals from aqueous solution. Eur. Polym. J. 2003;39:2337–2344. doi: 10.1016/S0014-3057(03)00150-2. [DOI] [Google Scholar]
- 39.Pal K., Banthia A. K., Majumdar D. K. Development of carboxymethyl cellulose acrylate for various biomedical applications. Biomed. Mater. 2006;1:85–91. doi: 10.1088/1748-6041/1/2/006. [DOI] [PubMed] [Google Scholar]
- 40.Singh B., Chauhan G. S., Sharma D. K., Chauhan N. The release dynamics of salicylic acid and tetracycline hydrochloride from psyllium and polyacrylamide based hydrogels (II) Carbohydr. Polym. 2007;67:559–565. doi: 10.1016/j.carbpol.2006.06.030. [DOI] [Google Scholar]
- 41.Torris D., Garccia-Encin G., Seij B., Villa Jat L. L. Formulation and in vitro Evaluation of HPMCP-microencapsulated Drug resin complexes for Sustained release Of Diclofenac. Int. J. Pharm. 1995;121:239–243. doi: 10.1016/0378-5173(95)00020-J. [DOI] [Google Scholar]
- 42.Nokhodchi A., Farid D. J., Najafi M., Andrangui M. Studies on controlled release Formulation of Diclofenac sodium. Drug Dev. Ind. Pharm. 1997;23:1019–1023. doi: 10.3109/03639049709150490. [DOI] [Google Scholar]
- 43.Rani M., Mishra B. Effect of Admixed Polymers on Diclofenac Sodium Release from Matrix Tablets. Pharm. Pharmacol. Lett. 2001;11:76–78. [Google Scholar]