Abstract
The objective was to investigate the suitable polymeric films for the development of diltiazem hydrochloride (diltiazem HCl) transdermal drug delivery systems. Hydroxypropyl methylcellulose (HPMC) and ethylcellulose (EC) were used as hydrophilic and hydrophobic film formers, respectively. Effects of HPMC/EC ratios and plasticizers on mechanical properties of free films were studied. Effects of HPMC/EC ratios on moisture uptake, in vitro release and permeation through pig ear skin of diltiazem HCl films were evaluated. Influence of enhancers including isopropyl myristate (IPM), isopropyl palmitate (IPP), N-methyl-2-pyrrolidone, oleic acid, polyethylene glycol 400, propylene glycol, and Tween80 on permeation was evaluated. It was found that addition of EC into HPMC film produced lower ultimate tensile strength, percent elongation at break and Young’s modulus, however, addition of EC up to 60% resulted in too hard film. Plasticization with dibutyl phthalate (DBP) produced higher strength but lower elongation as compared to triethyl citrate. The moisture uptake and initial release rates (0–1 h) of diltiazem HCl films decreased with increasing the EC ratio. Diltiazem HCl films (10:0, 8:2 and 6:4 HPMC/EC) were studied for permeation because of the higher release rate. The 10:0 and 8:2 HPMC/EC films showed the comparable permeation-time profiles, and had higher flux values and shorter lag time as compared to 6:4 HPMC/EC film. Addition of IPM, IPP or Tween80 could enhance the fluxes for approx. three times while Tween80 also shorten the lag time. In conclusion, the film composed of 8:2 HPMC/EC, 30% DBP and 10% IPM, IPP or Tween80 loaded with 25% diltiazem HCl should be selected for manufacturing transdermal patch by using a suitable adhesive layer and backing membrane. Further in vitro permeation and in vivo performance studies are required.
Key words: diltiazem, ethylcellulose, hydroxypropyl methylcellulose, permeation, release, transdermal
INTRODUCTION
Transdermal drug delivery (TDD) is an alternative route for systemic drug delivery. It provides several advantages over conventional drug therapy including avoids first-pass biotransformation and metabolism, minimizes absorption and metabolism variations, increases bioavailability and efficacy of drugs, provides good patient compliance, and enables fast drug delivery termination by removing the patch (1,2).
Diltiazem hydrochloride (diltiazem HCl), a calcium channel blocker, is widely used in the management of angina pectoris and hypertension (3). Because of its short biological half-life (3.5 h) and low oral bioavailability (40%) due to hepatic metabolism leading to high frequency drug dosing (4), the continuous delivery of diltiazem HCl is required. Therefore, development of TDD system for diltiazem HCl should be of great interest. Attempts had been made to develop diltiazem HCl TDD systems most of which based on the matrix diffusion controlled systems where the drug was dispersed in an inert polymer matrix (5–8). Rao and Diwan (5,6) formulated the ethylcellulose (EC)-polyvinyl pyrrolidone (PVP) films of diltiazem HCl for transdermal administration. According to the in vitro release and permeation studies through rat abdominal skin, the film composed of EC/PVP at ratio of 8:2 loaded with 20% w/w diltiazem HCl was selected as the polymeric film for diltiazem HCl transdermal patch. The in vivo study in rabbits revealed that this diltiazem HCl patch sustained the therapeutic activity over a study period of 24 h after transdermal administration and provided a five fold increase in the bioavailability compared to oral administration. Gupta and Mukherjee (7) reported that, based on the in vitro permeation of films loaded with 5% w/w diltiazem HCl through depilated freshly excised abdominal mouse skin, the film prepared with EC and PVP at the ratio of 2:1 should be selected for the development of TDD system of diltiazem HCl. Jain and others (8) also developed the matrix diffusion controlled TDD systems of diltiazem HCl using various combinations of hydrophobic polymers (Eudragit E100 and Eudragit L100) and hydrophilic polymers (PVP and polyethylene glycol 4000). It was stated that the drug release from polymeric matrix is governed by various factors including physicochemical properties of drug, dimensional parameters of films, polymer material and drug concentration in the film (5).
The objective of this study was to prepare and evaluate the matrix diffusion controlled system for diltiazem HCl by using hydroxypropyl methylcellulose (HPMC) and EC as hydrophilic and hydrophobic film formers, respectively. Basically, addition of plasticizer is necessary in order to produce a good appearance and desirable physical properties of the finished film. The selection of a suitable plasticizer has a profound influence on the mechanical properties (9,10). Furthermore, variations of the dimensional parameters of polymer matrix may altered moisture uptake, rate of drug release and permeation from the matrices (5,7). Therefore, effects of plasticizers (DBP and triethyl citrate [TEC]) and polymer ratios on the mechanical properties were evaluated. Effects of polymer ratios on moisture uptake, in vitro release and permeation through pig ear skin were studied. In addition, penetration enhancers may also be incorporated into the formulations in order to improve drug flux across the membranes (11,12). Influence of permeation enhancers on the in vitro permeation was thereby investigated.
MATERIALS AND METHODS
Materials
Diltiazem hydrochloride (diltiazem HCl; a gift sample from Siam Chemical Product, Bangkok, Thailand), ethylcellulose (EC; ETHOCEL® Standard 10 Premium), hydroxypropyl methylcellulose (HPMC; METHOCEL® K4M Premium EP, Dow Chemical, Midland, MI, USA), dibutyl phthalate (DBP; Merck-Schuchardt, Hohenbrunn, Germany), triethyl citrate (TEC; Fluka Chemie, Buchs, Switzerland), isopropyl myristate (IPM; S. Tong Chemicals, Bangkok, Thailand), isopropyl palmitate (IPP; Uniqema, Selango Darul Ehsan, Malaysia), N-methyl-2-pyrrolidone (NMP; ISP Pharmaceutical, USA), oleic acid (OA), polyethylene glycol 400 (PEG), propylene glycol (PG; Srichand United Dispensary, Bangkok, Thailand), Tween80 (B.L. Hua, Bangkok, Thailand).
Preparation of Films
Films composed of different ratios of HPMC and EC with or without plasticizers (DBP or TEC; Table I) were prepared by a plate casting method. HPMC and EC were weighted and dissolved in an equal volume of methylene chloride and methanol. The resultant solution was poured into a glass plate, which was then set at an ambient temperature for 24 h and subsequently oven-dried at 45 °C for 30 min to remove the residual organic solvents. In the case of diltiazem HCl films, DBP (30%) was added as a plasticizer. Diltiazem HCl (25% of dry weight of polymers) with or without enhancers (IPM, IPP, NMP, OA, PG, PEG and Tween80; 10% of dry weight of polymers) were incorporated into the polymer solution. The polymer mixture was poured into a glass plate and subsequently dried. The dry films were kept in a desiccator until used.
Table 1.
Mechanical Properties of Free Films as a Function of HPMC/EC Ratios and Plasticizer Types (DBP and TEC; n = 6)
| Formulation | Ingredients (ratio by weight) | Thickness (μm) | UTS (MPa) | % Elongation at break | Young's modulus (MPa) | |||
|---|---|---|---|---|---|---|---|---|
| HPMC | EC | DBP | TEC | |||||
| F0 | 10 | 0 | − | − | 42 ± 3 | 83.3 ± 9.9 | 38.5 ± 10.4 | 891.1 ± 47.4 |
| F1 | 10 | 0 | 3 | − | 100 ± 8 | 76.7 ± 7.8 | 107.0 ± 30.9 | 516.9 ± 77.1 |
| F2 | 8 | 2 | 3 | − | 101 ± 18 | 19.9 ± 3.4 | 17.7 ± 3.2 | 137.9 ± 25.7 |
| F3 | 6 | 4 | 3 | − | 118 ± 6 | 6.9 ± 7.4 | 22.1 ± 2.4 | 98.9 ± 14.7 |
| F4 | 4 | 6 | 3 | − | 104 ± 10 | 24.8 ± 3.4 | 27.6 ± 2.6 | 351.8 ± 34.9 |
| F5 | 8 | 2 | − | 3 | 121 ± 26 | 5.7 ± 8.2 | 33.2 ± 7.8 | 99.9 ± 23.6 |
| F6 | 6 | 4 | − | 3 | 106 ± 12 | 0.4 ± 0.3 | 29.2 ± 4.2 | 104.6 ± 12.9 |
Evaluation of Free Films
Thickness
The thickness of film specimen (rectangular shape, 0.5 × 4 cm) was measured at five different places using a dial thickness gauge (Peacock®, Labtek, USA). The average of the five values was calculated.
Mechanical Properties
Mechanical properties which are ultimate tensile strength (UTS), percent elongation at break and Young’s modulus were determined following the method modified from the ASTM standard D 882 (13,14) using an Instron 5500 Series (Instron Corporate Headquarters, MA). The cross-head speed was controlled at 10 mm/min and 1-kg-tension load cell was used. The UTS and percent elongation at break were calculated from Eqs. 1 and 2, respectively.
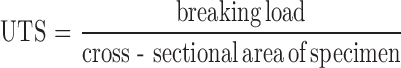 |
1 |
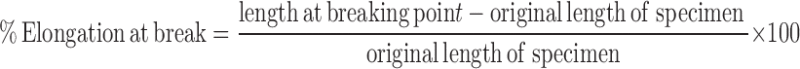 |
2 |
Evaluation of Diltiazem HCl Films
Moisture Uptake
A weighed film (1 × 1 cm) kept in a desiccator with silica gel for 24 h was taken out and transferred to a desiccator containing saturated sodium chloride solution (relative humidity 75%) at 25 °C. After equilibrium was attained, the film was taken out and weighed. Moisture uptake capacity was calculated based on the change in the weight with respect to initial weight of film.
Determination of Diltiazem HCl Content
A known weight of diltiazem HCl film was dissolved and diluted with an equal volume of methylene chloride and methanol. The diltiazem HCl content was determined by an HPLC system (CLASS-VP Software, Shimadzu, Japan) consisting of a UV detector (SPD-10A), a pump (LC-10AD), an automatic injector (SIL-10AD) and a reversed-phase column (Spherisorb ODS column, 5 μm, 250 mm length and 4.6 mm inner diameter, Waters Corporation, Milford, MA). The wave length of the UV detector was 240 nm. The mobile phase consisted of acetonitrile and phosphate buffer (pH 3.0) at a volume ratio of 1:1. The flow rate was 1 ml/min.
In Vitro Release of Diltiazem HCl
In vitro release of diltiazem HCl films was investigated by the paddle-over-disk method (apparatus 5, USP 30) (15) using dissolution tester (Erweka DT6 Dissolution tester, Erweka, Heusenstamm, Germany). Film specimens (12.57 cm2) were fixed over the disk assemblies with a pharmaceutical grade transfer adhesive (Cotran™ PGTA, No.9871, 3M Pharmaceuticals, MN). The disk assemblies were immersed in 500 ml deionized water (32 ± 1 °C). The paddle speed was set at 50 rpm. At predetermined time intervals, 5-ml medium was withdrawn and replaced with fresh medium. The concentration of diltiazem HCl was determined spectrophotometrically at 236 nm (UV-Vis spectrophotometer model V-530, Jasco, Japan).
In Vitro Permeation of Diltiazem HCl through Pig Ear Skin
Skin Preparation
Porcine ears were obtained from a local slaughter house. The ears were cleaned with water to remove bloodstains. The epidermis was prepared by soaking the ear in water at 60 °C for 45 s (16). The intact epidermis was subsequently teased off from dermis with forceps, washed with water and kept in the refrigerator at −40 °C.
The in vitro permeation of diltiazem HCl from the films through pig ear skin was conducted using the modified Franz diffusion cell with the diffusion area of 1.81 cm2. Phosphate buffer saline (PBS) pH 7.4 (14 ml) was used as receptor medium. The system was connected to a water bath maintained the temperature at 37 ± 1 °C. A thawed skin was mounted between the donor and receptor compartments with a clamp (the dermis side of skin contact with PBS) and was hydrated in PBS for 1 h. The receptor compartment was then replaced with freshly prepared receptor medium (37 ± 1 °C). The diltiazem HCl film with or without enhancer was placed over the stratum corneum side of skin and then securely clamped. At predetermined times, 1.0-ml sample was taken from the receptor compartment and equal volume of PBS was immediately added after each sampling. The concentration of diltiazem HCl was analyzed by HPLC assay as mentioned above. The cumulative amount of drug permeated was plotted against time.
Data Analysis
A cumulative amount of drug released per unit area (Q) versus square root of time (t1/2) at steady state from the polymer matrix diffusion-controlled TDD system is obtained from the following Eq. 3 (17).
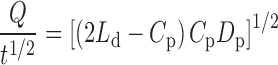 |
3 |
where Ld is the initially drug loading in polymer matrix; Cp and Dp are the solubility and diffusivity of drug in polymer matrix, respectively. Because only the drug species dissolved in the polymer can be released, Cp is thereby practically equal to the drug concentration in receptor compartment.
The steady state flux (Jss), permeability coefficient of skin (Ps), partition coefficient from TDD system onto stratum corneum (K), apparent diffusivity through skin (Dss), and lag time (Tlag) are defined by Eqs. 4 and 5.
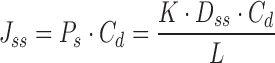 |
4 |
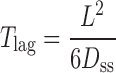 |
5 |
where, Cd is the concentration in donor phase; L is the thickness of skin.
Statistical Analysis
Each experiment was replicated at least four times. Results are expressed as the mean±SD. Kruskal–Wallis one way analysis of variance was used to test the statistical significance of differences among groups. Statistical significance in the differences of the means was determined by Mann–Whitney rank sum test.
RESULTS AND DISCUSSIONS
Evaluation of Free Films
Mechanical Properties
Selection of polymeric film as potential TDD system required knowledge of mechanical properties of the free film. Therefore, the mechanical properties of free films prepared from various ratios of HPMC and EC with and without plasticizer were characterized and are presented in Table I. The film made from HPMC alone without plasticizer (formulation F0) was very hard and brittle as expressed by the very high Young’s modulus value (891 MPa). Addition of plasticizer was needed. Hence, DBP or TEC at a concentration of 30% w/w of dry weight of polymer was incorporated as a hydrophobic plasticizer [water solubility of DBP (18) at 20 °C, 0.04%] or a hydrophilic plasticizer [water solubility of TEC (18) at 20°C, 6.90%], respectively.
DBP or TEC (30% w/w) incorporation had no significant effect on the thickness of the resulting HPMC/EC film. For 8:2 HPMC/EC films, the thickness of the film plasticized with DBP (formulation F2) was not different from that of formulation F5 plasticized with TEC (p > 0.05). Plasticization with DBP provided the higher strength but lower elongation film as compared to those of the film plasticized with TEC. Formulation F2 had the higher UTS value, lower percent elongation at break as compared to those of formulation F5 (p < 0.05). Likewise, in the case of 6:4 HPMC/EC films (formulation F3 and F6), the thickness of the films was not affected significantly by the type of plasticizer (p > 0.05). Plasticization with DBP (formulation F3) resulted in the higher strength but lower elongation (p < 0.05) as compared to those of the film plasticized with TEC (formulation F6). Plasticization results in a decrease in the inter-molecular forces between polymer chains, generally causing a decrease in the glass transition temperature and tensile strength. It is well known that different plasticizers will affect the glass transition temperature and hence the mechanical properties to a different extent (19,20). Since the major part of the films were HPMC (80% and 60%) which is hydrophilic polymer, the hydrophilic plasticizer could reduce the glass transition temperature of the films more than the hydrophobic plasticizer. Thereby, DBP was chosen for manufacturing the diltiazem HCl films.
Effect of HPMC/EC ratio
The thicknesses of formulations F1 (10:0), F2 (8:2) and F4 (4:6) were not different (p > 0.05) while that of formulation F3 (6:4) showed higher value (p < 0.05) as compared to those of F1, F2 and F4. This difference might come from the film preparation step. Since the evaporation rate of mix solvent in film casting is very fast, the glass plate should be carefully covered. However, the 2:8 and 0:10 HPMC/EC films stuck to the plate and were not strong enough for testing of mechanical properties.
Addition of EC into the HPMC film resulted in the lower UTS, percent elongation at break and Young’s modulus. The molecular structure of EC contains the long chain β-anhydro glucose units linked together with acetal linkage. This kind of structure is hydrophobic in nature. Therefore, the presence of EC might have been responsible for the lower strength and elongation when compared to HPMC alone.
The addition of EC at 60% (formulation F4) resulted in too high of Young’s modulus indicating hard film. Thus, the formulations F2 and F3 were considered to be more suitable for using as the polymeric film for diltiazem HCl. However, incorporation of diltiazem HCl might affect the films properties. The mechanical properties of films composed of diltiazem HCl should be further studied.
Evaluation of Diltiazem HCl Films
Moisture Uptake
The moisture uptakes of diltiazem HCl films as a function of HPMC/EC ratios are presented in Table II. The percentage moisture uptakes of the films were affected by the HPMC/EC ratio and are in the order of 10:0 > 8:2 > 6:4 > 4:6 > 2:8 > 0:10, respectively. This could be attributed to the higher polydispersity index (3.02) and solubility parameter (24.4 MPa1/2) of HPMC as compared to those of EC (2.96 and 20.6, respectively) (21). Thereby, it has a high affinity for water and induces higher moisture uptake as the HPMC ratio in the films increased.
Table 2.
Effect of HPMC/EC Ratios on the Percentage Moisture Uptake and the Calculated Solubility Parameter of Diltiazem HCl Films Plasticized with DBP (30%)
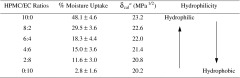
a δ cal is the solubility parameter calculated from δ cal = ∅HPMC δ HPMC + ∅EC δ EC + ∅DBP δ DBP, where ∅ is the weight fraction and δ is the solubility parameter of each ingredient.
The solubility parameters of the diltiazem HCl films as a function of HPMC/EC ratios were calculated and given in Table II. It can be seen that the calculated solubility parameter was decreased from 23.2 to 20.2 MPa1/2 when the ratio of EC increased from 0 to 10. These could be due to the hydrophilicity of the films was changed to higher hydrophobic property when the higher ratio of EC was added into the HPMC film. The water uptake or absorption behavior of the polymeric film plays an important role at the beginning stage of drug release from dosage form (22). Thus, the film with higher moisture uptake supposed to give higher drug release rate.
In Vitro Release of Diltiazem HCl Films
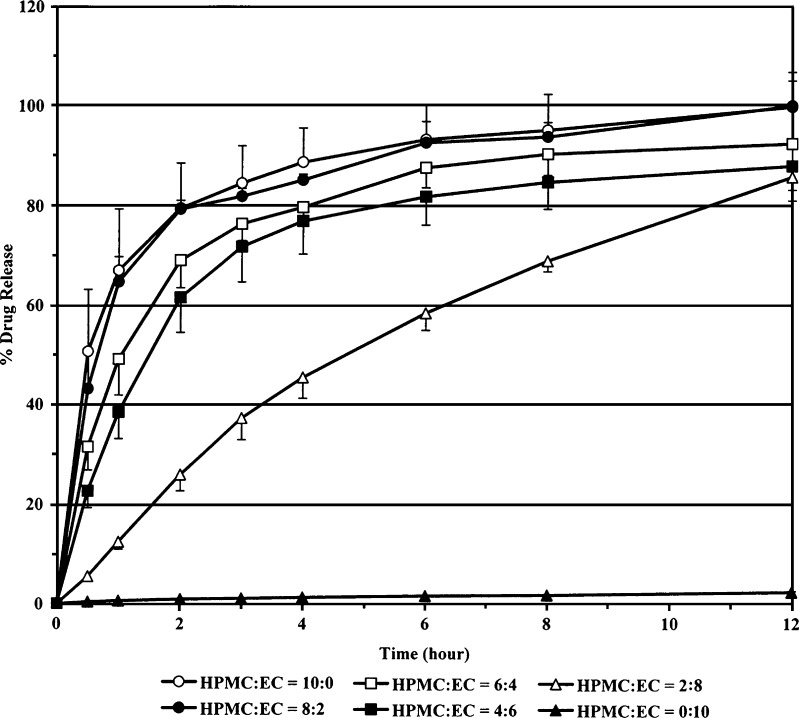
The in vitro release study was conducted to investigate the effect of polymer ratios on the release-time profiles of diltiazem HCl from the films. The typical release-time profiles are shown in Fig. 1. It clearly shows that diltiazem HCl release decreased when the ratio of EC in the film increased. The initial release rates, calculated over the study time range of 0–1 h, of diltiazem HCl films prepared with HPMC/EC ratio of 10:0, 8:2, 6:4, 4:6, 2:8 and 0:10 are approximately 67%/h, 65%/h, 49%/h, 39%/h, 13%/h, and 1%/h, respectively. The initial release rate within the first hour was found to be the same order (10:0 > 8:2 > 6:4 > 4:6 > 2:8 > 0:10) as the percentage moisture uptakes of the films (Table II). The film with higher moisture uptake property tended to give the higher initial release rate and the higher release-time profile. The presence of HPMC might have been responsible for this situation because of its hydrophilic property. As a result, diltiazem HCl films providing the highest release rate (HPMC/EC ratio of 10:0, 8:2 and 6:4) were chosen for further in vitro permeation study.
Fig. 1.
Effect of HPMC/EC ratios on percentage release-time profiles of diltiazem HCl films (n = 6)
In Vitro Permeation through Pig Ear Skin
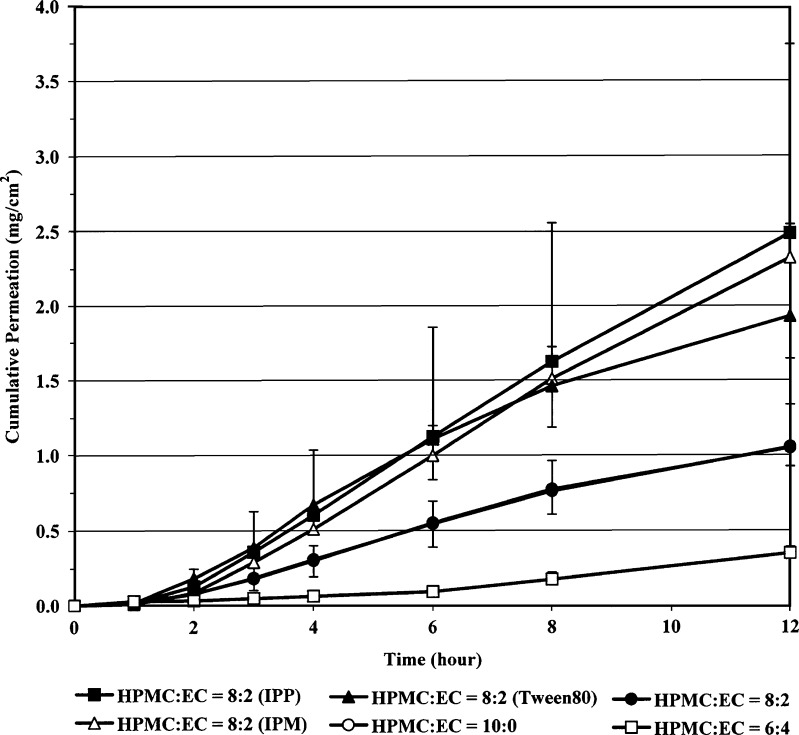
In vitro permeation through pig ear skin of diltiazem HCl films was studied by modified Franz diffusion cell using PBS pH 7.4 as a receiver medium. It should be noted that the solubility of diltiazem HCl at 32 °C in PBS pH 7.4 (461.9 mg/ml) was comparable to that in water (486.4 mg/ml). Figure 2 shows the permeation-time profiles of diltiazem HCl from the films prepared from HPMC and EC at the ratio of 10:0, 8:2 and 6:4. Table III presents the corresponding flux, lag time and coefficient of correlations according to Higuchi’s and zero order plots. The fluxes and lag times of the 10:0 and 8:2 HPMC/EC films were not different (p > 0.05), while the 6:4 HPMC/EC film gave the lower flux but longer lag time as compare those of 10:0 and 8:2 films. For the 10:0 HPMC/EC films, the correlation coefficient calculated from Higuchi’s was higher than that calculated from zero order plots (p < 0.05). However, in the case of 8:2 HPMC/EC films, the insignificant difference between the correlation coefficient values of these two plots was found (p > 0.05). Hence, the skin permeation of diltiazem HCl from the 8:2 HPMC/EC films might follow the Higuchi’s or the zero order models. Diltiazem HCl films made from HPMC/EC ratio of 8:2 was chosen to study the effect of enhancers on the in vitro permeation.
Fig. 2.
Effects of HPMC/EC ratios and enhancer types (IPM, IPP and Tween80) on cumulative permeation-time profiles of diltiazem HCl films
Table 3.
Effect of HPMC/EC Ratios on the Flux, Lag Time and Correlation Coefficient According to Zero Order and Higuchi’s Plots of Diltiazem HCl films
| HPMC/EC ratio | Flux‡ (μg/cm2 h) | Lag timea (h) | r 2 zero order plota | r 2 Higuchi’s plota |
|---|---|---|---|---|
| 10:0b | 97 ± 33 | 0.75 | 0.980 ± 0.017 | 0.997 ± 0.005 |
| 8:2b | 97 ± 9 | 0.73 | 0.985 ± 0.015 | 0.999 ± 0.011 |
| 6:4c | 34 ± 5 | 2.31 | 0.960 ± 0.031 | 0.913 ± 0.045 |
aCalculated from time range of 3–12 h
b n = 8
c n = 5
Effect of Permeation Enhancers on In Vitro Permeation through Pig Ear Skin
The corresponding flux, lag time and coefficient of correlations according to Higuchi’s and zero order plots of diltiazem HCl films prepared with 8:2 HPMC/EC with different enhancers (IPM, IPP and Tween80) are presented in Table IV. The permeation-time profiles are shown in Fig. 2. It was found that only IPP, IPM, Tween80, PEG, and NMP could significantly enhance the permeation of diltiazem HCl from the films as expressed by the higher flux values which are in the order of IPP > IPM > Tween80 > PEG > NMP > no enhancer. Interestingly, only Tween80 could shorten the lag time (0.03 h) as compared to the films containing no enhancer. Because of the higher flux value enhancement compared to other enhancers, IPM, IPP and Tween80 are the interesting permeation enhancers for diltiazem HCl films. Nevertheless, the studies in animal model must be further performed.
Table 4.
Effect of Different Enhancers on the Flux, Lag Time and Correlation Coefficients According to Zero Order and Higuchi’s Plots of Diltiazem HCl Films (HPMC/EC ratio, 8:2)
| Enhancers | Fluxa (μg/cm2.h) | Lag timea (h) | r 2 zero order plota | r 2 Higuchi’s plota |
|---|---|---|---|---|
| IPMb | 228 ± 17 | 1.67 | 0.998 ± 0.003 | 0.995 ± 0.006 |
| IPPb | 238 ± 109 | 1.39 | 0.998 ± 0.004 | 0.996 ± 0.006 |
| NMPb | 125 ± 13 | 2.93 | 0.989 ± 0.007 | 0.959 ± 0.013 |
| OAc | 90 ± 16 | 3.02 | 0.931 ± 0.037 | 0.877 ± 0.048 |
| PEGb | 144 ± 59 | 2.63 | 0.978 ± 0.034 | 0.940 ± 0.050 |
| PGc | 89 ± 19 | 2.82 | 0.939 ± 0.038 | 0.889 ± 0.057 |
| Tween80b | 169 ± 23 | 0.03 | 0.971 ± 0.023 | 0.995 ± 0.011 |
aCalculated from time range of 3–12 h
b n = 4
c n = 5
The coefficient of correlations of the 8:2 HPMC/EC film containing IPM, IPP and Tween80 calculated from Higuchi’s and zero order plots were not different (p > 0.05). The skin permeation of diltiazem HCl from the films containing these enhancers could possibly follow the Higuchi’s or the zero order models.
The corresponding release and permeation parameters (Q/t1/2, Ld, Cp, Dp, Jss, Tlag, and Dss) of diltiazem HCl films (8:2 HPMC/EC) with or without enhancers (IPM, IPP and Tween80) were calculated and summarized in Table V. Regarding to diltiazem HCl film containing no enhancer, addition of IPM and IPP resulted in increasing of drug diffusivity in the films (Dp) while decreasing of the apparent diffusivity in the skin (Dss). IPM addition could also improve the Q/t1/2 although the loading doses (Ld) were decreased. According to Eq. 4, these phenomena imply that the partition coefficients were increased. Hence, IPM and IPP may have the same mode of action in enhancing the permeation of diltiazem HCl from HPMC/EC films by improving the diffusivity of drug in the films and the partitioning between the films and the stratum corneum. Another enhancing effect of IPP may come from decreasing of the drug solubility in the film (Cp).
Table 5.
Effect of Enhancers on the Calculated Release and Permeation Parameters of Diltiazem HCl Films (HPMC/EC ratio, 8:2)
| Enhancers | Q/t 1/2 (mg/cm2 h1/2) | L d (mg/ml) | C p (mg/ml) | D p (cm2/h) | J ss (mg/cm2 h) | T lag (h) | D ss (cm2/h) |
|---|---|---|---|---|---|---|---|
| − | 3.328 | 25.355 | 0.085 | 2.568 | 0.097 | 0.73 | 0.009 |
| IPM | 3.500 | 18.700 | 0.086 | 3.833 | 0.228 | 1.67 | 0.004 |
| IPP | 2.980 | 15.550 | 0.072 | 3.949 | 0.238 | 1.39 | 0.005 |
| Tween80 | 3.000 | 25.851 | 0.075 | 2.327 | 0.169 | 0.03 | 0.229 |
In contrast to IPM and IPP, addition of Tween80 led to slightly decrease in the Dp, while the Dss was greatly increased for approximately 25 times as compared to the film containing no enhancer. The Q/t1/2 was also decreased. Therefore, the major effect of Tween80 in enhancing the flux of diltiazem HCl may be attributed to the improvement of drug diffusivity through the skin. Another effect could be due to the decrease in the solubility of drug in the film. There are two possible mechanisms by which the rate of transport is enhanced using nonionic surfactants. The surfactants may initially penetrate into the intercellular region of the stratum corneum, increase fluidity and eventually solubilize and extract lipid components. Secondly, penetration of the surfactant into the intercellular matrix followed by interaction and binding with keratin filaments may results in a disruption within the corneocyte (23,24). Tween80 is thought to enhance diltiazem HCl permeation via both the lipophilic and the hydrophilic molecular mechanisms, and to disrupt the lipid arrangements in the stratum corneum and to increase the water content of the proteins in the barrier. It contains the ethylene oxide and a long hydrocarbon chain. This structure imparts both lipophilic and hydrophilic characteristics to enhancer, allowing it to partition between lipophilic mortar substance and the hydrophilic protein domains. It may interact with the polar head groups of the lipids and the modification of H-bonding and ionic forces may occur.
In order to predict the permeation through human skin of diltiazem HCl from the polymeric films, the target flux which is the flux value sufficient to attain therapeutically effective plasma concentrations was calculated based on the pharmacokinetic parameters of diltiazem HCl (therapeutic level of diltiazem [Css], 50 ng/ml; total clearance in human [Clt], 60 l/h; standard human body weight [BW], 60 kg) (3) as follow (25).
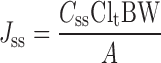 |
6 |
The maximum surface area of transdermal patch (A) is supposed to be 12.57 cm2. Therefore, the target flux for diltiazem HCl is 238.7 μg/cm2.h. It was found that the fluxes of diltiazem HCl films prepared with 8:2 HPMC/EC using IPM and IPP as plasticizers closely reached the target flux and might be sufficient to achieve the therapeutic concentration.
CONCLUSIONS
The ratio of hydrophilic and hydrophobic polymeric film formers affected the mechanical properties, percentage moisture uptake, rate of drug release and consequently the permeation of the diltiazem HCl films. Addition of permeation enhancer could profoundly improve the in vitro permeation of diltiazem HCl through pig ear skin. The results indicate that the polymeric film composed of HPMC and EC at the ratio of 8:2, DBP as a plasticizer and IPM, IPP or Tween80 as the permeation enhancer was suitable for developing a transdermal drug delivery system for diltiazem HCl. Further study respect to the in vitro permeation and in vivo performance after transdermal administration of such a diltiazem HCl patch in a suitable animal model is required.
Acknowledgements
The authors are grateful to the Graduate School, Chulalongkorn University, for its financial support of this study.
References
- 1.Chien Y. W. Advances in transdermal systemic medication. In: Chien Y. W., editor. Transdermal Controlled Systemic Medications. New York: Marcel Dekker; 1987. pp. 1–22. [Google Scholar]
- 2.Barry B. W. Novel mechanisms and devices to enable successful transdermal drug delivery. Eur. J. Pharm. Sci. 2001;14:101–114. doi: 10.1016/S0928-0987(01)00167-1. [DOI] [PubMed] [Google Scholar]
- 3.McEvoy G. K. Diltiazem hydrochloride. In: McEvoy G. K., Snow E. K., Kester L., Miller J., Welsh O. H., Litvak K., editors. AHFS Drug Information. Bethesda: American Society of Health-System Pharmacists; 2005. pp. 1835–1842. [Google Scholar]
- 4.Mazzo D. J., Obetz C. L., Shuster J. Diltiazem Hydrochloride. Analytical Profiles of Drug Substances, Vol. 23 . California: Academic Press; 1994. pp. 53–98. [Google Scholar]
- 5.Rao P. R., Diwan P. V. Formulation and in vitro evaluation of polymeric films of diltiazem hydrochloride and indomethacin for transdermal administration. Drug Dev. Ind. Pharm. 1998;24(4):327–336. doi: 10.3109/03639049809085627. [DOI] [PubMed] [Google Scholar]
- 6.Rao P. R., Diwan P. V. Comparative in vivo evaluation of diltiazem hydrochloride following oral and transdermal administration in rabbits. Indian J. Pharmacol. 1999;31:294–298. [Google Scholar]
- 7.Gupta R., Mukherjee B. Development and in vitro evaluation of diltiazem hydrochloride transdermal patches based on povidone–ethylcellulose matrices. Drug Dev. Ind. Pharm. 2003;29(1):1–7. doi: 10.1081/DDC-120016678. [DOI] [PubMed] [Google Scholar]
- 8.Jain S. K., Chourasia M. K., Sabitha M., Jain R., Jain A. K., Ashawat M. Development and characterization of transdermal drug delivery systems for diltiazem hydrochloride. Drug Delivery. 2003;10:169–177. doi: 10.1080/713840400. [DOI] [PubMed] [Google Scholar]
- 9.Rao P. R., Diwan P. V. Permeability studies of cellulose acetate free films for transdermal use: Influence of plasticizers. Pharm. Acta Helv. 1997;72:47–51. doi: 10.1016/S0031-6865(96)00060-X. [DOI] [PubMed] [Google Scholar]
- 10.Lin S., Cheng K., Run-Chu L. Organic esters of plasticizers affecting the water absorption, adhesive property, glass transition temperature and plasticizer permanence of Eudragit acrylic films. J. Control Release. 2000;68:343–350. doi: 10.1016/S0168-3659(00)00259-5. [DOI] [PubMed] [Google Scholar]
- 11.Leichtnam M. L., Rolland H., Wüthrich P., Guy R. H. Identification of penetration enhancers for testosterone transdermal delivery from spray formulations. J. Control Release. 2006;113:57–62. doi: 10.1016/j.jconrel.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 12.Nolana L. M. A., Corisha J., Corriganb O. I., Fitzpatrick D. Combined effects of iontophoretic and chemical enhancement on drug delivery II. Transport across human and murine skin. Int. J. Pharm. 2007;341(1–2):114–124. doi: 10.1016/j.ijpharm.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 13.The ASTM Committee on Standards . Standard test method for tensile properties of thin plastic sheeting (ASTM D 882–95a) In: The ASTM Committee on Standards, editor. An American National Standard. Philadelphia: The American Society for Testing and Materials; 1996. pp. 182–190. [Google Scholar]
- 14.Okhamafe A. O., York P. Studies of interaction phenomena in aqueous-based film coatings containing soluble additives using thermal analysis techniques. J. Pharm. Sci. 1987;77:438–443. doi: 10.1002/jps.2600770517. [DOI] [PubMed] [Google Scholar]
- 15.The United States Pharmacopeial Convention . The United States pharmacopeia. 30. Rockville: The United States Pharmacopeial Convention; 2007. [Google Scholar]
- 16.Bhatia K. S., Gao S., Singh J. Effect of penetration enhancers and iontophoresis on the FT-IR spectroscopy and LHRH permeability through porcine skin. J. Control Release. 1997;47:81–89. doi: 10.1016/S0168-3659(96)01618-5. [DOI] [PubMed] [Google Scholar]
- 17.Chien Y. W. Transdermal drug delivery and delivery systems. In: Chien Y. W., editor. Novel Drug Delivery Systems. 2. New York: Marcel Dekker; 1992. pp. 301–380. [Google Scholar]
- 18.Frohoff-Hülsmann M. A., Schmitz A., Lippold B. C. Aqueous ethyl cellulose dispersions containing plasticizers of different water solubility and hydroxypropyl methylcellulose as coating material for diffusion pellets: I. Drug release rates from coated pellets. Int. J. Pharm. 1999;177:69–82. doi: 10.1016/S0378-5173(98)00327-5. [DOI] [PubMed] [Google Scholar]
- 19.Bodmeier R., Paeratakul O. Mechanical properties of dry and wet cellulosic and acrylic films prepared from aqueous colloidal polymer dispersions used in the coating of solid dosage forms. Pharm Res. 1994;11(6):882–888. doi: 10.1023/A:1018942127524. [DOI] [PubMed] [Google Scholar]
- 20.Saettone M. F., Perini G., Rijli P., Rodriguez L., Cini M. Effect of different polymer–plasticizer combinations on ‘in vitro’ release of theophylline from coated pellets. Int. J. Pharm. 1995;126:83–88. doi: 10.1016/0378-5173(95)04099-4. [DOI] [Google Scholar]
- 21.Sakellariou P., Rowe R. C. The morphology of blends of ethylcellulose with hydroxypropyl methylcellulose as used in film coating. Int. J. Pharm. 1995;125:289–296. doi: 10.1016/0378-5173(95)00147-B. [DOI] [Google Scholar]
- 22.Golomb G., Fisher P., Rahamim E. The relationship between drug release rate, particle size and swelling of silicone matrices. J Control Release. 1990;12:121–132. doi: 10.1016/0168-3659(90)90088-B. [DOI] [Google Scholar]
- 23.Breuer M. M. The interaction between surfactants and keratinous tissues. J. Soc. Cosmet. 1979;30:41–64. [Google Scholar]
- 24.Walter K. A., Walker M., Olejnik O. Non-ionic surfactant effects on hairless mouse skin permeability characteristics. J Pharm Pharmacol. 1987;40:525–529. doi: 10.1111/j.2042-7158.1988.tb05295.x. [DOI] [PubMed] [Google Scholar]
- 25.Guy R. H., Hadgraft J. Selection of drug candidates for transdermal drug delivery. In: Hadgraft J., Guy R. H., editors. Transdermal Drug Delivery. New York: Marcel Dekker; 1989. pp. 59–81. [Google Scholar]