Abstract
The purpose of this research was to mask the bitter taste of Diphenhydramine Hydrochloride (DPH) using cation exchange resins. Indion 234 and Tulsion 343 that contained crosslinked polyacrylic backbone were used. The drug resin complexes (DRC) were prepared by batch process by taking drug: resin ratios 1:1, 1:2, and 1:3. The optimum drug: resin ratio and the time required for maximum complexation was determined. The drug resinates were evaluated for the drug content, taste, micromeritic properties drug release and X-ray diffraction (PXRD). Effervescent and dispersible tablets were developed from optimum drug: resin ratios of 1:2 and 1:1. The formulations were evaluated for uniformity of dispersion, disintegration time, and in vitro dissolution. The X-ray diffraction study confirmed the monomolecularity of entrapped drug in the resin beads. The taste evaluation depicted the successful taste masking of DPH with drug resin complexes. The drug release of 95% in 15 min was observed for effervescent and dispersible tablets.
Key words: diphenhydramine hydrochloride, ion exchange resins
INTRODUCTION
Administration of a oral drug with bitter taste and acceptable level of palatability has always been a in developing a formulation for pediatric and geriatric purpose. Taste masking has therefore become a potential tool to improve patient compliance (1). Taste masking can be done by using flavors, sweeteners, and amino acids (2–5); and by using various techniques such as lipophilic vehicles, Coating, inclusion complexation, ion exchange, effervescent agents, rheological modification, solid dispersion system, group alteration and prodrug approach, freeze drying Process, wet spherical agglomeration technique and continuous multipurpose melt technology (6–16).
Ion exchange resins (crosslinked water-insoluble polymer carrying ionizable functional groups) have been used as a drug carrier in pharmaceutical dosage form for taste masking and controlled release dosage forms (17–22). The sustained-release of drug can be obtained by using a mixture of uncoated and semi-permeable coated resinates (23–26). The drug resinates can also be used as reservoir that causes a change of the drug release in hydrophilic polymer tablets (27).
An attempt was made to mask the taste by using Indion 234 and Tulsion 343 weak acid cation exchange resins with a crosslinked polyacrylic backbone and carboxylic functional groups (30, 31) and to formulate and evaluate effervescent and dispersible oral formulations of DPH. Diphenhydramine Hydrochloride (DPH HCl) an antihistaminic drug with bitter taste and low dose (25 mg) (28, 29). Drug resin complexes are formed due to ion exchange reactions. The drug is diffused from such resinates in gastric fluid by exchanging ions.
MATERIAL AND METHODS
Materials
Diphenhydramine Hydrochloride, Indion 234 and Tulsion 343, Micro crystalline cellulose (MCC; Avicel PH 101), Polyvinlypyrolidone (PVP) K-30 was obtained as gift samples from Supriya Chemicals Limited, Mumbai, India, Ion Exchange Limited, Mumbai, India, Thermax Limited, Pune, India, Libra chemicals, Mumbai, India. Other AR grade chemicals were purchased.
Purification of Ion Exchange Resins
Indion 234 and Tulsion 343 were washed with distilled water. The wet resins were activated by 0.1 M HCl 300 ml followed by washing with distilled water and were dried overnight in hot air oven at 50°C and were stored in an air tight glass vial (32).
Preparation of Drug Resin complexes (DRC)
DPH was mixed separately with both the resins in the drug: resin ratio of 1:1, 1:2 and 1:3 (21). Two hundred ml of distilled water was added to the mixtures and stirred continuously on magnetic stirrer (Whirlmatic-mega, Spectralab, Mumbai, India), for 24 hours until the equilibrium was attained. Aliquots from the reaction mixture were withdrawn and filtered through Whatman filter paper no. 41 after every hour and were analyzed after appropriate dilution at 258 nm by UV/VIS spectrophotometer (JASCO-V530, Tokyo, Japan). The process was continued till the concentration values of two consecutive aliquots were almost constant. The readings were taken in triplicate. The resultant complex was filtered through Whatman filter paper no. 41, washed with water to remove the unreacted drug and oven dried at 50°C for 1 h and stored in air tight glass vial till the further use.
Evaluation of Drug–Resin Complex
Drug Content
The DPH content was determined by eluting the 100 mg of DRC with continuous stirring in 100 ml 1N HCl for 4 h to ensure complete elution (21). The solution was filtered. After suitable dilution the drug content was determined at 258 nm by UV/VIS spectrophotometer the readings were taken in triplicate.
Taste Evaluation
The optimum drug resinate complexes and DPH were subjected to taste evaluation (33). Taste evaluation was performed by testing the samples on 20 male volunteers in the age group 22–28 years. Each volunteer held DRC equivalent to 25 mg in the mouth for 15 s and then spit out. The scale used was (a) o-Tasteless, (b) 1-Slightly bitter, (c) 2-Bitter, and (d) 3-Very bitter.
Drug Release
The drug release studies were performed by USP Type II Tablet dissolution apparatus (TDT-082-Electrolab, Mumbai, India). DRC equivalent to a 25 mg of DPH was taken in 900 ml of 0.1 N HCl and Phosphate buffer pH 6.8. The temperature and speed of the apparatus were maintained at 37 ± 0.5°C and 100 rpm respectively. Aliquots were withdrawn after every 5 for 45 min and were filtered with Whatman filter paper no. 41 and were analyzed at 258 nm by UV/VIS spectrophotometer. The readings were taken in triplicate.
X-Ray Diffraction of Complex
The powder X-ray diffraction patterns (PXRD) of DPH, resins, DRC and the physical mixtures of drug and resins were recorded using Philips PW 1729 X-ray diffractometer. Samples were irradiated with monochromatized Cu Kαr radiation (1.542 A°) and analyzed between 50° and 2° (2θ). The voltage and current applied were 30 Kv and 30 mA, respectively, while the range of 5 × 103 cycle/s and chart speed of 100 mm/2θ were used.
Micromeritics
The DRCs evaluated for bulk density, tap density, compressibility, angle of repose and flow rate (34).
Bulk density
Ten grams of DRCs were placed into 100 ml measuring cylinder and volume noted. The bulk density was calculated by the following equation:
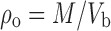 |
1 |
Where, ρo = bulk density,
- M
mass of the DRC and
- Vb
Volume of DRC
The experiment was performed in triplicate.
-
2.
Tap density
Ten grams of DRCs were placed into 100 ml measuring cylinder. The cylinder was then subjected to a fixed number of taps (100). The final volume was recorded and the tap density was calculated by the following equation:
 |
2 |
Where, ρt = tapped density,
- M
mass of the DRC and
- Vt
Volume of DRC on tapping
The experiment was performed in triplicate.
-
3.
Compressibility
Compressibility of the drug was found out using the following equation:
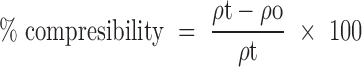 |
3 |
Where, ρt is the tapped density and
ρo is the bulk density.
-
4.
Angle of repose and flow rate
The angle of repose was determined by fixed funnel method. Five g of DRC was poured through the funnel (Borosil) and the pile diameter, height and flow rate were measured. The flow rate was measured as g/s.
Angle of repose:
 |
4 |
Where, h = height of the pile and
- D
diameter of the pile.
The experiments were performed in triplicate.
Preparation of Effervescent and Dispersible Tablet
The effervescent and dispersible tablets were prepared from the DRCs. The formula is presented in Table I. All the ingredients for effervescent tablets (E1 and E2) and dispersible tablets (D1 and D2) were sieved through mesh no. 100. The DRCs equivalent to 25 mg of DPH were mixed with the excipients of effervescent and dispersible tablets. The mixtures were kneaded with binder solution of PVP K30 until a moist consistency was achieved and the granules were obtained by sieving through the mesh no. 16 which were oven dried at 50°C for 1 h. The remaining super disintegrant, diluent, sweetener, lubricants and flavor were added to the dried granules that were compressed on rotary tablet machine (Karnavati-Minipress D-II Link, Mumbai, India.) using standard flat 8 mm punch. The die cavity was filled manually, after discarding initial 20 tablets the final 100 tablets were collected. The hardness was between 3–5 kg/cm2, friability was less then 1% and weight variation was in ±7.5% of the average weight of tablets.
Table I.
Composition for Effervescent and Dispersible Tablets
| Ingredients | Batch Code | |||
|---|---|---|---|---|
| E1 (mg) | E2 (mg) | D2 (mg) | D2 (mg) | |
| DRC equivalent to 25 mg with Tulsion 343 | 31.3 | – | 31.3 | – |
| DRC equivalent to 25 mg with Indion 234 | – | 35 | – | 35 |
| Sodium bicarbonate | 31 | 31 | – | – |
| Tartaric acid | 20 | 20 | – | – |
| Citric acid anhydrous | 9 | 9 | – | – |
| Dicalcium phosphate | 92.4 | 88.4 | – | – |
| Talc | 2.5 | 2.5 | – | – |
| Avicel 101 | – | – | 57.4 | 57.5 |
| Primojel | – | – | 8 | 8 |
| Mannitol | – | – | 141.5 | 137.7 |
| Aerosil | – | – | 1 | 1 |
| Sodium Saccharin | 1.5 | 1.5 | 0.3 | 0.3 |
| Poly vinyl pyrollidone (PVP K 30) | 8 | 8 | 8 | 8 |
| Magnesium stearate | 2.5 | 2.5 | 2.5 | 2.5 |
| Orange flavor | 1.8 | 2.1 | q.s. | q.s. |
| Total | 200 | 200 | 250 | 250 |
Evaluation of Effervescent and Dispersible Tablets
Uniformity of Dispersion
The dispersible tablets were subjected to uniformity of dispersion test. Two tablets were placed in 100 ml of distilled water with gentle stirring until completely dispersed that was passed through a sieve screen with a nominal mesh aperture of 710 μm (sieve no. 22).
Disintegration Time
Tablets were placed in digital disintegration test apparatus (Electrolab USP ED-2L, Mumbai, India.) at 25°C, after the gas around the tablet seizes, the content should either dissolve or disperse with no agglomerates of particles remaining, six tablets from each batch were tested and the readings were taken in triplicate.
In Vitro Dissolution Studies
Six tablets from each batch were studied for in vitro dissolution using USP type II dissolution testing apparatus. The media used were 0.1 N HCl and Phosphate buffer pH 6.8 (900 ml). The speed and temperature of the apparatus were maintained at 100 rpm and 37 ± 0.5°C respectively. The test was carried out for 45 min. The samples were withdrawn at predetermined time intervals and filtered with Whatman filter paper no. 41 and the absorbance was measured at 258 nm by UV/VIS spectrophotometer.
RESULTS AND DISCUSSION
Drug Content
As presented in Table II the complexation of drug with Indion 234 and Tulsion 343 gave efficient binding. The stirring time for all subsequent Complexation process was fixed to 4 h as the concentration values of aliquots between 4 and 5 h showed no significant change. Complexation of drug with Indion 234 and Tulsion 343 were studied for optimum drug: resin ratio for maximum loading. The values of percentage drug bound to the resins when complexed with drug: resin ratio 1:3 in Indion 234 and in Tulsion 1:2 and 1:3 did not indicate significant difference. The ratio of 1:2 for Indion 234 and 1:1 for Tulsion 343 were selected as the optimum and used for the further studies.
Table II.
Selection of Optimum Drug to Resin Ratio
| Resin | Resin to Drug Ratio | Percent Drug Bounda |
|---|---|---|
| Indion 234 | 1:1 | 64.29 ± 0.17 |
| 1:2 | 71.91 ± 0.53 | |
| 1:3 | 72.22 ± 0.13 | |
| Tulsion 343 | 1:1 | 80.00 ± 0.10 |
| 1:2 | 81.00 ± 0.58 | |
| 1:3 | 83.50 ± 0.29 |
aMean ± SD for n = 3
Taste Evaluation
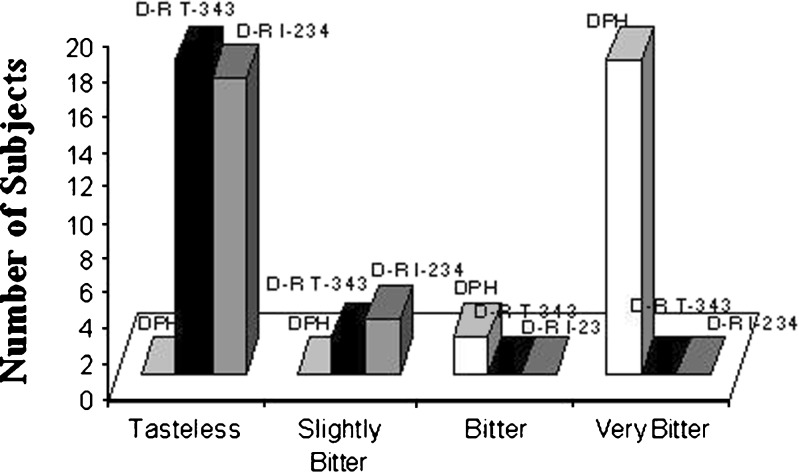
Figures 1 demonstrates the results of taste evaluation. The drug and the DRCs of optimized ratio were subjected to taste evaluation. Nineteen volunteers reported that the drug resinate prepared with Tulsion 343 (D-RT 343) was tasteless whereas 18 volunteers reported that the drug resinate prepared with Indion 234 (D-RI 234) was tasteless. The taste masking of DPH was excellent with both Indion 234 and Tulsion 343.
Fig. 1.
Taste evaluation of Diphenhydramine Hydrochloride, Drug-Resinate with Tulsion 343 and Drug-Resinate with Indion 234
Micromeritics
Table III presents the results of micromeritic studies for DRCs. The bulk and tapped densities of Indion 234 and Tulsion 343 were 0.625 ± 0.12, 0.787 ± 0.09; and 0.638 ± 0.18, 0.778 ± 0.09 g/ml respectively. The compressibility between 18–21% indicates fair compressibility. The angle of repose below 30° indicates good flow potential.
Table III.
Micromeritics Studies on DRCs
| Test | Results | |
|---|---|---|
| D-R with I-234 | D-R with T-343 | |
| Bulk density | 0.625 ± 0.12 g/ml | 0.638 ± 0.18 g/ml |
| Tap density | 0.778 ± 0.18 g/ml | 0.787 ± 0.09 g/ml |
| Compressibility | 19.66% | 18.93% |
| Angle of repose | 28.710 ± 1.10 | 26.940 ± 1.50 |
| Flow rate | 0.254 ± 0.57 g/s | 0.323 ± 0.83 g/s |
Mean ± SD for n = 3
Release Profile
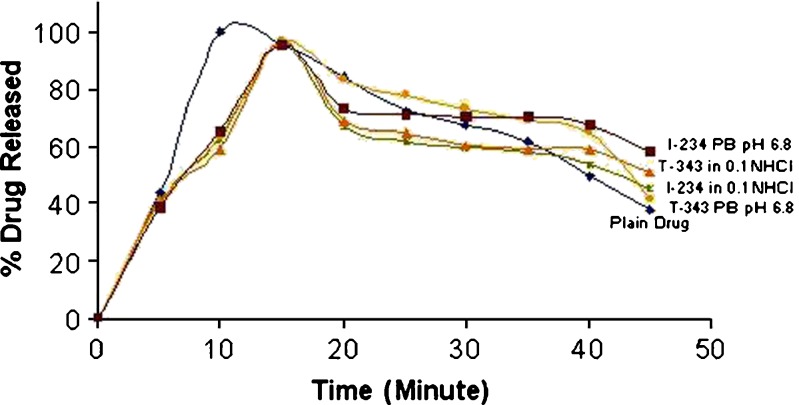
Figures 2 demonstrates the drug release studies of the DRCs. Highest release of 95.66% and 96.44% in 0.1 N HCl and 95.27% and 96.46% in Phosphate buffer pH 6.8 was achieved in 15 min. for drug resinate complex of DPH with Indion 234 and Tulsion 343 respectively. The results were compared with the plain conventional tablet of DPH where 99.7% drug release was observed in 10 min. The retarded drug release from the DRC can be attributed to the bound form of the drug. The ion exchange mechanism was the prime cause of drug release. By virtue of ion exchange resins, the benefits achieved of taste masking and better acceptance by patients might outweigh a slight delay in drug release.
Fig. 2.
Drug release from drug resinate complex in 0.1 N HCl and Phosphate Buffer pH 6.8 (PB pH 6.8) with Indion 234 and Tulsion 343
X-Ray Diffraction Studies
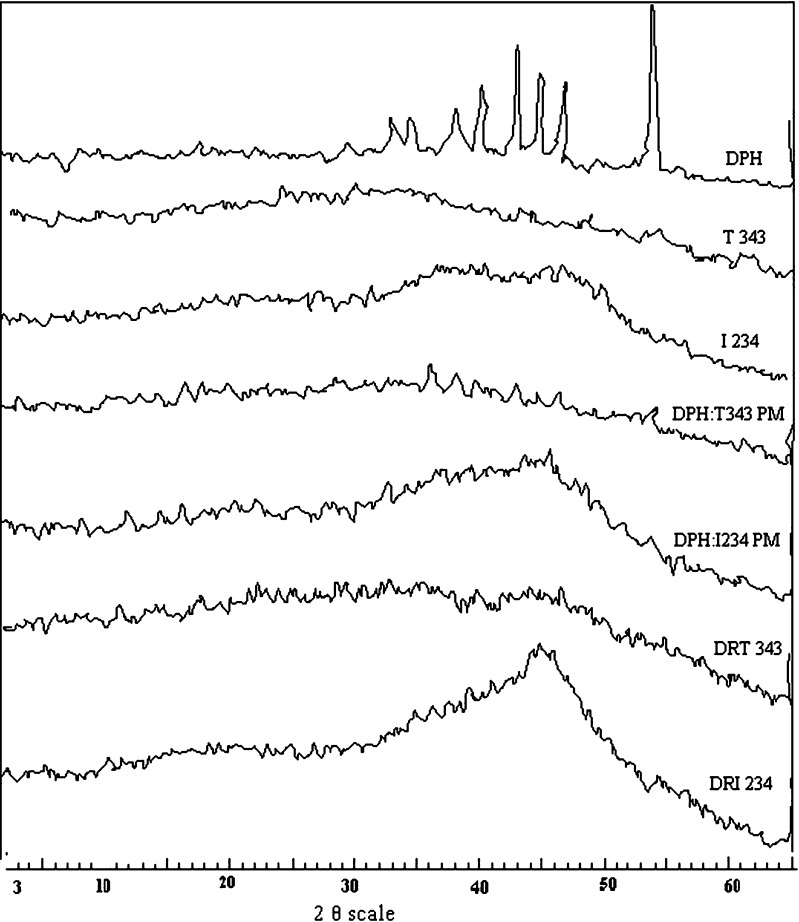
Figures 3 demonstrate the results of X-ray diffraction pattern (PXRD) of the drug (DPH), resins (T 343 and I 234), physical mixture (PM) and drug resinates (DR T343 and DR I234). DPH is crystalline in nature and 2θ were seen at 39, 42, 44, 46 and 53. The hollow pattern indicates that Indion 234 and Tulsion 343 are amorphous resins. PXRD of the physical mixture of drug and resins showed a sum of several sharp peaks owing to the crystalline nature of DPH and some diffused peak of the amorphous resin. The molecular state of the drug prepared as DRC showed a hollow diffused pattern and the absence of drug peaks. This finding confirmed that the entrapped drug was dispersed monomolecularly in the resin bead. In the case of physical mixture of drug and resins, drug molecules are outside the resin bead.
Fig. 3.
X- ray diffractograms
The effervescent and dispersible tablets of the DRCs were developed and evaluated for Uniformity of dispersion, Disintegration time and Drug release. The results are presented in Table IV. As seen from the results the tablets were disintegrated before 3 min, passed the uniformity of dispersion test and the drug release for both the formulation was above 90% in 15 min (Figs. 4 and 5).
Table IV.
Evaluation of Effervescent and Dispersible Tablets
| Parameter | Batch Code | |||
|---|---|---|---|---|
| E1 (T-343) | E2 (I-234) | D1 (T-343) | D2(I-234) | |
| Uniformity of dispersion | – | – | Passes through mesh no. 22 | Passes through mesh no. 22 |
| Disintegration time | 35 ± 2 s | 40 ± 2 s | 54 ± 2 s | 59 ± 2 s |
| Drug release | 95.07% (0.1 N HCl) and 94.48% (phosphate buffer pH 6.8) of drug dissolved in 15 min | 95.27% (0.1 N HCl) and 94.78% (phosphate buffer pH 6.8) of drug dissolved in 15 min | 95.25% (0.1 N HCl) and 94.46% (phosphate buffer pH 6.8) of drug dissolved in 15 min | 95.27% (0.1 N HCl) and 94.78% (phosphate buffer pH 6.8) of drug dissolved in 15 min |
Fig. 4.
Drug release from Effervescent Tablets of T-343 and I-234 in 0.1 N HCl and Phosphate Buffer pH 6.8 (PB pH 6.8). Mean ± SD for n = 3
Fig. 5.
Drug release from Dispersible Tablets of T-343 and I-234 in 0.1 N HCl and Phosphate Buffer pH 6.8 (PB pH 6.8). Mean ± SD for n = 3
CONCLUSION
The efficient taste masking can be obtained from drug–resin complexes that can be formulated as effervescent and dispersible tablets for better patient compliance.
Acknowledgement
The authors are thankful to Supriya Chemical Limited (Mumbai, India), Ion Exchange Limited (Mumbai, India), Thermax Limited (Pune, India) and Libra chemicals (Mumbai, India) for supplying gift sample of Diphenhydramine Hydrochloride, Indion resins, Tulsion resins, micro crystalline cellulose (Avicel PH 101) and polyvinlypyrolidone K-30. The authors also acknowledge to Faculty of Physics, Pune University (Pune, India) for the facility of PXRD.
References
- 1.Sohi H., Sultana Y., Khar R. K. Taste masking technologies in oral pharmaceuticals: recent developments and approaches. Drug Dev. Ind. Pharm. 2004;30:429–448. doi: 10.1081/DDC-120037477. [DOI] [PubMed] [Google Scholar]
- 2.T. Yakoo, and H. Hirohata. Composition for oral cavity. JP05, 000,931, January 8, 1993.
- 3.R. C. Fiusz. Taste masking of pharmaceutical floss with phenol, US Patent5, 028,632, July 02, 1992.
- 4.F. Wehling, and S. Schedule. Effervescent dosage form with microparticles. US Patent5, 178,878, January 12, 1993.
- 5.G. A. Depalomo. Composition based on ibuprofen, for oral dosage usage. Eur. Patent Appl. EP0560207, September 15, 1993.
- 6.W. G. Gowan, and R. D. Bruce. Aliphatic ester as a solventless coating pharmaceuticals. Can. Pat. Appl. CA2082137, November 4, 1993.
- 7.J. Block, A. Cassiere, and M. O. Christens. Galenicals Form. Ger. Offen DE3900811, July19, 1990.
- 8.R. W. Shen. Taste masking of ibuprofen by fluid bed coating. US Patent5, 55,152, September 3, 1996.
- 9.M. J. Poelinger, N. Rupp, and R, Buecheler M. D. Taste masked pharmaceuticals composition. Eur. Pat. Appl. EP0551820, July 21, 1993.
- 10.Lorenzo-Lamosa M. L., Kuna M., Vila Jato J. Development of microencapsulated form of Cefuroxime axetil using pH sensitive acrylic polymers. J. Microencapsule. 1997;14:660–616. doi: 10.3109/02652049709006813. [DOI] [PubMed] [Google Scholar]
- 11.Y. Ikezuki. Raw material for food and medicine and its production. JP02, 291,244, December 3, 1990.
- 12.Manek S. P., Kamat V. S. Evaluation of Indion CRP-244 and CRP-254 as sustained release and taste masking agents. Indian J. Pharm. Sci. 1981;43:209–212. [Google Scholar]
- 13.S. Niazi, and A. Shemesh. Chewing gum containing a medicament and taste masker. US Patent04, 639,368, January 27, 1987.
- 14.C. M. Blasé, and M. N. Shah. Taste masked pharmaceutical suspensions for pharmaceutical actives. Eur. Pat. Appl. EP0556057, August 18, 1993.
- 15.N. C. Diamini, and J. H. Tsau. Taste masked compositions. Eur. Pat. Appl. EP0212641, March 4, 1987.
- 16.Drugs and pharmaceuticals sciences. In M. J. Rathbone, J. Hadgraft, and M. S. Roberts (eds.), Drug Delivery Technology, Marcel Dekker, Inc., New York, 2003, pp. 191–202.
- 17.Borodkin S., Sundberg D. P. Polycarboxylic acid ion-exchange resin adsorbates for taste coverage in chewable tablets. J. Pharm. Sci. 1971;60:1523–1527. doi: 10.1002/jps.2600601018. [DOI] [PubMed] [Google Scholar]
- 18.Sriwongjanya M., Bodmeier R. Entrapment of drug loaded ion-exchange particles within polymer microparticles. Int. J. Pharm. 1997;158:29–38. doi: 10.1016/S0378-5173(97)00212-3. [DOI] [Google Scholar]
- 19.Cuna M., Jato J. L. V., Torres D. Controlled- release liquid suspensions based on ion- exchange particles within acrylic microcapsules. Int. J. Pharm. 2000;199:151–158. doi: 10.1016/S0378-5173(00)00379-3. [DOI] [PubMed] [Google Scholar]
- 20.Ichikawa H., Fujioka K., Adeyeye M. C., Fukamori Y. Use of ion-exchange resins to prepare 100µm- seized microcapsules with prolonged drug-release by the Wurster process. Int. J. Pharm. 2001;216:67–76. doi: 10.1016/S0378-5173(01)00573-7. [DOI] [PubMed] [Google Scholar]
- 21.Borodkin S. Ion exchange resins and sustained release. In: Swarbrick J., Boylan J. C., editors. Encyclopedia of pharmaceutical technology. Vol 8. Inc., New York: Marcel Dekker; 1993. pp. 203–216. [Google Scholar]
- 22.Notari R. E. Biopharmaceutics and clinical pharmaceutics, 4th ed. Inc., New York and Basel: Marcel Dekker; 1987. pp. 130–218. [Google Scholar]
- 23.Raghunathan Y., Amsel L., Hinsvark O. Sustained-released drug delivery system I: coated ion-exchange resins system for phenylpropanolamine and other drugs. J. Pharm. Sci. 1981;70:379–384. doi: 10.1002/jps.2600700409. [DOI] [PubMed] [Google Scholar]
- 24.Ogger K. E., Noory C., Gabay J., Shah V. P., Skelly J. P. Dissolution profiles of resins-based oral suspensions. Pharm. Technol. 1991;9:84–91. [Google Scholar]
- 25.Burke G. M., Mendes R. W., Jambhekar S. S. Investigation of the application of ion exchange resins as a sustained release drug delivery system for propranolol hydrochloride. Drug Dev. Ind. Pharm. 1986;12:713–732. doi: 10.3109/03639048609043487. [DOI] [Google Scholar]
- 26.Irwin W. J., Belaid K. A., Alpar H. O. Drug-delivery by ion-exchange. Part III: interaction of ester pro-drugs of propranolol with cationic exchange resins. Drug Dev. Ind. Pharm. 1987;13:2047–2066. doi: 10.3109/03639048709068706. [DOI] [Google Scholar]
- 27.Sriwongjanya M., Bodmeier R. Effect of ion exchange resins on the drug release form matrix tablets. Eur. J. Pharm. Biopharm. 1998;46:321–327. doi: 10.1016/S0939-6411(98)00056-3. [DOI] [PubMed] [Google Scholar]
- 28.British Pharmacopeia, Vol. II. Great Britain Published by The Stationary Office under license from the Controller of Her Majesty Stationary Office for the Department of Health on behalf of the Health Ministers, 2001:594–595.
- 29.The United Stated Pharmacopeia XX III/ National Formulary XVIII. U.S. Pharmacopoeial convention, Rockville, MD, 1980:532–533.
- 30.Ion exchange resins used in pharmaceutical industry. Available at: www.indion.com Accessed on June 30, 2006.
- 31.Ion exchange resins used in pharmaceutical industry. Available at: www.thermaxindia.com Accessed on June 30, 2006.
- 32.Akkaramongakolporn P., Terada K., Yonemochi E. Molecular properties of propranolol hydrochloride prepared as drug-resin complexes. Drug Dev. Ind. Pharm. 2001;27:359–364. doi: 10.1081/DDC-100103736. [DOI] [PubMed] [Google Scholar]
- 33.Pisal S., Zainuddin R., Nalawade P., Mahadik K., Kadam S. Drug release of polyethylene-glycol-treated ciprofloxacin-Indion 234 complexes. AAPS PharmSciTech. 2002;5:4. doi: 10.1208/pt050464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lachman L., Lieberman H. A., Kanig J. The Theory and Practice of Industrial Pharmacy, 3rd edition. Bombay: Varghese publishing house; 1987. [Google Scholar]