INTRODUCTION
Drug delivery across buccal mucosa offers a variety of advantages and is a good alternative to the conventional mode of drug administration such as the peroral and parenteral routes (1). The buccal mucosa has a stratified (multilayered) squamous epithelium, which forms the rate-limiting barrier to absorption across this membrane (2). The intercellular space is filled with about 50% polar lipids such as phospholipids and glycosylceramides (3). Based on the biochemical composition and structure of the buccal mucosa, drugs can permeate by the lipoidal and/or aqueous pathways (4). The lipoidal pathway encompasses both transcellular transport and transport through the intercellular lipids by partitioning. The water molecules entrapped by the polar head groups of intercellular lipids result in an aqueous pathway (5).
Drug lipophilicity is a very important descriptor governing permeation across a biological membrane (6). Lipophilicity is generally expressed quantitatively as the log10 of the partitioning of a neutral drug species between n-octanol and water (logP) and is the most widely used predictor for drug permeation. However, logP does not encompass the extent of ionization of ionizable molecules. As 95% of all drugs have ionizable groups, distribution coefficient (logD) that considers the extent of ionization as well as intrinsic lipophilicity may be a better descriptor that reflects the partitioning of a mixture of drug species as well as the actual drug lipophilicty at any given pH (7).
In this study, it was hypothesized that logD describes buccal permeation better than logP. Therefore, the aim of this study was to assess and compare the correlation of logP and logD with buccal permeability. Toward this purpose, permeabilities of 12 different drugs across porcine buccal mucosa were determined.
MATERIALS AND METHODS
Materials
Nimesulide was purchased from Alexis Biochemicals (Lausen, Switzerland). The remaining drugs (lidocaine HCl, propranolol HCl, caffeine, antipyrine, verapamil HCl, diltiazem HCl, amitriptyline HCl, naproxen, warfarin, metoprolol, and pindolol) were purchased from Sigma Chemicals (St. Louis, MO). HPLC grade solvents were purchased from Fisher Scientific (Bridgewater, NJ). All other reagents were of analytical grade and used as received. Deionized water was used in preparing the buffers and drug donor solutions. Phosphate buffers (pH 6.8 and 7.4) were used in the permeation studies.
Drugs and Descriptors
In this study 12 drugs were used as model compounds, with diverse structures and were shown to be stable under the experimental conditions. These included three acidic (naproxen, warfarin, nimesulide), seven basic (lidocaine, propranolol, verapamil, diltiazem, amitriptyline, metoprolol, pindolol), and two neutral (caffeine, antipyrine) molecules. LogP and logD6.8 (log10 of distribution coefficient at pH 6.8, which corresponds to salivary pH) values were obtained either from literature or were estimated using ChemIDplus (8–15). Drugs with a logD6.8 value of greater than −1.0 and lower than +1.0 were selected as model compounds to minimize the effects of unstirred water layer and drug accumulation in the membrane. In addition, a linear relationship between permeability and lipophilicity has been reported in this range (2).
Tissue Preparation
Porcine buccal tissue was obtained from a local ranch immediately after the pigs were slaughtered. Tissues were processed and prepared according to a previously described procedure (4). Briefly, the tissues were stored in phosphate buffer, pH 7.4 during transport and processing. Buccal epithelium was separated from the underlying connective tissue by trimming the latter to a thickness of 500 ± 50 μm. This thickness corresponds to buccal epithelial thickness, which contributes to the diffusional barrier (16). Permeation studies were initiated within 2 h of isolating the buccal tissue.
Permeation Studies
In vitro permeation studies were conducted at 37°C using horizontal, water-jacketed, side-by-side cells with a diffusional area of 0.68 cm2 (PermeGear Inc., Riegelsville, PA, USA). The tissue was mounted between donor and receiver chambers followed by equilibration with phosphate buffer solution (pH 6.8 in the donor chamber and pH 7.4 in the receiver chamber) for 30 min. A pH of 6.8 was used in the donor as it represents a mean value of the physiological oral cavity pH (2). The receiver pH was fixed at 7.4 to simulate in vivo plasma pH. After the equilibration period, the donor contents were replaced with drug solution in phosphate buffer, pH 6.8. Samples were withdrawn from the receiver chamber at different time points over a period of 5 to 8 hours depending on the drug and analyzed using HPLC. The drug concentration in the donor varied from one drug to the other. For drugs with poor solubility (diltiazem, amitriptyline, nimesulide, naproxen, warfarin), saturated donor drug solutions were used. In case of the remaining drugs, the initial drug concentration was 1.0 mg/ml (verapamil), 5.0 mg/ml (lidocaine, propranolol, caffeine, antipyrine), 7.5 mg/ml (metoprolol) and 10 mg/ml (pindolol). All permeation studies were performed in triplicate. The donor and receiver contents were stirred with magnetic stir bars to minimize unstirred water layers in the vicinity of the mucosal barrier.
The apparent permeability coefficient, Kp (centimeters per second) was calculated from the permeation studies using the following equation:
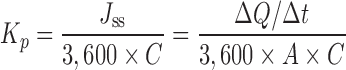 |
where, Jss is the steady-state flux (μg h−1 cm−2), ΔQ/Δt is the steady-state rate of appearance of the drug in the receiver chamber (μg/h), A is the diffusional area (cm2), and C is the initial drug donor concentration (μg/ml).
HPLC Analysis
HPLC methods were developed for all the drugs (Table I). The analytical methods were specific for the drug i.e., the drug peak was separated from peaks produced by the solvent and other impurities eluting out of the buccal tissue. The apparatus used for the HPLC analysis was a Waters system (Massachusetts, USA) equipped with a Waters 510 pump, Waters 717 plus autosampler, and a Shimadzu SPD-10A UV-Vis detector (Kyoto, Japan). A Zorbax SB-C18 column (4.6 × 150 mm) (Agilent technologies, Santa Clara, CA, USA) was used. The column was maintained at room temperature (25 ± 2°C). The mobile phase contained a mixture of 50 mM monobasic potassium phosphate (adjusted to pH 3.0 with phosphoric acid), acetonitrile, and methanol. The flow rate was set at 1.0 ml/min.
Table I.
Chromatographic Conditions for the Analysis of Various Drugs
| Drug | Mobile phasea | λ (nm) | Retention time (min) |
|---|---|---|---|
| Nimesulide | 60 A + 40 B | 300 | 5.1 |
| Naproxen | 60 A + 40 B | 224 | 5.7 |
| Warfarin | 60 A + 40 B | 210 | 7.2 |
| Caffeine | 20 A + 80 B | 274 | 4.5 |
| Antipyrine | 25 A + 75 B | 254 | 4.9 |
| Lidocaine | 25 A + 75 B | 224 | 4.8 |
| Propranolol | 40 A + 60 B | 224 | 5.7 |
| Metoprolol | 30 A + 70 B | 224 | 5.0 |
| Pindolol | 20 A + 80 B | 263 | 5.3 |
| Verapamil | 50 A + 50 B | 235 | 5.3 |
| Diltiazem | 50 A + 50 B | 237 | 3.7 |
| Amitriptyline | 50 A + 50 B | 252 | 6.6 |
aMobile phase consisted of a mixture of A acetonitrile + methanol (50/50) and B 50 mM KH2PO4 (pH 3.0) buffer
RESULTS AND DISCUSSION
Buccal Permeability of Model Drugs
The apparent permeability coefficients (Kp) for the 12 drugs covering a wide range of logP and logD6.8 values were experimentally determined. Of these drugs, nimesulide exhibited the highest permeability (Kp = 30 × 10−6 cm/s) while pindolol had the lowest permeability (Kp = 0.12 × 10−6 cm/s; Table II). The greater permeability of nimesulide can be attributed to the favorable logD6.8 of nimesulide. In contrast, pindolol had the lowest logD6.8 among the different compounds studied, which resulted in the lowest permeability.
Table II.
LogP, LogD 6.8 and Buccal Permeabilities of the Various Drugs Used in this Study
| Drug | LogP | LogD 6.8 | K p (×106; in cm/s)a | References |
|---|---|---|---|---|
| Lidocaine | 2.10 | 1.20 | 17.0 ± 1.8 | (9, 10) |
| Propranolol | 3.48 | 1.20 | 14.0 ± 1.7 | (8, 11) |
| Verapamil | 3.79 | 1.72 | 25.1 ± 3.6 | (8) |
| Diltiazem | 2.79 | 1.04 | 7.3 ± 0.7 | (8) |
| Amitriptyline | 5.04 | 1.64 | 13.4 ± 1.8 | (8, 12) |
| Metoprololb | 1.95 | −0.56 | 1.3 ± 0.2 | (13, 15) |
| Pindololb | 1.83 | −0.90 | 0.12 ± 0.01 | (13, 15) |
| Nimesulide | 1.94 | 1.69 | 30.0 ± 6.0 | (8) |
| Naproxenc | 3.18 | 0.60 | 3.8 ± 0.3 | (8) |
| Warfarinc | 2.60 | 0.70 | 1.6 ± 0.2 | (8) |
| Caffeined | −0.07 | −0.07 | 9.0 ± 0.5 | (8) |
| Antipyrined | 0.39 | 0.39 | 5.4 ± 0.9 | (14) |
Correlation of logP and logD with Buccal Permeability
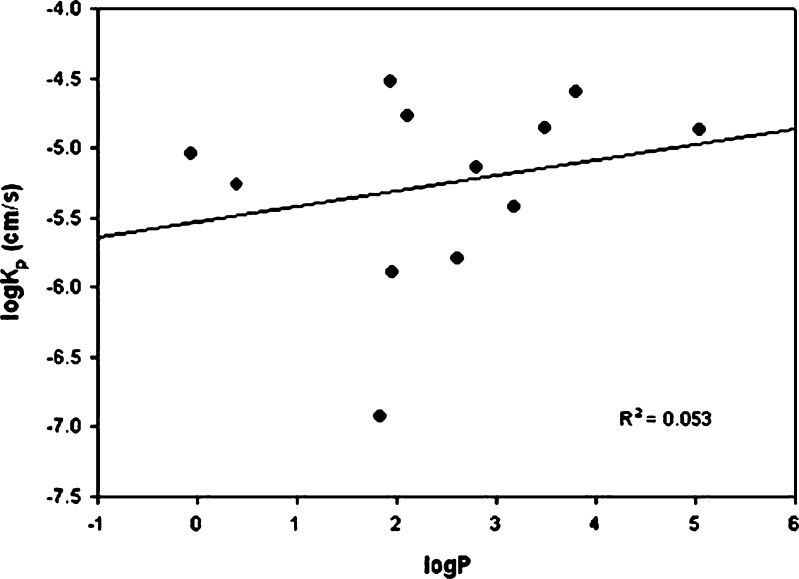
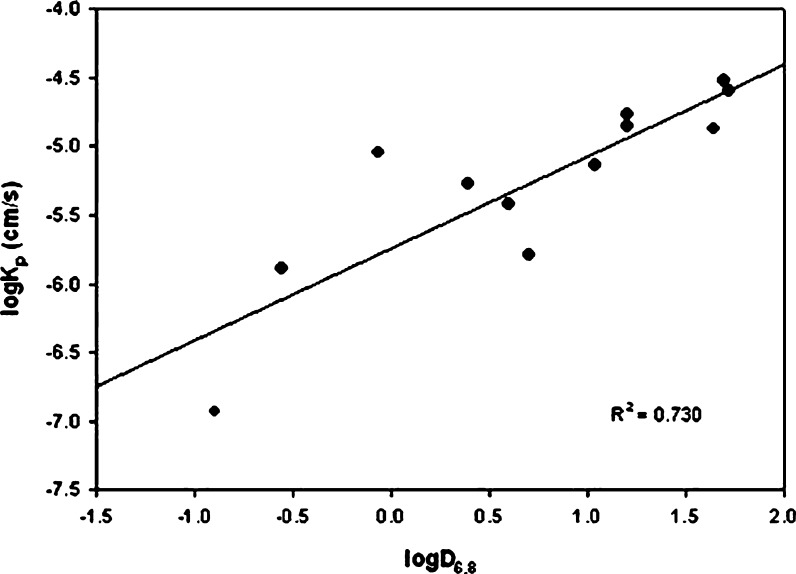
A plot of logKp and logP (R2 = 0.053; Fig. 1) as shown in Fig. 1, showed poor correlation. However, the correlation improved significantly when logD6.8 was plotted instead of logP (R2 = 0.730; Fig. 2). Therefore, when drug ionization was considered along with the intrinsic lipophilicity, the correlation improved.
Fig. 1.
Correlation between logK p and logP
Fig. 2.
Correlation between logK p and logD 6.8
Previous studies in our laboratory have demonstrated a good buccal permeability for ionized acidic (nimesulide) and basic (bupivacaine) drugs (17). It was observed that the permeability of the ionized drug form was only four-fold lower than the unionized drug permeability. It is likely that the presence of about 35% of phospholipids in the buccal mucosa accounts for a better permeation of ionized drug forms than expected (18). This is possibly due to significant contribution by the polar pathway that exists in this tissue to the total drug transport. Another possible reason for good ionized species permeability is a greater partitioning of the polar molecules into the phospholipid-filled buccal barrier. This observation is in agreement with the study by Avdeef et al., which demonstrated that the presence of phospholipid in n-dodecane PAMPA models resulted in better permeability predictions for polar molecules (19). Based on these results, the partitioning of both the ionized and unionized forms needs to be considered for a reliable estimate of buccal permeability. Also, a recent study has proposed the use of logD instead of logP in permeability screening techniques such as the ‘Rule of 5’ (7). In addition, other studies have demonstrated better correlation of caco-2 permeability with logD in comparison to logP (20).
SUMMARY AND CONCLUSIONS
LogP and logD are widely considered as effective molecular descriptors capable of predicting drug permeability and absorption. This prompted a study that explores the correlation between these descriptors and buccal permeability. The results demonstrated that logD6.8 gives better correlation with buccal permeability than logP. Therefore, a predictive model for buccal permeability should include logD6.8 rather than logP as a lipophilicity marker. The involvement of additional descriptors that might contribute to buccal permeability is being currently investigated.
References
- 1.Rathbone M., Drummond B., Tucker I. The oral cavity as a site for systemic drug delivery. Adv. Drug Del. Rev. 1994;13:1–22. doi: 10.1016/0169-409X(94)90024-8. [DOI] [Google Scholar]
- 2.Le Brun P. P. H., Fox P. L. A., de Vries M. E., Bodde H. E. In vitro penetration of some b-adrenoreceptor blocking drugs through porcine buccal mucosa. Int. J. Pharm. 1989;49:141–145. doi: 10.1016/0378-5173(89)90113-0. [DOI] [Google Scholar]
- 3.Squier C. A., Cox P., Wertz P. W. Lipid content and water permeability of skin and oral mucosa. J. Invest. Dermatol. 1991;96(1):123–126. doi: 10.1111/1523-1747.ep12515931. [DOI] [PubMed] [Google Scholar]
- 4.Shojaei A. H., Berner B., Li X. Transbuccal delivery of acyclovir: I. In vitro determination of routes of buccal transport. Pharm. Res. 1998;15(8):1182–1188. doi: 10.1023/A:1011927521627. [DOI] [PubMed] [Google Scholar]
- 5.Wertz P. W., Squier C. A. Cellular and molecular basis of barrier function in oral epithelium. Crit. Rev. Ther. Drug Carrier Syst. 1991;8(3):237–269. [PubMed] [Google Scholar]
- 6.Malkia A., Murtomaki L., Urtti A., Kontturi K. Drug permeation in biomembranes: in vitro and in silico prediction and influence of physicochemical properties. Eur. J. Pharm. Sci. 2004;23(1):13–47. doi: 10.1016/j.ejps.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 7.Bhal S. K., Kassam K., Peirson I. G., Pearl G. M. The rule of five revisited: applying log d in place of log p in drug-likeness filters. Mol. Pharm. 2007;4(4):556–560. doi: 10.1021/mp0700209. [DOI] [PubMed] [Google Scholar]
- 8.ChemIDplus. Available at: http://chem.sis.nlm.nih.gov/chemidplus/, accessed August 10, 2006.
- 9.Fredholt K., Larsen D. H., Larsen C. Modification of in vitro drug release rate from oily parenteral depots using a formulation approach. Eur. J. Pharm. Sci. 2000;11(3):231–237. doi: 10.1016/S0928-0987(00)00104-4. [DOI] [PubMed] [Google Scholar]
- 10.Hadgraft J., Valenta C. pH, pK(a) and dermal delivery. Int. J. Pharm. 2000;200(2):243–247. doi: 10.1016/S0378-5173(00)00402-6. [DOI] [PubMed] [Google Scholar]
- 11.Hendriksen B. A., Felix M. V., Bolger M. B. The composite solubility versus pH profile and its role in intestinal absorption prediction. AAPS PharmSci. 2003;5(1):E4. doi: 10.1208/ps050104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Manzo R. H., Olivera M. E., Amidon G. L., Shah V. P., Dressman J. B., Barends D. M. Biowaiver monographs for immediate release solid oral dosage forms: amitriptyline hydrochloride. J. Pharm. Sci. 2006;95(5):966–973. doi: 10.1002/jps.20615. [DOI] [PubMed] [Google Scholar]
- 13.Avdeef A., Artursson P., Neuhoff S., Lazorova L., Grasjo J., Tavelin S. Caco-2 permeability of weakly basic drugs predicted with the double-sink PAMPA pKa(flux) method. Eur. J. Pharm Sci. 2005;24(4):333–349. doi: 10.1016/j.ejps.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 14.Higaki K., Asai M., Suyama T., Nakayama K., Ogawara K., Kimura T. Estimation of intradermal disposition kinetics of drugs: II. Factors determining penetration of drugs from viable skin to muscular layer. Int. J. Pharm. 2002;239(1–2):129–141. doi: 10.1016/S0378-5173(02)00084-4. [DOI] [PubMed] [Google Scholar]
- 15.Schoenwald R. D., Huang H. S. Corneal penetration behavior of beta-blocking agents I: Physiochemical factors. J. Pharm. Sci. 1983;72(11):1266–1272. doi: 10.1002/jps.2600721108. [DOI] [PubMed] [Google Scholar]
- 16.Sudhakar Y., Kuotsu K., Bandyopadhyay A. K. Buccal bioadhesive drug delivery—a promising option for orally less efficient drugs. J. Control. Release. 2006;114(1):15–40. doi: 10.1016/j.jconrel.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 17.Kokate, A., Singh, P., Li, X., Jasti, B. Effect of thermodynamic activities of the unionized and ionized species on drug flux across buccal mucosa. J. Pharm. Sci. (2008), in press. [DOI] [PubMed]
- 18.Wertz P. W., Swartzendruber D. C., Squier C. A. Regional variation in the structure and permeability of oral mucosa and skin. Adv. Drug Deliv. Rev. 1993;12:1–12. doi: 10.1016/0169-409X(93)90037-5. [DOI] [Google Scholar]
- 19.Avdeef A., Tsinman O. PAMPA-A drug absorption in vitro model 13. Chemical selectivity due to membrane hydrogen bonding: In combo comparisons of HDM-, DOPC-, and DS-PAMPA models. Eur. J. Pharm. Sci. 2006;28:43–50. doi: 10.1016/j.ejps.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 20.Hou T. J., Zhang W., Xia K., Qiao X. B., Xu X. J. ADME evaluation in drug discovery. 5. Correlation of Caco-2 permeation with simple molecular properties. J. Chem. Inf. Comput. Sci. 2004;44(5):1585–1600. doi: 10.1021/ci049884m. [DOI] [PubMed] [Google Scholar]