Abstract
The purpose of this study was to prepare wax-incorporated pectin-based emulsion gel beads using a modified emulsion-gelation method. The waxes in pectin–olive oil mixtures containing a model drug, metronidazole, were hot-melted, homogenized and then extruded into calcium chloride solution. The beads formed were separated, washed with distilled water and dried for 12 h. The influence of various types and amounts of wax on floating and drug release behavior of emulsion gel beads of calcium pectinate was investigated. The drug-loaded gel beads were found to float on simulated gastric fluid if the sufficient amount of oil was used. Incorporation of wax into the emulsion gel beads affected the drug release. Water-soluble wax (i.e. polyethylene glycol) increased the drug release while other water-insoluble waxes (i.e. glyceryl monostearate, stearyl alcohol, carnauba wax, spermaceti wax and white wax) significantly retarded the drug release. Different waxes had a slight effect on the drug release. However, the increased amount of incorporated wax in the formulations significantly sustained the drug release while the beads remained floating. The results suggest that wax-incorporated emulsion gel beads could be used as a carrier for intragastric floating drug delivery.
Key words: calcium pectinate, emulsion gel beads, floating, intragastric drug delivery, pectin, wax
INTRODUCTION
Oral administration is the most preferable route of drug delivery to the systemic circulation. A problem frequently encountered with conventional sustained release dosage forms is the inability to increase the residence time in an absorption window, i.e. stomach and proximal portion of the small intestine (1). Retention of drug delivery systems in the stomach prolongs the overall gastrointestinal transit time, thereby resulting in improved oral bioavailability of poorly soluble drugs (2). These systems are also appropriate for drugs which are locally active to the gastric mucosa in the stomach, for example, antibiotic administration for Helicobacter pylori eradication in the treatment of peptic ulcer disease (3). The local delivery of antibiotic by a means of intragastric floating drug delivery may overcome the inefficiency of conventional oral administration of the antibiotic, e.g. low concentration of the antibiotic reaching the bacteria under the mucosa and short residence time of antibiotic in the stomach.
Methods for prolonging the gastric retention of drugs or dosage forms have been attempted based on different mechanisms such as floating, expansion/plug type, high density, or adhesion to mucosa (2,4). The floating system in particular has been extensively researched, mainly because the floating system does not adversely affect the motility of the GI tract. Immediate floating can be achieved if the density of the device is low at the beginning, for example, being provided by the entrapment of air (5) or by the incorporation of low density materials such as oils (6) and foam powders (7).
Calcium pectinate is a result of complexation of the polygalacturonate sequences by calcium ions (8), known to be insoluble and resistant to acidic media. Calcium pectinate gel (CaPG) beads has been used as a vehicle for controlled release of drugs (9,10) and for targeting drugs to the colon (11–13). The benefits include cheap and abundant sources, excellent biocompatibility, and total degradation without hazardous by-products (14). Recently, CaPG beads have been used as a floating drug delivery system, e.g. oil-entrapped CaPG beads (15,16) and CaPG beads containing carbonate salts (17,18). The morphology, floating properties, and drug release behavior of floating CaPG beads were studied. The effect of various release modification methods on drug release including the use of additives in the starting solution prior to bead formation, hardening with glutaraldehyde, and coating with polymer has been also investigated.
The purpose of the present study was to investigate the influence of incorporated wax, which prepared by hot-melt extrusion along with ionotropic gelation, on the drug release behavior of the drug-loaded emulsion gel beads of calcium pectinate. Metronidazole, an antibiotic used for eradication of H. pylori, was used as a model drug for intragastric floating drug delivery system.
MATERIALS AND METHODS
Materials
Low methoxy pectin with a degree of esterification of 28% and a degree of amidation of 20% (GENU pectin type LM-104 AS-FS) was the generous gift of CP Kelco (Lille Skensved, Denmark). Metronidazole (i.e. MZ), glyceryl monostearate (i.e. GMS; P.C. Drug Center, Bangkok, Thailand), calcium chloride, polyethylene glycol 10000 (i.e. PEG; E. Merck, Darmstadt, Germany), olive oil, carnauba wax, spermaceti wax, white wax and stearyl alcohol (Nam Siang Co., Ltd., Bangkok, Thailand) were used as received. All other chemicals were of standard pharmaceutical grade.
Preparation of Conventional CaPG Beads and Emulsion Gel Beads
Conventional CaPG beads were prepared by the ionotropic gelation method that was previously described (9,10). Briefly, 4 g of pectin were dispersed in water with agitation, and then 4 g of MZ (80-mesh sieved) were dispersed in pectin solution to make a 100-g solution. The dispersion was then extruded through a plastic needle into 0.34 M calcium chloride which was gentle stirred at room temperature. The gel beads formed were allowed to stand in the solution for 20 min before being separated and washed with distilled water. The beads were dried at 37°C for 12 h.
The emulsion gel beads of calcium pectinate were prepared by emulsion-gelation method as previously reported (15,16). Four grams of pectin were dissolved in water with agitation. Thirty grams of olive oil were added to the mixture of pectin and MZ (1:1) to make 100-g mixtures and homogenized using a homogenizer (Type X1020, Ystral GmbH, Dottingen, Germany), at 3,000 rpm for 5 min. The emulsion gel beads were treated in the same manner as conventional CaPG beads.
Preparation of Wax-incorporated Emulsion Gel Beads
Various amounts of different waxes (i.e. PEG, GMS, white wax, carnauba wax, spermaceti wax and stearyl alcohol) were melted in water bath at 60–85°C, depending on the melting ranges of the waxes used. The molten wax was dispersed in the homogenized emulsion mixture of pectin, oil and MZ which already heated to same temperature, and then mixed until the homogenous mixture was obtained. The hot-melted mixture was extruded into 0.34 M calcium chloride (cooled at 5°C). The wax-incorporated emulsion gel beads obtained were treated in the same manner as conventional CaPG beads. The formulations studied are shown in Table I.
Table I.
Formulation, Shape and Mean Diameter of the MZ-Loaded Conventional Beads, Emulsion Gel Beads and Wax-Incorporated Emulsion Gel Beads Made of Calcium Pectinate
| Formulation and designation | Composition (g /100 g of mixture) | Shape | Mean diameter, mm±SD (n = 50) | Buoyancya (n = 20) | |||
|---|---|---|---|---|---|---|---|
| Pectin | MZ | Olive oil | Wax | ||||
| Conventional beads | |||||||
| CaPG beads | 4 | 4 | 0 | 0 | Oval | 2.45 ± 0.14 | S |
| Emulsion gel beads | |||||||
| EB/20 | 4 | 4 | 20 | 0 | Spherical | 2.21 ± 0.11 | S |
| EB/30 | 4 | 4 | 30 | 0 | Spherical | 2.25 ± 0.05 | F |
| Wax-incorporated emulsion gel beads | |||||||
| Polyethylene glycol 10000 (melting range = 53–55°C) | |||||||
| PEG4/20 | 4 | 4 | 20 | 4 | Spherical | 2.34 ± 0.09 | F |
| Glyceryl monostearate (melting range = 58–63°C) | |||||||
| GMS4/20 | 4 | 4 | 20 | 4 | Spherical | 2.35 ± 0.21 | F |
| GMS4/30 | 4 | 4 | 30 | 4 | Spherical | 2.44 ± 0.08 | F |
| GMS12/30 | 4 | 4 | 30 | 12 | Spherical | 2.36 ± 0.15 | F |
| Stearyl alcohol (melting range = 55–57°C) | |||||||
| SA4/20 | 4 | 4 | 20 | 4 | Spherical | 2.33 ± 0.14 | F |
| SA4/30 | 4 | 4 | 30 | 4 | Spherical | 2.41 ± 0.42 | F |
| SA12/30 | 4 | 4 | 30 | 4 | Spherical | 2.23 ± 0.08 | F |
| Carnauba wax (melting range = 78–80°C) | |||||||
| CW4/20 | 4 | 4 | 20 | 4 | Spherical | 2.35 ± 0.20 | S |
| CW4/30 | 4 | 4 | 30 | 4 | Spherical | 2.46 ± 0.11 | F |
| CW12/30 | 4 | 4 | 30 | 12 | Spherical | 2.41 ± 0.14 | F |
| CW16/30 | 4 | 4 | 30 | 16 | Spherical | 2.40 ± 0.07 | F |
| Spermaceti wax (melting range = 43–53°C) | |||||||
| SW4/20 | 4 | 4 | 20 | 4 | Drop-like | 2.34 ± 0.17 | S |
| SW4/30 | 4 | 4 | 30 | 4 | Drop-like | 2.45 ± 0.07 | F |
| SW12/30 | 4 | 4 | 30 | 12 | Drop-like | 2.37 ± 0.10 | F |
| SW16/30 | 4 | 4 | 30 | 16 | Drop-like | 2.30 ± 0.20 | F |
| White wax (melting range = 62–65°C) | |||||||
| WW4/20 | 4 | 4 | 30 | 4 | Spherical | 2.29 ± 0.18 | S |
| WW4/30 | 4 | 4 | 30 | 4 | Spherical | 2.38 ± 0.07 | F |
| WW12/30 | 4 | 4 | 30 | 12 | Spherical | 2.25 ± 0.12 | F |
| WW12/30 | 4 | 4 | 30 | 16 | Spherical | 2.20 ± 0.11 | F |
MZ metronidazole
aBuoyancy test in simulated gastric fluid, and S sink, F float (immediately, and still afloat for at least 24 h).
Study of Particle Size and Morphology of Gel Beads
The mean diameter of 50 dried beads was determined by optical microscopy (BH-2, Olympus, Japan). The microscope eyepiece was fitted with a micrometer by which the size of the beads could be determined.
Morphological examination of the surface and internal structure of the dried beads was carried out using a scanning electron microscope (Model Maxim-2000, CamScan Analytical, Cambridge, England) equipped with secondary electron detector at an accelerating voltage of 15 keV. The samples were coated with gold to a thickness of about 30 nm in a vacuum evaporator. The internal structure of the beads was examined by cutting them in half with a steel blade.
Buoyancy of Gel Beads
The gel bead samples (n = 20) were placed in the Erlenmeyer flask filled with 50 ml of simulated gastric fluid USP without pepsin (SGF) test solution. The flask was shaken in a shaking incubator. The shaking speed was 100 rpm and the temperature was maintained at 37°C. Their buoyancy was observed for 24 h. The preparation was considered to have buoyancy in the test solution only when all of the gel beads floated in it (3,16).
Determination of Drug Release
The in vitro release of MZ from the different formulations was examined using a USP dissolution apparatus 1 (Erweka, Germany) with 1000 ml of SGF (pH 1.2) and the basket rotation at 100 rpm. The temperature was controlled at 37 ± 0.1°C. Samples were taken at appropriate time intervals and assayed spectrophotometrically (UV-spectrophotometer, Hitachi U-2000, Japan) at 277 nm (15,16). All dissolution runs were performed in triplicate.
RESULTS AND DISCUSSION
Preparation and Morphology of Gel Beads
The emulsion gel beads containing pectin could be prepared by emulsion-gelation method as described in previous reports (15,16). Pectin helped to emulsify the mixture of aqueous drug solution and oil phases during the homogenization process. It is probably due to the surface active ability of pectin to reduce the interfacial tension between an oil phase and a water phase (19), or steric and mechanical stabilization mechanisms similar to other polysaccharides such as cellulose, guar gum and locust bean gum (20). To investigate the effect of selected waxes (with different melting temperatures) in the formulation on drug release behavior, a modified emulsion-gelation method, by which the emulsion containing pectin was gelled by the action of calcium, was used. The molten wax was dispersed in the homogenized emulsion mixture of pectin, oil and MZ which already heated to the same temperature, and then mixed until the homogenous mixture was obtained. The hot-melted mixture was extruded into calcium chloride solution. The spherical beads of wax-incorporated emulsion gel were obtained by hot-melt extrusion and ionotropic gelation.
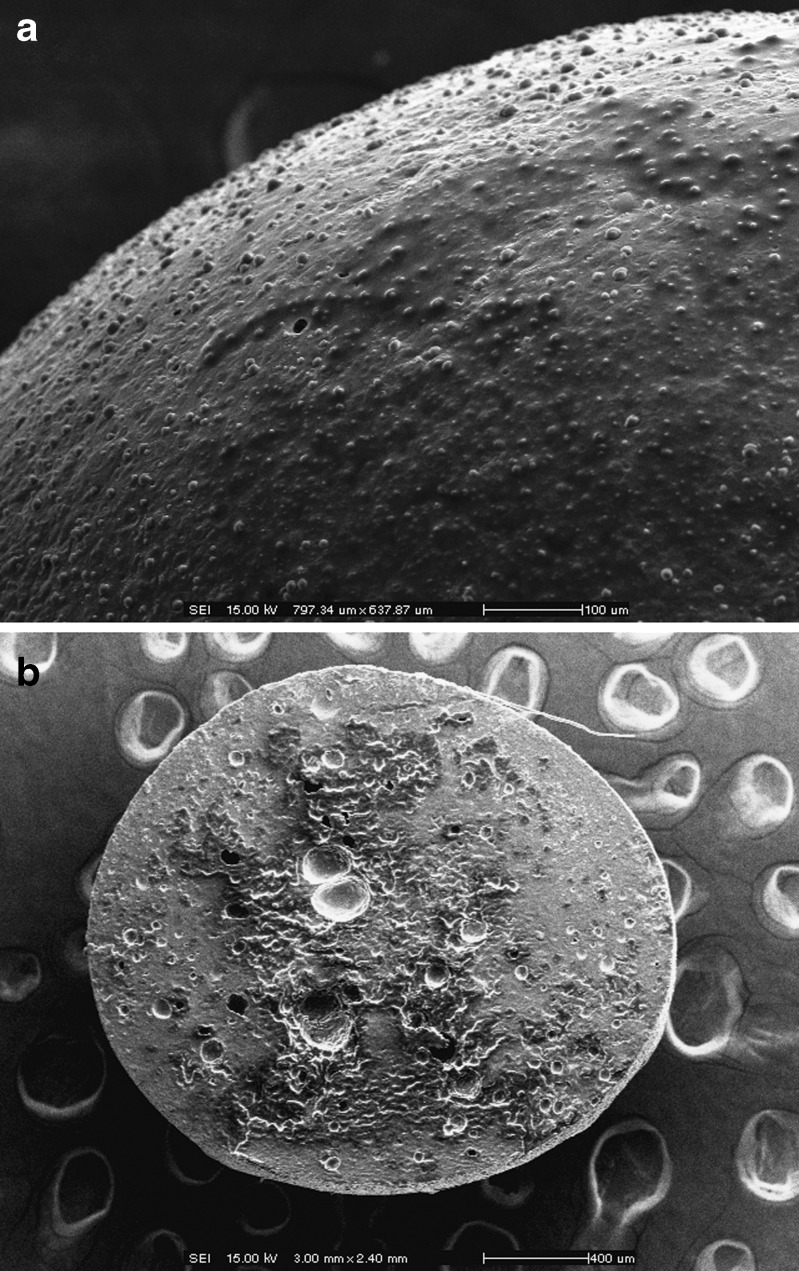
The conventional CaPG beads were oval in shape but the most of the emulsion gel beads were spherical after drying (Table I). The exception is for the emulsion gel beads containing spermaceti wax which were drop-like shape. Figure 1 shows the typical SEM images illustrating the external and internal structures of the MZ-loaded emulsion gel beads. The external surface of gel beads was rough as the oil droplets were distributed all over the bead surface. The internal (cross-sectional) structure also demonstrated the distribution of the oil droplets within the structure of the calcium cross-linked gel beads. The morphology of all other emulsion gel beads was similar to that shown in Fig. 1.
Fig. 1.
Scanning electron micrographs of a external and b internal structures of emulsion gel beads of calcium pectinate containing olive oil (30%). Magnifications and scale bars are shown on the individual micrographs
Mean Diameter of Gel Beads
The mean diameter of the drug-loaded CaPG beads, emulsion gel beads and wax-incorporated emulsion gel beads is shown in Table I. The mean diameter of the MZ-loaded gel beads ranges between 2.2 and 2.5 mm. The size of the obtained beads resulted from the inner diameter of the extruded needles that used in the study. A pendant droplet, which is formed by the flow of a liquid through a needle, continuously grew until its mass achieved a critical value, at which moment the droplet detached from the tip of the needle and fell in a receiving solution (21). The mean size of the beads insignificantly changed when the amount of oil and/or wax was changed. This agreed with the previous report which the type of additives used (e.g. waxes or polymers) insignificantly influenced the mean diameter of the emulsion gel beads (16).
Buoyancy of Gel Beads
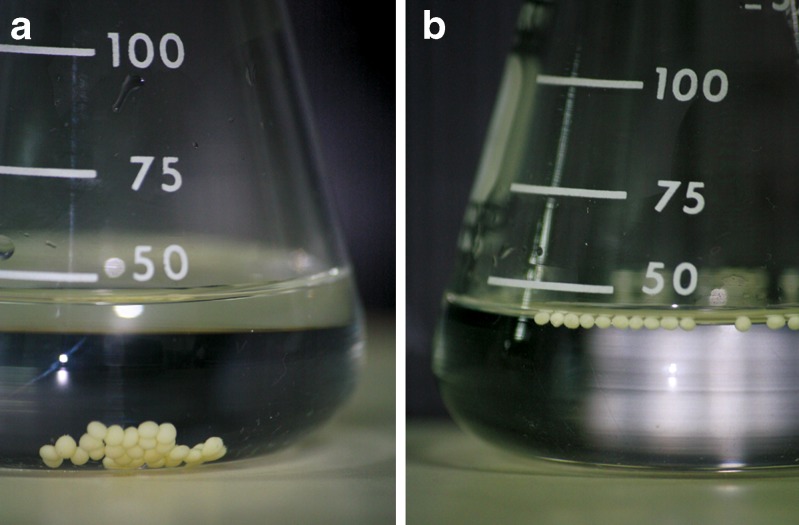
The conventional CaPG beads made of low methoxy pectin (without oil) did not float in SGF. This is not surprising as the apparent density of the conventional CaPG beads was higher than the density of the test medium (15). If a sufficient amount of oil was added, the emulsion gel beads could float immediately (Table I) and remained floating for 24 h (Fig. 2). Incorporation of various waxes with different melting temperatures did not significantly influence the floating behavior of the wax-incorporated emulsion gel beads. The beads floated with the oil amount of 30% w/w. The exception is for the PEG, GMS and SA in which the oil amount of 20% w/w could induce floating of the beads. Increasing the amount of wax in the formulation also resulted in the floated beads. Good in vitro floating behavior in SGF was observed in most of the formulations. The floating properties of the emulsion gel beads and wax-incorporated emulsion gel beads may be attributed to the low apparent density of the beads containing oils. This implied that the beads will have the tendency to exhibit a good in vivo floating effect.
Fig. 2.
Photo images showing the floating of a) conventional calcium pectinate gel beads, and b) emulsion gel beads containing 30% w/w olive oil and 4% w/w white wax
In vitro Release of MZ from Gel Beads
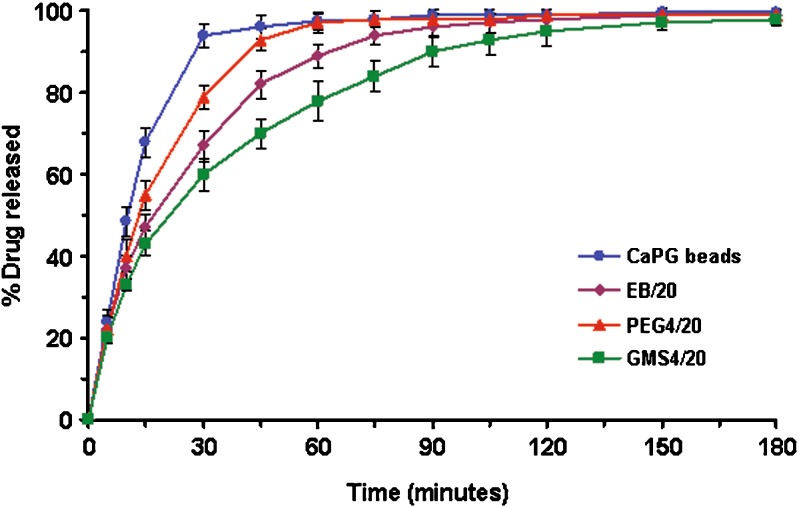
Drug release studies were performed, only when the beads floated in SGF, in order to examine the suitability of the beads as an intragastric floating drug delivery system. The drug release profiles were presented by plotting the amount of MZ released against time. Figure 3 shows the drug release profiles of CaPG (conventional) beads, and emulsion gel beads containing 20% w/w olive oil without and with 4% w/w wax (either PEG or GMS). No lag time was observed in any of the formulations studied. As MZ is freely soluble in acid medium, the release of MZ from the formulations in SGF is quite rapid. About 80% of MZ was released within 20–30 min from CaPG beads. The emulsion gel beads containing 20% w/w olive oil released the drug slower due to the fact that the oil, which is dispersed in the structure of emulsion gel beads, obstructed the dissolution channel of MZ. Incorporation of hydrophilic wax (i.e. PEG) in the formulation, the drug release was faster than the emulsion gel beads without wax. It is likely that the PEG enhanced water uptake into the beads, and thus increased the dissolution of MZ. Moreover, PEG could dissolve out and create porous matrix which enhanced drug release (16,22). In contrast, the drug release was slower when a hydrophobic wax (i.e. GMS) was incorporated (Fig. 3). The addition of GMS gave the matrix with more hydrophobicity which consequently delayed the diffusion of drug from the beads. Also, it could be seen from Fig. 3 that the incorporation of GMS could significantly retard the drug release (i.e. the time for 80% release of drug was about two-time slower), compared to that using PEG.
Fig. 3.
The release profiles of metronidazole from conventional calcium pectinate gel (CaPG) beads, emulsion gel beads containing 20% w/w olive oil (EB/20) and emulsion gel beads containing 20% w/w olive oil and 4% w/w wax (PEG4/20 and GMS4/20). The designations of different formulations were clarified in Table I
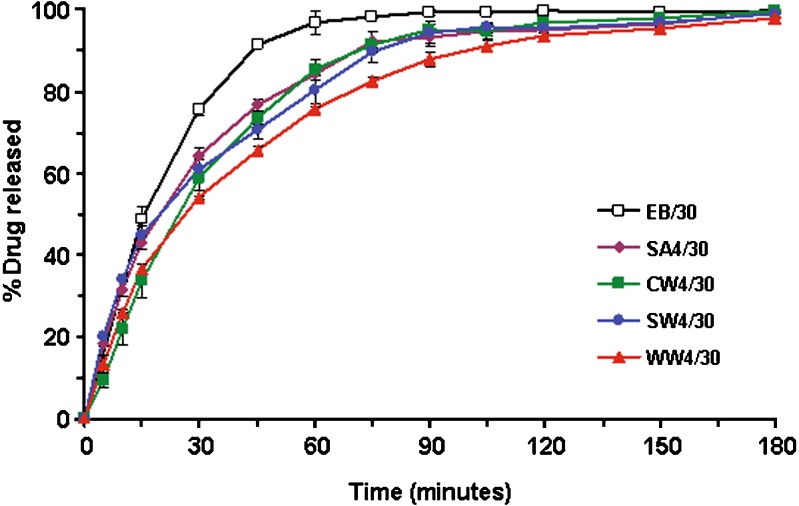
Various types of hydrophobic wax were then examined in order to observe the effect on the drug release. Figure 4 shows the effect of type of wax on drug release from the wax-incorporated emulsion gel beads containing 30% w/w olive oil and 4% w/w wax. Obviously, the incorporation of wax could retard the drug release from emulsion gel beads. However, there is only a slight effect on drug release when using different types of hydrophobic wax. It seems that there is no relation between the melting temperature of various waxes (see Table I) and the ranking order of drug release. Though the waxes used in this study are insoluble in water (except PEG), it is known that stearyl alcohol, which is a fatty alcohol prepared from stearic acid, is somewhat hydrophilic with the tendency of being hydrated in aqueous medium, compared to other waxes (white wax, carnauba wax, and spermaceti wax). The drug release from beads containing stearyl alcohol was slightly faster than those of the beads containing other hydrophobic waxes. Although white wax and carnauba wax had similar structure, they showed different release rates, i.e. the drug release from formulation containing white wax was slower than that containing carnauba wax. Both waxes are natural, complex lipid materials, consisting of different amounts of primarily acid esters, free acids, fatty alcohols, and hydrocarbons. White wax contains a higher percentage of free fatty acid but a lower percentage of fatty esters than carnauba wax. The difference in chemical characteristics mentioned above would influence in the faster drug release from white wax-based formulation. These data fit well with those of other studies (23) with the exception of Ozyazici and colleagues (24) that reported more drug release with the carnauba wax than the beeswax.
Fig. 4.
Effect of the type of waxes on drug release from wax-incorporated emulsion gel beads containing 30% w/w olive oil and 4% w/w wax. The designations of different formulations were clarified in Table I
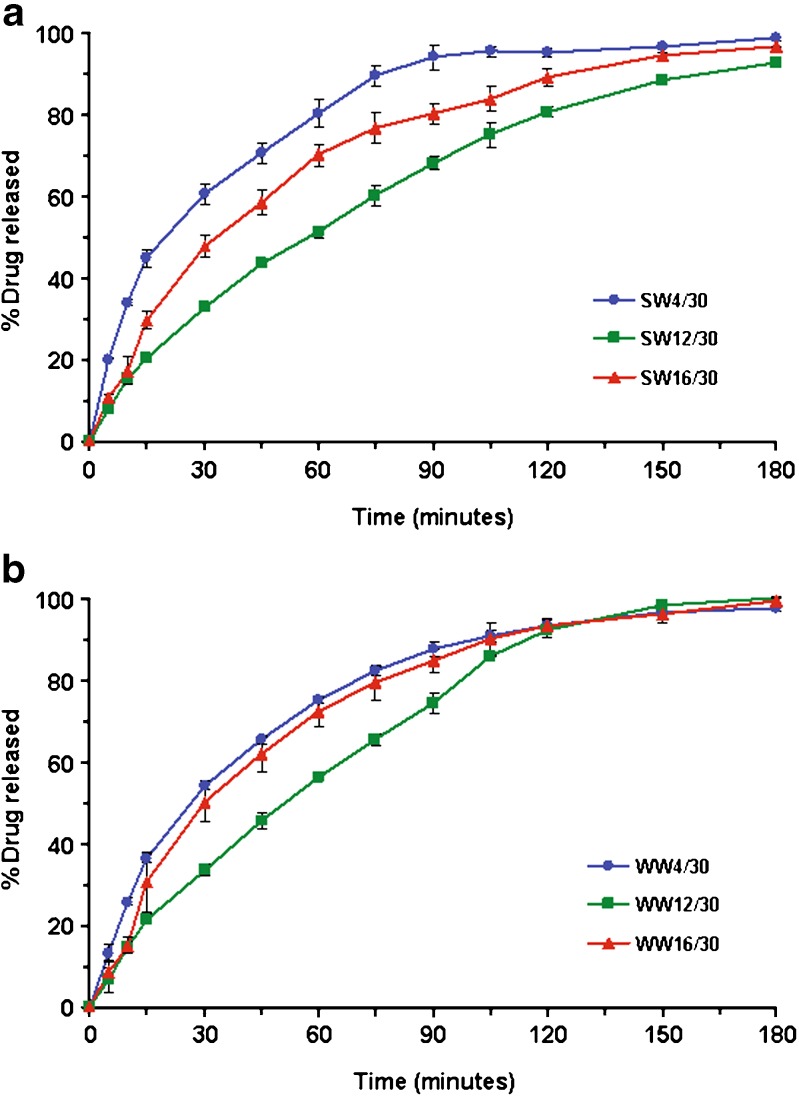
Increasing the amount of wax significantly slowed the drug release. Figure 5 shows the effect of the amount of incorporated wax on drug release from wax-incorporated emulsion gel beads containing 30% w/w olive oil and 4% w/w wax. The effect of the increased amount of wax (i.e. from 4 to 16% w/w) on drug release was more apparent when using spermaceti wax (Fig. 5a) than white wax (Fig. 5b) and other waxes (data not shown). The release rate decreased due to the water resistance when the amount of wax was increased. The same influence of the increased amount of the wax used on drug release from pellets based on the combination of wax, starch and maltodextrin was observed (25). Cao et al. (26) also demonstrated that the release rate from lipophilic matrix tablets decreased when the amount of lipophilic excipients (stearyl alcohol, stearic acid and/or carnauba wax) was increased. However, the drug release from all beads was completed within 3 h. It is likely that the incorporation of hydrophobic wax in the hydrophilic carrier (i.e. pectin beads) was not efficient for sustaining the release rate of a highly soluble drug (in acid environment).
Fig. 5.
Effect of the amount of incorporated wax on drug release from wax-incorporated emulsion gel beads containing 30% w/w olive oil and 4% w/w wax; a) spermaceti wax and b) white wax. The designations of different formulations were clarified in Table I
CONCLUSION
Intragastric floating drug delivery system was prepared by incorporating low density materials, e.g. oils and/or waxes. Incorporation of wax into the beads influenced the drug release, i.e. hydrophilic wax increased the drug release while other hydrophobic waxes significantly retarded the drug release. Increasing the amount of hydrophobic wax in the formulation significantly prolonged the drug release but was insufficient for sustaining the release of highly water-soluble drug.
ACKNOWLEDGEMENTS
The authors thank K. Kuakoon for laboratory assistance and Food & Cosmetic System Co., Ltd. (Thailand) for supplying pectin samples manufactured by CP Kelco (Denmark). Financial support from Faculty of Pharmacy, Silpakorn University is greatly appreciated.
References
- 1.Streubel A., Siepmann J., Bodmeier R. Drug delivery to the upper small intestine window using gastroretention technologies. Curr Opin Pharmacol. 2006;6:501–508. doi: 10.1016/j.coph.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 2.Moes A. J. Gastroretentive dosage forms. Crit Rev Ther Drug Carrier Syst. 1993;10:143–195. [PubMed] [Google Scholar]
- 3.Cooreman M. P., Krausgrill P., Hengels K. J. Local gastric and serum amoxycilline concentrations after different oral application forms. Antimicrob Agents Chemother. 1993;37:1506–1509. doi: 10.1128/aac.37.7.1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davis S. S. Formulation strategies for absorption windows. Drug Discov Today. 2005;10:249–257. doi: 10.1016/S1359-6446(04)03351-3. [DOI] [PubMed] [Google Scholar]
- 5.Krogel I., Bodmeier R. Development of a multifunctional matrix drug delivery system surrounded by an impermeable cylinder. J Control Release. 1999;61:43–50. doi: 10.1016/S0168-3659(99)00096-6. [DOI] [PubMed] [Google Scholar]
- 6.Desai S., Bolton S. A floating controlled release drug delivery system: in-vitro – in-vivo evaluation. Pharm Res. 1993;10:1321–1325. doi: 10.1023/A:1018921830385. [DOI] [PubMed] [Google Scholar]
- 7.Streubel A., Siepmann J., Bodmeier R. Floating microparticles based on low density foam powder. Int J Pharm. 2002;241:279–292. doi: 10.1016/S0378-5173(02)00241-7. [DOI] [PubMed] [Google Scholar]
- 8.Grant G. T., Morris E. R., Rees D. A., Smith P. J. A., Thom D. Biological interactions between polysaccharides and divalent cations: The egg-box model. FEBS Lett. 1973;32:195–198. doi: 10.1016/0014-5793(73)80770-7. [DOI] [Google Scholar]
- 9.Sriamornsak P., Nunthanid J. Calcium pectinate gel beads for controlled release drug delivery: I. Preparation and in-vitro release studies. Int J Pharm. 1998;160:207–212. doi: 10.1016/S0378-5173(97)00310-4. [DOI] [Google Scholar]
- 10.Sriamornsak P., Nunthanid J. Calcium pectinate gel beads for controlled release drug delivery: II. Effect of formulation and processing variables on drug release. J Microencapsul. 1999;16:303–313. doi: 10.1080/026520499289031. [DOI] [PubMed] [Google Scholar]
- 11.Sriamornsak P. Investigation of pectin as a carrier for oral delivery of proteins: using calcium pectinate gel beads. Int J Pharm. 1998;169:213–220. doi: 10.1016/S0378-5173(98)00129-X. [DOI] [Google Scholar]
- 12.Sriamornsak P. Effect of calcium concentration, hardening agent and drying condition on release characteristics of oral proteins from calcium pectinate gel beads. Eur J Pharm Sci. 1999;8:221–227. doi: 10.1016/S0928-0987(99)00010-X. [DOI] [PubMed] [Google Scholar]
- 13.Chambin O., Dupuis G., Champion D., Voilley A., Pourcelot Y. Colon-specific drug delivery: Influence of solution reticulation properties upon pectin beads performance. Int J Pharm. 2006;321:86–93. doi: 10.1016/j.ijpharm.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 14.Sriamornsak P. Chemistry of pectin and its pharmaceutical uses: A review. Silpakorn Univ Int J. 2003;3:206–228. [Google Scholar]
- 15.P. Sriamornsak, N. Thirawong, and S. Puttipipatkhachorn. Morphology and buoyancy of oil-entrapped calcium pectinate gel beads. AAPS J. 2004;6(3):article 24. (http://www.aapsj.org) [DOI] [PMC free article] [PubMed]
- 16.Sriamornsak P., Thirawong N., Puttipipatkhachorn S. Emulsion gel beads of calcium pectinate capable of floating on the gastric fluid: effect of some additives, hardening agent or coating on release behavior of metronidazole. Eur J Pharm Sci. 2005;24:363–373. doi: 10.1016/j.ejps.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 17.Badve S. S., Sher P., Korde A., Pawar A. P. Development of hollow/porous calcium pectinate beads for floating-pulsatile drug delivery. Eur J Pharm Biopharm. 2007;65:85–93. doi: 10.1016/j.ejpb.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 18.Sriamornsak P., Sungthogjeen S., Puttipipatkhachorn S. Use of pectin as a carrier for intragastric floating drug delivery: Carbonate salt contained beads. Carbohydr Polym. 2007;67:436–445. doi: 10.1016/j.carbpol.2006.06.013. [DOI] [Google Scholar]
- 19.Leroux J., Langendorff V., Schick G., Vaishnav V., Mazoyer J. Emulsion stabilizing properties of pectin. Food Hydrocolloids. 2003;17:455–462. doi: 10.1016/S0268-005X(03)00027-4. [DOI] [Google Scholar]
- 20.Garti N., Reichman D. Hydrocolloids as food emulsifiers and stabilizers. Food Structure. 1993;12:411–426. [Google Scholar]
- 21.Poncelet D., Babak V. G., Neufeld R. J., Goosen M. F. A., Burgarski B. Theory of electrostatic dispersion of polymer solutions in the production of microgel beads containing biocatalyst. Adv Colloid Interface Sci. 1999;79:213–228. doi: 10.1016/S0001-8686(97)00037-7. [DOI] [Google Scholar]
- 22.Kumar M. K., Shah M. H., Ketkar A., Mahadik K. R., Paradkar A. Effect of drug solubility and different excipients on floating behaviour and release from glyceryl monooleate matrices. Int J Pharm. 2004;272:151–160. doi: 10.1016/j.ijpharm.2003.12.025. [DOI] [PubMed] [Google Scholar]
- 23.Rodriguez L., Passerinim N., Cavallari C., Cini M., Sancin P., Fini A. Description and preliminary evaluation of a new ultrasonic atomizer for spray-congealing processes. Int J Pharm. 1999;183:133–143. doi: 10.1016/S0378-5173(99)00076-9. [DOI] [PubMed] [Google Scholar]
- 24.Ozyazici M., Gokce E. H., Ertan G. Release and diffusional modelling of metronidazole lipid matrices. Eur J Pharm Biopharm. 2006;63:331–339. doi: 10.1016/j.ejpb.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 25.Zhou F., Vervaet C., Remon J. P. Matrix pellets based on the combination of waxes, starches and maltodextrins. Int J Pharm. 1996;133:155–160. doi: 10.1016/0378-5173(95)04431-0. [DOI] [Google Scholar]
- 26.Cao Q. R., Kim T. W., Lee B. J. Photoimages and the release characteristics of lipophilic matrix tablets containing highly water-soluble potassium citrate with high drug loadings. Int J Pharm. 2007;339:19–24. doi: 10.1016/j.ijpharm.2007.04.016. [DOI] [PubMed] [Google Scholar]