Abstract
The present investigation was undertaken with the objective of formulating TC containing fast dissolving films for local delivery to oral cavity. Various film forming agents, film modifiers and polyhydric alcohols were evaluated for optimizing the composition of fast dissolving films. The potential of poloxamer 407 and hydroxypropyl-β- cyclodextrin (HPBCD) to improve solubility of TC was investigated. Fast dissolving films containing hydroxypropyl methylcellulose (HPMC), xanthan gum, and xylitol were formulated. Use of poloxamer 407 and HPBCD resulted in significant improvement in the solubility of TC. Fast dissolving films containing TC-HPBCD complex and TC-Poloxamer 407 were formulated and were evaluated for the in vitro dissolution profile and in vitro microbiological assay. Films containing TC-Poloxamer 407 exhibited better in vitro dissolution profile and in vitro antimicrobial activity as compared to the films containing TC-HPBCD complex. Effect of incorporation of eugenol on the in vivo performance of TC-Poloxamer 407 containing films was evaluated in human volunteers. Eugenol containing films improved the acceptability of TC-Poloxamer 407 films with respect to taste masking and mouth freshening without compromising the in vivo dissolution time.
Key words: fast dissolving films, hydroxypropyl-β-cyclodextrin, poloxamer 407, triclosan
INTRODUCTION
Triclosan (TC) is a broad spectrum antimicrobial agent that exhibits activity against a wide range of gram-negative and gram positive bacteria, molds, yeasts and even parasites responsible for malaria and toxoplasmosis. Due to its broad spectrum of activity, it is being used in several personal care products such as toothpaste, mouthwashes, body washes, anti-microbial creams, lotions and hand soaps (1–3). TC is one of most preferred agents for treating diseases of oral cavity such as plaque, caries and gingivitis due to its potent activity against Streptococcus mutans, Streptococcus sanguis, Streptococcus salivarius and Actinomycetes species (4–6). Furthermore, low buccal absorption of TC makes it a drug of choice for the local treatment of diseases of oral cavity (7).
One of the most popular formulations of TC is mouthwash. However, the utility of mouthwashes is often limited by problems related to handling, inconvenience of use during traveling, stability aspects of liquid formulations in certain cases and patient noncompliance due to its conspicuous nature. The need of hour is to have a solid oral hygiene product that would be inconspicuous, easy to handle and carry and would allow rapid release of TC for instant local action.
Fast dissolving films fulfill all the aforementioned requirements of potential solid oral dosage form for local delivery of TC. Fast dissolving film when placed in the oral cavity quickly gets hydrated, sticks onto the site of application and then disintegrates to release the drug (8). Thus, a fast dissolving film is a unique solid oral dosage form and has valuable advantages (9).
Initial investigations were focused on the development of placebo fast dissolving films with good peelability, mouth feel and in vivo dissolution time in human volunteers. After choosing the components for the placebo film, TC loaded films were formulated. Although, fast dissolving films is an attractive dosage form for the delivery of TC, the poor water-solubility of TC (10 μg/ml) (10) and its bitter taste are real challenges in the development of fast dissolving films. In order to facilitate incorporation of TC in the films and also to improve its in vivo solubility, we evaluated potential of hydroxypropyl-β-cyclodextrin (HPBCD) and Poloxamer 407 in the present investigation. Poloxamer 407 was chosen mainly due to its bland taste unlike other commonly used solubilizers which would be advantageous for the development of oral films. The fast dissolving films based on TC-HPBCD complex and TC-Poloxamer 407 dispersion were formulated and evaluated for in vitro and in vivo performance.
MATERIALS AND METHODS
Materials
Triclosan (Johnson and Johnson Ltd., Mumbai, India.), Methocel E15, E5, E3 (Colorcon Asia Pvt Ltd., Goa, India), HPBCD (Cerestar Inc., Cedar Rapids, IA), Poloxamer 407 (BASF, Mumbai, India) and Streptococcus mutans ATCC 700610 culture (Colgate Palmolive India Ltd., Mumbai, India) were received as gift samples. Eugenol (S. H. Kelkar Co., Mumbai, India), carrageenan (Sigma Chemicals, USA), guar gum and d-sorbitol (s.d. Fine-chem. Ltd., Mumbai, India), xylitol, mannitol and aspartame (Sigma-Aldrich, Saint Louis, MO), propylene glycol and glycerin (Qualigens Fine Chemicals, Mumbai, India) were purchased for carrying out various experiments. All the chemicals used were of analytical grade.
Screening of the Components for Formulation of Blank Fast Dissolving Films
Hydroxypropylmethyl cellulose (HPMC) is known for its good film forming properties and has excellent acceptability. Hence, various grades of HPMC namely Methocel E3, Methocel E5 and Methocel E15 Premium LV were evaluated as a primary film former. For the fabrication of films, propylene glycol was used as a plasticizer, glycerin as humectant and aspartame was used as a sweetener. Initially, films containing various film modifiers such as xanthan gum, guar gum and carrageenan and various polyhydric alcohols such as mannitol, sorbitol and xylitol were formulated. The composition of various films is shown in Table I. The films were prepared by solvent-casting method. Briefly, propylene glycol, glycerin, aspartame and various polyhydric alcohols were dissolved in a 5 ml of 50% v/v ethanol. HPMC and film modifiers (xanthan gum or guar gum or carrageenan) were soaked in water for 4 h and then uniformly dispersed to obtain a dispersion. Alcoholic solution and the polymeric dispersion were mixed to obtain a homogenous dispersion and 20 ml of the dispersion was cast onto each polypropylene petri plate (Tarsons, Mumbai, India). The dispersion was dried in a tray drier (Sapphire Machines, Mumbai, India) at 40–45 °C. The films were carefully removed from petri plates and cut into strips of dimensions 2 × 1.5 cm and stored in an air tight glass bottle. The films were evaluated for imperfections and cuts, peelability without rupturing, folding and cracking endurance and surface roughness.
Table I.
Composition of Various Placebo Films
| Components | Quantities | Formulae | ||||||
|---|---|---|---|---|---|---|---|---|
| F1 | F2 | F3 | F4 | F5 | F6 | F7 | ||
| Methocel E5 | 2.2% w/v | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Propylene glycol | 1.35% w/v | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Glycerine | 1.6% w/v | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Aspartame | 0.125% w/v | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Ethanol | 15% v/v | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Xanthan Gum | 0.4% w/v | ✓ | – | – | ✓ | ✓ | ✓ | ✓ |
| Guar gum | 0.4% w/v | – | ✓ | – | – | – | – | – |
| Carageenan | 0.4% w/v | – | – | ✓ | – | – | – | – |
| Mannitol | 0.5% w/v | – | – | – | ✓ | – | – | – |
| Sorbitol | 0.5% w/v | – | – | – | – | ✓ | – | – |
| Xylitol | 0.5% w/v | – | – | – | – | – | ✓ | – |
| Xylitol | 1.6% w/v | – | – | – | – | – | – | ✓ |
| Water to make | 100 ml | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
Complexation of TC with HPBCD
The TC-HPBCD complex (CM) was prepared by co grinding method (11). The complex formation was evaluated by various techniques such as differential scanning calorimetry (DSC), Infra Red (IR) spectroscopy, X-ray diffraction (XRD) studies and 1H Nuclear Magnetic Resonance (NMR) studies.
Equilibrium Solubility Study
The equilibrium solubility study of TC, TC-HPBCD complex and TC in the presence of 0.5% w/v poloxamer 407 solution was carried out in buffer pH 6.4. Briefly, excess of TC or TC-HPBCD complex was added to a vial containing 5 ml of pH 6.4 buffer. For studying the solubilization capacity of poloxamer 407, an excess amount of TC was added to a vial containing 5 ml of 0.5% w/v of poloxamer 407 solution in pH 6.4 buffer. After sealing the vials, mixtures were shaken in a water bath shaker (Remi Equipments, Mumbai, India) for 48 h at 37 ± 0.5 °C. The mixtures were centrifuged at 10,000 rpm for 20 min in RM12C micro centrifuge (Remi Equipments, Mumbai, India). The supernatant was suitably diluted with pH 6.4 buffer and the equilibrium solubility was determined by measuring the absorbance of the supernatant at 283 nm in a UV-Visible double beam spectrophotometer (Shimadzu 160A, Kyoto, Japan). The equilibrium solubility values were evaluated by a non-paired, two tailed Student’s ‘t’ test (GraphPad InStat Demo Version). Differences were considered statistically significant at P < 0.05.
Preparation of the TC Containing Fast Dissolving Films
TC containing fast dissolving films were fabricated as per the method described for the fabrication of blank fast dissolving films. Briefly, TC-HPBCD complex or TC and poloxamer 407 were incorporated in 5 ml of 50% w/v ethanol and the rest of the procedure was same as that for the fabrication of blank fast dissolving films. The composition of TC containing films is shown in Table II. TC concentration in the final polymeric dispersion was 0.080% w/v.
Table II.
Compositions of TC Containing Fast Dissolving Films
| Components | Quantities | Formulae | ||
|---|---|---|---|---|
| F8 | F9 | F10 | ||
| Methocel E5 | 2.2% w/v | ✓ | ✓ | ✓ |
| Propylene glycol | 1.35% w/v | ✓ | ✓ | ✓ |
| Glycerine | 1.6% w/v | ✓ | ✓ | ✓ |
| Aspartame | 0.125% w/v | ✓ | ✓ | ✓ |
| Ethanol | 15% v/v | ✓ | ✓ | ✓ |
| Xanthan Gum | 0.4% w/v | ✓ | – | – |
| Poloxamer 407 | 0.5% w/v | ✓ | ✓ | – |
| TC | 0.08% w/v | ✓ | – | ✓ |
| TC-HPBCD complex (1:1 molar ratio) | 0.46% w/v | – | ✓ | – |
| Eugenol | 0.3% v/v | – | – | ✓ |
| Xylitol | 1.6% w/v | ✓ | ✓ | ✓ |
| Water to make | 100 ml | ✓ | ✓ | ✓ |
Evaluation of the TC Containing Films
Determination of Weight, Thickness, Density and Moisture Content
Films of formula F8 and F9 (n = 30) were weighed on analytical balance (A&D Company Ltd., GR200, Tokyo, Japan). Thickness of films of formula F8 and F9 (n = 12) was measured using a micrometer screw gauge (Mitutoyo, Kawasaki, Japan). The density of the films was determined by using a formula
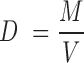 |
1 |
Where, D = density of films in g/cm3, M = mass of films in g and V = volume of films in cm3 which is product of thickness and area.
The moisture uptake by the films of formula F8 and F9 (n = 5) was determined by exposing them to an environment of 40°C/75% relative humidity (RH) for 1 week. The uptake of moisture by the films was measured as percent increase in weight. The differences in the moisture uptake values were evaluated by a non-paired, two tailed Student’s ‘t’ test (GraphPad InStat Demo Version). Differences were considered statistically significant at P < 0.05.
DSC Thermograms of Films
DSC thermograms of the TC, blank films and TC containing films were recorded on a thermal analyzer (Shimadzu DT-40, Kyoto, Japan). The samples were heated from 30 to 300 °C at a heating rate of 10 °C/min in an inert nitrogen atmosphere.
X-ray Diffractograms of Films
The X-ray diffractograms of the blank and TC loaded films were recorded on diffractometric system (Ital Structures APD 2000, Riva del Garda, Italy) at 1.5 mA and 30 KV over the range 2θ = 5° to 50° at rate of 2θ = 5°/min.
Determination of TC Content
For determination of TC content, films (n = 10) were crushed to obtain a fine powder and the TC was extracted from the films by using propan-2-ol. The extract was centrifuged at 6,000 rpm in RM12C micro centrifuge (Remi Equipments, Mumbai, India) for 15 min. The amount of the TC present in the supernatant was determined by measuring the absorbance at 283 nm against a blank film extract in a UV-Visible double beam spectrophotometer (Shimadzu 160A, Kyoto, Japan).
In Vitro TC Release from the Films
For in vitro dissolution studies, each film was placed with the help of forceps in a 50 ml glass beaker containing 20 ml of phosphate buffer pH 6.4. The beaker was suspended in a water bath of a USP-XXIII dissolution apparatus (Labindia, Mumbai, India) and agitation was provided by the shaft of the USP-XXIII type1 apparatus (Labindia, Mumbai, India) at 100 rpm without the basket attached to it. The temperature of the dissolution media was maintained at 37 ± 0.5 °C. During the study, 4 ml of aliquots were withdrawn at 7, 14, 21, 28, 35 and 42 min and were replaced by fresh buffer. The aliquots were centrifuged at 10,000 rpm in a microcentrifuge (RM12C, Remi Equipments, Mumbai, India) for 10 min and the amount of TC released in the media was determined by a UV-Visible Spectrophotometer (Shimadzu 160A, Kyoto, Japan) at 283 nm. The in vitro TC release data were evaluated by a non-paired, two tailed Student’s ‘t’ test (GraphPad InStat Demo Version). Differences were considered statistically significant at P < 0.05.
In Vitro Microbiological Studies
In vitro microbiological studies were carried out to confirm that the antimicrobial activity of TC is retained after getting released from the films. For this purpose, the aliquot withdrawn at the end of 35 min of in vitro drug release experiment (the time at which films show complete dissolution) for each film of formula F8 and F9 was evaluated for its antimicrobial activity. Antimicrobial activity was evaluated against Streptococcus mutans, which is a common causative organism of dental plaque and caries (12). The protocol for the antimicrobial studies was in accordance to the study reported by Jagtap and Karkera (13). The effective zone of inhibition at the end of 24 hours was calculated as a difference between diameters of zone of inhibition of TC loaded film and that of blank film. The values of zone of inhibition observed with the films were evaluated by a non-paired, two tailed Student’s ‘t’ test (GraphPad InStat Demo Version). Differences were considered statistically significant at P < 0.05.
Formulation of TC Fast Dissolving Films Containing Eugenol
TC loaded films containing eugenol (Formula F10, Table II) were prepared. The films of formula F10 had same composition as that of F8. During the preparation of films of formula F10, eugenol was dissolved in the 5 ml of 50% alcohol and the rest of the procedure was similar to that described for the preparation of TC containing films.
Evaluation of Films of Formulae F8 and F10 in Human Volunteers
The films of formula F8 and F10 were evaluated in healthy human volunteers with their consent (n = 9; 7males and 2 females) for mouth feel, bitter taste masking, mouth freshening and in vivo dissolution time in oral cavity. The protocol for the human studies was approved by the Ethical Committee of Bombay College of Pharmacy. Each volunteer was randomly given film of formula F8 or F10 (single blind design) with a potable water rinse at start. The volunteers were asked to place the film on the tongue. Volunteers were not restricted later on with respect to tongue movement. The films used in the study had dimensions of 2 × 1.5 cm and average weight of 61 ± 3 mg. The in vivo dissolution time observed for the films was evaluated by a non-paired, two tailed Student’s ‘t’ test (GraphPad InStat Demo Version). Differences were considered statistically significant at P < 0.05.
Stability Studies
Films of formulae F8 and F9 were stored at two different storage conditions namely 30 °C/60% RH and 40 °C/75% RH. Each film was wrapped in a butter paper followed by aluminium foil and placed in an aluminium pouch, which was heat-sealed at the end. The films were evaluated for appearance, weight, TC content and in vitro drug release after storage for 30, 60 and 90 days. The f1 (difference factor) and f2 (similarity factor) values for in vitro TC release from the films were calculated and were compared for change in the dissolution profile (14). The films of formula F10 (containing eugenol) were evaluated for in vivo dissolution and taste in human volunteers (n = 5), at the end of 30 and 90 days.
RESULTS AND DISCUSSION
Screening of the Components for Formulation of Blank Fast Dissolving Film
HPMC, a polymer with excellent film forming ability, was used as a primary film former in films (15). Initial studies indicated that amongst various grades of HPMC, Methocel E5 Premium LV gave films with the most desired properties at the concentration of 2.2% w/v. Amongst various film modifiers, xanthan gum showed best ability to improve film forming properties of HPMC as compared to the other film modifiers like guar gum and carrageenan. Incorporation of mannitol in films resulted in white patches whereas xylitol and sorbitol containing films had good film characteristics. However, xylitol was selected for the film formation due to its more negative heat of solution and lesser hygroscopicity as compared to sorbitol. The agents with more negative heat of solution are expected to give more cooling sensation in the mouth. The films of formula F6 and F7 differ only in concentration of xylitol (Table I). Both the films were evaluated in human volunteers (n = 5) for in vivo dissolution time and mouth feel. It was observed that film F7 had quicker dissolution time and good mouth feel as compared to F6 (data not shown). Hence, for fabrication of TC loaded films, composition of film F7 was used.
Studies on Complexation of TC with HPBCD
It was observed that the HPBCD formed a complex with TC by co-grinding method. The various characterization studies such as DSC, XRD and NMR confirmed the complex formation between TC and HPBCD. It was observed that the complexation process rendered TC in a partially amorphic form (data not shown).
Equilibrium Solubility Studies
Equilibrium solubility studies were carried out in phosphate buffer pH 6.4 as it is reported that the pH of saliva is in the range of 5.8–8.4 with a mean of 6.4 (16). It was observed that the plain TC had very less solubility in the pH 6.4 buffer (9.96 ± 0.44 μg/ml). The complexation of TC with HPBCD significantly improved its solubility in pH 6.4 buffer (63.45 ± 02.98 μg/ml; P < 0.05) confirming that there is a strong association between TC-HPBCD as reported earlier (17). Surfactants are known to increase the aqueous solubility of the drug by either improved wetting, co-solvency or by forming micelles depending on their concentration used (18). Poloxamer 407 resulted in significant increase in the TC solubility in pH 6.4 buffer as compared to plain drug as well as TC-HPBCD complex (369.33 ± 2.42 μg/ml; P < 0.05). Thus, both the components selected for the investigation confirmed their potential in improving TC solubility which would be useful for the successful development of fast dissolving films that can quickly release the drug in solubilized form.
Preparation of the TC Containing Fast Dissolving Films
The incorporation of Poloxamer 407 (Formula F8) and HPBCD (Formula F9) did not adversely affect peelablity, flexibility, tensile strength and appearance of the film. The concentration of TC in the final dispersion used for the formula F8 and F9 was 0.08% w/v. Increasing the concentration of the TC beyond 0.08% w/v affected the peelability of the films.
Evaluation of the TC Containing Films
Determination of Weight, Thickness, Density and Moisture Content
The average weight, thickness and density values for the films of formula F8 and F9 are shown in Table III. The films could not be differentiated on the basis of moisture uptake. Maximum moisture uptake by a film in 7 days was 4.2% w/w. The moisture uptake could be attributed to the presence of excipients like polyhydric alcohols, HPBCD and Poloxamer 407 which can take up moisture.
Table III.
Weight, Thickness and Density of the Films of Formulae F8 and F9 (n = 30)
| Film formula | Weight (mg) | Thickness (mm) | Density (g/cm3) |
|---|---|---|---|
| F8 | 62.06 ± 7.23 | 0.166 ± 0.05 | 1.36 ± 0.30 |
| F9 | 61.09 ± 7.48 | 0.158 ± 0.05 | 1.39 ± 0.26 |
DSC Thermograms of Films
The DSC thermograms of TC, films of formulae F8 and F9 and placebo films of formulae F8 and F9 (BF8 and BF9) are shown in Fig. 1. The thermograms pure TC shows melting endotherm at 61 °C and another endothermic event at 260 °C. From the figure, it is evident that the blank fast dissolving films (BF8 and BF9) and TC containing films did not show any melting endotherm for TC. This could be due to the conversion of the TC to its amporhic form which can be attributed to the process of complexation and/or to the components of the films such as HPMC and poloxamer 407 which are known crystallization inhibitors.
Fig. 1.
The DSC thermograms of TC and films, formulae F8, F9, BF8 and BF9
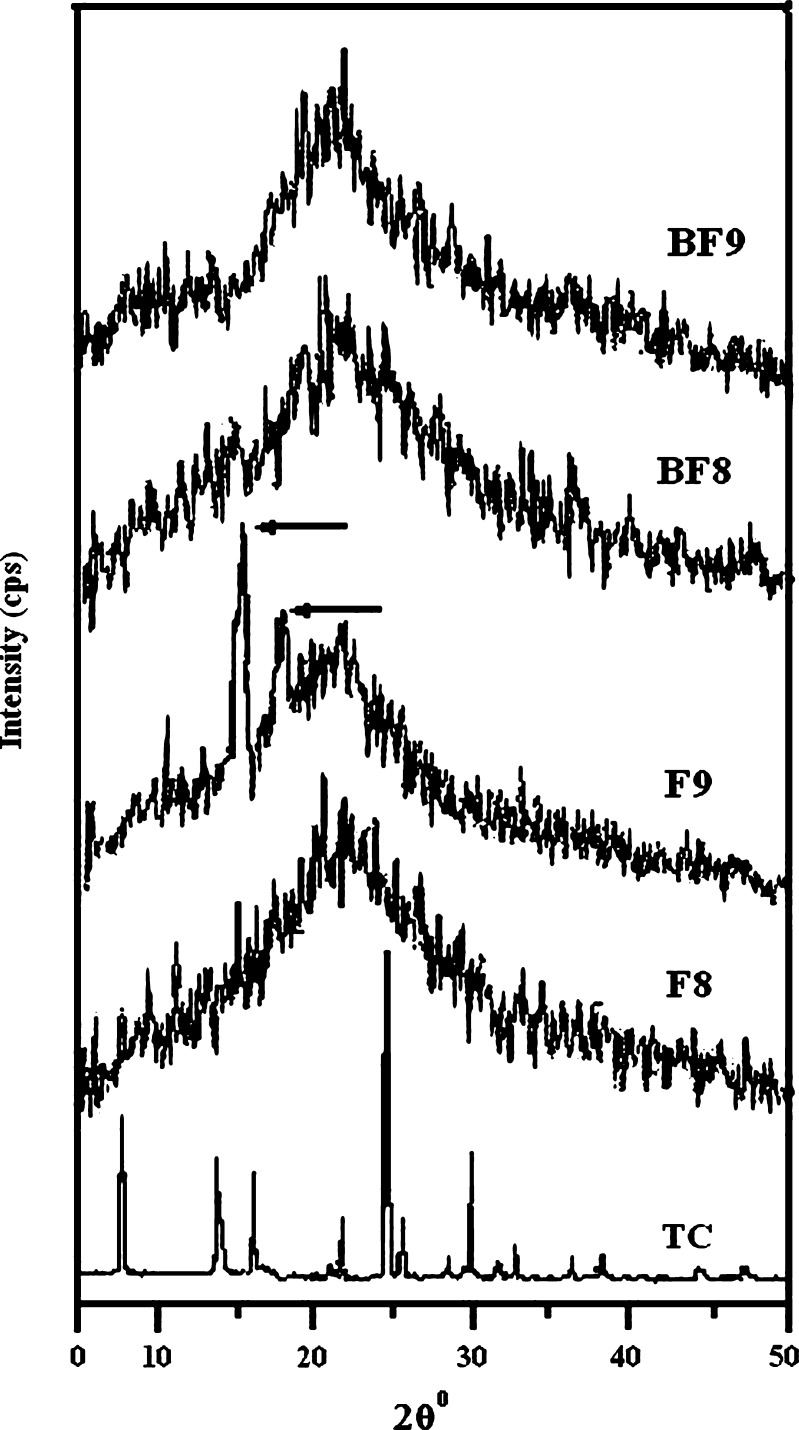
X-ray Diffractograms of Films
The X-ray diffractograms of TC, films of formulae F8 and F9 and blank fast dissolving films of formulae F8 and F9 (BF8 and BF9) were recorded for obtaining further information about the process of amorphization of TC in the films. The X-ray diffractograms are shown in Fig. 2. The characteristic peaks of the drug are not visible in the diffractograms of F8 whereas diffractogram of F9 shows two small peaks; one at 2θ value of 14° and the other at 2θ value of 16° which are characteristic peaks of the TC and correspond to peaks of pure TC, at 2θ values of 13.8° and 16° (Fig. 2). The peak intensity is indeed quite less in diffractogram of F9. This indicates that TC is partially amorphized in F9 whereas it is completely amorphized in F8. Hence, the film F9 is likely to demonstrate better in vitro and in vivo performance of TC.
Fig. 2.
X ray diffractograms of TC and films, formulae F8, F9, BF8 and BF9
Determination of TC Content
The theoretical TC content in F8 and F9 was 625 μg/62 mg of the film. Considering this content, as 100%, the TC content in the F8 and F9 was evaluated. The TC content in F8 and F9 was found to be 99.42 ± 2.56 and 99.01 ± 1.49% respectively (n = 7). The TC content in the films was optimum for antimicrobial activity. It is known that the MIC of the TC against Streptococcus mutans is 16 μg/ml (19). The normal saliva volume is 0.7 ml (20). Hence, each film after oral administration would yield TC content in the mouth that is significantly higher than its MIC value.
In Vitro TC Release from the Films
The release profile of TC from the films of formulae F8 and F9 in phosphate buffer pH 6.4 is shown in Fig. 3. Pure TC containing films showed very negligible dissolution in the medium even after 90 min (data not shown).
Fig. 3.
In Vitro drug release from films, formulae F8 and F9 (n = 14)
One of the objectives of in vitro release studies was to discriminate between the F8 and F9. The films dissolved almost completely in about 25 to 32 min. The TC release pattern of both the films (F8 and F9) was similar. In the initial period, the films remained intact and a small amount of TC was released which could be due to slow agitation employed in the experiment and also due to the absence of the drug on the surface. As the films got hydrated, they began to disintegrate and release the drug. After about 14 min, the films became thinner and around 34–35% of TC was released. However, at the end of 35 min, the amount of TC released from F8 was significantly higher than that of F9 (P < 0.05). The reason could be insufficient solubilization of TC in the films of formula F9 as compared to that of F8. This corroborates the observations of equilibrium solubility study and XRD data observed for film F9. Poloxamer 407 (present in F8) was found to be better in solubilizing TC as compared to that of TC-HPBCD complex (present in F9). Although at the end of 35 min, matrix of F8 and F9 was completely disintegrated, the contents in the beakers that contained F9 had a milky tinge. This also supports that the dissolution of TC was incomplete in case of F9 whereas the contents in the beakers containing F8 had no such tinge at the end of 35 min. XRD results also showed that some crystallinity of TC was retained in F9 but not in F8 which could support the inferior dissolution profile of TC from F9.
In Vitro Microbiological Studies
The ability of the TC available from films to inhibit the growth of Streptococcus mutans was evaluated by measuring the zone of inhibition observed with aliquots of release medium taken at the end of 35 min of in vitro dissolution studies for formulae F8 and F9. The effective zone of inhibition due to the aliquot of F8 was 6.83 ± 0.62 mm and that of F9 was 4.67 ± 0.62 mm (n = 5). As expected from the in vitro drug release profile, F8 demonstrated significantly larger zones of inhibition than that of F9 (P < 0.05). Considering this and earlier observations, F8 was selected for the further studies.
Formulation of TC Fast Dissolving Films Containing Eugenol
Eugenol is a main component of clove oil and is known to give good mouth feel and freshness. It can also be used to mask the bitter after taste of the drug due to its mild local anesthetic effect. Hence, eugenol was decided to be used as a flavoring agent. Incorporation of eugenol in the films did not affect the properties such as transparency, strength and peelability.
Evaluation of Films of Formula F8 and F10 in Human Volunteers
Films of formulae F8 and F10 were evaluated in the human volunteers. The results of the in vivo studies are shown in Table IV (key in Table V). It is evident that films of formula F10 (containing eugenol) were better than that of F8 with respect to taste masking of TC and mouth freshening. However, films of formulae F8 and F10 did not differ significantly with respect to in vivo dissolution time (P > 0.05) and mouth feel. Volunteers preferred the flavored films (F10) to the unflavored film and reported that the films were fast dissolving. This indicates that the incorporation of eugenol considerably improved the acceptability of the films. It is also noteworthy that the in vivo dissolution time was considerable lesser than that of the in vitro dissolution time. This could be due to the fact that there is much higher degree of agitation in the mouth than in the beaker. Another reason for its quick dissolution time reported by volunteers in vivo could be due to the small pieces of the film which may not be felt in the mouth due to smooth mouth feel, which might give, the impression that the film has dissolved.
Table IV.
In Vivo Performance of Films, Formulae F8 and F10
| Volunteer | After Taste | Mouth Feel | Mouth Freshening | In vivo Dissolution Time (s) | ||||
|---|---|---|---|---|---|---|---|---|
| F8 | F10 | F8 | F10 | F8 | F10 | F8 | F10 | |
| 1 | +++ | 0 | ++ | ++ | 0 | 0 | 19.45 | 31.00 |
| 2 | ++ | 0 | ++ | ++ | 0 | + | 38.00 | 18.27 |
| 3 | ++ | 0 | ++ | ++ | 0 | ++ | 25.09 | 14.47 |
| 4 | ++ | + | +++ | +++ | 0 | + | 24.34 | 16.15 |
| 5 | ++ | 0 | + | + | 0 | + | 14.61 | 29.49 |
| 6 | +++ | 0 | ++ | ++ | 0 | + | 34.2 | 23.89 |
| 7 | +++ | + | ++ | ++ | 0 | ++ | 31.29 | 30.00 |
| 8 | ++ | 0 | ++ | + | 0 | + | 23.85 | 29.07 |
| 9 | ++ | + | + | + | 0 | + | 23.61 | 39.5 |
Table V.
Key to Table IV
| Parameter | 0 | + | ++ | +++ |
|---|---|---|---|---|
| After taste | Not bitter | Bitter | Very bitter | Extremely bitter |
| Mouth feel | Not good | Good | Very good | Excellent |
| Mouth freshness | Not present | Slightly present | Present | Predominantly present |
Stability Studies
The films did not show any significant change in appearance and weight loss on storage and TC content (data not shown). The f1 values for the films ranged from 2.07 to 12.87 and f2 values ranged from 67.55 to 87.55, which indicated that the in vitro TC release profiles of films of both the formulae (F8 and F9) was not affected after storage. The films of formula F10 did not show any alteration in the acceptability by the human volunteers and their scoring with respect to taste and mouth feel (data not shown). The in vivo dissolution time was also not affected as seen in Table VI.
Table VI.
In Vivo Dissolution Time for Films, Formula F10 When Subjected to Stability Studies (n = 5)
| Stability Time Point with Conditions | In Vivo Dissolution Time (s) |
|---|---|
| 0 days | 25.44 ± 4.93 |
| 30 days, 30 °C and 60% RH | 22.90 ± 4.58 |
| 30 days, 40 °C and 75% RH | 29.48 ± 5.04 |
| 90 days, 30 °C and 60% RH | 26.00 ± 8.83 |
| 90 days, 40 °C and 75% RH | 26.66 ± 8.80 |
CONCLUSION
The fast dissolving films could be formulated with the easily available components such as HPMC and xanthan gum. Triclosan, a poorly water soluble and bitter drug could be successfully incorporated in the fast dissolving films with the help of solubilizers such as HPBCD and poloxamer 407 and the bitter taste of the TC could successfully be masked by the use of poloxamer 407 and eugenol in the films. In vitro and in vivo evaluation of the films confirmed their potential as an innovative dosage form to improve delivery of TC.
Acknowledgements
The authors wish to acknowledge (1) Miss Harsha Uskaikar, Lecturer and Dr. Rane, Professor of the Department of Chemistry, Goa University for XRD analysis, (2) The National Facility for High Field NMR at Tata Institute of Fundamental Research, Mumbai for performing NMR studies, (3) Johnson and Johnson Ltd., for gift sample of triclosan, (4) Colgate Palmolive India for S. mutans culture sample (5) Colocon-Asia Ltd and BASF India for providing gift sample of the various excipients and (6) Mr. Abhijit Date for helping in the preparation and revision of the manuscript.
Contributor Information
Aditya Dinge, Phone: +91-222-6670871, FAX: +91-222-6670816.
Mangal Nagarsenker, Phone: +91-222-6670871, FAX: +91-222-6670816, Email: mangal_nag511@yahoo.co.in.
References
- 1.Nissen H. P., Ochs D. Triclosan an antimicrobial active ingredient with anti-inflammatory activity. Cosm. Toilet. 1998;113:61–64. [Google Scholar]
- 2.Surolia N., Surolia A. Triclosan offers protection against blood stages of Malaria by inhibiting enoyl ACP reductase of Plasmodium falciparum. Nat. Med. 2001;7:167–173. doi: 10.1038/84612. [DOI] [PubMed] [Google Scholar]
- 3.Regos J., Hitz H. R. Antimicrobial spectrum of triclosan: a broad spectrum antimicrobial agent. Zbl. Bakt. Hyg. A Orig. A. 1974;226:390–401. [PubMed] [Google Scholar]
- 4.Jackson R. J. Metal salts, essential oils and phenols- old or new. Periodontol. 1997;15:63–73. doi: 10.1111/j.1600-0757.1997.tb00106.x. [DOI] [PubMed] [Google Scholar]
- 5.Scheie A. Modes of action of currently known chemical anti plaque agents other than chlorhexidine. J. Dent. Res. 1989;68:1609–1616. [Google Scholar]
- 6.Bradshaw D. J., Marsh P. D., Watson G. K., Cummins D. Effects of triclosan and zinc citrate, alone and in combination, on a community of oral bacteria grown in vitro. J. Dent. Res. 1993;72:25–30. doi: 10.1177/00220345930720010301. [DOI] [PubMed] [Google Scholar]
- 7.Lin Y. J. Buccal absorption of triclosan following topical mouthrinse application. Am. J. Dent. 2000;13:215–217. [PubMed] [Google Scholar]
- 8.S. B. Borsadia, D. O’Halloran, and J. L. Osborne. Quick-dissolving films—a novel approach to drug delivery. Drug Delivery Technology. 2(2) (2003). Available at http://www.drugdeliverytech.com/cgi-bin/articles.cgi?idArticle. Accessed July 3, 2003.
- 9.Liang A. C., Chen L. Fast dissolving intraoral drug delivery systems. Expert Opin Ther Pat. 2001;11:981–986. doi: 10.1517/13543776.11.6.981. [DOI] [Google Scholar]
- 10.Loftsson T., Leeves N., Bjonsdottir B., Duffy L., Masson M. Effect of cyclodextrins and polymers on triclosan availability and substantivity in toothpastes in vivo. J. Pharm. Sci. 1999;88:1254–1258. doi: 10.1021/js9902466. [DOI] [PubMed] [Google Scholar]
- 11.Nagarsenker M. S., Ramprakash G. Influence of preparation methodology on solid-state properties of an acidic drug-cyclodextrin system. J. Pharm. Pharmacol. 2004;56:725–733. doi: 10.1211/0022357023538. [DOI] [PubMed] [Google Scholar]
- 12.Hamada S., Slade H. D. Immunology and Cariogenicity of Streptococcus mutans. Microbiol. Rev. 1980;44:331–384. doi: 10.1128/mr.44.2.331-384.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jagtap A. G., Karkera S. G. Extract of Juglandaceae regia inhibits growth, in-vitro adherence, acid production and aggregation of Streptococcus mutans. J. Pharm. Pharmacol. 2000;52:235–242. doi: 10.1211/0022357001773751. [DOI] [PubMed] [Google Scholar]
- 14.Polli J. E., Rekhi G. S., Augsburger L. L., Shah V. P. Methods to compare dissolution profiles and a rationale for wide dissolution specifications for metoprolol tartrate tablets. J. Pharm. Sci. 1997;86:690–700. doi: 10.1021/js960473x. [DOI] [PubMed] [Google Scholar]
- 15.Peh K. K., Wong C. F. Polymeric films as vehicle for buccal delivery: swelling, mechanical, and bioadhesive properties. J. Pharm. Pharmcol. Sci. 1999;2:53–61. [PubMed] [Google Scholar]
- 16.D. M. Brahmankar, and S. B. Jaiswal. Biopharmaceutics and Pharmacokinetics: A Treatise. Vallabh Prakashan, New Delhi, India, 2003, pp. 178–203.
- 17.Grove C., Liebenberg D. J., Yang W., de Villiers M. M. Improving the aqueous solubility of triclosan by solubilization, complex formation and in situ salt formation. J. Cosmet. Sci. 2003;54:537–550. [PubMed] [Google Scholar]
- 18.Strickley R. G. Solubilizing excipients in oral and injectable formulations. Pharm. Res. 2004;21:201–229. doi: 10.1023/B:PHAM.0000016235.32639.23. [DOI] [PubMed] [Google Scholar]
- 19.Coenye T., Honraet K., Rigole P., Jimenez P. M., Nelis H. J. In vitro inhibition of streptococcus mutans biofilm formation on hydroxyapatite by subinhibitory concentrations of anthraquinones. Antimicro. Agents Chemother. 2007;51:1541–1544. doi: 10.1128/AAC.00999-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goodson J. M. Pharmacokinetic principles controlling of oral therapy. J. Dent. Res. 1989;68:1625–1632. [Google Scholar]