Abstract
The present investigation aims at studying the effect of mixed surfactant system of sodium lauryl sulphate (SLS) and alkyl polyglucosides (C10APG, C12APG and C12/14APG) on dissolution rate enhancement of poorly water soluble drug. Aceclofenac—a non-steroidal anti-inflammatory agent was used as a model drug as it has limited water solubility. The influence of the surfactant concentration in various blends on dissolution rate of Solid Dispersion (SD), prepared using solution method with ethanol as the solvent was studied and the advantage of mixed surfactant systems over the individual surfactants was illustrated by differences in the in-vitro dissolution profiles of SD. Physico chemical evaluation (critical micellar concentration, zeta potential and β-parameter calculations) was carried out to study the mixed surfactant systems. Solid mixtures were characterized by Infrared spectroscopy (FT-IR); X-ray diffraction studies (XRD) and scanning electron microscopy (SEM). It was seen that the dissolution rate of aceclofenac from SD increased with the increase in the APG proportion relative to SLS with the optimum ratio of 0.2 SLS:0.8 APG showing the best effect in all cases. Results obtained from physico-chemical evaluation (the decrease in the value of critical micelle concentration and higher negative value of β-parameters) suggested the existence of synergism between surfactants blends. The observed results in the dissolution rate enhancement could be attributed to the drug—surfactant interactions as evident from FT-IR, SEM and XRD results.
Key words: aceclofenac, β-parameter, critical micelle concentration values, dissolution, FT-IR, solid dispersion, XRD, zeta potential
INTRODUCTION
The poor dissolution characteristics of relatively insoluble drugs have long been and still remain a problem to pharmaceutical industry because the dissolution rate could be the rate-limiting process in the absorption of drug from solid dosage form. Therefore, together with permeability, the solubility and dissolution behavior of drug are key determinants of its oral bioavailability. There have been numerous efforts to improve drug dissolution rate, out of which solid dispersions constitutes a commonly employed technique (1–3). Selecting a carrier in solid dispersion is one of the most important considerations that determine the performance of the system. Among many known carriers, polymeric surfactants (Poloxamers) are widely used for preparation of solid dispersions (3–4). Thus, it can be assumed that surfactants can be used as carriers in solid dispersions.
Mixtures of surfactants solution form mixed micelle aggregates that exhibit characteristic properties, which are superior to those of the individual components (5). Synergistic behavior of mixed surfactant systems may be exploited to reduce the total amount of surfactants used in particular applications (6–7). This might be of significant advantage in case of pharmaceuticals, which emphasizes more on the limited use of surfactants owing to toxicity related complications.
Sodium lauryl sulphate (SLS) is an anionic surfactant with a high solubilizing potential. It is commonly used as excipient in oral formulation for the purpose of increasing aqueous solubility of poorly water soluble drugs. However, the amount of surfactant that can be incorporated in a formulation is limited owing to its charged nature and irritation potentials (8). Alkyl polyglucosides (APGs) are nonionic surfactants derived from renewable raw materials especially starch and their derivatives (9). APGs constitute a broad class of bio-surfactants and are categorized based on the chain length of fatty alcohol in their structure (C10, C12, and C14). Although APGs have been known for 100 years, ways of producing APGs economically was discovered only few years ago. The advent of more economical production of APGs and because of their safer ecological and technical properties, APGs are now been considered for applications in drug delivery systems 9.
Literature has reported a favorable interaction of APG and SLS, wherein the former leads to reduction of the irritation potential of SLS, attributed to the overall reduction of total charge on the molecule (8). Consequently, this fact can be exploited for formulating a mixed surfactant system of APG: SLS to meet the requirement of reduction of total amount of SLS required for solubilization and also to overcome the irritation related disadvantages of SLS.
This article explores the possibility of using mixed surfactant systems of SLS and APG as a carrier in solid dispersions. Aceclofenac [2-[[2-[2-[(2,6 dichlorophenyl)amino] phenyl]acetyl]oxy] acetic acid] (Fig. 1), a non-steroidal anti-inflammatory agent, was selected as model drug as it was considered as an appropriate molecule for study, owing to its poor water solubility (10–11).
Fig. 1.
Structure of aceclofenac
Mixed surfactant systems of SLS and APG were studied for its contribution in solubilizing effect on poorly soluble drug aceclofenac. The drug-carrier interaction was further characterized using dissolution test, X-ray diffraction (XRD), zeta potential, scanning electron microscopy (SEM) and FT-IR spectroscopy.
MATERIALS AND METHODS
Materials
Aceclofenac (USP grade) was gifted by Aarti Drugs (Mumbai, India). APG based on C10, C12 and C12/14 fatty alcohols (linear saturated fatty alcohols) were prepared in the laboratory by modified Koenig and Knorr method (12). SLS was obtained from S. D. fine chemicals (Mumbai, India). Solvents used were analytical grade and solutions were prepared using double distilled water.
Methods
Synthesis of APGs
The method has been explained in detail elsewhere (12). Briefly, 1-Bromo, 2,3,4,6-tetra acetyl β-d-glucopyranoside was reacted with fatty alcohols (C10–C16) in presence of lithium carbonate to get gylcosidic linkage. Further, hydrolysis was carried out using sodium methoxide in methanol to obtain desired APGs.
Surface Tension Measurements
The surface tension was measured by a ring method using du Nuoy tensiometer (Sigma 703, KSV, NY, USA) at room temperature (13). The critical micelle concentrations (CMC) of single and mixed surfactants were determined by surface tension measurements at varying total surfactant concentration in aqueous solution at different mole fractions.
Preparation of Solid Dispersions
Co-evaporates of aceclofenac with the surfactants carriers like SLS, C10APG, C12APG, C12/14APG (1:1 mixture of C12APG with C14APG) and mixed surfactant systems like SLS/C10APG, SLS/C12APG, SLS/C12/14APG (0.8:0.2; 0.6:0.4; 0.4:0.6; 0.2:0.8 Molar ratios) were used to prepare solid dispersions by solvent evaporation method. The concentration of surfactant carrier was kept at observed critical micelle concentration (Table I) in all cases. The ratio of drug:carrier was kept constant (1:0.5 w/w) in all cases. Briefly, co-evaporates were prepared by dissolving drug (40 gm) in 20 ml ethanol and separately dissolving 20 gm of APG/SLS mixtures in 10 ml of distilled water. The alcoholic solution of aceclofenac was then poured into the minimum aqueous solution of carriers under continuous stirring (using overhead stirrer at 50 rpm). The mixture was then subjected to mild heat (50°C) to affect solvent evaporation. The moist mass obtained was dried overnight at 40°C (in temperature oven), pulverized and passed though sieve no. 60 (BSS mesh 60) and kept in screw capped vials until use.
Table I.
Properties of surfactants
| Surfactant | Observed CMCa (mM) | Calculated CMCb (mM) | β | f 1 c | f 2 c |
|---|---|---|---|---|---|
| SLS | 8.23 | ||||
| C10APG | 2.20 | ||||
| C12APG | 0.19 | ||||
| C12/14APG | 0.40 | ||||
| (0.8)SLS:(0.2)C10APG | 2.10 | 3.80 | −1.62 | 0.704 | 0.628 |
| (0.6)SLS:(0.4)C10APG | 2.00 | 3.72 | −1.85 | 0.521 | 0.736 |
| (0.4)SLS:(0.6)C10APG | 1.80 | 3.02 | −2.63 | 0.319 | 0.741 |
| (0.2)SLS:(0.8)C10APG | 1.10 | 2.54 | −4.97 | 0.104 | 0.603 |
| (0.8)SLS:(0.2)C12APG | 0.46 | 0.85 | −3.49 | 0.180 | 0.730 |
| (0.6)SLS:(0.4)C12APG | 0.24 | 0.45 | −4.60 | 0.083 | 0.723 |
| (0.4)SLS:(0.6)C12APG | 0.17 | 0.31 | −5.40 | 0.043 | 0.738 |
| (0.2)SLS:(0.8)C12APG | 0.15 | 0.23 | −5.66 | 0.023 | 0.820 |
| (0.8)SLS:(0.2)C12/14APG | 0.70 | 1.58 | −3.95 | 0.217 | 0.567 |
| (0.6)SLS:(0.4)C12/14APG | 0.54 | 0.92 | −3.54 | 0.166 | 0.745 |
| (0.4)SLS:(0.6)C12/14APG | 0.44 | 0.64 | −3.50 | 0.118 | 0.845 |
| (0.2)SLS:(0.8)C12/14APG | 0.32 | 0.49 | −4.80 | 0.048 | 0.815 |
aCMC was calculated from plot of surface tension versus logarithm of surfactant concentration
bCMC calculated using Eq. 1
c f 1 and f 2 are the activity coefficients of surfactants 1 and 2, respectively, in the mixed micelles
In Vitro Dissolution Studies
The dissolution studies were performed in 900 ml of distilled water using USP Apparatus II, paddle method. Samples equivalent to 100 mg of aceclofenac (150 mg solid dispersion) were used for dissolution studies. The stirring speed employed was 50 rpm, and the temperature was maintained at 37 ± 0.5°C. Five milliliter aliquots of dissolution medium were withdrawn at predetermined time intervals (5, 15, 30, 45, 60 and 75 min) and replaced by 5 ml of fresh dissolution medium. Samples were filtered through 0.45 mm filters and the filtrates were then analyzed for the content of drug using UV spectrophotometry (JASCO 530 S Spectrophotometer, Tokyo, Japan) at 275 nm. It was ascertained from previous tests that there was no interference in the UV absorption due to the presence of carriers dissolved in dissolution medium.
Dissolution studies were also carried out for plain drug in dissolution medium containing dissolved surfactant and surfactant blends (amount equivalent to the full amount present in solid dispersions), to ascertain that the dissolution enhancement of aceclofenac was due to formation of solid dispersion and not due to the mere presence of surfactant in the medium. Each experiment was performed in triplicate; the coefficient of variation associated with each measurement was never >3%.
Solubility Measurement
Equilibrium solubility measurement was carried out to find the effect of surfactant blend on the solubility of aceclofenac. The study was carried out as follows: 25 ml of each of the medium (with and without surfactants) were measured and transferred to stoppered conical flask followed by addition of excess of acelcofenac (1 g). Formed suspensions were then shaken using mechanical shaker at 25°C for 48 hrs. After reaching equilibrium, each sample was filtered through membrane filter (0.45 μm, 13 mm, Whatman, USA) and aliquots were then used for quantification of drug using UV spectroscopy at 275 nm.
X-ray Diffraction
X-ray diffraction patterns were obtained by using a Diano X-ray diffractometer (Woburn, USA) equipped with Co Kα. The tube operated at 45 kV, 9 mA and the scan range used was 10–30° of the diffraction angle 2θ. Equal amount of samples was accurately weighed and used in the study.
Fourier-Transform Infrared Spectroscopy
Fourier transform infrared (FT-IR) spectra of the samples were obtained in the range of 400 to 4000 cm−1 using a Jasco-FT-IR spectrophotometer (Jasco, Essex, UK) by the KBr disc method.
Scanning Electron Microscopy
SEM analysis was performed using a Philips XL-30 SEM (Basel, The Netherlands). Before examination, samples were gold-sputter coated using SEM Gold Sputtering System 6 (coating thickness 20 nm), to render them electrically conductive.
Zeta Potential Measurement
Zeta potential measurement was varied out using Zeta-Meter System 3.0 system (Zeta Meter Inc., NY, USA). Zeta potential measurement was done to identify the surface charge phenomena. The net surface charge of solid dispersion was measured to derive the zeta potential. Briefly, charged colloid was placed between two electrodes having a DC voltage across them, and the velocity of the movement of colloid in presence of voltage difference was measured giving us the values of zeta potential.
Statistical Analysis
Experiments for in vitro evaluation (solubility, dissolution and zeta potential measurement) were conducted in triplicate and the results were reported in terms of mean ± standard deviation. An unpaired Student’s t test was used to compare the means for each study to assess the statistical significance. Results were considered statistically significant at P < 0.05.
RESULTS AND DISCUSSION
Surface Tension Measurement
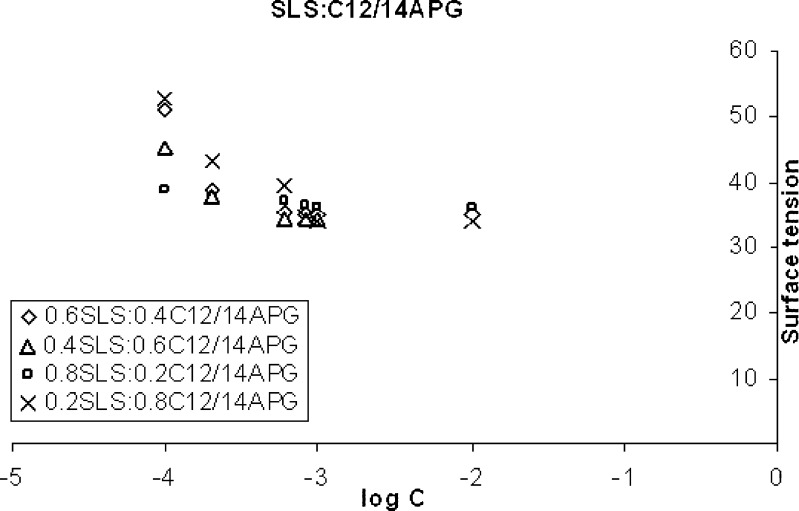
Representative values of the plots of surface tension (γ) versus logarithm of various surfactants concentrations (log C) (Fig. 2), were used to calculate critical micelle concentration of pure and mixed surfactants and the result of the same is shown in Table I. The reproducibility of (γ) measurements was within ± 0.1 dynes/cm. The values were calculated from the slope of plots of surface tension versus log C. Slope of the plots were found out using regression analysis. The nature and strength of interaction between two surfactants can be determined by Rubingh’s theory (13). According to Rubingh, the mixed critical micelle concentration (C12) for a binary surfactant system obtained by mixing two surfactants is given by the equation,
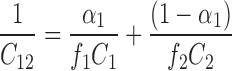 |
1 |
α1 is the mole fraction of surfactant 1 in total mixed solute C1 and C2, where C1and C2 are the critical micelle concentration values of surfactant 1 and 2 respectively, f1 and f2 are the activity coefficients of surfactant 1 and 2 respectively. In case of ideal behavior, f1 = f2 = 1, hence Eq. 1 changes to the Eq. 2, as proposed in literature for ideal mixed micelles (13).
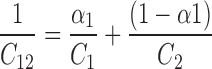 |
2 |
Fig. 2.
Surface tension (dyne/cm) vs log C (mM) plots for SLS and C12/14APG
However, in the present study the condition (f1 = f2 = 1) for ideal state was not satisfied and hence CMC values were calculated using Eq. 1. The critical micelle concentration values obtained experimentally (Table I) were found to be lower than the values calculated from Eq. 1.
The nature and strength of the interaction between the two surfactants in mixed systems are determined by calculating the values of β parameter from plot of surface tension (γ) versus concentration (C) of aqueous solutions of the individual surfactants and their mixtures. The β values calculated for all anionic–nonionic mixed surfactant systems by using Eq. 3 (derived from phase separation model by Rubingh) (12).
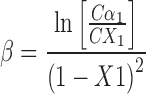 |
3 |
where, X1 = mole fraction of surfactant.
The β values calculated for SLS+C10APG, SLS+C12APG and SLS+C12/14APG systems were negative thus indicating a considerable interaction between nonionic and anionic surfactants. The synergistic behavior can be explained, by assuming strong interaction between head groups of nonionic–ionic surfactants and hydrophobic–hydrophobic attraction of two surfactants (14). From the X1 values, it was convenient to calculate the quantities f1 and f2 using the Eqs. 4 and 5.
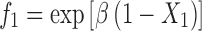 |
4 |
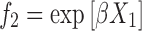 |
5 |
f1 and f2 are the activity coefficients of surfactants 1 and 2, respectively in the mixed micelles. The very low activity coefficient values of mixed micelle probably indicate that these components in the mixed micelle were far away from their standard states, where the activity coefficient should be unity.
In Vitro Dissolution Studies
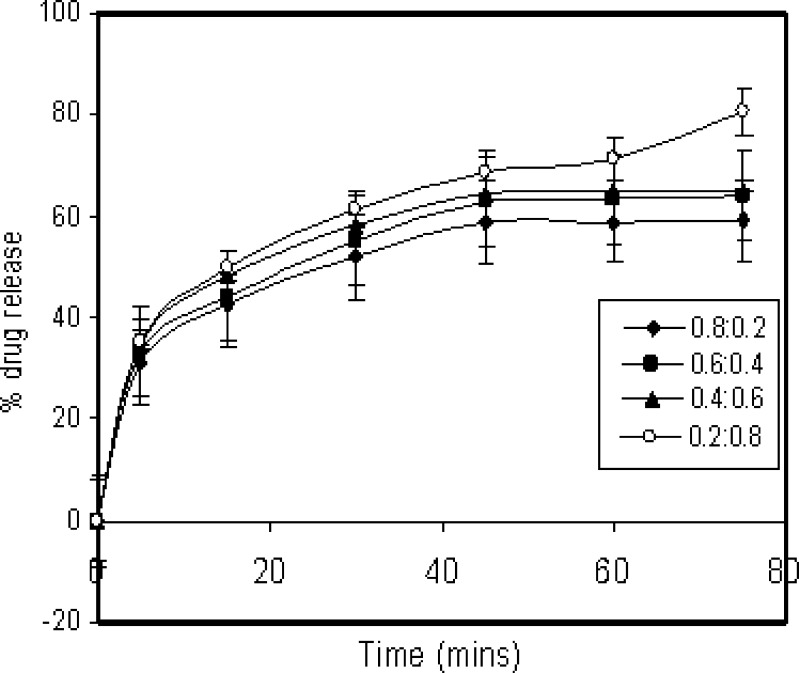
The release rate of aceclofenac from solid dispersions was analyzed and the results are presented in Table II where terms Q5 min and Q15 min represent % drug dissolved within 5 and 15 min respectively. It can be seen that dissolution results that mere presence on surfactants in the medium did not show a major effect on the drug release with maximum drug release of less that 5%. The improvement in the dissolution rate in SD can be attributed to the physical state of SD obtained on co-evaporation of solvent, which increases the effective surface and in-turn, affects the wettability of the drug considerably. It was observed that there was a sudden increase in the initial rate for drug dissolution. This could be attributed to the probable transition of dissolved drug into solubilized mixed micelles formed due to the presence of surfactants. Among single surfactant system SLS was found to give best results while C10APG, C12APG, C12/14APG showed moderate dissolution enhancement (Figs. 3 and 4). Among mix surfactant systems, SLS/C12/14APG was found to give best results. SLS/C12APG and SLS/C10APG showed slightly lesser dissolution rate enhancement than SLS/C12/14APG systems. This could be explained from the fact that C12/14APG has the lowest critical micelle concentration attributed to its longer hydrophobic tail compared to C10APG and C12APG.
Table II.
Percent drug dissolved within 5 and 15 min (Q 5 min and Q 15 min) of aceclofenac-surfactants systems in water (standard deviation in all cases was less than 5%)
| Surfactant | a Q 5 min | Q 5 min | a Q 15 min | Q 15 min |
|---|---|---|---|---|
| No surfactant | 0.5 ± 0.01 | 0.5 ± 0.01 | 0.5 ± 0.08 | 0.5 ± 0.08 |
| SLS | 2.04 ± 0.11 | 26.54 ± 0.19 | 2.25 ± 0.12 | 42.85 ± 0.41 |
| C10APG | 1.01 ± 0.02 | 18.63 ± 0.92 | 2.51 ± 0.14 | 24.56 ± 0.12 |
| C12APG | 1.32 ± 0.12 | 19.36 ± 0.62 | 2.66 ± 0.03 | 25.63 ± 0.79 |
| C12/14APG | 1.52 ± 0.10 | 24.63 ± 0.32 | 2.02 ± 0.01 | 28.36 ± 0.32 |
| (0.8)SLS:(0.2)C10APG | 3.04 ± 0.02 | 31.00 ± 0.66 | 3.91 ± 0.04 | 42.41 ± 0.42 |
| (0.6)SLS:(0.4)C10APG | 3.41 ± 0.14 | 33.00 ± 0.71 | 4.37 ± 0.21 | 44.36 ± 0.56 |
| (0.4)SLS:(0.6)C10APG | 3.99 ± 0.18 | 34.90 ± 0.01 | 4.23 ± 0.19 | 48.43 ± 0.96 |
| (0.2)SLS:(0.8)C10APG | 3.05 ± 0.21 | 35.51 ± 0.88 | 4.97 ± 0.22 | 50.17 ± 0.74 |
| (0.8)SLS:(0.2)C12APG | 2.21 ± 0.17 | 33.81 ± 0.02 | 3.03 ± 0.19 | 47.53 ± 0.95 |
| (0.6)SLS:(0.4)C12APG | 3.12 ± 0.27 | 36.02 ± 0.84 | 3.17 ± 0.36 | 50.17 ± 0.36 |
| (0.4)SLS:(0.6)C12APG | 3.24 ± 0.36 | 38.24 ± 0.66 | 3.28 ± 0.33 | 54.21 ± 0.63 |
| (0.2)SLS:(0.8)C12APG | 4.77 ± 0.26 | 39.07 ± 0.06 | 4.82 ± 0.34 | 69.85 ± 0.43 |
| (0.8)SLS:(0.2)C12/14APG | 4.82 ± 0.41 | 39.52 ± 0.61 | 4.93 ± 0.36 | 49.21 ± 0.76 |
| (0.6)SLS:(0.4)C12/14APG | 4.84 ± 0.15 | 40.83 ± 0.16 | 4.96 ± 0.02 | 54.26 ± 0.02 |
| (0.4)SLS:(0.6)C12/14APG | 4.25 ± 0.07 | 41.20 ± 0.06 | 4.57 ± 0.16 | 60.57 ± 0.66 |
| (0.2)SLS:(0.8)C12/14APG | 4.12 ± 0.05 | 45.66 ± 0.65 | 4.94 ± 0.52 | 74.84 ± 0.32 |
a Q 5 min and Q 15 min are values for percent drug dissolved of pure drug in medium containing exactly the same amount used in respective solid dispersion
Fig. 3.
Dissolution pattern of aceclofenac with SLS (1 mM), C10APG (1 mM), C12APG (1 mM) and C12/14APG (1 mM)
Fig. 4.
Dissolution pattern of aceclofenac in presence of mixed surfactants
Further, possible explanations of the increased dissolution rate of solid dispersions can be assumed to be due to following factors: reduction of drug crystallite size, a solubilization effect of the carrier, absence of aggregation of drug crystallite size, improved wettability and dispersibility of the drug, dissolution of the drug in the hydrophilic carrier and finally a combination of these.
Effect of Surfactant on Solubility
Table III lists aceclofenac solubility in the presence and absence of surfactants. It was seen that the mixed surfactant blends showed higher solubility enhancement as compared to the individual surfactants. Drug solubility increases relatively with increase in APG concentration in the mixed surfactant blend as expected. The highest concentration of APG in SLS/C10APG, SLS/C12APG and SLS/C12/14APG shows 90-, 99- and 105-fold enhancement in solubility respectively. Further, with the increase in the alkyl chain length of APG, there is a relative increase in the solubility of aceclofenac, attributed to the lowering of CMC with increase in the alkyl chain length. As discussed earlier, the interaction of surfactants leads to synergism in the properties and performance in mixed surfactant mixture. Likewise, the lower solubilization efficacy of single surfactants compared to that obtained with mixed surfactant systems is attributed to absence of these interactions.
Table III.
Solubility of aceclofenac in the presence and absence of surfactants
| Surfactant | Total surfactant concentration (mM) | Aceclofenac solubility (mM) | f f a | f m a |
|---|---|---|---|---|
| No surfactant | – | 0.00254 | 1 | 0 |
| SLS | 1.10 | 0.157 ± 0.005 | 0.016 | 0.984 |
| C10APG | 2.20 | 0.115 ± 0.002 | 0.022 | 0.978 |
| C12APG | 0.19 | 0.118 ± 0.001 | 0.021 | 0.978 |
| C12/14APG | 0.40 | 0.131 ± 0.002 | 0.019 | 0.980 |
| (0.8)SLS:(0.2)C10APG | 2.10 | 0.166 ± 0.003 | 0.015 | 0.984 |
| (0.6)SLS:(0.4)C10APG | 2.00 | 0.180 ± 0.004 | 0.014 | 0.985 |
| (0.4)SLS:(0.6)C10APG | 1.80 | 0.183 ± 0.002 | 0.013 | 0.986 |
| (0.2)SLS:(0.8)C10APG | 1.10 | 0.227 ± 0.008 | 0.011 | 0.989 |
| (0.8)SLS:(0.2)C12APG | 0.46 | 0.192 ± 0.003 | 0.013 | 0.986 |
| (0.6)SLS:(0.4)C12APG | 0.24 | 0.197 ± 0.004 | 0.012 | 0.987 |
| (0.4)SLS:(0.6)C12APG | 0.17 | 0.211 ± 0.005 | 0.012 | 0.988 |
| (0.2)SLS:(0.8)C12APG | 0.15 | 0.244 ± 0.006 | 0.010 | 0.989 |
| (0.8)SLS:(0.2)C12/14APG | 0.70 | 0.194 ± 0.001 | 0.013 | 0.987 |
| (0.6)SLS:(0.4)C12/14APG | 0.54 | 0.223 ± 0.009 | 0.011 | 0.988 |
| (0.4)SLS:(0.6)C12/14APG | 0.44 | 0.241 ± 0.008 | 0.010 | 0.989 |
| (0.2)SLS:(0.8)C12/14APG | 0.32 | 0.270 ± 0.007 | 0.009 | 0.990 |
a f f and f m are fractions of drug that free in solution (f f) and micelle-incorporated (f m)
The values of fraction of micelle-incorporated (fm) and free drug in solution (ff) are listed in Table III. It is a general observation that the higher surfactant concentrations leads to greater fm and smaller ff. In the present study, the vast majority of aceclofenac was micelle-bounded in all mixed surfactant systems (SLS/C10APG, SLS/C12APG, and SLS/C12/14APG). Thus, this could explain the increased solubility of aceclofenac in the mixed surfactant systems.
Scanning Electron Microscopy
SEM images (Fig. 5) of solid dispersions shows that there is a reduction in the crystallinity of aceclofenac. The reduced crystallinity of aceclofenac in different solid mixtures was further confirmed from the results of XRD patterns and FT-IR spectrophotometry.
Fig. 5.
SEM photographs of a pure aceclofenac b aceclofeanac + 0.2 SLS:0.8 C10APG c aceclofeanac + 0.2 SLS:0.8 C12APG d aceclofeanac + 0.2 SLS:0.8 C12/14 APG
X-ray Diffraction (XRD)
Numerous distinct peaks in diffraction spectrum of pure aceclofenac indicate crystalline nature of drug. The characteristic peaks for pure aceclofenac, mixture of aceclofenac with surfactants are shown in Fig. 6. From XRD graph, Intensity, Sin2θ, values of h2 + k2 + l2, Miller Indices (hkl), unit cell parameter (a) and inter planner spacing (d value) can be calculated. In the case of pure drug, the major peaks were observed at 2θ and d-spacing of 25.96 (3.4323 Ǻ) and 11.48 (7.709 Ǻ). Intensity ratios (I/Io) were observed for 2θ at 25.96, 17.48 and 22.26. All the principal peaks from pure drug, pure drug + SLS, Pure dug + SLS + C10APG, Pure dug + SLS + C12APG and Pure dug + SLS + C12/14APG were present in their respective solid dispersions, although with lower intensity, but no new peaks could be observed, suggesting the absence of any chemical interaction between the drug and the carrier. The prominent peak from pure aceclofenac at 2θ of 17.48° was almost seen at the same position in the solid dispersions but its intensity was remarkably decreased as the concentration of the surfactants blend changed. Several other 2θ values of pure drug and mixed surfactants system show almost same values while intensity was moderately changed. From these observations we can conclude that the crystalline nature of the drug was still maintained, but the relative reduction of diffraction intensity of aceclofenac in surfactant blends preparation at these angles suggests that the crystallinity was reduced. Same results can be concluded from the SEM images. Pure drug XRD shows drug has cubic structure which is maintain through out solid dispersion micelle formation. Further, the average of unit cell parameter (a) is almost constant for both i.e. pure drug and surfactant systems. Moreover, the miller indices remained same for single surfactant (SLS) as well as mixed surfactant systems. Thus, the average of unit cell parameter (a) and miller indices (hkl) suggest that pure drug as well as micelle bounded solid dispersed drug both have cubic geometry.
Fig. 6.
Powder X-ray diffraction patterns of solid dispersions of drug with different carriers
Fourier-Transform Infrared Spectroscopy
Fourier-transform infrared (FT-IR) spectroscopy was used to further characterize to ascertain possible interactions between the drug and the carrier in the solid state. From the structures of aceclofenac, SLS, C10APG, C12APG and C12/14APG it can be assumed that possible interaction could occur between the carboxylic acid and secondary amine groups of aceclofenac sulfate and hydroxyl groups of drug and carriers. In this case any sign of interaction would be reflected by a change in N–H and O–H vibration, depending on the extent of interaction. In the case of SLS, the site of interaction is expected to be at the SO3- group. From the knowledge of structures of aceclofenac, SLS and APG the potential interaction could occur between basic function (N–H) of drug and the acidic group (SO3-) of the carriers. In the case of APG, the site of interaction is expected to be at the C=O group of aceclofenac and OH group of carriers. Hence any sign of interaction would be reflected by a change in the position of C=O, N–H, S=O and O–H stretching. Figure 7 shows FT-IR spectrums of the various carriers with aceclofenac. The spectrum of aceclofenac shows peaks assigned to the O–H, N–H, C=O, C–O and Cl groups that were found at 3300 to 3600, 3319.6, 1717.1, 1150.2 to 1055.9,750 to 700 cm−1 respectively. The spectrum of aceclofenac and SLS shows, all the important functional group peaks including aliphatic C–H starching at 2905.7 and 2853.8 cm−1 which are characteristic peaks for long chain fatty alkyl group of surfactants. The incorporation of carriers (SLS, C10APG, C12APG, C12/14APG) did not modify their peaks position and trends. These results further indicate the absence of well-defined interaction between aceclofenac and SLS, C10APG, C12APG, C12/14APG which was already confirmed from the powder X-ray diffraction study.
Fig. 7.
FT-IR spectra of solid dispersions of drug with different carriers
Zeta Potential Measurement
Zeta potential measurement was done to identify the surface charge phenomena. The net surface charge of solid dispersion is easily measured because charged colloid will move when the suspension is placed between two electrodes that have a DC voltage across them, and its velocity will be proportional to the zeta potential. Table IV gives the values for the zeta potential of various mixed surfactant blends. Zeta potential of pure SLS is −74.55 mV and as the APG is increased the zeta potential is decreased up to −68.45 mV for 0.2 SLS + 0.8 APG. Exceptional values were obtained for 0.2 SLS + 0.8 APG mole ratios owing to a strong synergistic interaction among the components of the surfactant blends. The results here are in agreement with the values obtained for the interaction parameter β which shows negative values indicating strong interaction between anionic surfactant (SLS) and nonionic surfactant (APG).
Table IV.
Zeta potential values in mV
| No. | Surfactants blends | Average ZP (in mV) |
|---|---|---|
| 1 | SLS | −74.5 ± 0.07 |
| 2 | 0.2 SLS and 0.8 C12/14APG | −68.4 ± 0.04 |
| 3 | 0.4 SLS and 0.6 C12/14APG | −68.9 ± 0.01 |
| 4 | 0.6 SLS and 0.4 C12/14APG | −53.2 ± 0.02 |
| 5 | 0.8 SLS and 0.2 C12/14APG | −71.4 ± 0.08 |
CONCLUSION
Results obtained in the present investigation confirmed that mixed surfactant systems (APG: SLS) can be efficiently used as a carrier system for preparation of solid dispersion.
References
- 1.Valizadeh H., Nokhochi A., Qarakhani N., Zakeri-Milani P., Azarmi S., Hassanzadeh D., Lobenberg R. Physicochemical characterization of solid dispersions of Iodomethacin with PEG 6000, Myrj 52, Lactose, Sorbitol, Dextrin, and Eudragit E100. Drug Dev. Ind. Pharm. 2004;30:303–311. doi: 10.1081/DDC-120030426. [DOI] [PubMed] [Google Scholar]
- 2.Sekiguchi K., Obi N. Studies on absorption of eutectic mixture. I. A comparison of the behavior of eutectic mixture of sulfathiazole and that of ordinary sulfathiazole in man. Chem. Pharm. Bull. 1961;9:866–872. [Google Scholar]
- 3.Chiou W. L., Reigelman S. Pharmaceutical applications of solid dispersion systems. J. Pharm. Sci. 1971;60:1281–1302. doi: 10.1002/jps.2600600902. [DOI] [PubMed] [Google Scholar]
- 4.Leuner C., Dressman J. Improving drug solubility for oral delivery using sold dispersions. Eur. J. Pharm. Biopharm. 2000;50:47–60. doi: 10.1016/S0939-6411(00)00076-X. [DOI] [PubMed] [Google Scholar]
- 5.Behavior and applications of surfactant mixtures: Future perspectives. In J. F. Scameehorn (ed.), Phenomena in Mixed Surfactant Systems. ACS Symposium series; American Chemical Society: Washington, DC, 1986, pp. 324–330.
- 6.Blankschtein D., Shilach A. Predicting micellar solution properties of binary surfactant mixtures. Langmuir. 1998;14:1618–1636. doi: 10.1021/la971151r. [DOI] [Google Scholar]
- 7.P. Mukharjee, and K. J. Mysels. Critical micelle concentrations of aqueous surfactant system. Nat. Stand. Ref. Data. (USA), Washington, DC, 1971.
- 8.Zhang J., Li G.-Z. Phase behavior of APG/Alcohol/Alkane/Water mixture. J. Disper. Sci. Tech. 2004;25:27–34. doi: 10.1081/DIS-120027665. [DOI] [Google Scholar]
- 9.Abe M., Ogino K. Mixed Surfactant Systems; Surfactant Science Series. New York: Marcel Dekker; 1992. [Google Scholar]
- 10.Hinz B., Auge D., Rau T., Rietbrock S., Brune K., Werner U. Simultaneous determination of aceclofenac and three of its metabolites in human plasma by high-performance liquid chromatography. Biomed. Chromatogr. 2003;17:268–275. doi: 10.1002/bmc.243. [DOI] [PubMed] [Google Scholar]
- 11.Martin-Mola E., Gijon-Banos J., Ansoleaga J. J. Aceclofenac in comparison to Ketoprofen in the treatment of rheumatoid arthritis. Rheumatol. Int. 1995;15:111–116. doi: 10.1007/BF00302127. [DOI] [PubMed] [Google Scholar]
- 12.Joshi V. Y., Sawant M. R. A convenient stereo selective synthesis of b-d- Glucopyranosides. Ind. J. Chem. 2006;45:461–467. [Google Scholar]
- 13.Rubingh D. N. Mixed micelle solution. In: Mittal K. L., editor. Solution Chemistry of Surfactants. New York: Plenum Press; 1979. pp. 337–354. [Google Scholar]
- 14.Clint J. H. Micellization of mixed nonionic surface active agents. J. Chem. Soc. Faraday Trans. 1975;73:1327–1332. [Google Scholar]