Abstract
The identification of the bacterial endotoxin receptors for innate immunity, most notably the Toll-like receptor 4 (TLR4), has sparked great interest in therapeutic manipulation of innate immune system. We have recently developed synthetic molecules that have been shown to inhibit TLR4 activation in vitro and in vivo. Here we present the synthesis and the biological characterization of a new molecule, the cationic amphiphile 3,4-bis(tetradecyloxy)benzylamine, with a structure strictly related to the previously developed TLR4 modulators. This compound is able to inhibit in a dose-dependent manner the LPS-stimulated TLR4 activation in HEK cells. In order to characterize the mechanism of action of this compound, we investigated possible interactions with the extracellular components that bind and shuttle LPS to TLR4, namely LBP, CD14, and MD-2. This compound inhibited LBP/CD14-dependent LPS transfer to MD-2.TLR4, resulting in reduced formation of a (LPS-MD-2-TLR4)2 complex. This effect was due to inhibition of the transfer of LPS from aggregates in solution to sCD14 with little or no effect on LPS shuttling from LPS/CD14 to MD-2. This compound also inhibited transfer of LPS monomer from full length CD14 to a truncated, polyhistidine tagged CD14. Taken together, our findings strongly suggest that this compounds inhibits LPS-stimulated TLR4 activation by competitively occupying CD14 and thereby reducing the delivery of activating endotoxin to MD-2.TLR4.
Keywords: TRL4, CD14, LPS, endotoxin, septic shock, inflammation
1. Introduction
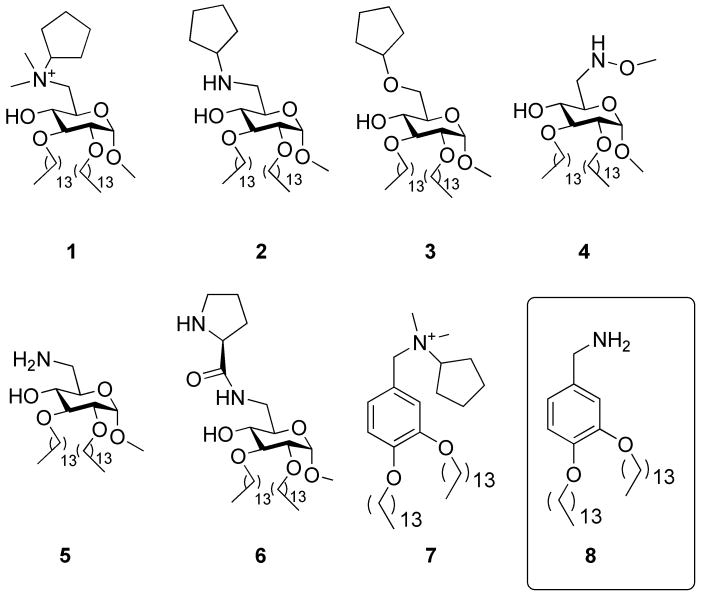
The ability of an organism to protect itself from microbial challenge requires the rapid organization of “pathogen associated molecular patterns” that are recognized by a variety of pathogen sensors, in particular Toll-like receptor 4 (TLR4) that activate the host response by rapidly triggering pro-inflammatory processes [1-3]. Among microbial components, lipopolysaccharides (LPS) and lipooligosaccharides (LOS) and their bioactive portions, the lipodisaccharide lipid A, commonly defined as endotoxin (E), are potent stimulants of immune responses [4]. The induction of inflammatory responses by LPS is achieved by the coordinate and sequential action of four principal endotoxin-binding proteins: the lipopolysaccharide binding protein (LBP), the cluster differentiation antigen (CD14), the myeloid differentiation protein (MD-2) and Toll-like receptor 4 (TLR4) [5]. LBP interacts with endotoxin-rich bacterial membranes and purified endotoxin aggregates, catalyzing extraction and transfer of E monomers to CD14 that in turn transfers endotoxin to MD-2 and to MD-2-TLR4 heterodimer [6]. The role of CD14 seems to be fundamental when endotoxin has very low concentration and when TLR4 is stimulated by the smooth LPS (LPS with the entire O-chain oligosaccharide), while the rough chemotype of LPS can stimulate efficiently TLR4 even in the absence of CD14 [7]. Besides being an essential chaperone or matchmaker assisting the TLR4-MD-2-endotoxin complex formation, CD14 very likely has an important role in cellular pathways not necessarily related to the TLR4 signaling. It has been recently discovered that CD14 regulates dendritic cells life cycle after LPS exposure through a signal pathway based on NFAT activation, totally independent from the TLR4-activated intracellular pathway [8]. Moreover, although the role of CD14 in LPS response is well established, the participation of CD14 to other TLR-dependent ligands has been less well studied. In particular, it has been shown that CD14 contributes to innate immune response activated by other, non-LPS ligands, including many TLR1-TLR2 ligands [9,10]. Some recent studies also suggest that therapies targeting CD14 may also interfere with TLR3 activation by viral nucleic acids, thus holding out the possibility that these agents may be effective in the control of viral as well as bacterial diseases in which excess immune responsiveness damages the host [11]. With the aim to develop new lipid A mimetics containing a non-natural, non-hydrolizable glycosidic bond, we serendipitously found that amino glycolipids and aromatic ammonium salts (Figure 1) are active in inhibiting lipid A and LPS-promoted cytokines production in innate immunity cells such as macrophages or dendritic cells [12]. Molecule 1 and compounds with a similar chemical structure (Figure 1) inhibit LPS-induced TLR4 activation on HEK/TLR4 cells and LPS-induced septic shock in mice [13].
Figure 1.
Cationic amphiphiles derived from D-glucose (compounds 1-6) and from benzylamine (compounds 7 and 8).
Our structure-activity studies point out that amphiphilic compounds with a positively charged ammonium or protonatable nitrogen, such as compounds 1, 2, 5, 6 and 7 are active in blocking TLR4-mediated endotoxin stimulus, while very similar compounds lacking the positive charge (molecules 3 and 4) are totally inactive [13]. These molecules showed in vivo activity as inhibitors of LPS-induced lethality [13] and are also able to combat other pathologies caused by TLR4 activation, such as inflammation and neuropathic pain [14,15]. Interestingly, if the cyclic pyranose scaffold of the sugar is replaced by an aromatic ring, the biological activity is retained, and compound 7 (Figure 1) showed to be highly potent as antisepsis agent [13]. The mechanism of action of bioactive molecules 1, 2, 5, 6, 7 has been investigated and it was found that these compounds inhibit TLR4 activation by endotoxin by competitively occupying CD14 and thereby reducing the delivery of activating endotoxin to MD-2/TLR4 [16]. In this paper we present the synthesis and the characterization of the biological activity of 3,4-bis(tetradecyloxy)benzylamine 8, whose chemical structure is strictly related to the ammonium salt 7 (Figure 1). The rationale to design this compound is based on the following points. First, when replacing the alkyl ammonium group of 1 with the primary amine of 5 in sugar-derived compounds, the synthesis is simplified and the biological activity is retained in vitro, or even increased in vivo [13]. We therefore projected to do the same functional group change on compound 7, thus obtaining 8. Second, compounds containing alkyl ammonium groups are generally toxic, especially in the case of N-methyl ammonium ions present in compound 7, that are supposed to be methylating agents in vivo. Compound 8 was therefore designed with the aim to reduce the in vitro and in vivo toxicity. In this paper we present the synthesis and the biological characterization of compound 8 in terms of activity on cells, toxicity and binding to purified receptors.
2. Materials and methods
2.1. Chemistry, synthesis of 3,4-bis(tetradecyloxy)benzylamine
2.1.1. Tert-butyl 3,4-dihydroxybenzylcarbamate
To a solution of 3,4-dihydroxybenzylamine (90 mg, 0.41 mmol) in dry pyridine (4 mL) at 0°C, di-tert-butyldicarbonate (88 mg, 0.41 mmol) was added and the mixture was reacted for 4 h at 0°C. The solution was concentrated in vacuo and the residue was dissolved in AcOEt and washed with HCl 1 N solution and brine then dried with anhydrous sodium sulphate and evaporated in vacuo. The crude product was purified by flash chromatography on silica gel (EtP/AcOEt 7:3) to afford the tert-butyl 3,4-dihydroxybenzylcarbamate (78 mg, 78%) as a white solid. Rf (AcOEt/EtP 8:2): 0.65. 1H NMR (400 MHz, CD3OD): δ= 6.70 (s, 1 H, ArH-2), 6.68 (d, 1 H, ArH-6, J= 9.8 Hz), 6.56 (dd, 1 H, ArH-5, J= 8.0, 1.6 Hz), 4.05 (s, 2 H, -CH2N), 1.43 (s, 9 H, -C(CH3)3). 13C NMR (100 MHz, CD3OD): δ= 156.08, 149.02, 148.12, 135.14, 122.42, 118.92, 118.26, 47.48, 31.55.
2.1.2. Tert-butyl 3,4-bis(tetradecyloxy)benzylcarbamate
Tert-butyl 3,4-dihydroxybenzylcarbamate (50 mg, 0.21 mmol) was dissolved in dry DMF (5 mL) and potassium carbonate (60 mg, 0.42 mmol) was added. After 1 h at room temperature, tetradecanoyl bromide (174 mg, 0.62 mmol) was added dropwise and the mixture was reacted at 80 °C overnight. The solvent was concentrated in vacuo, the residue was dissolved in AcOEt and washed with water and brine. The organic phase was dried with anhydrous sodium sulphate and evaporated in vacuo and the crude product was purified by flash chromatography on silica gel (EtP/AcOEt 9:1) to afford tert-butyl 3,4-bis(tetradecyloxy)benzylcarbamate (110 mg, 83%) as a white solid. Rf (EtP/AcOEt 9:1): 0.40. 1H NMR (400 MHz, CDCl3): δ= 6.79 (m, 3 H, ArH-2,5,6), 4.21 (s, 2 H, -CH2N), 3.97 (t, 4 H, CH2α, J= 5.6 Hz), 1.79 (m, 4 H, CH2β), 1.46 (s, 9 H, -C(CH3)3), 1.25 (m, 44 H, CH2) 0.88 (t, 6 H, CH3, J= 6.0 Hz). 13C NMR (100 MHz, CD3OD): δ= 156.08, 149.50, 148.63, 131.79, 120.08, 114.10, 113.54, 69.62, 69.44, 63.93, 32.15, 29.93, 29.89, 29.86, 29.81, 29.66, 29.59, 29.51, 29.44, 28.62, 26.48, 23.01, 14.35.
2.1.3. 3,4-bis(tetradecyloxy)benzylamine
Tert-butyl 3,4-bis(tetradecyloxy)benzylcarbamate (750 mg, 1.27 mmol) was dissolved in dry CH2Cl2 under argon atmosphere, trifluoroacetic acid (3 mL, 39.17 mmol) was added dropwise and the solution was reacted for 2 h at room temperature. The mixture was concentrated in vacuo, the residue was dissolved in CH2Cl2 and washed with NaOH 0.1 M solution then the organic layer was dried with anhydrous sodium sulphate and evaporated in vacuo to afford 3,4-bis(tetradecyloxy)benzylamine (675 mg, quantitative) as white powder. Rf (AcOEt/MeOH/TEA 9:1:0.1): 0.42.1H NMR (400 MHz, CD3OD): δ= 6.95 (s, 1 H, ArH-2), 6.86 (m, 2 H, ArH-5,6), 3.97 (m, 4 H, CH2α), 3.71 (s, 2 H, CH2NH2), 1.76 (m, 4 H, CH2β), 1.28 (m, 44 H, CH2), 0.88 (t, 6 H, CH3, J= 6.0 Hz). 13C NMR (100 MHz, CD3OD): δ= 149.51, 148.21, 136.39, 119.43, 114.27, 113.18, 69.70, 69.45, 46.49, 32.17, 29.95, 29.91, 29.89, 29.68, 29.61, 29.57, 26.29, 22.94, 14.37.
2.2. Biology: Materials
Tritiated lipooligosaccharide ([3H]LOS, 25 000 cpm/pmol) was metabolically labeled and isolated from an acetate auxotroph of Neisseria meningitidis serogroup B as described. LBP and sCD14 were gifts from Xoma (Berkley, CA) and Amgen Corp. (Thousand Oaks, CA), respectively. Human serum albumin (HSA) was obtained as an endotoxin-free, 25% stock solution (Baxter Health Care, Glendale, CA). Chromatography matrices (Sephacryl HR S200 and S300) were purchased from GE Healthcare and metal chelation matrix, HisLink is from Promega. ESF921 medium for High Five insect cells was purchased from Expressions Systems. In the assays described below, stocks of molecule 8 were freshly prepared in a 75% DMSO/ 25% EtOH solution and then further diluted to the appropriate concentration as needed in PBS, pH 7.4, 0.1 % HSA, or DMEM 0.1 % HSA when used for cellular assays.
2.2.1. Preparation of recombinant proteins
Preparative amounts of sMD-2 and tCD14-His6 were generated from infections of High Five insect cells with baculovirus containing the cDNA for human MD-2-His6 inserted into pBAC3 or for human tCD14 (amino acids 1-156) inserted into pBAC11 as described previously [17]. Conditioned medium containing MD-2 was stored at -80 °C until needed. tCD14-His6 was purified using Ni FF Sepharose resin (GE Healthcare) with an imidazole gradient; purified tCD14-His6 was stored at 4 °C [17].
Conditioned medium containing MD-2 associated with TLR4 ectodomain (TLR4ECD) proteins (MD-2/TLR4ECD) was produced in HEK293T cells as described previously [6]. Expression vectors containing DNA of interest for production of FLAG-TLR4ECD, amino acids 24-631, (pFLAG-CMV-TLR4) and MD-2-FLAG-His (pEF-BOS) have been previously described and characterized [6]. Media containing secreted proteins were concentrated 10-20-fold using Millipore Centricon-10 before use. Conditioned medium containing secreted MD-2/TLR4ECD proteins maintained activity to react with [3H]LOS·sCD14 for at least 6 months when stored at 4 °C.
2.2.2. Preparation of [3H]LOSagg and [3H]LOS·sCD14 complex
[3H]LOSagg and [3H]LOS·sCD14 complex were prepared as previously described [6]. Briefly, [3H]LOSagg (Mr > 20×106) were obtained after hot phenol extraction of [3H]LOS followed by ethanol precipitation of [3H]LOSagg, and ultracentrifugation. Monomeric [3H]LOS·CD14 complexes (Mr~60 000) were prepared by treatment of [3H]LOSagg for 30 min at 37 °C with substoichiometric LBP (molar ratio 200:1 LOS:LBP) and 1-1.5 molar excess sCD14 followed by gel exclusion chromatography (Sephacryl S200, 1.6×70 cm column) in PBS, pH 7.4, 0.03% HSA to isolate monomeric [3H]LOS·sCD14 complex. Radiochemical purity of [3H]LOS·sCD14 was confirmed by S200 chromatography [6,17].
2.2.3. Preparation of [3H]LOS·Protein(s) complexes in the presence of molecule 8
[3H]LOS·MD-2·TLR4 complex was generated by a three-step process. sCD14 (0.8 nM, final concentration) was pre-incubated with LBP (4 pM, final concentration) ± molecule 8 (0-20 μM) for 30 min at 37 °C in PBS, pH 7.4, 0.1% HSA (0.875 uL). After this incubation, [3H]LOSagg (up to 5 uL for a final concentration of 0.8 nM) was added; the mixture was incubated for 30 min at 37 °C and then supplemented with conditioned HEK293T cell heterodimer [6] (125 uL for an estimated final medium containing preformed MD-2·TLR4ECD concentration of 0.2 nM) and incubated again for 15 min at 37 °C. Analysis of the reaction products was determined by gel size exclusion chromatography as described below. To examine the ability of the molecule to affect LOS-sCD14 transfer, sCD14 (0.8 nM, final concentration)was pre-incubated with molecule 8 (10 μM in PBS, pH 7.4, 0.1% HSA) for 30 min at 37 °C then [3H]LOS. (0.8 nM, final concentration) was added and the solution was incubated for 30 min at 37 °C. Products of the reactions were analyzed by size exclusion chromatography (Mr [3H]LOS.CD14~25 000 Da) and by co-capture to Ni chelating resin.
2.2.4. Size exclusion chromatography
Sephacryl S300 and S200 columns used for determination of apparent Mr of the [3H]LOS containing products were calibrated with the following Mr standards: blue dextran (2×106, V0), thyroglobulin (650 000), ferritin (440 000), catalase (232 000), IgG (158 000), HSA (66 000), ovalbumin (44 500), myoglobin (17 500), vitamin B12 (1200, Vi). The reaction mixture for the generation of [LOS·MD-2·TLR4ECD]2 was applied in a volume of 0.9 mL to a Sephacryl HR S300 column (1.6×70 cm) pre-equilibrated in PBS, pH 7.4, 0.1% HSA and eluted in the same buffer at a flow-rate of 0.5 mL/min at room temperature using AKTA Purifier or Explorer 100 fast protein liquid chromatography (GE Healthcare), with the collection of 1 mL fractions. Radioactivity in collected fractions was analyzed by liquid scintillation spectroscopy (Beckman LS liquid scintillation counter). Total recovery of [3H]LOS was ≥ 70% in all experiments. For formation of [3H]LOS·CD14 complexes, the chromatographic analyses were carried out, using a Sephacryl HR S200 column (1.6×30 cm) with the same experimental conditions.
2.2.5. Evaluation of transfer of [3H]LOS to His6-tagged proteins by co-capture to metal chelating resin
tCD14-His6 (10 uL for an estimated concentration of 1.6 nM) or His6-sMD-2 (final concentration of 1.2 nM in the reaction mixture) was pre-incubated in 0.3 mL PBS, 0.1% HSA in presence or absence of LBP for 30 min at 37 °C ± different concentrations of synthetic compounds. [3H]LOSagg or [3H]LOS·sCD14 was added followed by another incubation for 30 min at 37 °C. The reaction mixture was then incubated with HISLINK resin (20 uL) for 15 min at 25 °C, allowing His-tagged tCD14 and sMD-2 to be adsorbed onto beads. The resin was spun down for 2 min at 2000 g, supernatant was removed, and the resin was washed twice with 300 uL PBS, 1% HSA using the same procedure. The [3H]LOS absorbed onto the beads or recovered in the supernatant was quantified by liquid scintillation spectroscopy. Capture of [3H]LOS in the absence of His-tagged proteins was less than 12%.
2.2.6. HEK cell activation assay
293/hTLR4A-MD2-CD14 cells and parental HEK, as negative control, have been purchased by Invivogen and cultured as described [18]. For cell activation assays, 293/hTLR4A-MD2-CD14 or parental HEK cells were grown to confluency in 96-well plates. Cell monolayers were washed twice with warm PBS and incubated overnight at 37 °C, 5% CO2, and 95% humidity in 200 μL DMEM supplemented with molecule 8 (0-10 μM), and LPSagg (1 nM, from Sigma-Aldrich™) as stimulus. The final organic solvent (DMSO/ethanol) concentration per well is less then 0.5%. After the incubation, plates were centrifuged at 1000 rpm for 5 min and supernatants were collected. Activation of HEK cells was assessed by measuring the accumulation of extracellular IL-8 by ELISA according to supplier protocol (BD Clontech, Inc., Palo Alto, CA).
2.2.7. HEK-Blue™ assay
HEK-Blue™-4 cells (HEK-Blue™ LPS Detection Kit, InvivoGen) were cultured according to manufacturer’s instructions. Briefly, cells were cultured in DMEM high glucose medium supplemented with 10% fetal bovine serum (FBS), 2 mM glutamine, 1X Normocin™ (InvivoGen), 1X HEK-Blue™ Selection (InvivoGen). HEK-Blue™-4 cells were detached by the use of a cell scraper and the cell concentration was estimated by using a cell counter. The cells were diluted in DMEM high glucose medium supplemented with DMEM high glucose medium supplemented with 10% fetal bovine serum (FBS), 2 mM glutamine, 1X Normocin™ (InvivoGen), 1X HEK-Blue™ Selection (InvivoGen) and 200 μL of cell suspension (20 000 cells) were added to each well. After O.N. incubation at 37 °C in a CO2 incubator cells reached confluency and supernatant removed, cell monolayers were washed twice with warm PBS without Ca2+ and Mg2+ and incubated overnight at 37 °C, 5% CO2, and 95% humidity in 200 μL DMEM supplemented with molecule 8 (0-20 μM), and LPSagg (1 nM) as stimulus. After the incubation, plates were centrifuged at 1000 rpm for 5 min and supernatants were collected. 5μL of each sample were added to 95 μL PBS, pH 8, 0.84 mM pNpp for a final concentration of 0.8 mM pNpp. Plates were incubated for 2 h in the dark at RT then the plate reading was assessed by using a spectrophotometer at 405 nm. As a control, we treated the cells with ± LPS (1 nM) alone.
2.2.8. MTT cell viability assay® (Sigma-Aldrich™)
Parental HEK cells were seeded at a density of 2×104 cells/mL in 100 μL medium (DMEM without red phenol) per well in 96-well-plate. Compound 8 was diluted with PBS and added to the cells to yield a final concentration of 20 μM. 24 h after incubation at 37 °C, the medium was removed, monolayer washed two times with warm PBS and 100 μL of DMEM without phenol red + 0.05 mg MTT was added per well. After 2 h incubation at 37 °C and 5% CO2, formazan crystals were dissolved by adding 100 μL of MTT solubilization solution (anhydrous isopropanol 0.1 N HCl with 10% Triton X-100). Formazan concentration in the wells was determined measuring the absorbance at 570 nm. As positive control for cytotoxicity (C+), we set up triplicate wells containing cells treated with a compound known to be toxic to the cells.
3. Results
3.1. Synthesis of bis(tetradecyloxy)benzylamine 8
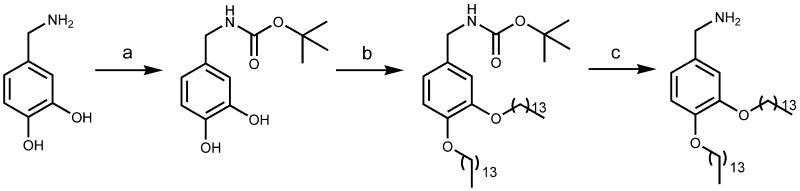
Bis(tetradecyloxy)benzylamine 8 was synthesized starting from commercially available 3,4-dihydroxybenzylamine according to Scheme 1. The amino group was protected as tert-butyloxycarbonyl (Boc) derivative using Boc anhydride in pyridine, then the tertbutyl 3,4-dihydroxybenzylcarbamate was reacted with tetradecanoyl bromide in the presence of potassium carbonate to afford the tertbutyl 3,4-bis(tetradecyloxy)benzylcarbamate. The amine deprotection using trifluoroacetic acid in dichloromethane gave finally bis(tetradecyloxy)benzylamine 8.
Scheme 1.
Synthesis of bis(tetradecyloxy)benzylamine 8. Reagents and conditions: (a) Boc2O, dry pyridine, 0°C, 4 h (78%); (b) tetradecanoyl bromide, K2CO3, dry DMF, 80°C, overnight (83%); (c) trifluoroacetic acid, RT, 2 h, quantitative yield.
3.2. Compound 8 inhibits LPS-stimulated TLR4 activation in HEK-293/hTLR4A-MD2-CD14 and HEK-Blue™-4 cells
The activity of compound 8 in modulating the LPS signaling through the TLR4 receptor was tested in vitro using HEK cells. Two type of cells were used: the HEK-293/hTLR4A-MD2-CD14 cells stably transfected with TLR4, MD2, and CD14 genes and HEK-Blue™-4 cells that, in addition, stably express an optimized alkaline phosphatase gene engineered to be secreted (sAP), placed under the control of a promoter inducible by several transcription factors such as NF-kB and AP-1. This reporter gene allows to monitor the activation of TLR4 signal pathway by endotoxin.
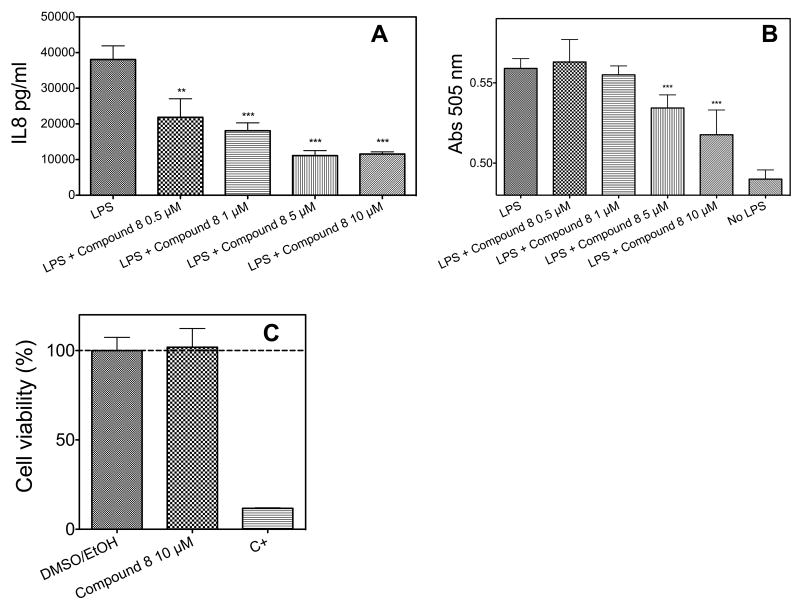
HEK-293/hTLR4A-MD2-CD14 cells were stimulated with LPS (Sigma-Aldrich) at a concentration of 1 nM after pre-exposure to the molecule 8, at four different concentrations ranging from 0.5 to 10 μM, and the production of the cytokine IL-8 was monitored (Figure 2A). A concentration-dependent inhibition of IL-8 production is observed. HEK-Blue™ -4 were treated with LPS after exposition to four concentrations of molecule 8, from 0.5 to 10 μM (Figure 2B). In this case too, a dose-dependent inhibition of phosphatase activity was observed, indicating an inhibition of TLR4 activation. In this latter case, the inhibitory effect of compound 8 is more evident, giving a 57% reduction of TLR4 activation and phosphatase activity when LPS reaches the concentration of 10 μM. To asses if molecule 8 could provide a change in IL-8 production in absence of TLR4 receptor complex, we treated parental HEK cells (that do not express TLR4) with increasing amount of the synthetic compound. No change in IL-8 production is observed in this latter case (Figure 2C). A viability assay was performed to verify if reduction in LPS-stimulated IL-8 by compound 8 is not due to a general cytotoxic effect. MTT test (Figure 2C) clearly showed that molecule 8 is not toxic.
Figure 2.
(A) Co-administration of LPS and increasing amount of molecule 8 (0-10 μM) to 293/hTLR4A-MD2-CD14 cells. Cell activation was measured as extracellular production of IL-8 through an ELISA assay. (B) Measurement of activated NF-κB level in HEK-Blue™ cells when increasing amount of molecule 8 (0-10 μM) and supplemented with LPS as stimulus. (C) MTT assay of parental HEK cells (that do not express TLR4) in the presence of 10 μM molecule 8, of 0.5 % DMSO/EtOH and of a positive control.
Results shown represent the mean SEM of three independent experiments, each in triplicate. The asterisks indicate a significant statistical difference (P<0.01; ANOVA, Dunnett’s test) between data in the analyzed group and reference data (LPS alone for panel A and DMSO/EtOH for panel C).
3.3 Compound 8 inhibits the formation of tritiated lipooligosaccharide-CD14 ([3H]LOS-CD14) and [3H]LOS-MD-2-TLR4 complexes
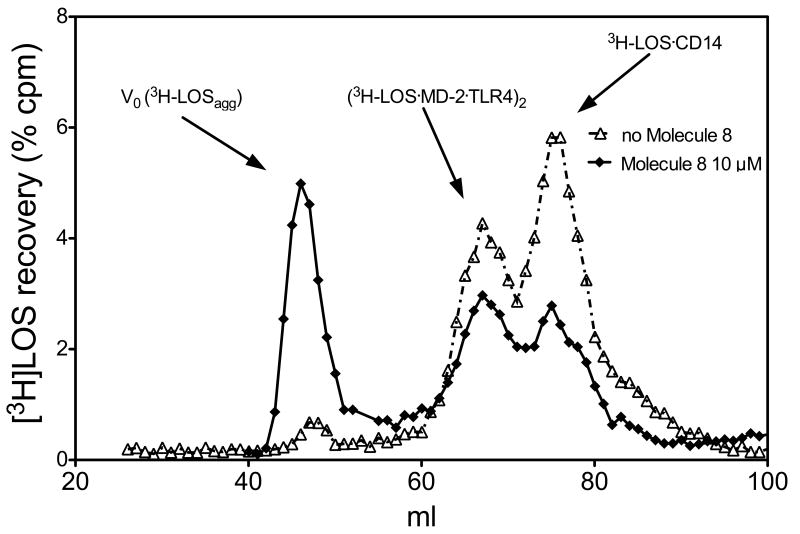
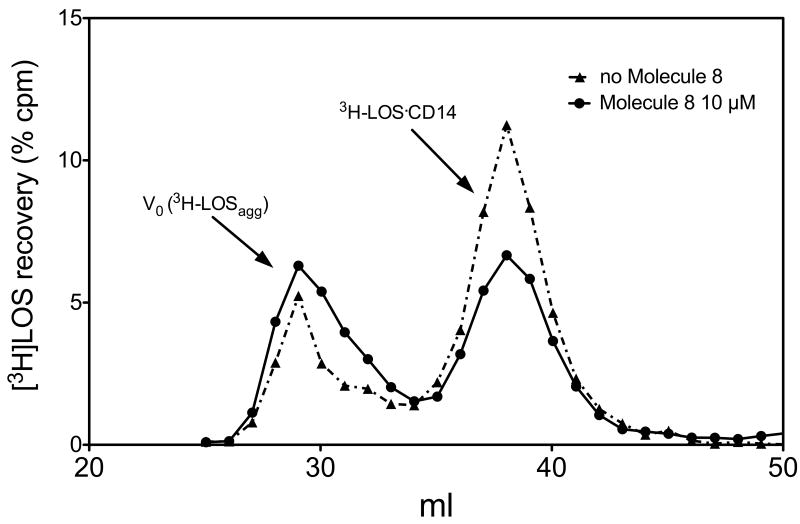
We then wanted to verify if the inhibition of lipooligosaccharide (LOS) signal by our compound is due to an interference with the formation of (LOS_MD-2_TLR4)2 complex. We have previously shown that both reactions of monomeric LOS-sCD14 with MD-2-TLR4ECD and of monomeric LOS.MD-2 with TLR4 ectodomain (TLR4ECD) produced a complex of apparent Mr 190 000 containing LOS, MD-2 and TLR4ECD that nearly matches the mass of a dimer of a 1:1:1 complex of LOS.MD-2.TLR4 [6]. We then modified the protocol of (LOS-MD-2-TLR4)2 complex formation for evidencing a possible interference of compound 8 in this process [16]. Soluble CD14 (sCD14) was pre-incubated with LBP in the presence or absence of molecule 8 (10 μM), then were added and incubated, successively, [3H]LOS and the preformed MD-2-TLR4 dimer. Size-exclusion chromatography by gel-filtration of the reaction mixture (Figure 3) showed that the presence of compound 8 inhibited the formation the peak corresponding to an Mr of about 190 KDa, corresponding to the (LOS.MD-2.TLR4)2 complex, while increasing the earlier eluting peak corresponding to high molecular weight LOS aggregates.
Figure 3.
Molecule 8 inhibits the formation of the [[3H]LOS-MD-2-TLR4ECD]2 complex. The incubation mixture containing [3H]-LOSagg, LBP, sCD14 and MD-2-TLR4ECD ± the synthetic compound (10 μM) were analyzed by gel-sieving analysis using a Sephacryl S300 as described in Materials and Methods section. Profiles shown are representative of 3 experiments for each condition (both in presence or absence of molecule 8).
Interestingly, also the peak corresponding to the mass of LOS-sCD14 complex is reduced when incubation is done in the presence of compound 8 (Figure 3).
3.4. Compound 8 inhibits the endotoxin loading on CD14
While being an interesting preliminary indication, the observed effect of inhibition of 190 KDa complex formation by molecule 8 could derive from the interference of this compound at different levels in the sequence of reactions leading to the formation of the complex. To gain further information, we divided the process into two main steps: (1) the formation of dimeric [3H]LOS-CD14 complex and (2) the subsequent transfer of LOS from the complex with CD14 to MD2. The first step is the LBP-aided formation of LOS.CD14 from LOS aggregates. We formed the complex by incubating LOS and CD14 in the presence or in the absence of 8 (10 μM). After incubation, the reaction mixture was analyzed by gel sieving chromatography using a Sephacryl HR S200 resin and the elution profile showed a reduction in the formation of the [3H]LOS-CD14 peak (Figure 4).
Figure 4.
Transfer of [3H]-LOS monomers to sCD14 is inhibited by 10 μM of molecule 8. Transfer of tritiated endotoxin from aggregates to sCD14 and formation of [3H]-LOS-sCD14 complex is monitored with Sephacryl S200 chromatografy. Profiles shown are representative of 3 independent experiments.
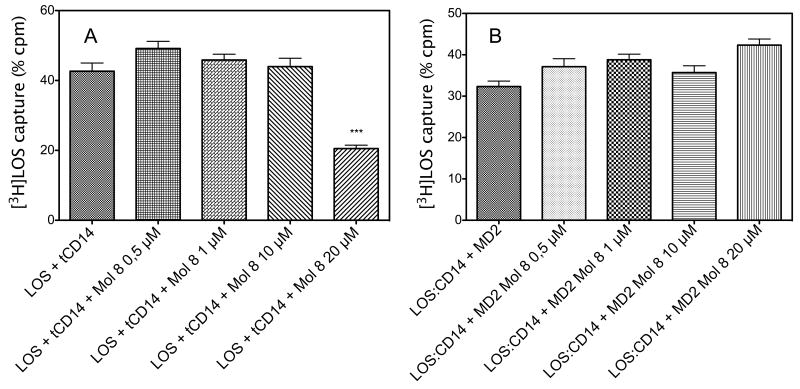
To further test this hypothesis, we performed an experiment of direct antagonism between 8 and LOS for CD14 binding. We have shown that LOS can be rapidly and efficiently transferred from LOS_CD14 to free CD14 [19]. A capture experiment was carried out in which [3H]LOS is transferred to His-tagged tCD14 from CD14 complex according to the following equilibrium: [3H]LOS-CD14 + tCD14 ↔ CD14 + [3H]LOS-tCD14. Once formed, the [3H]LOS.tCD14 complex is efficiently captured by Ni++-coated HISLINK resin and the resulting radioactivity quantified [16]. Unlike previous experiments, in which LOSagg were used, in this experiment LOS is present in monomeric form associated to CD14 in the [3H]LOS-CD14 complex. A preformed [3H]LOS.CD14 complex was incubated with His-tagged tCD14 ± molecule 8, the complex captured with resin (Figure 5A).
Figure 5.
(A) Dose dependent inhibition of [3H]-LOS transfer to His tagged tCD14. This capture assay shows the dependency between molecule 8 concentration and the inhibition of [3H]-LOS-sCD14 complex formation. (B) Molecule 8 does not inhibit [3H]-LOS transfer from CD14 to MD2. [3H]-LOS–MD-2 complex formation is not inhibited if MD-2 is pre-incubated with molecule 8 suggesting that the compound could not compete with LOS for MD-2 binding. Data shown are representative of 3 experiments, each in duplicate, and are expressed as mean of percent of radioactivity captured in comparison with total radioactivity measured ± SEM. The asterisks indicate a significant statistical difference (P<0.01; ANOVA, Dunnett’s test) between data in the analyzed group and reference data (sample without molecule 8).
The percents of radioactivity captured in different conditions are depicted in Figure 5 showing that 8 inhibits the transfer of monomeric LOS from CD14 complexes to soluble tagged CD14. A control capture experiment in which the endotoxin is transferred from [3H] LOS.CD14 to His-tagged MD-2 confirmed that molecule 8 does not inhibit this reaction (Figure 5B).
4. Discussion
Compound 8 is active in inhibiting in a dose-dependent way LPS-stimulated TLR4 activation in HEK cells stably transfected with TLR4, CD14, and MD-2 genes. After pre-incubation with compound 8, and subsequent treatment with LPS, we observed a reduction in the production of the IL-8 (Figure 2A) and, in the case of HEK-Blue™-4 cells, a dose-dependent inhibition of the NF-kB induced production of alkaline phosphatase (Figure 2B). Compound 8 did not present detectable cellular toxicity (Figure 2C). In order to clarify the mechanism of action at a molecular level, and identify its molecular target, we tested the effect of molecule 8 on the sequential extraction and transfer of tritiated lipooligosaccharide ([3H]LOS) monomers from [3H]LOSagg to LBP, CD14, and MD-2(·TLR4ECD), reactions that are key to efficient delivery of activating endotoxin to MD-2-TLR4 [18,6].
To do this, we performed an experiment of sequential receptor incubation in the presence and absence of compound 8, followed by mass determination of formed complexes by gel sieving chromatography. sCD14 was pre-incubated with LBP in the presence or absence of molecule 8, and then incubated with tritiated LOS in form of aggregates ([3H]LOS) followed by conditioned medium containing preformed MD-2-TLR4ECD heterodimer. Size-exclusion chromatography of the reaction mixture showed that, in the absence of molecule 8, virtually all [3H]LOS aggregates (LOSagg) were converted to later eluting species (i.e., smaller [3H]LOS-containing complexes) corresponding to, from the left, [[3H]LOS·MD-2·TLR4ECD]2 (Mr~190 000) and [3H]LOS·sCD14 (Mr~60 000). The identity of these complexes was confirmed by co-capture of [3H]LOS with immobilized monoclonal antibodies to CD14 [20] or to epitope tags in His6-MD-2 or in Flag-TLR4ECD [6] and by co-elution with purified, defined [3H]LOS·protein complexes.
In contrast, in the presence of 10 μM molecule 8, accumulation of both [3H]LOS·sCD14 and [[3H]LOS·MD-2.-TLR4ECD]2 was markedly reduced and most [3H]LOS was recovered in the void volume presumably as large [3H]LOS aggregates (Figure 3). The inhibition of accumulation of [3H]LOS·sCD14 suggested a primary effect of molecule 8 on LBP/sCD14-dependent extraction and transfer of [3H]LOS monomers from [3H]LOSagg to [3H]LOS·sCD14. To test this hypothesis more directly, we examined the effect of molecule 8 on LBP/sCD14-dependent conversion of [3H]LOSagg to [3H]LOS·sCD14. As shown in Figure 4, molecule 8 caused a clear reduction in conversion of [3H]LOSagg to [3H]LOS·sCD14, with decreased accumulation of [3H]LOS·sCD14 accompanied by increased recovery of [3H]LOS as high Mr LOS aggregates.
The above experiments demonstrate an ability of molecule 8 to inhibit extraction and transfer of [3H]LOS monomers from [3H]LOS aggregates to CD14. This targeted inhibitory effect could be explained by a selective effect on extraction of LOS monomers from aggregates that is needed for generation of [3H]LOS·sCD14 or a selective effect on net transfer of [3H]LOS monomers to sCD14 vs. sMD-2. To test the latter possibility more directly, we took advantage of the ability of monomeric E·sCD14 complexes to serve as a donor of E monomers to either CD14 or MD-2. For this purpose, we used [3H]LOS-sCD14 (full-length sCD14, no His tag) as [3H]LOS donor and either His6-tagged truncated sCD14 (tCD14; residues 1-156) or His6-tagged sMD-2 as [3H]LOS acceptors. Thus, [3H]LOS transfer from [3H]LOS·sCD14 to tCD14-His6 or to His6-sMD-2 could be readily measured by assay of co-capture of [3H]LOS to HISLINK resin as described in Methods.
Molecule 8 markedly inhibited transfer of [3H]LOS from full-length sCD14 (no His tag) to tCD14-His6 (Figure 5A) but did not inhibit transfer of [3H]LOS to His6-sMD-2 (Figure 5B). Our findings demonstrate that molecule 8 affects this multi-step pathway in a relatively selective manner, acting mainly to inhibit transfer to and/or stable occupation of CD14 by lipooligosaccharide ([3H]LOS) monomers. Similar inhibitory effects of molecule 8 were seen when the transfer of [3H]LOS from either [3H]LOS aggregates or from monomeric [3H]LOS·sCD14 was examined (Figures 3, 4 and 5), indicating that an additional effect of molecule 8 on interactions between LBP and endotoxin-rich interfaces was unlikely and not necessary for the inhibitory action of molecule 8. Remarkably, shuttling of LOS monomers from CD14 to MD-2 is unaffected by molecule 1. The apparently selective targeting of CD14 by this compounds is very similar to that observed for compounds 1 and 7 [16]. The functional properties described suggest that molecule 8 could be considered lead compounds in the development of new anti-endotoxic agents selectively targeting CD14. Increasing evidence is emerging that TLR4 and CD14 are implicated in other important pathologies, such as neuropathic pain, related to the microglia TLR4 activation, [21] or Alzheimer disease [22,23]. The broader role of CD14 in other biological recognition/response pathways may further expand the potential pharmacological applications of this compound.
Acknowledgments
We acknowledge NIH/NIAID, grant number [1R01AI059372] “Regulation of MD-2 function and expression”.
Abbreviations
- TLR
Toll-like receptor
- LPS
lipopolysaccharide
- E
endotoxin
- LBP
lipopolysaccharide binding protein
- CD14
Cluster Differentiation antigen 14
- MD-2
Myeloid Differentiation protein
- HEK
Human Embryonic Kidney cells
- AcOEt
Ethyl Acetate
- EtP
Petroleum Ether
- TEA
Triethylamine
- MeOH
Methanol
- HSA
Human Serum Albumin
- DMSO
Dimethyl sulfoxyde
- EtOH
Ethanol
- PBS
Phosphate Buffer
- DMEM
Dulbecco modified Eagle Medium
- His
Histidine
- TLR4ECD
TLR4 ectodomain
- LOSagg
LOS aggregates
- IgG
Immuno-globulin G
- IL-8
Interleukin-8
- ELISA
Enzyme-Linked Immunoabsorbent Assay
- FBS
fetal bovine serum
- pNpp
para-nitrofenil phosphate
- MTT
1-(4,5-Dimethylthiazol-2-yl)-3,5-diphenylformazan
- Boc
tert-butyloxycarbonyl
- sAP
secreted Alkalyne Phosphatase
- sCD14
solubile CD14
- tCD14
truncated CD14
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Beutler B. TLR4 as the mammalian endotoxin sensor. Curr Top Microbiol Immunol. 2002;270:109–20. doi: 10.1007/978-3-642-59430-4_7. [DOI] [PubMed] [Google Scholar]
- 2.Beutler B, Jiang Z, Georgel P, Crozat K, Croker B, Rutschmann S, Du X, Hoebe K. Genetic analysis of host resistance: toll-like receptor signaling and immunity at Large. Annu Rev Immunol. 2006;24:353–89. doi: 10.1146/annurev.immunol.24.021605.090552. [DOI] [PubMed] [Google Scholar]
- 3.Palsson-McDermott EM, O’Neill LAJ. Signal transduction by the lipopolysaccharide receptor, Toll-like receptor-4. Immunology. 2004;113:153–62. doi: 10.1111/j.1365-2567.2004.01976.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Freudenberg MA, Tchaptchet S, Keck S, Fejer G, Huber M, Schutze N, Beutler B, Galanos C. Lipopolysaccharide sensing an important factor in the innate immune response to Gram-negative bacterial infections: benefits and hazards of LPS hypersensitivity. Immunobiology. 2008;213:193–203. doi: 10.1016/j.imbio.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 5.Jerala R. Structural biology of the LPS recognition. Int J Med Microb. 2007;297:353–63. doi: 10.1016/j.ijmm.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 6.Prohinar P, Re F, Widstrom R, Zhang D, Teghanemt A, Gibson BW, Weiss JP. Specific high affinity interactions of monomeric endotoxin-protein complexes with Toll-like receptor 4 ectodomain. J Biol Chem. 2007;282:1010–17. doi: 10.1074/jbc.M609400200. [DOI] [PubMed] [Google Scholar]
- 7.Huber M, Kalis C, Keck S, Jiang Z, Georgel P, Du X, et al. R-form LPS, the master key to the activation of TLR4/MD-2-positive cells. Eur J Immunol. 2006;36:701–11. doi: 10.1002/eji.200535593. [DOI] [PubMed] [Google Scholar]
- 8.Zanoni I, Ostuni R, Capuano G, Collini M, Caccia M, Ronchi AE, et al. CD14 regulates the dendritic cell life cycle after LPS exposure through NFAT activation. Nature Lett. 2009;460:264–8. doi: 10.1038/nature08118. [DOI] [PubMed] [Google Scholar]
- 9.Nakata T, Yasuda M, Fujita M, Kataoka H, Kiura K, Sano H, Shibata K. CD14 directly binds to triacylated lipopeptides and facilitates recognition of the lipopeptides by the receptor complex of Toll-like receptors 2 and 1 without binding to the complex. Cell Microbiol. 2006;8:1899–909. doi: 10.1111/j.1462-5822.2006.00756.x. [DOI] [PubMed] [Google Scholar]
- 10.Manukyan M, Triantafilou K, Mackie A, Nilsen N, Espevik T, Wiesmuller KH, Ulmer AJ, Heine H. Binding of lipopeptide to CD14 induces physical proximity of CD14, TLR2 and TLR1. Eur J Immunol. 2005;35:911–21. doi: 10.1002/eji.200425336. [DOI] [PubMed] [Google Scholar]
- 11.Lee HK, Dunzendorfer S, Soldau K, Tobias PS. Double-Stranded RNA-Mediated TLR3 Activation Is Enhanced by CD14. Immunity. 2006;24:153–63. doi: 10.1016/j.immuni.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 12.Peri F, Granucci F, Costa B, Zanoni I, Marinzi C, Nicotra F. Amino and hydroxylamino monosaccharides inhibit lipid A stimulated activation of human dendritic cells and macrophages. Angew Chem Int Ed. 2007;46:3308–12. doi: 10.1002/anie.200604932. [DOI] [PubMed] [Google Scholar]
- 13.Piazza M, Rossini C, Della Fiorentina S, Pozzi C, Comelli F, Bettoni I, Fusi P, et al. Glycolipids and benzylammonium lipids as novel anti-sepsis agents: synthesis and biological characterization. J Med Chem. 2009;52:1209–13. doi: 10.1021/jm801333m. [DOI] [PubMed] [Google Scholar]
- 14.Peri F, Nicotra F, Granucci F, Costa B. Lipid A antagonists with anti-septic shock, antiinflammatory, anti-ischemia and analgesic activity. PCT/EP2007/002279 [Google Scholar]
- 15.Bettoni I, Comelli F, Rossini C, Granucci F, Giagnoni G, Peri F, Costa B. Glial TLR4 receptor as new target to treat neuropatic pain: efficacy of a new receptor antagonist in A model peripheral nerve injury in mice. Glia. 2008;56:1312–19. doi: 10.1002/glia.20699. [DOI] [PubMed] [Google Scholar]
- 16.Piazza M, Yu L, Teghanemt A, Gioannini T, Weiss J, Peri F. Evidence of a specific interaction between new synthetic antisepsis agents and CD14. Biochemistry. 2009;48:12337–44. doi: 10.1021/bi901601b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gioannini TL, Teghanemt A, Zhang D, Prohinar P, Levis EN, Munford RS, Weiss JP. Endotoxin-binding proteins modulate the susceptibility of bacterial endotoxin to deacylation by acyloxyacyl hydrolase. J Biol Chem. 2007;282:7877–84. doi: 10.1074/jbc.M605031200. [DOI] [PubMed] [Google Scholar]
- 18.Gioannini TL, Teghanemt A, Zhang D, Coussens NP, Dockstader W, Ramaswamy S, Weiss JP. Isolation of an endotoxin-MD-2 complex that produces Toll-like receptor 4-dependent cell activation at picomolar concentrations. Proc Natl Acad Sci USA. 2004;101:4186–91. doi: 10.1073/pnas.0306906101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Teghanemt A, Prohinar P, Gioannini TL, Weiss JP. Transfer of monomeric endotoxin from MD-2 to CD14. J Biol Chem. 2007;282:36250–6. doi: 10.1074/jbc.M705995200. [DOI] [PubMed] [Google Scholar]
- 20.Gioannini TL, Zhang DS, Teghanemt A, Weiss JP. Essential role for albumin in the interaction of endotoxin with lipopolysaccharide-binding protein and sCD14 and resultant cell activation. J Biol Chem. 2002;277:47818–25. doi: 10.1074/jbc.M206404200. [DOI] [PubMed] [Google Scholar]
- 21.Watkins LR, Hutchinson MR, Rice KC, Maier SF. The “Toll” of opioid-induced glial activation: improving the clinical efficacy of opioids by targeting glia. Trends in Pharmacol Sci. 2009;30:581–91. doi: 10.1016/j.tips.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rodriguez-Rodriguez E, Sanchez-Juan P, Mateo I, Infante J, Sanchez-Quintana C, Garcia-Gorostiaga I, Berciano J, Combarros O. Interaction between CD14 and LXRbeta genes modulates Alzheimer’s disease risk. J Neurol Sci. 2008;264:97–9. doi: 10.1016/j.jns.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 23.Fassbender K, Walter S, Kuhl S, Landmann R, Ishii K, Bertsch T, et al. The LPS receptor (CD14) links innate immunity with Alzheimer’s disease. FASEB J. 2004;18:203–5. doi: 10.1096/fj.03-0364fje. [DOI] [PubMed] [Google Scholar]