Abstract
Experimental models of permeability in animals, excised tissues, cell monolayers, and artificial membranes are important during drug discovery and development as permeability is one of several factors affecting the intestinal absorption of oral drug products. The utility of these models is demonstrated by their ability to predict a drug’s in vivo intestinal absorption. Within the various permeability models, there are differences in the performance of the assays, along with variability in animal species, tissue sources, and cell types, resulting in a variety of experimental permeability values for the same drug among laboratories. This has led to a need for assay standardization within laboratories to ensure applicability in the drug development process. Method suitability provides a generalized approach to standardize and validate a permeability model within a laboratory. First, assay methodology is optimized and validated for its various experimental parameters along with acceptance criteria for the assay. Second, the suitability of the model is demonstrated by a rank order relationship between experimental permeability values and human extent of absorption of known model compounds. Lastly, standard compounds are employed to classify a test drug’s intestinal permeability and ensure assay reproducibility and quality. This review will provide examples of the different aspects method suitability for in situ (intestinal perfusions), ex vivo (everted intestinal sacs, diffusion chambers), and in vitro (cell monolayers, artificial membranes) experimental permeability models. Through assay standardization, reference standards, and acceptance criteria, method suitability assures the dependability of experimental data to predict a drug’s intestinal permeability during discovery, development, and regulatory application.
Key words: artificial membranes, drug permeability, ex vivo perfusion, in situ perfusion, in vitro cell monolayers, method suitability
INTRODUCTION
The physicochemical properties of a drug substance and its product, the physiological functions of gastrointestinal tract, and the biochemical and physical properties of the epithelial barrier all influence the complex process of intestinal drug absorption. Since oral delivery is the preferred route of administration, successful therapy requires sufficient intestinal absorption to ensure that the drug is available at its intended target site. Good oral bioavailability takes place when the drug has maximal permeability and solubility at the site of absorption. Consequently, in vivo extent of absorption (fa) can be predicted based on solubility and permeability measurements (1). The fundamental relationship between the rate of drug absorption measured as a permeability coefficient and extent of absorption has led to the use of experimental models as a surrogate for predicting the absorption of oral drug products (2,3).
Permeability models can assist in the drug candidate selection for in vivo clinical studies at early stages in drug discovery and development, along with submission of regulatory applications (4–7). They serve as tools in the decision-making process to prevent the loss of drug candidates in later, more costly, clinical phases due to poor pharmacokinetics (8). Since drug absorption is an important selection criterion in drug discovery and development, there is a need for reliable and appropriate screening methods to assess intestinal permeability (4–6).
The dependability of an experimental permeability model is revealed by its ability to accurately predict a drug’s in vivo intestinal absorption. Experimental conditions need to be first optimized and controlled for the physiological environment that drugs encounter in the intestinal tract to obtain satisfactory in vitro–in vivo correlations (IVIVC) (8–12). To make the models useful in drug discovery and development, there is a need to establish a correlation between experimental and in vivo absorption with standardized methods for the quantification of permeability data (9,13). Standardization of the assays reduces intra-laboratory variations in permeability results and thus improves the predictive potential of the assay. The validity of the assays is reflected in their ability to predict the behavior of a drug at the in vivo intestinal barrier (14).
METHOD SUITABILITY
Different experimental effective (Peff) or apparent (Papp) permeability values for the same drug between laboratories are the result of differences in the performance of in situ, ex vivo, and in vitro permeability assays, along with variability in animal species, tissue sources, and cell types (15–20). Numerous factors influence the performance of the permeability models from the choice of animal species, tissue source, and cell line, how the permeation experiment is conducted within a laboratory, and how the data are analyzed (7,21,22). Method suitability provides a practical and generalized approach to standardize and validate a permeability model within a laboratory. It also accounts for intra- and inter-laboratory variability, allows for improvements in technology, and is applicable to a variety of tissues, cell lines, and membranes (23,24).
Method suitability provides a framework to utilize different permeability models and protocols involving human studies, intact animals, intestinal tissue, or epithelial cells (23–25). There are three stages which compromise method suitability (Table I), including method development (optimization, standardization), demonstrating assay suitability (IVIVC), and permeability classification of new drugs (23). The assay is first optimized and standardized for the parameters that influence its experimental outcome to increase predictivity and throughput (26–29). Additionally, the assay can be characterized for the presence of functional active transporters (e.g., amino acids, di/tripeptides, monocarboxylic acids, organic anions and cations) and drug efflux mechanisms. Acceptance criteria are defined for selected standard compounds and measurements (e.g., viability, resistance, integrity). These criteria are used to assess the permeability assay’s functionality (29).
Table I.
Method Suitability
| Method development | • Establish assay protocol |
| • Optimize and standardize assay parameters | |
| • Set acceptance criteria | |
| Assay suitability | • Rank order relationship between experimental permeability values and human intestinal absorption |
| • Define HP-IS | |
| Permeability classification | • HP-IS to classify new compound |
| • Reference standard to demonstrate assay reproducibility | |
| • Molecular markers for tissue, cell or membrane integrity |
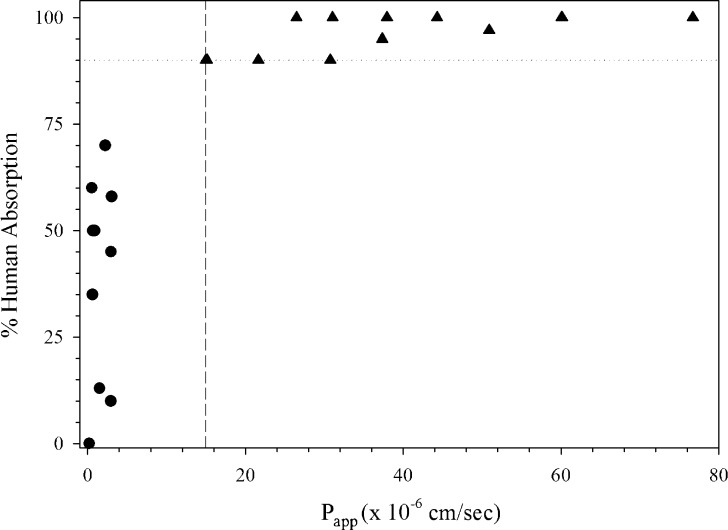
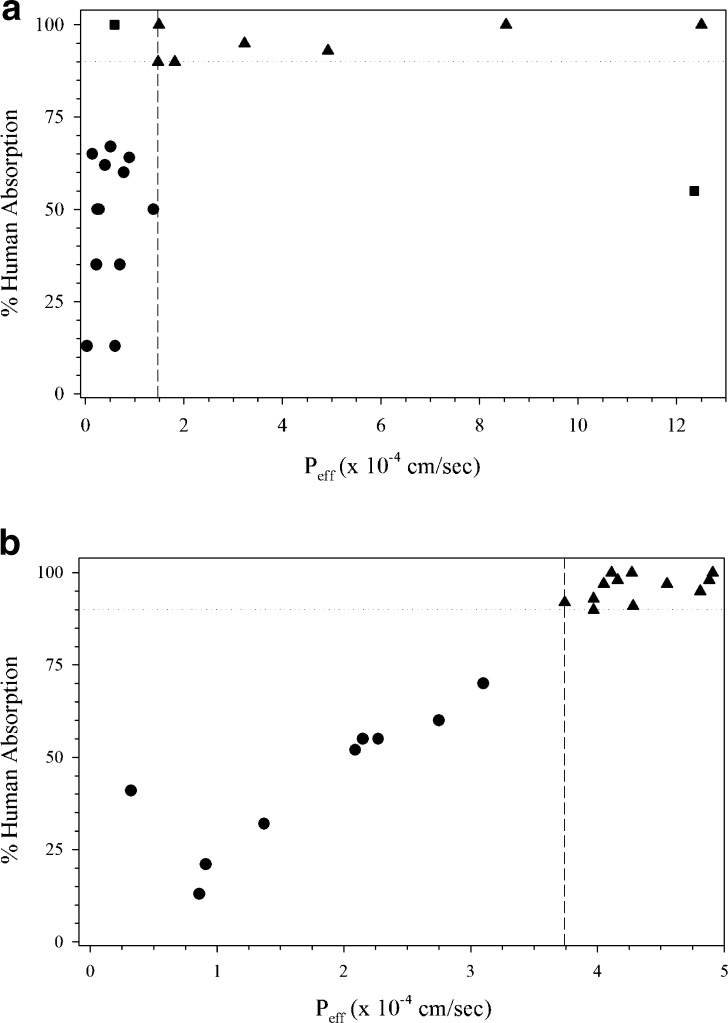
Employing an optimized assay protocol, a rank order relationship is established between Peff or Papp permeability values and the extent of intestinal absorption in humans with a sufficient number of passively absorbed model drugs that are not subject to active or efflux transport (20,25) (Fig. 1). This relationship should clearly differentiate between high (HP, fa ≥ 90%) and low (LP, fa < 90%) permeability drug substances such as that according to the Biopharmaceutics Classification System (BCS) (25) (Fig. 1). Model drugs, evaluated at clinically relevant concentrations (27,28), should ideally represent an extent of absorption range of <50%, 50–89%, and ≥90%. Even though investigators have utilized a wide variety of drugs to demonstrate assay suitability, many include similar HP (e.g., antipyrine, ketoprofen, naproxen, metoprolol, propranolol) and LP (e.g., atenolol, ranitidine, hydrochlorothiazide) model drugs in their experiments. An evenly distributed data set according to absorption improves permeability predictions since bias toward completely absorbed model compounds in the assay tend to predict HP well but lack precision to predict LP drugs (5). A highly permeable internal standard (HP-IS), with permeability in close proximity to the high/low class boundary, is selected from the model drugs (Fig. 1) (30).
Fig. 1.
Demonstration of method suitability from a 21-day Caco-2 assay in 12-well format (30). Triangle HP drugs, Circle LP drugs, Square HP-IS, Dashed line LP/HP boundary, Dotted line 90% absorption
Employing the same assay protocol to demonstrate assay suitability, test drugs are classified as HP or LP. Standard compounds (reference, internal, marker) are used to monitor intra-laboratory variation, active and efflux transporters, and barrier integrity in the experimental assays (31,32). The standards are also used to establish permeability class membership, assure reproducibility, and enhance the rank order of a drug series (24,26,27). The routine use of standard compounds generates acceptance criteria which can be monitored on a regular basis (4,10,14,26,33–36). Furthermore, repeated investigation of the transport of standards at regular time intervals facilitates intra- and inter-laboratory comparisons (26,37).
The reference standards should be known LP and HP drugs, along with substrates for active transporters and/or efflux mechanisms to monitor intra-laboratory variability (25). Internal standards are used to classify a test drug as low or high permeability. The flux of a low or zero permeability paracellular marker molecule (e.g., mannitol, polyethylene glycol (PEG), dextran, inulin, Lucifer yellow) is used in each study to provide evidence for tissue or cell layer integrity. These markers demonstrate the presence of an intact physical barrier for drug transport (38,39). The permeability results for standard compounds (reference, internal, marker) should not differ considerably over multiple experiments, including those that demonstrated assay suitability. Along with each reference compounds, the laboratory sets in-house specifications and acceptance criteria for each assay system. Lastly, the HP-IS is utilized to facilitate the classification of a test drug substance. If the test drug’s experimental Papp or Peff value is equal to or greater than that of the HP-IS, it is classified as highly permeable (25,40).
Drug stability and solubility are other considerations in the permeability models (27). It is important that the drug is stable in the aqueous buffer solutions for the time, temperature, and pH conditions of the transport experiment to ensure that the Papp or Peff results are not biased from drug loss due to instability. Another issue is that the drug is not lost due to adherence to the experimental apparatus or retained in the cells or tissues (27,41). Drug solubility can be limiting since the experiments are conducted in an aqueous buffer.
Method suitability is a process to optimize and validate permeability assays for drug classification (23). The use of acceptance criteria demonstrates the functionality of the assay, while reference standards ensure reproducibility and high-quality results. Such assays become tools for decision making in the discovery and early development of new drugs. The advantages of method suitability is that it accounts for inter-laboratory variability and differences in assay protocols, allows for the incorporation of improved technologies, and is applicable to in situ, ex vivo, and in vitro permeability assays (23,24,30).
PERMEABILITY ASSAYS
There is a variety of methods that have been developed to assess drug permeation across the gastrointestinal tract. The methods include in situ perfusion through isolated intestinal segments, ex vivo diffusion across tissues, and in vitro permeation through cell monolayers or artificial membranes. Each assay has it own advantages and limitations to be considered when developing models for permeability classification (Table II). Furthermore, due to inter-assay differences in the experimental Papp or Peff values, cross-system permeability comparison ought to rely solely upon evaluations generated relative to specific standard compounds that have been well characterized across the model systems.
Table II.
Advantages and Limitation of Permeability Assays
| In situ perfusion | |
| Advantages | Closest to in vivo anatomy; retains blood flow and innervation; assay requires surgery and anesthesia; low throughput |
| Limitations | Animal usage; not a screening tool |
| Ex vivo tissue diffusion | |
| Advantages | Retains gut architecture; regional differences; human or animal tissue; mechanistic and directional transport |
| Limitations | Limited tissue viability; suboptimal stirring conditions |
| In vitro cell monolayers | |
| Advantages | Transcellular and paracellular passive diffusion, active transport, and efflux; mechanistic studies; human or animal cell lines; can be automated |
| Limitations | Inter-laboratory variability due to culture conditions; labor-intensive; low expression of transporters; lack of mucus layer |
| Artificial membranes | |
| Advantages | Relatively simple and high throughput; can be automated; tolerates wider pH ranges and higher solubilizer concentrations |
| Limitations | Transport dependent upon lipid composition and pH; membrane retention of lipophilic compounds; no active transport |
In Situ Perfusion
The in situ perfusion model allows for the measurement of drug permeability in the intact intestine by single-pass and recirculating methods (42–44) (Table III). For the single-pass (open loop) perfusion method, a section of intestine in an anesthetized animal is cannulated proximally and distally, rinsed with buffer solution, and then perfused with a drug solution. The amount of drug in the perfusate is measured at defined time points. The perfusion assays are normalized for inlet drug concentration, flow rate, and drug aqueous diffusion coefficient. Intestinal permeability (Peff) is calculated from the difference between solute concentration entering and leaving the cannulated region. Falgerholm et al. (9) reported on a single-pass in situ perfusion study in rats with comparison to in vivo perfusion values in humans, finding that jejunal Peff estimates for passively absorbed compounds correlated well and could be used to predict human in vivo absorption. Alternatively, carrier-mediated transport required scaling between models as there were differences in transport maximums and/or substrate specificity (9).
Table III.
In Situ Perfusion
| Parameters to standardize | • Animal species and age |
| • Fed/fast status of animal | |
| • Anesthesia regimen | |
| • Time to equilibrium | |
| • Intestinal region | |
| • Perfusion buffer composition, osmolarity and pH | |
| • Perfusion rate | |
| • Drug analysis and P eff calculation | |
| Acceptance criteria | • P eff of non-absorbable marker |
| • P eff of HP marker | |
| • P eff of active transport marker |
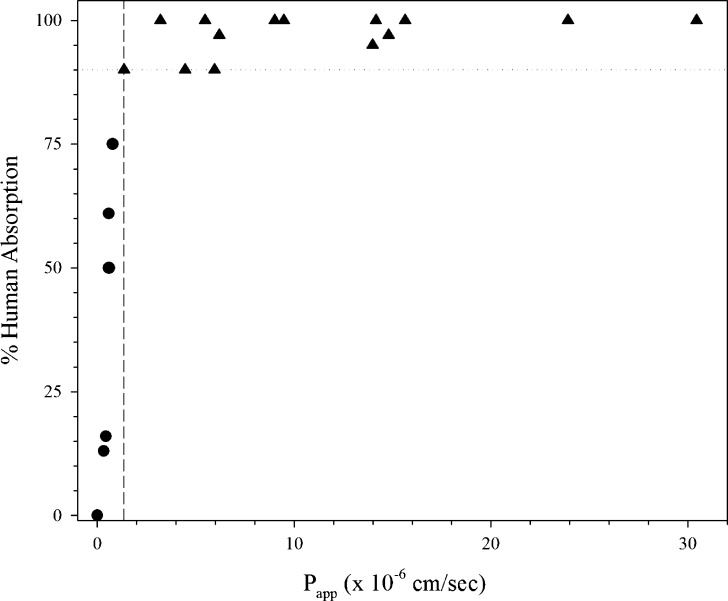
Kim et al. (20) demonstrated the suitability of a rat single-pass perfusion method to classify 20 drugs according to the BCS (Fig. 2a). In this study, the initial drug concentration was based on the highest dose strength (HDS) in 250 mL, and PEG 4000 was utilized as a non-absorbable marker to measure water flux (20). This rat single-pass perfusion method categorized the selected test drugs into the proper permeability class in comparison to human absorption with metoprolol at the HP/LP boundary (Fig. 2a) (20). Zakeri-Milani et al. (45) determined Peff values in ligated jejunal segment in rats with a similar assay to classify compounds based upon permeability (Fig. 2b). Metoprolol was also at the HP/LP boundary in this smaller data set (45). Furosemide, a low permeability drug, had a Peff value the same as metoprolol. It is possible that the efflux of furosemide was decreased in this system, increasing its absorption.
Fig. 2.
Rat in situ perfusion assays from a Kim et al. (20) and b Zakeri-Milani et al. (45). Triangle HP drugs, Circle LP drugs, Dashed line LP/HP boundary, Dotted line 90% absorption, Square outlier drug
Ex Vivo Diffusion Chambers
In ex vivo diffusion chambers, a small section of the intestine is removed from an animal or human and then opened to form a flat epithelial sheet which is mounted between two chambers containing oxygenated buffer. Drug solution is added to the buffer in the donor chamber and its appearance is measured over time in the receiver chamber. The method allows for the measurement of drug transport across the tissue as a function of time in the absorptive (mucosal to serosal) and secretive (serosal to mucosal) directions. Flux, or apparent permeability (Papp), is defined as the rate of drug accumulation in the receiver chamber normalized for tissue surface area. Acceptance criteria for this assay can include electrical measures of barrier function (e.g., potential difference, short circuit current, resistance), along with markers of passive and active transport and tissue viability (Table IV) (6,18,46).
Table IV.
Ex Vivo Tissue Diffusion
| Parameters to standardize | • Animal species and age |
| • Fed/fast status of animal | |
| • Anesthesia regimen | |
| • Stripped or unstripped tissue | |
| • Intestinal region | |
| • Time to equilibrium | |
| • Diffusion buffer composition, osmolarity, and pH | |
| • Monitoring of viability and integrity | |
| • Oxygenation of buffer and mixing process | |
| • Sink conditions and sampling method | |
| • Drug analysis and P app calculation | |
| Acceptance criteria | • Measure of tissue viability/integrity |
| • Potential difference, tissue resistance, and/or short circuit current | |
| • P app of non-absorbable marker | |
| • P app of HP and LP markers | |
| • P app of active transport marker |
Lennernäs (47) found that rat permeability in jejunal segments was comparable to human in vivo perfusion. There was a similar rank order for passively absorbed drugs in the two assays, although human in vivo Peff was five to six times higher than rat ex vivo Papp due to differences in available intestinal surface area, experimental methods (e.g., oxygenation), tissue viability, and stirring (47). 3?>.
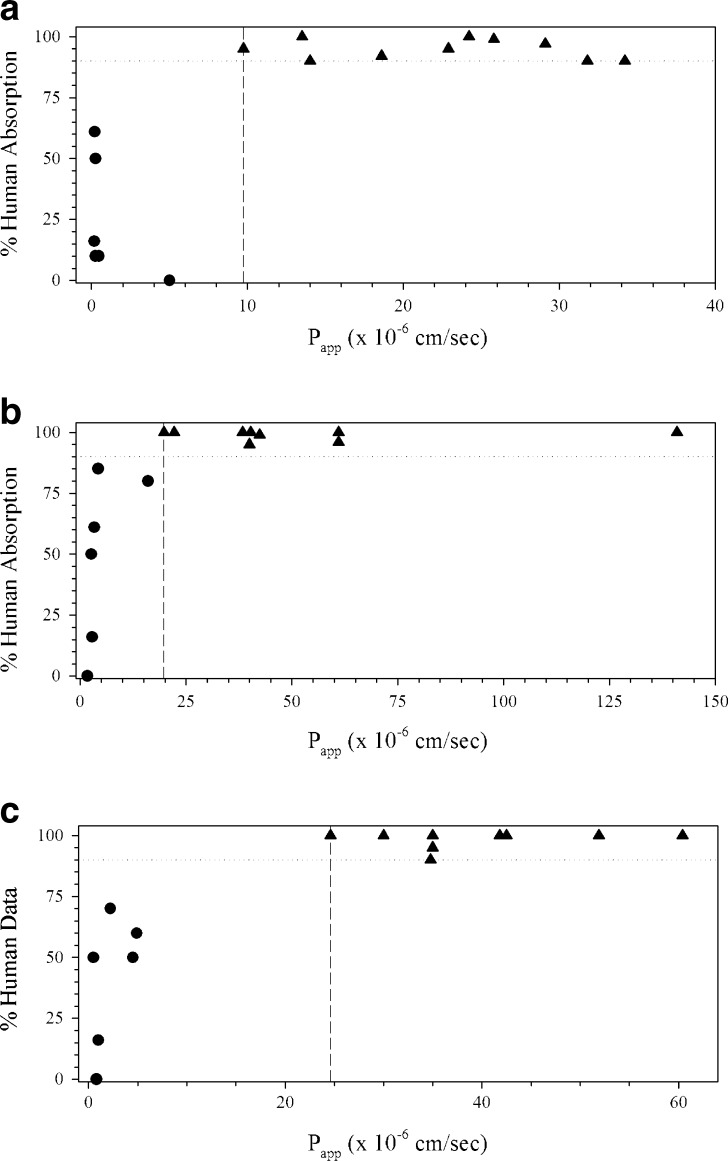
Fig. 3.
Rat ex vivo diffusion assay from Ungell et al. (18) in the a jejunum, b ileum, and c colon. Triangle HP drugs, Circle LP drugs, Square outlier drugs, Dashed line LP/HP boundary, Dotted line 90% absorption
Ungell et al. (18) were able to classify compounds as HP or LP according to method suitability in stripped rat proximal jejunum, ileum, and colon tissue (Fig. 3). Tissue viability was monitored by potential difference and transsegmental electrical resistance as a quality control measure. Hydrophilic LP drugs had Papp values of 0.9–8.3 × 10−6 and 11.4–100.3 × 10−6 cm/s for HP hydrophobic drugs (18). Erythritol (fa = 90%) was at the HP/LP boundary for the three tissues, although the Papp for erythritol decreased from the jejunum to colon. Creatinine, raffinose, and foscarnet may be considered as outliers in the colon, but these were only slightly greater than the HP-IS. Creatinine was properly classified in the jejunum (18) and both foscarnet and raffinose were correctly classified in the same assay in the ileum and jejunum (18). Regional differences in drug permeability were observed as the Papp of LP drugs decreased down the intestinal tract (jejunum > ileum > colon), while it increased for HP drugs (jejunum < ileum < colon) (18).
Ex Vivo Gut Sacs
In the everted gut (or intestinal) sac model, a section of the intestine is removed from an anesthetized animal, flushed with buffer, and everted over a rod or tube. The intestine is divided into 2- to 4-cm sacs which are tied at each end, filled with oxygenated buffer, and placed in a container of well-mixed oxygenated buffer containing the test drug. After a specified time period, the amount of drug in the sac is measured and Papp is normalized based on sac protein content. Alternatively, the intestine is not everted and the drug solution is placed in the sac. The sac is then placed in a container with oxygenated buffer and the drug is measured over time from the container. The applicability of a non-everted rat intestinal sacs method was validated and demonstrated for 11 marketed compounds (46). The study showed a good relationship between the permeability of the model drugs and their corresponding human fa data. The mean permeability values of the series of drugs examined range from 1.08 × 10−6 cm/s for acyclovir to 15.66 × 10−6 cm/s for caffeine (46).
Trapani et al. (16) found a rank order relationship for BCS classification between human extent of absorption and Papp calculated from a non-everted frog intestinal sac assay (Fig. 4). The assay used a 5-cm segment from the intestinal tract in frog Ringer’s solution at pH 8.2 without oxygenation for the assays which was agitated during the experiment. Metoprolol was used as a reference standard. In this assay with method suitability, HP drug had Papp values >1.1 × 10−6 cm/s and with LP drug Papp values <1 × 10−6 cm/s based on propranolol as the HP-IS (16).
Fig. 4.
Frog ex vivo everted gut sac assay from Trapani et al. (16). Triangle HP drugs, Circle LP drugs, Dashed line LP/HP boundary, Dotted line 90% absorption
In Vitro Cell Monolayers
Various cell lines are grown on a semi-porous filter to form monolayers that morphologically and functionally resemble the intestinal epithelium with barrier properties (Table V). The monolayer is placed in diffusion apparatus containing apical (AP) and basolateral (BL) chambers that represent the mucosal (lumen) and serosal (blood) surfaces of the intestine, respectively. Drugs are added to the AP or BL chamber and its appearance measured over time in the BL (absorption) or AP (efflux) chamber, respectively. Papp is measured as the rate of drug accumulation in the receiver chamber normalized for the filter area and chamber volumes. A number of researchers have found a correlation between Caco-2 and Madin–Darby canine kidney (MDCK) cell assay with human in vivo drug absorption (50–52).
Table V.
In Vitro Cell Monolayers
| Parameters to standardize | • Cell clone and passage number |
| • Culture media composition | |
| • Filter type, diameter, pore size | |
| • Initial seeding density | |
| • Feeding regimen | |
| • Monolayer age | |
| • Transport buffer composition and pH | |
| • Transport temperature and time | |
| • Co-solvent effects on cells | |
| • Sink conditions and stirring process | |
| • Sampling method | |
| • Drug analysis and P app calculation | |
| Acceptance criteria | • Measure of monolayer integrity |
| • P app of non-absorbable marker | |
| • P app of HP and LP markers | |
| • Efflux of substrate compound |
Volpe et al. (30) validated a traditional 21-day, 12-well Caco-2 cell assay with over 20 model drugs with an initial drug concentration based on the HDS in 250 mL. Acceptance criteria were set for TEER values of the monolayers and metoprolol, FITC-dextran, and rhodamine 123 were standards to monitor high permeability, monolayer integrity, and efflux, respectively. There was good IVIVC between the drugs’ Papp values and extent of human absorption, resulting in a Spearman rank correlation coefficient of 0.89 (Fig. 1) (30). Labetalol was determined to be the HP-IS which was then employed to correctly classify four fluoroquinolone drugs in comparison to known human absorption (40). Ciprofloxacin was classified as a LP drug, while levofloxacin, lomefloxacin, and ofloxacin were classified as HP drugs (40).
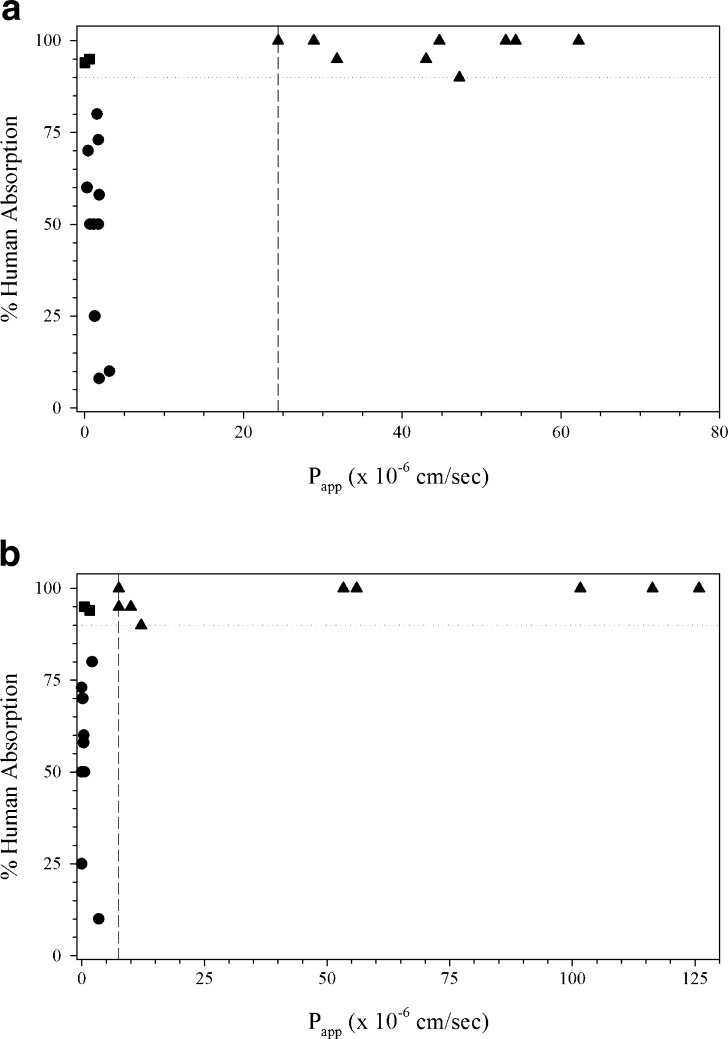
Bock et al. (14) validated another 12-well assay Caco-2 assay for permeability classification. Validation studies included inter-day precision, transport direction, monolayer age, and cell passage number for classification in terms of intestinal permeability. Acceptance criteria were based on passage number, monolayer age, transport of LP (fluorescein) and HP (propranolol) standards, efflux ratio (rhodamine 123), and barrier tightness (TEER) (14). There was a good correlation between human %fa and Papp for the test compounds with clonidine at the LP/HP boundary (Fig. 5a). Lentz et al. (53) developed a rapid, reduced serum assay with Caco-2 cells for application to the BCS. Caco-2 monolayers were grown in a six-well format with 2% iron-supplemented calf serum for 4 days. Cell morphology and differentiation were evaluated along with TEER to characterize the assay. The permeability of mannitol, metoprolol, and taurocholate were monitored along with the presence of efflux pumps (53). The assay classified drug as HP or LP with nicaripine at the HP/LP boundary (Fig. 5b).
Fig. 5.
Caco-2 cell monolayers from a Bock et al. (14) in a 12-well, 21-day assay, b Lentz et al. in a six-well, 4-day assay (53), and c Withington (54) in a 24-well, 3-day assay. Triangle HP drugs, Circle LP drugs, Dashed line LP/HP boundary, Dotted line 90% absorption
Withington (54) compared a 21-day Caco-2 cell assay to an accelerated (3-day) BioCoat™ assay in a 24-well format according to the BCS. The assay was optimized for culture conditions, including cell harvest and cell seeding density, along with investigation of monolayer morphology and biochemistry. The Papp values correlated well with human absorption, with theophylline at the HP/LP boundary in this accelerated assay (Fig. 5c) (54). Alsenz and Haenel (55) developed a 7-day, 96-well Caco-2 assay which was optimized for monolayer age, seeding density, feeding conditions, sample analysis, and transport buffer pH effects. Transport was compared with the apical buffer pH at 7.4 or 6.5, while the basolateral buffer remained at pH 7.4. Ketoprofen and desipramine were the HP-IS in the pH 7.4 and 6.5 conditions, respectively (Fig. 6). Highly permeable, but actively transported, cimetidine and amoxicillin were classified as low permeability in both the pH 7.4 and 6.5 assays (55).
Fig. 6.
Caco-2 cell monolayers in a 96-well, 7-day assay from Alsenz et al. (55) with AP buffer at a pH 7.4 or b pH 6.5. Triangle HP drugs, Circle LP drugs, Square outlier drug, Dashed line LP/HP boundary, Dotted line 90% absorption
In a 24-well cell assay with MDCKII-MDR1 cells, Thiel-Demby et al. (56) demonstrated a rank order relationship for BCS permeability classification. The investigators standardized the method according to cell type, pH conditions, transport direction, incubation time, drug concentration, and reference standards. Labetalol was the HP reference standard, Lucifer yellow as a paracellular marker for monolayer integrity, and amprenavir as an efflux substrate. Metoprolol, pindolol, labetalol, and ranitidine were reference standards to show assay reproducibility (56). The assay classified the permeability of model drugs whether the AP buffer pH 6.5 or 7.4 with minoxidil as the HP-IS (Fig. 7) (56). Labetalol, a basic drug, was an outlier in the pH 6.5 assay, which was due to a large decrease in Papp at the lower pH as compared to pH 7.4. The actively transported amoxicillin had a lower than expected permeability in pH 7.4 conditions. While the assay was predictive in both pH conditions, it demonstrates how pH conditions during the transport experiment can affect Papp values.
Fig. 7.
MDCK-MDR1 cell monolayers in a 3- to 4-day, 24-well assay from Thiel-Demby et al. (56) at a pH 7.4 or b pH 5.5. Triangle HP drugs, Circle LP drugs, Square outlier drugs, Dashed line LP/HP boundary, Dotted line 90% absorption
In Vitro Artificial Membranes
The non-cellular parallel artificial membrane assay (PAMPA) is comprised of two aqueous buffer solution chambers separated by a porous filter that contains a lipid in an organic solvent. Drug transport is assessed much like the cell monolayer assays where a drug is added to the donor chamber and its appearance measured over time in the receiver chamber. The assay is usually performed in a 96-well microtiter format, allowing for high-throughput screening of passive permeability (Table VI). Kansy et al. (57) validated a higher throughput assay in a 96-well plate that categorized drugs as well absorbed (fa = 70–100%), having a flux of 25–100%, intermediate absorption (fa = 30–70%), with a flux of 5–25%, and low absorption (fa = 0–30%), having a flux of <5%. Wohnsland and Faller (58) developed another 96-well model with hexadecane on a polycarbonate filter that had a good correlation for over 30 compounds between in vitro permeability and human intestinal absorption.
Table VI.
Artificial Membranes
| Parameters to standardize | • Lipid composition |
| • Organic solvent in membrane | |
| • Filter type, diameter, and pore size | |
| • Transport buffer composition and pH | |
| • Transport temperature and time | |
| • Co-solvents | |
| • Stirring process and sink conditions | |
| • Sampling method | |
| • Drug analysis and P app calculation | |
| Acceptance criteria | • P app of non-absorbable marker |
| • P app of HP and LP markers |
Flaten et al. (59) validated a vesicle membrane PAMPA model where egg phosphatidylcholine liposomes were dispersed into the pores and on the surface of 24-well mixed cellulose ester filters. A tight barrier was demonstrated by calcein permeability and TEER measurements followed by model validation with over 30 compounds. Both timolol and acetylsalicylic acid were at the HP/LP boundary (Fig. 8a). The only compounds outside the classification were tranexamic acid and salicylic acid, due to analytical difficulties and active transport, respectively (59). Corti et al. (60) demonstrated the method suitability and general applicability of a dynamic diffusion cell artificial membrane method that had been optimized for the filter support (acetate–nitrate cellulose) and lipid phase composition (cholesterol and Lipoid® E80 in n-octanol) (61). Lucifer yellow was used as a paracellular marker in this model. There was a linear correlation for 20 drugs between Papp and fraction drug absorbed in humans (r2 = 0.957) with pindolol as the HP-IS (Fig. 8b) (60).
Fig. 8.
Artificial membrane assays from a Flaten et al. (vesicle membrane) (59) and b Corti et al. (diffusion cell) (60). Triangle HP drugs, Circle LP drugs, Square outlier drugs, Dashed line LP/HP boundary, Dotted line 90% absorption
CONCLUSION
Method suitability is a generalized approach to standardize and validate permeability methods within a laboratory to investigate and classify the intestinal absorption of drugs. It includes the optimization of model methodology to improve permeability predictions (Tables III, IV, V, and VI). Method suitability establishes a correlation between experimental permeability values and the extent of absorption in humans in an assay that is characterized by reference standards and acceptance criteria (23). Literature examples with in situ perfusion, ex vivo tissue chambers and gut sacs, cell monolayers, and in vitro artificial membranes demonstrate the applicability and feasibility of method suitability in devising intestinal drug permeability models (14,16,18,20,30,45,53–56,59,60). Method suitability, with its reliance on assay standardization and validation, reference standards, and acceptance criteria, enhances the consistency of experimental data to predict a drug’s intestinal permeability during discovery, development, and regulatory application.
Footnotes
Disclaimer: The findings and conclusions in this article have not been formally disseminated by the Food and Drug Administration and should not be construed to represent any Agency determination or policy.
References
- 1.Egan W, Lauri G. Prediction of intestinal permeability. Adv Drug Deliv Rev. 2002;54:273–89. doi: 10.1016/S0169-409X(02)00004-2. [DOI] [PubMed] [Google Scholar]
- 2.Sinko P, Leesman JD, Amidon GL. Predicting fraction dose absorbed in humans using a macroscopic mass balance approach. Pharm Res. 1991;8:979–88. doi: 10.1023/A:1015892621261. [DOI] [PubMed] [Google Scholar]
- 3.Burton PS, Goodwin JT, Vidmar TJ, Amore BM. Predicting drug absorption: how nature made it a difficult problem. J Pharmacol Exp Ther. 2002;303:889–95. doi: 10.1124/jpet.102.035006. [DOI] [PubMed] [Google Scholar]
- 4.Hidalgo IJ. Assessing the absorption of new pharmaceuticals. Curr Top Med Chem. 2001;1:385–401. doi: 10.2174/1568026013395010. [DOI] [PubMed] [Google Scholar]
- 5.Matsson P, Bergström CAS, Nagahara N, Tavelin S, Norinder U, Artursson P. Exploring the role of different drug transport routes in permeability screening. J Med Chem. 2005;48:604–13. doi: 10.1021/jm049711o. [DOI] [PubMed] [Google Scholar]
- 6.Bohets H, Annaert P, Mannens G, van Beijsterveldt L, Anciaux K, Verboven P, Meuldermans W, Lavrijsen K. Strategies for absorption screening in drug discovery and development. Curr Top Med Chem. 2001;1:367–83. doi: 10.2174/1568026013394886. [DOI] [PubMed] [Google Scholar]
- 7.Abrahamsson B, Lennernäs H. Application of the biopharmaceutics classification system now and in the future. Methods Princ Med Chem. 2003;18:495–531. [Google Scholar]
- 8.Ungell AL. Transport studies using intestinal tissue ex vivo. In: Lehr CM, editor. Cell culture models of biological barriers: in vitro test systems for drug absorption and delivery. London: Taylor and Francis; 2002. pp. 164–88. [Google Scholar]
- 9.Fagerholm U, Johansson M, Lennernäs H. Comparison between permeability coefficients in rat and human jejunum. Pharm Res. 1996;13:1336–42. doi: 10.1023/A:1016065715308. [DOI] [PubMed] [Google Scholar]
- 10.Lee KL, Johnson N, Castelo J, Sinko PJ, Grass G, Holme K, Lee YH. Effect of experimental pH on the in vitro permeability in intact rabbit intestines and Caco-2 monolayer. Eur J Pharm Sci. 2005;25:193–200. doi: 10.1016/j.ejps.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 11.Borchardt RT. Assessment of transport barriers using cell and tissue culture systems. Drug Dev Ind Pharm. 1990;16:2595–612. doi: 10.3109/03639049009058549. [DOI] [Google Scholar]
- 12.Hillgren KM, Kato A, Borchardt RT. In vitro systems for studying intestinal drug absorption. Med Res Rev. 1995;15:83–109. doi: 10.1002/med.2610150202. [DOI] [PubMed] [Google Scholar]
- 13.Borchardt RT, Smith PL, Wilson G. General principles in the characterization and use of model systems for biopharmaceutical sciences. In: Borchardt RT, Smith PL, Wilson G, editors. Models for assessing drug absorption and metabolism. New York: Plenum; 1996. pp. 1–11. [Google Scholar]
- 14.Bock U, Flötotto T, Haltner E. Validation of cell culture models for the intestine and the blood brain barrier and comparison of drug permeation. ALTEX. 2004;21(Suppl. Linz03):57–64. [PubMed] [Google Scholar]
- 15.Fagerholm U, Lindahl A, Lennernäs H. Regional intestinal permeability in rats of compounds with different physicochemical properties and transport mechanisms. J Pharm Pharmacol. 1997;49:687–90. doi: 10.1111/j.2042-7158.1997.tb06093.x. [DOI] [PubMed] [Google Scholar]
- 16.Trapani G, Franco M, Trapani A, Lopedota A, Latrofa A, Gallucci E, Micelli S, Liso G. Frog intestinal sac: a new in vitro method for the assessment of intestinal permeability. J Pharm Sci. 2004;93:2909–19. doi: 10.1002/jps.20180. [DOI] [PubMed] [Google Scholar]
- 17.Nejdfors P, Ekelund M, Jeppsson B, Weström BR. Mucosal in vitro permeability in the intestinal tract of the pig; the rat; and man: species- and region-related differences. Scand J Gastroenterol. 2000;35:501–7. doi: 10.1080/003655200750023769. [DOI] [PubMed] [Google Scholar]
- 18.Ungell AL, Nylander S, Bergstrand S, Sjoberg A, Lennernäs H. Membrane transport of drugs in different regions of the intestinal tract of the rat. J Pharm Sci. 1998;87:360–6. doi: 10.1021/js970218s. [DOI] [PubMed] [Google Scholar]
- 19.Volpe DA. Variability in Caco-2 and MDCK cell-based intestinal permeability assays. J Pharm Sci. 2008;97:712–25. doi: 10.1002/jps.21010. [DOI] [PubMed] [Google Scholar]
- 20.Kim JS, Mitchell M, Kijek P, Tsume Y, Hilfinger J, Amidon GL. The suitability of an in situ perfusion model for permeability determinations: utility for BCS class I biowaiver requests. Mol Pharm. 2006;3:686–94. doi: 10.1021/mp060042f. [DOI] [PubMed] [Google Scholar]
- 21.Ungell AL. In vitro absorption studies and their relevance to absorption from the gastrointestinal tract. Drug Dev Ind Pharm. 1997;23:879–92. doi: 10.3109/03639049709148694. [DOI] [Google Scholar]
- 22.Deferme S, Annaert P, Augustijns P. In vitro screening models to assess intestinal drug absorption and metabolism. In: Ehrhardt C, Kim KJ, editors. Drug absorption studies—in situ, in vitro and in silico models. New York: Springer; 2008. pp. 182–215. [Google Scholar]
- 23.Volpe DA. Drug absorption studies in regulatory biowaiver applications. In: Ehrhardt C, Kim KJ, editors. Drug absorption studies—in situ, in vitro and in silico models. New York: Springer; 2008. pp. 665–80. [Google Scholar]
- 24.Volpe DA, Faustino PJ, Yu LX, Hussain AS. Towards the standardization of an in vitro method of drug absorption. Pharm Forum. 2001;27:2916–22. [Google Scholar]
- 25.Center for Drug Evaluation and Research; Food and Drug Administration. Guidance for Industry. Waiver of in vivo bioavailability and bioequivalence studies for immediate release solid oral dosage forms based on a biopharmaceutics classification system. 2000. http://www.fda.gov/cder/guidance/3618fnl.pdf.
- 26.Ingels F, Deferme S, Delbar N, Oth M, Augustijns P. Implementation of the Caco-2 cell model as a predictive tool for the oral absorption of drugs: in-house evaluation procedures. J Pharm Belg. 2002;57:153–8. [PubMed] [Google Scholar]
- 27.Griffin B, O’Driscoll C. Models of the small intestine. In: Ehrhardt C, Kim KJ, editors. Drug absorption studies—in situ, in vitro and in silico models. New York: Springer; 2008. pp. 34–76. [Google Scholar]
- 28.Berggren S, Hoogstraate T, Falgerholm U, Lennernäs H. Characterization of jejunal absorption and apical efflux of ropivacaine, lidocaine, and bupivacaine in the rat using in situ and in vitro absorption models. Eur J Pharm Sci. 2004;21:553–60. doi: 10.1016/j.ejps.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 29.Keogh JP, Jeevan R, Kunta JR. Development, validation and utility of an in vitro technique for assessment of potential clinical drug–drug interactions involving P-glycoprotein. Eur J Pharm Sci. 2006;27:543–54. doi: 10.1016/j.ejps.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 30.Volpe DA, Faustino PJ, Ciavarella AB, Asafu-Adjaye EB, Ellison CD, Yu LX, Hussain AS. Classification of drug permeability with a Caco-2 cell monolayer assay. Clin Res Regul Aff. 2007;24:39–47. doi: 10.1080/10601330701273669. [DOI] [Google Scholar]
- 31.Dowty ME, Dietsch CR. Improved prediction of in vivo peroral absorption from in vitro intestinal permeability using an internal standard to control for intra- and inter-rat variability. Pharm Res. 1991;14:1792–7. doi: 10.1023/A:1012148300807. [DOI] [PubMed] [Google Scholar]
- 32.Faustino PJ, Volpe DA, Knapton AD, Ellison CD, Hussain AS. Value of an internal standard approach for determining internal permeability class membership of drugs. AAPS PharmSci. 1999;1(4):abstract 2183. [Google Scholar]
- 33.Artursson P, Borchardt RT. Intestinal drug absorption and metabolism in cell culture: Caco-2 and beyond. Pharm Res. 1997;14:11655–8. doi: 10.1023/A:1012155124489. [DOI] [PubMed] [Google Scholar]
- 34.Lee CP, deVrueh RL, Smith PL. Selection of development candidates base on in vitro permeability measurements. Adv Drug Deliv Rev. 1997;23:47–62. doi: 10.1016/S0169-409X(96)00425-5. [DOI] [Google Scholar]
- 35.Hidalgo IJ, Windisch V, Hu H, Furukawa D, Kardos P. Importance of establishing acceptance criteria for Caco-2 monolayers used in permeability studies. AAPS PharmSci. 1998;1(1):abstract 1197. [Google Scholar]
- 36.Sulzbacher A, Jarosch A, Schuler R, Acerbi D, Ventura P, Puccini R, Woodcock BG. Validation of a Caco-2 cell monolayer culture for drug transport studies. Int J Clin Pharmacol Ther. 1998;36:86–9. [PubMed] [Google Scholar]
- 37.Artursson P, Palm K, Luthman K. Caco-2 monolayers in experimental and theoretical predictions of drug transport. Adv Drug Deliv Rev. 1996;22:67–84. doi: 10.1016/S0169-409X(96)00415-2. [DOI] [PubMed] [Google Scholar]
- 38.Borchardt RT. Rational delivery strategies for the design of peptides with enhanced oral delivery. Drug Deliv Indust Pharm. 1994;20:469–83. doi: 10.3109/03639049409038313. [DOI] [Google Scholar]
- 39.Hidalgo IJ, Raub TJ, Borchardt RT. Characterization of the human colon carcinoma cell line (Caco-2) as a model system for intestinal epithelial permeability. Gastroenterology. 1989;96:736–49. [PubMed] [Google Scholar]
- 40.Volpe DA. Permeability classification of representative fluoroquinolones by a cell culture method. AAPS PharmSci. 2004;6:article 13. doi: 10.1208/ps060213. [DOI] [PubMed] [Google Scholar]
- 41.Palmgrén JJ, Mönkkönen T, Korjamo T, Hassinen A, Auriola S. Drug adsorption to plastic containers and retention of drugs in cultured cells under in vitro conditions. Eur J Pharm Biopharm. 2006;64:369–78. doi: 10.1016/j.ejpb.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 42.Schanker LS, Tocco DJ, Brodie BB, Hogben CAM. Absorption of drugs from the rat small intestine. J Pharmacol Exp Ther. 1958;123:81–8. [PubMed] [Google Scholar]
- 43.Doluisio JT, Billups NF, Dittert LW, Sugita ET, Swintosky JV. Drug absorption. I. An in situ rat gut technique yielding realistic absorption rates. J Pharm Sci. 1969;58:1196–200. doi: 10.1002/jps.2600581006. [DOI] [PubMed] [Google Scholar]
- 44.Komiya I, Park JY, Kamani A, Ho NFH, Higuchi WI. Quantitative mechanistic studies in simultaneous fluid flow and intestinal absorption using steroids as model solutes. Int J Pharm. 1980;4:249–62. doi: 10.1016/0378-5173(80)90140-4. [DOI] [Google Scholar]
- 45.Zakeri-Milani P, Valizadeh H, Tajerzadeh H, Islambulchilar Z. The utility of rat jejunal permeability for biopharmaceutics classification system. Drug Dev Ind Pharm. 2009;35:1496–502. doi: 10.3109/03639040903037199. [DOI] [PubMed] [Google Scholar]
- 46.Ruan LP, Chen S, Yu BY, Zhu DN, Cordell GA, Qiu SX. Prediction of human absorption of natural compounds by the non-everted rat intestinal sac model. Eur J Med Chem. 2006;41:605–10. doi: 10.1016/j.ejmech.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 47.Lennernäs H. Human jejunal effective permeability and its correlation with preclinical drug absorption models. J Pharm Pharmacol. 1997;49:627–38. doi: 10.1111/j.2042-7158.1997.tb06084.x. [DOI] [PubMed] [Google Scholar]
- 48.Braun A, Hämmerle S, Suda K, Rothen-Rutishauser B, Günthert M, Krämer SD, Wunderli-Allenspach H. Cell cultures as tools in biopharmacy. Eur J Pharm Sci. 2000;11(Suppl 2):S51–S60. doi: 10.1016/S0928-0987(00)00164-0. [DOI] [PubMed] [Google Scholar]
- 49.Ungell AL, Karlsson J. Cell cultures in drug discovery: an industrial perspective. In: van de Waterbeen H, Lennernäs H, editors. Drug bioavailability: estimation of solubility, permeability, absorption and bioavailability. Weinheim: Wiley-WCH; 2003. pp. 90–131. [Google Scholar]
- 50.Artursson P, Karlsson J. Correlation between oral drug absorption in humans and apparent drug permeability coefficients in human intestinal epithelial (Caco-2) cells. Biochem Biophys Res Commun. 1991;175:880–5. doi: 10.1016/0006-291X(91)91647-U. [DOI] [PubMed] [Google Scholar]
- 51.Yazdanian M, Glynn SL, Wright JL, Hawi A. Correlating partitioning and Caco-2 cell permeability of structurally diverse small molecular weight compounds. Pharm Res. 1998;15:1490–4. doi: 10.1023/A:1011930411574. [DOI] [PubMed] [Google Scholar]
- 52.Irvine JD, Takahashi L, Lockhart K, Cheong J, Tolan JW, Selick HE, Grove JR. MDCK (Madin–Darby canine kidney) cells: a tool for membrane permeability screening. J Pharm Sci. 1999;88:28–33. doi: 10.1021/js9803205. [DOI] [PubMed] [Google Scholar]
- 53.Lentz KA, Hayashi J, Lucisano LJ, Polli JE. Development of a more rapid, reduced serum culture system for Caco-2 monolayers and application to the biopharmaceutics classification system. Int J Pharm. 2000;200:41–51. doi: 10.1016/S0378-5173(00)00334-3. [DOI] [PubMed] [Google Scholar]
- 54.Withington L. High-throughput epithelial cell culture systems for screening drug intestinal permeability. In: Lehr CM, editor. Cell culture models of biological barriers: in vitro test systems for drug absorption and delivery. London: Taylor and Francis; 2002. pp. 94–111. [Google Scholar]
- 55.Alsenz J, Haenel E. Development of a 7-day; 96-well Caco-2 permeability assay with high-throughput direct UV compound analysis. Pharm Res. 2003;20:1961–9. doi: 10.1023/B:PHAM.0000008043.71001.43. [DOI] [PubMed] [Google Scholar]
- 56.Thiel-Demby VE, Humphreys JE, St. John Williams LA, Ellens HM, Shah N, Ayrton AD, Polli JW. Biopharmaceutics classification system: validation and learning of an in vitro permeability assay. Mol Pharm. 2009;6:11–18. doi: 10.1021/mp800122b. [DOI] [PubMed] [Google Scholar]
- 57.Kansy M, Senner F, Gubernator K. Physicochemical high throughput screening: parallel artificial membrane permeability assay in the description of passive absorption process. J Med Chem. 1998;41:1007–10. doi: 10.1021/jm970530e. [DOI] [PubMed] [Google Scholar]
- 58.Wohnsland F, Faller B. High-throughput permeability pH profile and high-throughput alkane/water log P with artificial membranes. J Med Chem. 2001;44:923–30. doi: 10.1021/jm001020e. [DOI] [PubMed] [Google Scholar]
- 59.Flaten GE, Dhanikula AB, Luthman K, Brandl M. Drug permeability across a phospholipid vesicle based barrier: a novel approach for studying passive diffusion. Eur J Pharm Sci. 2006;27:80–90. doi: 10.1016/j.ejps.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 60.Corti G, Maestrelli F, Cirri M, Zerrouk N, Mura P. Development and evaluation of an in vitro method for prediction of human drug absorption. II. Demonstration of the method suitability. Eur J Pharm Sci. 2006;27:354–62. doi: 10.1016/j.ejps.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 61.Corti G, Maestrelli F, Cirri M, Furlanetto S, Mura P. Development and evaluation of an in vitro method for prediction of human drug absorption. I. Assessment of artificial membrane composition. Eur J Pharm Sci. 2006;27:346–53. doi: 10.1016/j.ejps.2005.11.004. [DOI] [PubMed] [Google Scholar]