Abstract
The low bioavailability of genistein has impeded its development into a therapeutic agent. Our earlier studies indicate that glucuronidation is one of the major barriers to genistein oral bioavailability. This study will determine how sulfotransferases and efflux transporters affect its intestinal disposition. A rodent intestinal perfusion model and S9 fractions were used. Sulfate excretion rates were comparable to glucuronide excretion in mouse small intestine but significantly higher than glucuronide excretion in mouse colon, which is different from rat intestinal disposition but similar to disposition in Caco-2 cells. To define efflux transporter(s) involved in sulfate excretion, two organic anion inhibitors (estrone sulfate and dihydroepiandrosterone sulfate) or a multidrug resistance protein inhibitor (MK-571) were used but neither was able to decrease the excretion of genistein sulfates. In contrast, the excretion of genistein sulfate decreased substantially (>90%) in small intestine of breast cancer resistance protein (BCRP) knockout mice and became undetectable in colon of the knockout mice. The excretion rates of genistein glucuronide in the small intestine of BCRP knockout mice were also significant decreased (78%). This study shows clearly that BCRP facilitates the cellular genistein sulfate excretion by removing sulfates to prevent their backward hydrolysis and to limit substrate inhibition, indicating that BCRP plays a dominant role in genistein sulfate excretion and a significant role in genistein glucuronide excretion in the mouse intestine.
Key words: genistein, sulfotransferases, efflux transporters, breast cancer resistance protein (BCRP), multidrug resistance protein 2 (MRP2)
INTRODUCTION
Genistein, a soy isoflavone, has been reported to have anticancer activities (1,2). In vitro studies have shown that it can inhibit cancer cell growth via a variety of mechanisms (3–5). However, the bioavailability of genistein is poor (usually less than 5%) (6,7). The in vivo plasma concentration of genistein is typically in the range of 0.01 to 0.1 μM after administering genistein-containing dietary supplements (1). Poor bioavailability of genistein is a serious concern because these in vivo concentrations are significantly less than the IC50 or EC50 value of 5 μM to 50 μM reported for its anticancer and other beneficial effects in vitro (8–10).
Genistein is rapidly absorbed, but undergoes extensive phase II metabolism in the intestine (11). In rats, mice, and humans, the major metabolites of genistein in plasma are genistein glucuronides and genistein sulfates (12–15). Both sulfate and glucuronide conjugates are much more hydrophilic than the parent compound, and therefore, cannot pass through the intestinal epithelial cell membrane by passive diffusion. Previous studies in our laboratory have shown that phase II conjugates of flavonoids were effluxed out of the intestine by yet-to-be determined transporters that were sensitive to estrone sulfate and MK-571 (16–19).
Previous published data from this laboratory also showed extensive phase II conjugation of various flavonoids including genistein in intestine using the Caco-2 cell culture model and the perfused rodent intestinal model (16–19). In the Caco-2 transport study, the excretion rates of genistein sulfates and glucuronides were rapid and transporter-mediated (17). In the rat intestinal perfusion study, mainly flavonoid (including genistein) glucuronides were excreted to the lumen by the intestinal epithelial cells, whereas little or no sulfates were excreted (16,20). Moreover, the intestinal glucuronides were secreted at rates much slower than their optimal formation rates (11,16,19). Unlike rats, both sulfate and glucuronide conjugates of formononetin, an isoflavone analog of genistein with significant phytoestrogen-like properties and found in abundance in the plant red clover, were observed in the mouse intestinal perfusate (21). The excretion rates of formononetin sulfate were higher than the formation rates in mouse intestinal homogenates, whereas the excretion rates of formononetin glucuronide were much lower than the formation rate (21).
Recent data showed that the excretion rate of genistein glucuronide is affected by both UDP-glucuronosyltransferases (UGTs) activities and activities of efflux transporters (22). However, it is not well understood if excretion rate of genistein sulfate is determined by sulfotransferases activities or efflux transporters or both, and which efflux transporter is involved in this process. Therefore, the main objective of the present study was to determine how sulfotransferases and efflux transporters function together to remove genistein sulfates from the intestinal epithelial cells back to the lumen. Another important objective was to determine the transporters involved in the excretion of genistein sulfates.
MATERIALS AND METHODS
Genistein was purchased from Indofine Chemicals (Somerville, New Jersey, USA). 3′-Phosphoadenosine 5′-phosphosulfate (PAPS), MK-571 (sodium salt) was purchased from Cayman Chemicals (Ann Arbor, Michigan, USA). Estrone sulfate (E1S), dihydroepiandrosterone sulfate, uridine diphosphoglucuronic acid (UDPGA), alamethicin, D-saccharic-1,4-lactone monohydrate, magnesium chloride, phenylmethylsulfonyl fluoride (PMSF), and Hanks’ balanced salt solution (HBSS, powder form) were purchased from Sigma-Aldrich (St. Louis, Missouri, USA). All other materials were analytical grade or better.
Animals
Male CB6F1 mice, male FVB mice (9–11 weeks old) weighing between 26 and 34 g and male Sprague-Dawley rats (70–110 days old) weighing between 260 and 350 g were purchased from Harlan Laboratories (Madison, Wisconsin, USA). Male BCRP knockout mice (9–11 weeks old) weighing between 26 and 34 g were purchased from Taconic Farm, Inc. (Hudson, New York, USA). Mice and rats were fed with Teklad F6 rodent diet (W) from Harlan Laboratories (Madison, Wisconsin, USA) for at least 1 week prior to experiments in our animal facility. All mice and rats were fasted overnight before surgery.
Perfusion Models
Rodent Surgery
Four mice or rats were used for each experimental group. The rat intestinal surgical procedures, approved by University of Houston Institutional Animal Care and Use Committee, are the same as those described previously (16). In rats, four segments (duodenum, jejunum, ileum, and colon) of the intestine were perfused (a four-site single-pass intestinal perfusion model). The mouse intestinal surgical procedures were modified from the rat model, in that only two segments of intestine (upper small intestine and colon) was perfused (a two-site single-pass intestinal perfusion model) because of the shorter length of mouse intestine (total ∼25 cm) when compared to the length of the rat intestine (total ∼80 cm).
Transport and Metabolism Experiments in the Perfused Rodent Intestinal Models
This is a single-pass perfusion method. Four segments of the rat intestine (duodenum, upper jejunum, terminal ileum, and colon) or two segments of the mouse intestine (upper small intestine and colon) were perfused simultaneously with a perfusate containing 2, 10, or 40 μM genistein in the HBSS supplemented with 20 mM sodium chloride using an infusion pump (Harvard Apparatus, Cambridge, Massachusetts, USA) at a flow rate of 0.191 or 0.382 ml/min. Inhibitors, when used, were added to the perfusate before the experiment. After a 30-min washout period, which is usually sufficient to achieve steady-state absorption, intestinal perfusate samples were collected from the outlet cannulae every 30 min. The perfusate was also collected from a segment of plastic tubing every 30 min as the control. After perfusion, the length of the intestine was measured as described previously (23,24). A 50-μl solution of 94% acetonitrile/6% glacial acetic acid containing 100 μM testosterone as the HPLC/UPLC internal standard were then added into 200 μl samples solutions. Concentrations of genistein and its metabolites in the luminal perfusate were determined by HPLC/UPLC.
Subcellular Models
Rat Intestinal and Hepatic S9 Fraction Preparation
This method was adapted from a previously published method to prepare microsomes (16). Briefly, rats that were fasted overnight with access to water only were euthanized with urethane (1,500 mg/kg). Four segments of rat intestines (duodenum, jejunum, ileum, and colon) were cut out, separated, and flushed with ice-cold saline containing 1 mM dithiothreitol. After segmented intestine were pooled from eight rats, they were then washed twice with the ice-cold washing solution (consisted of 8 mM KH2PO4, 5.6 mM Na2HPO4, 1.5 mM KCl, 96 mM NaCl, 27 mM sodium citrate, and 0.04 mg/ml phenylmethylsulfonyl fluoride or PMSF). The intestinal strips were then blot dried with paper and cut open lengthwise, and mucosal cells were scraped off in a 4°C cold room. The scraped mucosal cells were centrifuged at 900 × g for 5 min and washed twice in 12 ml of the homogenization buffer (consisted of 10 mM pH 7.4 KH2PO4, 250 mM sucrose, 1 mM EDTA, and 0.04 mg/ml PMSF). The cells were resuspended in 2 ml of the homogenization buffer. In the mean time, the livers of eight rats were collected in the 4°C cold room and cut into tiny pieces and suspended in 100 ml of the homogenization buffer. Both intestinal and hepatic cells were homogenized with a motorized Teflon/glass homogenizer (four strokes). After 15-min centrifugation in 9,000 × g at 4°C, the fat layer and pellet were discarded and the supernatant was collected, aliquoted, and stored at −80°C until use.
Mouse Intestinal and Hepatic S9 Fractions Preparation
CB6F1 mouse intestinal and hepatic S9 fractions were prepared using the same procedure as the preparation of rat intestinal and hepatic S9 fraction with modification. Two segments of mouse intestines (small intestine and colon) were used instead of four segments in the rats. The volume of each solution was modified based on the differences between mice and rats in pooled cell amounts (mg) harvested from the respective organs.
Protein Concentration Measurement
Protein concentrations of S9 fractions were determined using a protein assay kit (Bio-Rad, Hercules, California, USA), with bovine serum albumin serving as the standard.
Sulfation and Glucuronidation of Genistein in Intestinal or Hepatic S9 Fractions
Sulfation of genistein by S9 fractions was measured using procedures described previously with minor modifications (25). Briefly, S9 fractions (final protein concentration at ∼1 mg/ml) were mixed with genistein in 50 mM potassium phosphate buffer (pH 7.4), and 0.1 mM PAPS solution was added last to the reaction mixture (total volume 200 μl). The mixture was incubated in a 37°C water bath for 60 or 120 min.
Glucuronidation of genistein by S9 fractions was also measured using procedures described previously (16,18). S9 fractions (final protein concentration at ∼1 mg/ml) were mixed with magnesium chloride (0.88 mM), saccharolactone (4.4 mM), and alamethicin (0.022 mg/ml). Genistein in 50 mM potassium phosphate buffer (pH 7.4) was then added. A solution of 3.5 mM UDPGA was added last to the reaction mixture (total volume 200 µl), and the mixture was incubated in a 37°C water bath for 60 min.
The sulfation and glucuronidation reactions were stopped by addition of 50 µl solution of 94% acetonitrile/6% glacial acetic acid containing 100 μM testosterone as the internal standard. We also performed the same experiment but added water instead of PAPS or UDPGA as the negative control.
Hydrolysis Reaction of Genistein Sulfates in Intestinal S9 Fractions
A mixture containing genistein sulfate and genistein were added to mouse S9 fractions (final concentrations about 1 mg/ml total protein concentration) to make a final concentration of 14 μM genistein sulfates and 0.75 μM of genistein. It was then incubated in a 37°C shaking water bath for up to 4 h. The reaction was stopped by addition of 50 μl solution of 94% acetonitrile/6% glacial acetic acid containing 100 μM testosterone as the internal standard. We also performed the same experiment but using 50 mM potassium phosphate buffer (pH 7.4) instead of mouse S9 fractions as the negative control.
Genistein Sulfation in the Presence of Genistein Sulfate
Genistein sulfate was added to mouse S9 fractions (final concentrations about 0.5 mg/ml total protein concentration) to make two solutions containing 2.5 or 5 μM genistein sulfates, respectively. Genistein was then added to each of the solution to make a final concentration of 2.5 or 10 μM genistein, respectively. The sulfation reaction cofactor, PAPS, was then added to the reaction system and incubated in a 37°C shaking water bath for 60 min as previously described. The reaction was stopped by addition of 50 μl solution of 94% acetonitrile/6% glacial acetic acid containing 100 μM testosterone as the internal standard. We also performed the same sulfation experiment in mouse S9 fractions without the addition of genistein sulfates as the control.
Sample Analysis
Genistein and its conjugates (sulfates and glucuronides) were quantified using HPLC with UV detector (for data in Table I, Figs. 3, 4a and b, and 6) or UPLC with DAD detector (for data in Table II, Figs. 1, 2, 4c and d, and 5). Identification of the metabolites was achieved using UPLC-MS/MS.
Table I.
Formation and Excretion Rates of Genistein Metabolites in Rat and Mouse Intestinal Models
| Rate (pmol/min/10 cm) | Genistein glucuronide | Genistein sulfate | |||
|---|---|---|---|---|---|
| Small intestine | Colon | Small intestine | Colon | ||
| Rat | Formation | 3,268 ± 94 | 957 ± 10 | 3.07 ± 0.11 | 1.37 ± 0.03 |
| Excretion | 236 ± 93 | 174 ± 36 | n.d. | n.d. | |
| Mouse | Formation | 1,709 ± 35 | 518 ± 11 | 60.7 ± 0.6 | 20.1 ± 0.5 |
| Excretion | 150 ± 69 | n.d. | 272 ± 31 | 158 ± 33 | |
Formation rates of the genistein conjugates were obtained using the sulfation and glucuronidation rates of 10 μm genistein in the rat or mouse intestinal S9 fractions (n = 3) and normalized to the length (10 cm) of intestine. Excretion rates of genistein conjugates were obtained from the rat or mouse intestinal perfusion (n = 4). Perfusate containing 10 μM genistein was used to perfuse four segments of rat intestine (duodenum, jejunum, ileum, and colon) or two segments of mouse intestine (upper small intestine and colon) simultaneously at a flow rate of 0.191 ml/min. Excretion rates of the metabolites were expressed as pmol of metabolites excreted per minute per 10 cm segment in the intestinal perfusion models. Rates were presented as mean ± SD
n.d. not detected or less than 1 pmol/min/10 cm based on the current analytical method
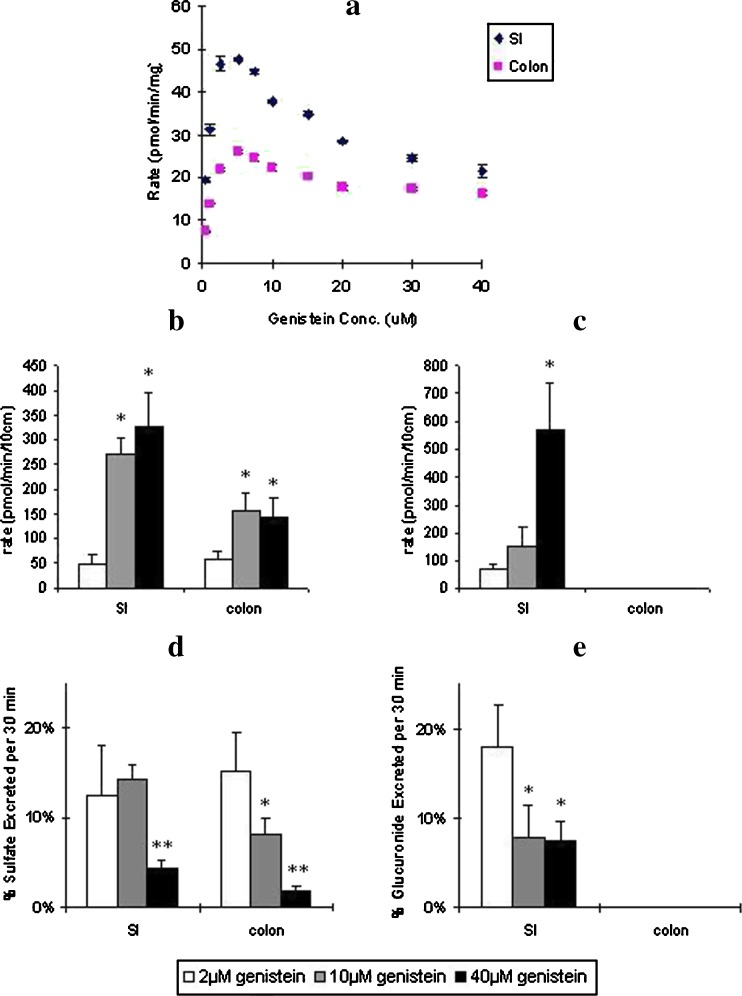
Fig. 3.
a Effects of genistein concentration on its sulfation in CB6F1 mouse small intestine and colon S9 fractions. Also shown are the effects of genistein concentration on excretion rates of b genistein sulfates and c genistein glucuronides and d percent genistein sulfates and e genistein glucuronides excretion in mouse intestine. In Fig. 4a, sulfation rates were determined from 0.5 to 40 μM of genistein concentration with a reaction time of 60 or 120 min. The diamonds (blue) represent sulfation rate in small intestine and squares (pink) represent sulfation rate in colon. The error bars represent the standard deviation of the mean. In Fig. 4b, c, d, and e, excretion rates of genistein sulfates and glucuronides were obtained from the mouse intestinal perfusion (n = 4). Perfusate solutions containing 2 (open bars), 10 (gray bars), or 40 μM (black bars) genistein were used, and two segments of mouse (small intestine and colon) were perfused simultaneously at a flow rate of 0.191 ml/min. In Fig. 4b and c, excretion of b sulfates or c glucuronides was expressed as amount excreted per minute per 10 cm segment. Excretion of genistein glucuronides was not detected in colon (less than 1 pmol/min/10 cm). The error bars represent the standard deviation of the mean. In Fig. 4d and e, excretion of d sulfates or e glucuronides was expressed as percent excreted per 30 min per 10 cm segment in the mouse intestinal perfusion models. The error bars represent the standard deviation of the mean. The symbols “*” (or p < 0.05) mean the differences of percent excreted between 10 and 40 μM genistein perfusion and 2 μM genistein perfusion in mouse small intestine and colon are significant according to Student’s t test
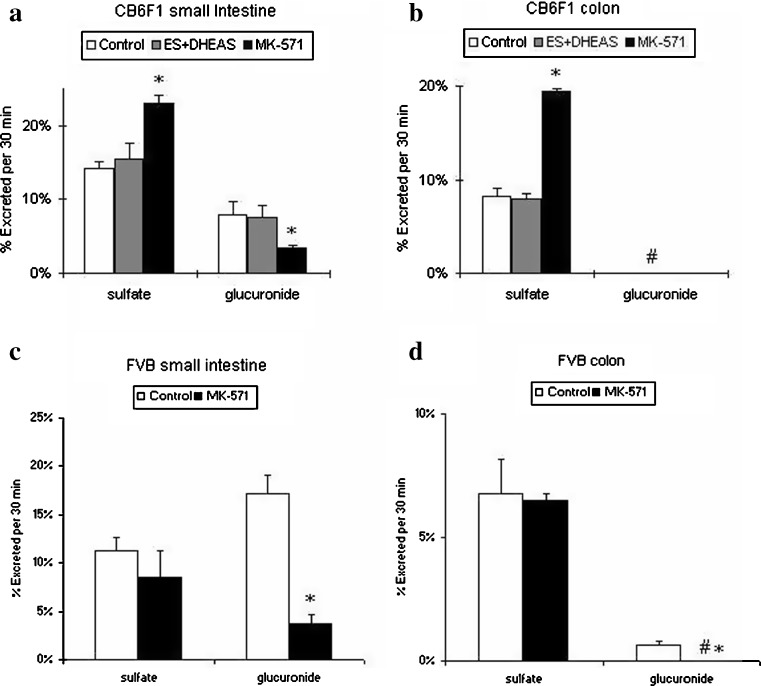
Fig. 4.
Effects of OAT inhibitor and MRP inhibitor on genistein conjugates excretion in a small intestine and b colon of CB6F1 mouse intestine. Also shown are the effects of MRP inhibitor on genistein conjugates excretion in the c small intestine and d colon in FVB mouse intestine (n = 4). For Fig. 5a and b, perfusate containing 10 μM genistein (white bar), 10 μM genistein plus 1 mM estrone sulfate plus 1 mM dihydroepiandrosterone (DHEA) sulfate (gray bar), and 10 μM genistein plus 50 μM MK-571 (black bar) were used, and two segments of CB6F1 mouse (small intestine and colon) were perfused simultaneously at a flow rate of 0.191 ml/min. For Fig. 5c and d, perfusate containing 10 μM genistein (open bar) and 10 μM genistein plus 50 μM MK-571 (black bar) were used, and two segments of FVB mouse (small intestine and colon) were perfused simultaneously at a flow rate of 0.382 ml/min. Percentage of conjugates excreted during perfusion, normalized to 10 cm intestinal length, were obtained as described in the “MATERIALS AND METHODS” section. The symbol “#” means the percentage of genistein glucuronide excretion in colon was not detected (or less than 0.5% based on our analytical method). The error bars represent the standard deviation of the mean. The symbol “*” means the difference of percent excretion of sulfates or glucuronides between genistein perfusion with inhibitor and control genistein perfusion is significant (or p < 0.05) according to Student’s t test
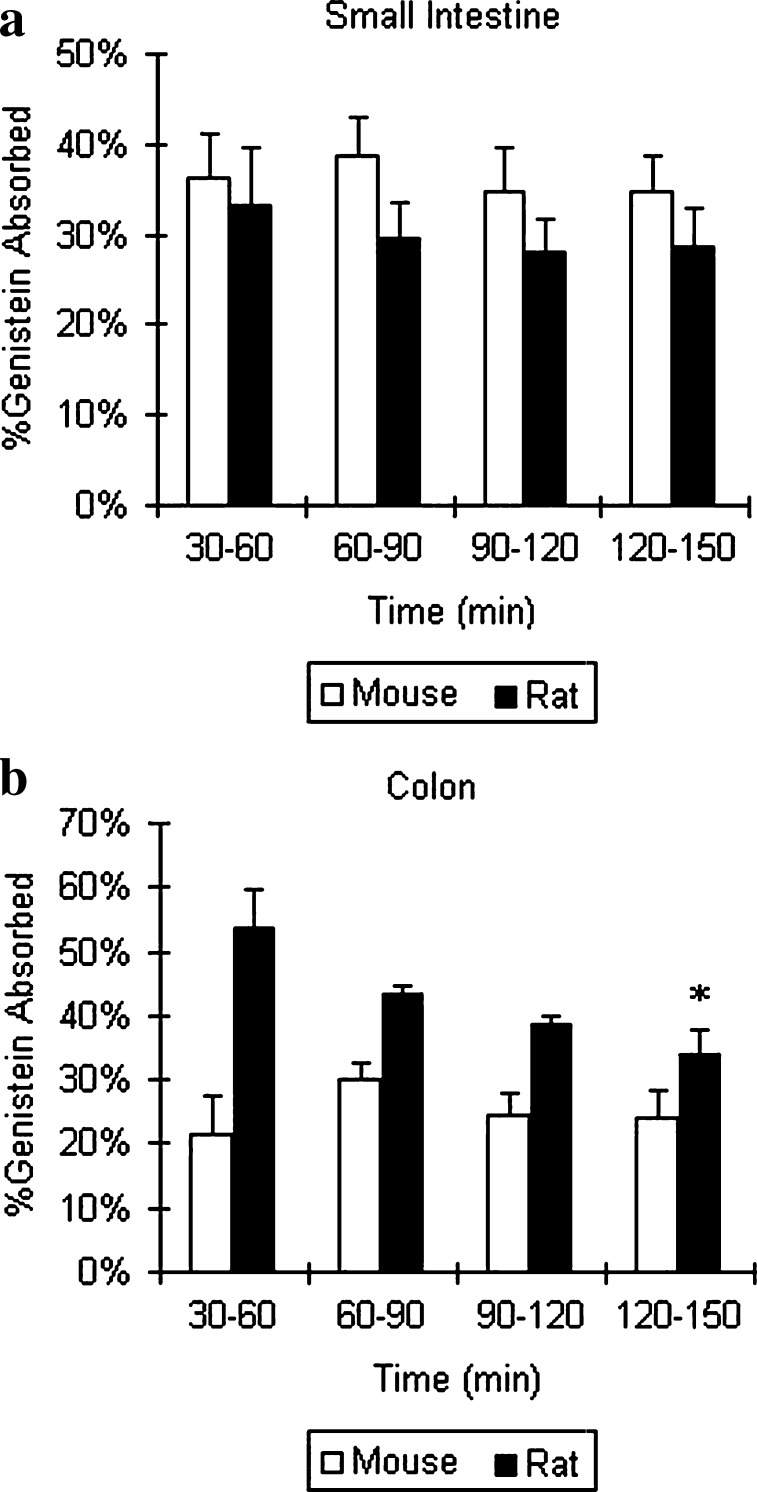
Fig. 6.
Genistein absorption in a small intestine and b colon in mouse and rat perfusion models (n = 4). Perfusate containing 10 μM genistein was used, and two segments of mouse (small intestine and colon) and four segments of rat (duodenum, jejunum, ileum, and colon) were perfused simultaneously at a flow rate of 0.191 ml/min. Percentage of genistein absorbed during 150 min perfusion, normalized to 10 cm intestinal length, was obtained as described in the “MATERIALS AND METHODS” section. The error bars represent the standard deviation of the mean. The open bars and black bars represent percentages of genistein absorption in mouse and in rat intestine, respectively. More than 15% of absorption is considered that genistein was well absorbed. The symbol “*” means the difference of percent absorption between mouse colon and rat colon during 150 min perfusion is significant (or p < 0.05) according to one-way ANOVA
Table II.
Effect of Genistein Sulfate on Sulfation of Genistein by Mouse Intestinal S9 Fraction
| Genistein concentration | Genistein (μM) | Genistein sulfate (μM) | ||
|---|---|---|---|---|
| Control | Sulfate | Control | Sulfate | |
| 2.5 µM | 2.08 ± 0.05 | 2.03 ± 0.03 | 3.21 ± 0.10 | 3.07 ± 0.03 |
| 10 µM | 6.41 ± 0.07 | 6.45 ± 0.04 | 8.46 ± 0.08 | 8.24 ± 0.10 |
The reaction was conducted at 2.5 or 10 μM genistein, respectively (n = 3). Concentrations of genistein and its sulfate were obtained after sulfation reaction in the absence (control) or the presence (sulfate) of equal concentration of genistein sulfates at the beginning of the reaction. The reaction time was 30 min. The results indicated that presence of sulfate did not affect genistein sulfation rates. Concentrations were presented as mean ± SD
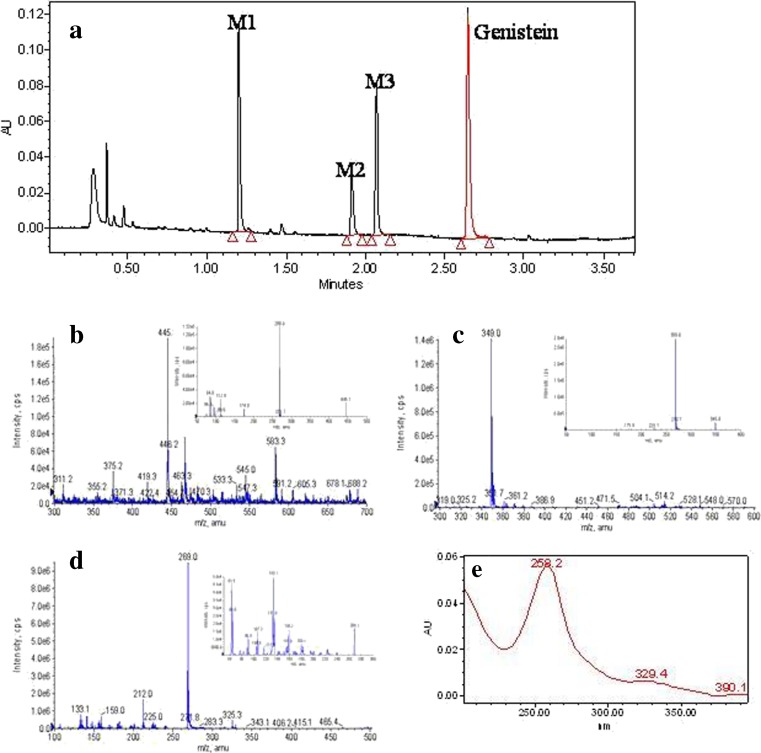
Fig. 1.
UPLC chromatogram and mass spectra of genistein and its conjugated metabolites. a Genistein and its three main conjugates (M1–M3) in a sample spiked with genistein and its three metabolites. b, c, d MS full scan spectra of genistein glucuronide (M1, b), genistein sulfate (M2 and M3, c), and genistein (d), respectively. The small windows in each panel show the MS2 full scan for the respective analyte. e Online UV spectra of genistein and not shown are similar online spectra of its conjugated metabolites
Fig. 2.
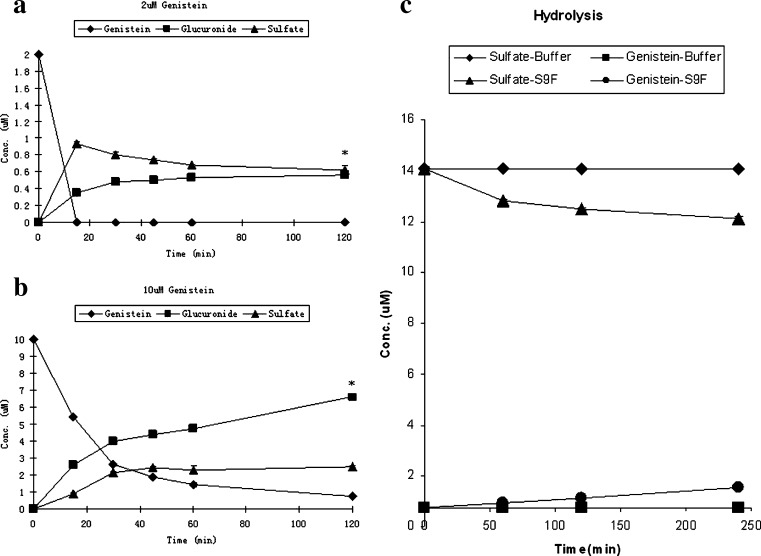
Simultaneous glucuronidation and sulfation reactions of genistein at concentrations of a 2 and b 10 μM (n = 3). Also shown is the c hydrolysis of genistein sulfate in FVB mouse small intestinal S9 fractions (n = 3). In a and b, squares, triangles, and diamonds represent concentrations of glucuronides, sulfates, and genistein, respectively. The error bar is the standard deviation of the mean. The symbol “*” means the differences between concentration of glucuronides and sulfates are significant (or p < 0.05) according to one-way ANOVA analysis. In Fig. 3c, concentrations of genistein and its sulfate were obtained from incubation of genistein sulfate with in vitro mouse small intestinal S9 fractions or buffer for a period up to 240 min. In Fig. 3c, triangles and circles represent concentrations of genistein sulfates and genistein in which S9 fractions were present whereas diamonds and squares represent concentrations of genistein sulfates and genistein in which S9 fractions were absent (i.e., only buffer used). The error bar is the standard deviation of the mean, which was derived from an average of three determinations
Fig. 5.
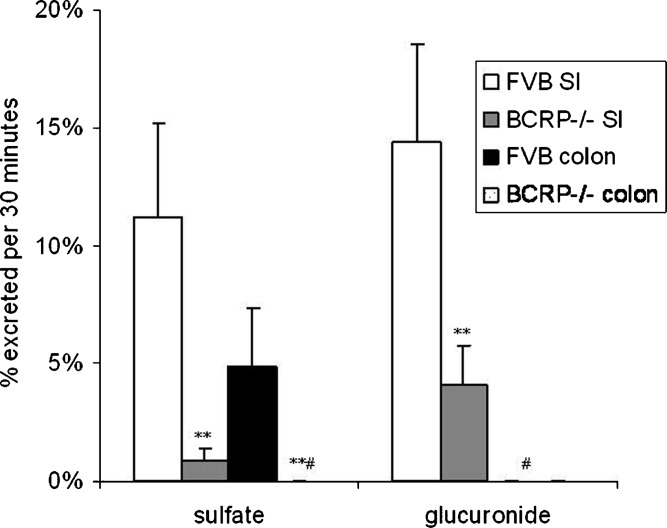
Effects of BCRP on genistein conjugates excretion in small intestine (SI) and colon of mouse (n = 4). Perfusate containing 10 μM genistein were used in wild-type FVB mouse or BCRP−/− mouse, and two segments of mouse (small intestine and colon) were perfused simultaneously at a flow rate of 0.191 ml/min. Percentage of sulfates (left panel) and glucuronides (right panel) excreted during perfusion, normalized to 5 cm intestinal length, were obtained as described in the “MATERIALS AND METHODS” section. The symbol “#” means the percentage of genistein glucuronide excretion in FVB and BCRP−/− mice colon and percentage of genistein sulfate excretion in BCRP−/− mice colon were not detected (or less than 0.5% based on our analytical method). The error bars represent the standard deviation of the mean. The symbol “**” means the difference of percent excretion of genistein sulfates or glucuronides excreted in small intestine and colon between BCRP−/− mice and FVB mice is significant (or p < 0.01) according to Student’s t test
Identification of Genistein and its Conjugates
An API 3200 QTrap triple quadrupole mass spectrometer (Applied Biosystem/MDS SCIEX, Foster City, California, USA) equipped with a TurboIonSpray™ source was operated in negative ion mode to perform the qualitative UPLC-MS/MS analysis. The main working parameters for the mass spectrometers were set as follows: ionspray voltage, −4.0 kV; ion source temperature, 700°C; the nebulizer gas (gas1), nitrogen, 40 psi; turbo gas (gas2), nitrogen, 40 psi; curtain gas, nitrogen, 10 psi. Genistein, genistein sulfates, and genistein glucuronides were identified by MS full scan and MS2 full scan modes (Fig. 1). Two sulfates were combined in the following quantitation, primarily because these two sulfates are not separated in HPLC.
UPLC Analysis of Genistein and its Conjugates with a DAD Detector
Waters Acquity™ UPLC System with DAD detector was used to quantitatively analyze genistein and its conjugates. The conditions were: Waters Acquity with a binary pump, an autosampler, and a 2996 DPA diode array detector (DAD; Waters, Milford, Massachusetts, USA); column, Acquity UPLC BEH C18 column (50 × 2.1 mm I.D., 1.7 μm, Waters, Milford, Massachusetts, USA); mobile phase A: 2.5 mM ammonium acetate (pH 7.5); mobile phase B: 100% acetonitrile; gradient: 0 to 2.5 min, 5% to 20% B, 2.5 to 3.0 min, 20% to 45% B, 3.0 to 3.5 min, 45% to 5% B, 3.5 to 3.7 min, 5% B; column temperature: 40°C; injection volume: 10 μl; flow rate: 0.5 ml/min; UV detector wavelength: 254 nm. Typical precision and accuracy was usually within 9%. Linear response range was 0.32–50 μM.
HPLC Analysis of Genistein and its Conjugates
HPLC was used to analyze genistein and its conjugates prior to acquisition of the UPLC, and this method measured only one major glucuronide and one major sulfate. HPLC conditions were essentially the same as those published previously (16,17). The actual conditions for quantitatively analyzing genistein and its conjugates were: system, Agilent 1050 with UV detector and ChemStation; column, Aqua (Phenomenex, Gilroy, California, USA, 5 μm, 150 × 4.6 mm); mobile phase A, 100% acetonitrile; mobile phase B, 0.04% (v/v) phosphoric acid plus 0.06% (v/v) triethylamine in water (pH 2.9); gradient, 0–3 min, 98% B, 3–35 min, 98–50% B, 35–39 min, 50% B, 39–40 min, 50–98% B, 40–43 min, 98% B; injection volume, 200 μl; UV detector wavelength: 254 nm. Typical precision and accuracy was usually within 10%. Linear response range was 0.5–50 μM.
Data Analysis
Data Analysis in the Rat and Mouse Intestinal Perfusion Model
Absorption of genistein from the perfusate was measured by determining the rate of disappearance from the perfusate which was described previously (16,21). Excretion rates of the metabolites were expressed as amounts of metabolites excreted per minute per 10 cm segment (pmol/min/10 cm), in which excretion of total sulfates (sulfate 1 + sulfate 2) was presented. Absorption was also normally expressed as %absorption per 10 cm or as pmol/min/10 cm. Occasionally, absorption was so rapid that it exceeded 50% per 5 cm, which necessitate the use of %absorbed per 5 cm or amount absorbed per 5 cm.
Data Analysis in the Rat and Mouse Intestinal S9 Fraction Model
Rates of metabolite (either sulfate or glucuronide) formation in intestinal S9 fractions were expressed as amounts of metabolites formed per minute per 10 cm segment (pmol/min/10 cm), in which formation rates of total sulfates (sulfate 1 + sulfate 2) was presented. We normalized the formation rates measured in S9 fractions to length of the intestine by utilizing the relationship between the total length of intestine (from eight to ten mice or rats) needed to make a certain volume of S9 fractions of a particular protein concentration. This normalized formation rates facilitate the comparison between formation and excretion rates. We realized that the formation rates may be underestimated since we are bound to lose a small amount of enzymes during the preparation process. But we estimated the loss at less than 30% since we are able to recover almost all activities using the same procedures in Caco-2 cells, where harvest of cells can easily reach nearly 100%.
Statistical Analysis
Independent Student’s t test and one-way ANOVA followed by Tukey post hoc test were used to analyze the data. The level of significance was set at 5% or p < 0.05.
RESULTS
Identification of Phase II Metabolites of Genistein in Mouse Intestinal Perfusate
In the sample chromatogram (Fig. 1a), genistein and its three metabolite peaks that have the same UV absorbance profile (only one is shown) were shown (Fig. 1). The retention time of genistein was 2.7 min and that of the metabolite peaks were 1.2 min (M1), 1.9 min (M2), and 2.1 min (M3), respectively.
These metabolites were then characterized by mass spectrometer. Metabolite M1 has the pseudomolecular ion [M–H]− at m/z 445 (Fig. 1b), which was 176 Da higher (characteristic of the addition of glucuronic acid) than that of genistein, whose pseudomolecular ion appeared at m/z 269 (Fig. 1d). The base peak at m/z 269 in the MS2 spectra of M1 corresponded to the [M–H]− ion of genistein. Based on these data, M1 was identified as the glucuronide conjugates of genistein.
Metabolite M2 and M3 gave a pseudomolecular ion at m/z 369 (Fig. 1c), which was 80 Da higher (characteristic of the addition of a sulfate) than that of genistein (m/z 269). The MS2 spectrum of m/z 369 gave a base peak at m/z 269 (−80 Da). The results suggested that M2 and M3 were both genistein sulfates.
Regional Metabolism of Genistein in the Mouse and Rat Intestinal Perfusion Models
Significant differences in the excretion of phase II conjugates were observed between mice and rats and between different segments of the intestine. For example, only genistein glucuronides were detected in the rat intestinal perfusate. On the other hand, both sulfates and glucuronides were found in the mouse intestinal perfusate. Furthermore, the amounts (pmol/min/10 cm) of genistein sulfate excreted in the mouse small intestine (272.4 ± 31.1) were significantly higher (p < 0.05) than the amount of genistein glucuronide excreted (150 ± 70). Similarly, more genistein sulfates than genistein glucuronides were excreted into mouse colon (157.7 ± 33.1 pmol/min/10 cm), where glucuronide was not detected in either species (Table I).
We also determined the formation rates and attempted to compare the formation rates and excretion rates here although we understood we have two experimental systems and some enzyme may be lost during S9 fraction preparations. The purpose here is to contrast the difference between mice and rats. As can be seen from the formation rates, rat S9 fraction glucuronidated genistein 2× faster than mouse S9 fraction, whereas mouse intestinal S9 fraction sulfated genistein 20× faster than rat S9 (Table I). On the other hand, rat intestinal excretion of glucuronides was approximately two times faster, whereas mouse intestinal excretion was at least 150× faster. Thus, sulfate and glucuronide excretion displayed rather unusual differences that we did not expect to see based on difference in formation rates. Hence, it was apparent that there was no correlation between formation rate and excretion rate of genistein conjugates in mouse and rat intestinal models. We have chosen mice for the remaining studies since sulfate excretion is only apparent in the mouse intestine, and in human Caco-2 cells. Previously, we have used rats for studying the glucuronidation pathway.
Simultaneous Glucuronidation and Sulfation Reactions in Mouse S9 Fractions
To further investigate the relative contribution of sulfation and glucuronidation reaction of genistein in mouse small intestine, we conducted the glucuronidation and sulfation reactions simultaneously in S9 fractions at two concentrations of genistein: 2 and 10 μM. At 2 μM concentration, genistein glucuronide concentration increased but was always lower than sulfate concentration during the 120 min experiment (Fig. 2a). Surprisingly, at 10 μM, the glucuronidation reaction was about 2- to 3-folds faster than sulfation reaction during 120 min experiment (Fig. 2b). We would have predicted faster sulfation rates since in 10 μM genistein intestinal perfusion study, genistein sulfates were excreted faster than glucuronides in mouse small intestine (272.4 pmol/min/10 cm vs. 149.9 pmol/min/10 cm) and colon (157.7 pmol/min/10 cm vs. less than 1 pmol/min/10 cm; Table I).
Genistein Sulfate Hydrolysis Reaction in Mouse S9 Fractions
Unexpectedly, we observed that the sulfate concentration of genistein in simultaneous reaction either decreased (Fig. 2a) or became stable after reaching the peak (Fig. 2b), whereas glucuronide concentration continued to increase with time. We thought that the presence of sulfatase in the S9 fractions might have caused this because sulfatase inhibitors were not added into the reaction system whereas a glucuronidase inhibitor (saccharolactone) was. To confirm the presence of sulfatase, we incubated genistein sulfates with FVB mouse small intestinal S9 fractions, and we found that genistein sulfates were lost and genistein was formed during the incubation, confirming the presence of sulfatases (Fig. 2c).
Effects of Genistein Concentration Changes on Sulfate Formation Rates in Mouse Small Intestinal and Colonic S9 Fractions
In order to understand how efflux transporter(s) facilitate the sulfation of genistein in mouse intestine, we conducted a kinetic study for genistein sulfation in mouse intestinal and colonic S9 fractions using different concentrations of genistein. Sulfation rates were determined from 0.5 to 40 μM of genistein concentration. We found the sulfation rates increased with concentration from 0.5 to 5 μM, after which the sulfation rates decreased with a further increase in genistein concentration (Fig. 3a).
Effect of Concentration Change on Excretion of Genistein Sulfates and Glucuronides in the Mouse Perfusion Model
Using different concentrations of genistein solution as perfusate (2, 10, or 40 μM), we calculated the amount and the percentages of genistein sulfates and glucuronides excreted in mouse small intestine and colon. We found the amount of genistein sulfates excreted increased from 2 to 10 μM and was unchanged from 10 to 40 μM in both small intestine and colon (Fig. 3b). The amount of genistein glucuronides excreted increased stepwise from 2 to 40 μM (Fig. 3c). We also found that the percentage excretion of genistein sulfates was unchanged from 2 to 10 μM and decreased from 10 to 40 μM in small intestine. The percentage excretion of genistein sulfates decreased stepwise from 2 to 40 μM in colon (Fig. 3d). The percentage of genistein glucuronides excreted decreased from 2 to 10 μM and was unchanged from 10 to 40 μM in small intestine, and no glucuronide was excreted in colon (Fig. 3e).
Genistein Sulfation in the Presence of Genistein Sulfates
To determine if genistein sulfation was caused by product inhibition, pure genistein sulfates were added to the genistein sulfation reaction system. The results showed that genistein sulfation rates did not decrease with the addition of genistein sulfates at two initial substrate concentrations (Table II). Rather, we showed significant hydrolysis of genistein sulfates by sulfatases (Fig. 2c).
Effects of Organic Anion Transporter or MRP Inhibitors on Excretion of Genistein Conjugates in the Mouse Perfusion Model
Excretion rates of genistein sulfates in the absence or presence of anion transporter inhibitors were determined to identify the possible involvement of efflux transporter(s) on the disposition of genistein conjugates. Two organic anion transporter (OAT) inhibitors (1 mM estrone sulfate plus 1 mM dihydroepiandrosterone [DHEA] sulfate) were added together to the 10 μM genistein perfusate used for CB6F1 mouse perfusion. The results indicated that these two inhibitors did not significantly change the percentage excretion of genistein conjugates in either mouse small intestine or colon (Fig. 4a and b).
We then tested the effect of 50 μM MRP2 inhibitor MK-571 on genistein disposition in both CB6F1 mice and FVB mice. Surprisingly, the percent excretion of genistein sulfate significantly increased in both small intestine and colon of CB6F1 mice (p < 0.01), whereas the percent excretion of genistein sulfate slightly (but no significantly) decreased in FVB mouse small intestine and colon. The percent excretion of genistein glucuronides significantly decreased in small intestine of both mouse strains (p < 0.05; Fig. 4a–d).
Role of BCRP on Absorption and Metabolism of Genistein in the Mouse Perfusion Model
In BCRP−/− mice, we found that the percent excretion of genistein sulfates in small intestine decreased about 12-fold (from 11.2% to 0.9%, a 92% decrease), and the percent excretion of genistein glucuronides decreased about 3.5-fold (from 14.4% to 4.1%, a 78% decrease) compare to wild-type FVB mice (p < 0.01). We also found that %excretion of genistein sulfates was not detectable (less than 0.5%, >90% decrease) in BCRP−/− mice colon. In wild-type FVB mice, the %excretion of sulfates was about 4.8% (Fig. 5).
Regional Absorption of Genistein in the Mouse and Rat Intestinal Perfusion Model
Absorption of genistein (10 μM) in intestine was rapid (>15%) with minimal effects on water flux (i.e., water flux <0.5%/cm of perfused segment), in both mouse and rat intestinal models. The percentage of genistein absorbed in mouse small intestine was slightly higher than in rat small intestine, but the difference was not significant (p > 0.05). However, the percentage of genistein absorbed in mouse colon was significantly lower than that in rat colon during perfusion (p < 0.05; Fig. 6).
DISCUSSION
In this study, we found that BCRP played a dominant role in facilitating genistein sulfate excretion and a major role in enabling genistein glucuronide excretion in the mouse intestine. BCRP facilitated the efflux of genistein sulfate, which is critical to maintain the intracellular concentration of sulfates and aglycones, both of which are important to cellular production of sulfates. Because sulfate formation catalyzed by sulfotransferases is reversible by the action of sulfatases, the results clearly showed that the coordinated action (or coupling) of BCRP and sulfotransferases is essential for rapid sulfate formation and subsequent excretion. Hence, interfering with this coupling mechanism will diminish the coordinated action and negatively impact intestinal genistein disposition via the sulfation pathway in the BCRP−/− mice.
Our results showed that BCRP is critical for intestinal disposition of genistein (a flavonoid with rapid absorption (Fig. 6)) via the sulfation pathway in intestine because genistein sulfate excretion by enterocytes was almost completely demolished in BCRP−/− (knockout) mice (Fig. 5). This is one of the rare instances when we observed that a deficiency in single transporter almost completely diminished the cellular excretion of its substrate, in this case a sulfate. Previously, Adachi et al. showed that excretion of 4-methylumbelliferone sulfate was also similarly decreased in BCRP knockout mice (26). In addition, BCRP also played a major role in the excretion of genistein glucuronide, as its excretion was decreased 78% in the knockout mice (Fig. 5). This is also consistent with the observation that excretion of 4-methylumbelliferone glucuronide was also diminished in BCRP knockout mice (26). Moreover, another study showed a 36% and 17% decrease for the apical excretion of benzo[a]pyrene-3-sulfate in Caco-2 cells and TC7 cells, respectively, in the presence of Ko 143 (a specific BCRP inhibitor) (27), although the differences were not as distinct as shown in this paper. On the other hand, many other investigators only saw marginal effects or no effect at all (28).
Our results showed that MK-571, an MRP inhibitor, decreased glucuronide excretion in the intestine of CB6F1 and FVB mice (Fig. 4). Previously, 50 μM MK-571 has been shown to inhibit both UGT and MRPs, which are transporters involved in the apical and basolateral efflux of phase II conjugates in Caco-2 cells (17). Therefore, same effects of MK-571 on UGT and MRPs are expected in mouse intestine. We were surprised that the use of MK-571 caused a surge (61%) in luminal excretion of genistein sulfates in CB6F1 mouse intestine (Fig. 4a and b). The same compound did not cause this stimulation in FVB mice (Fig. 4c and d), however, suggesting that these stimulating effects may be highly specific to the strain of mice used. We are uncertain as to why it happens only in the CB6F1 mice but we believe that it is probably the result of compensatory responses to decreases (44%) in glucuronide excretion, and/or to the inhibition of basolateral efflux via MRP1 and/or MRP3 (29,30). More detailed studies are needed to explain this strain difference. Taken together, this result further supports our conclusion that BCRP is probably the exclusive and the dominant apical efflux transporter of genistein sulfates. On the other hand, MRP2 appears to play an important role in limiting glucuronide excretion. In contrast, an estrone/DHEA sulfates sensitive OAT, which was shown to be involved in flavonoid sulfate efflux in Caco-2 cells (17), did not appear to be involved in the mouse intestinal excretion of genistein sulfates or glucuronides (Fig. 4).
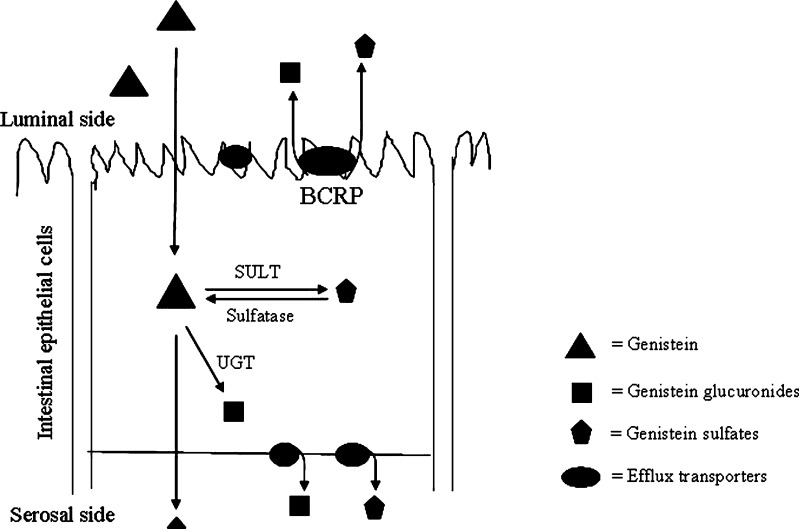
The results of this study lend support to the hypothesis that an important physiological role of BCRP is to facilitate the intestinal disposition of some flavonoids via sulfotransferase pathway, which is schematically represented in Fig. 7. This facilitating action of BCRP is enabled by rapidly removing the sulfates from the cytosolic domain because sulfates can be hydrolyzed back to aglycones by sulfatases (Fig. 2c). If sulfates are not removed, they will be hydrolyzed back to aglycones and ultimate results in often more production of glucuronides (Fig. 2a and b). Therefore, we proposed that BCRP works in a coordinated style with SULTs to maintain the intracellular concentration of genistein at a low level, so that substrate inhibition seen in S9 fraction (Fig. 3a) is less likely to occur in vivo. Taken together, this proposed physiological role of BCRP to rapidly metabolize food-borne flavonoids may be related to the transporter’s endogenous function in humans, since humans have had flavonoid-rich diets for thousands of years. Previously, BCRP has been shown to be important to protect stem cell functions and in fact has been used as a marker protein for sorting out stem cells (31,32). Whether these two facts are related or just coincidence remain to be determined.
Fig. 7.
Schematic diagram of genistein disposition in intestine. Absorption of genistein into the epithelial cells is fast, while the aglycone undergoes extensive phase II metabolism in the cells results in low bioavailability in vivo. BCRP facilitates the cellular genistein sulfate excretion by rapidly removing sulfates from the cytosolic domain to the lumen to prevent their backward hydrolysis which is catalyzed by sulfatases. If sulfates are not removed from the cells, they will be hydrolyzed back to aglycones and ultimate results in often more production of glucuronides (catalyzed by UGTs)
Our results also show that it is possible to take advantage of what we know about sulfate production and excretion to limit their intestinal conjugation. The results showed that sulfotransferases and BCRP-mediated efflux of sulfates were both saturable at high concentrations (Fig. 3a, d, and e). Therefore, this metabolic barrier (via sulfation pathway) can be overwhelmed by using a high concentration of flavonoid. In fact, many people are interested in delivering large quantities of beneficial natural polyphenols such as curcumin (33), by overwhelming the barrier even though the biological consequence of barrier obliteration remains unknown.
Last but not the least, the results of this study clearly show the differences of genistein sulfation and thereby sulfate excretion between different species. While no excreted sulfates were detected in rat intestine, significant amount of sulfates were excreted in mouse intestine (Table I). Pharmacokinetics studies of genistein showed that even though genistein glucuronides were the major metabolites presented in human plasma, genistein sulfate conjugates represented about 10% to 20% of the total genistein equivalents in human plasma, where only 1% presented as aglycone (34,35). Furthermore, previous transport study of genistein in Caco-2 cells showed rapid apical and basolateral excretion of genistein sulfates and glucuronides (17). These data suggested a significant contribution of genistein sulfation to the overall metabolism of genistein in humans. Therefore, mouse intestine appears to be a better model to study genistein sulfation and the interplay between BCRP and sulfotransferases in humans.
In conclusion, this is the first report that clearly demonstrates the fact that BCRP facilitates the function of sulfotransferases. This facilitating function can be termed as interplay or coupling and together they (BCRP and SULT) determine the disposition of genistein via the sulfation pathway in the mouse intestine. The results also show that BCRP played a major role in limiting the glucuronide excretion from the mouse intestine. Therefore, our results support the hypothesis that an important physiological function of BCRP is to facilitate the conjugation of food-borne flavonoids and phenolics, which humans have experienced in their diets for thousands of years.
REFERENCES
- 1.Kurzerand MS, Xu X. Dietary phytoestrogens. Annu Rev Nutr. 1997;17:353–381. doi: 10.1146/annurev.nutr.17.1.353. [DOI] [PubMed] [Google Scholar]
- 2.Lamartiniere CA, Cotroneo MS, Fritz WA, Wang J, Mentor-Marcel R, Elgavish A. Genistein chemoprevention: timing and mechanisms of action in murine mammary and prostate. J Nutr. 2002;132:552S–558S. doi: 10.1093/jn/132.3.552S. [DOI] [PubMed] [Google Scholar]
- 3.Cao F, Jin TY, Zhou YF. Inhibitory effect of isoflavones on prostate cancer cells and PTEN gene. Biomed Environ Sci. 2006;19:35–41. [PubMed] [Google Scholar]
- 4.Gu Y, Zhu CF, Iwamoto H, Chen JS. Genistein inhibits invasive potential of human hepatocellular carcinoma by altering cell cycle, apoptosis, and angiogenesis. World J Gastroenterol. 2005;11:6512–6517. doi: 10.3748/wjg.v11.i41.6512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim EJ, Shin HK, Park JH. Genistein inhibits insulin-like growth factor-I receptor signaling in HT-29 human colon cancer cells: a possible mechanism of the growth inhibitory effect of Genistein. J Med Food. 2005;8:431–438. doi: 10.1089/jmf.2005.8.431. [DOI] [PubMed] [Google Scholar]
- 6.Setchell KD, Brown NM, Desai P, Zimmer-Nechemias L, Wolfe BE, Brashear WT, et al. Bioavailability of pure isoflavones in healthy humans and analysis of commercial soy isoflavone supplements. J Nutr. 2001;131:1362S–1375S. doi: 10.1093/jn/131.4.1362S. [DOI] [PubMed] [Google Scholar]
- 7.Busby MG, Jeffcoat AR, Bloedon LT, Koch MA, Black T, Dix KJ, et al. Clinical characteristics and pharmacokinetics of purified soy isoflavones: single-dose administration to healthy men. Am J Clin Nutr. 2002;75:126–136. doi: 10.1093/ajcn/75.1.126. [DOI] [PubMed] [Google Scholar]
- 8.Gopalakrishnan A, Xu CJ, Nair SS, Chen C, Hebbar V, Kong AN. Modulation of activator protein-1 (AP-1) and MAPK pathway by flavonoids in human prostate cancer PC3 cells. Arch Pharm Res. 2006;29:633–644. doi: 10.1007/BF02968247. [DOI] [PubMed] [Google Scholar]
- 9.Fotsis T, Pepper M, Adlercreutz H, Fleischmann G, Hase T, Montesano R, et al. Genistein, a dietary-derived inhibitor of in vitro angiogenesis. Proc Natl Acad Sci USA. 1993;90:2690–2694. doi: 10.1073/pnas.90.7.2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vermaand SP, Goldin BR. Effect of soy-derived isoflavonoids on the induced growth of MCF-7 cells by estrogenic environmental chemicals. Nutr Cancer. 1998;30:232–239. doi: 10.1080/01635589809514669. [DOI] [PubMed] [Google Scholar]
- 11.Liuand Y, Hu M. Absorption and metabolism of flavonoids in the caco-2 cell culture model and a perused rat intestinal model. Drug Metab Dispos. 2002;30:370–377. doi: 10.1124/dmd.30.4.370. [DOI] [PubMed] [Google Scholar]
- 12.King RA, Broadbent JL, Head RJ. Absorption and excretion of the soy isoflavone genistein in rats. J Nutr. 1996;126:176–182. doi: 10.1093/jn/126.1.176. [DOI] [PubMed] [Google Scholar]
- 13.Rimbach G, Weinberg PD, de Pascual-Teresa S, Alonso MG, Ewins BA, Turner R, et al. Sulfation of genistein alters its antioxidant properties and its effect on platelet aggregation and monocyte and endothelial function. Biochim Biophys Acta. 2004;1670:229–237. doi: 10.1016/j.bbagen.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 14.Barnes S, Sfakianos J, Coward L, Kirk M. Soy isoflavonoids and cancer prevention. Underlying biochemical and pharmacological issues. Adv Exp Med Biol. 1996;401:87–100. doi: 10.1007/978-1-4613-0399-2_7. [DOI] [PubMed] [Google Scholar]
- 15.Allred CD, Twaddle NC, Allred KF, Goeppinger TS, Churchwell MI, Ju YH, et al. Soy processing affects metabolism and disposition of dietary isoflavones in ovariectomized BALB/c mice. J Agric Food Chem. 2005;53:8542–8550. doi: 10.1021/jf051246w. [DOI] [PubMed] [Google Scholar]
- 16.Chen J, Lin H, Hu M. Metabolism of flavonoids via enteric recycling: role of intestinal disposition. J Pharmacol Exp Ther. 2003;304:1228–1235. doi: 10.1124/jpet.102.046409. [DOI] [PubMed] [Google Scholar]
- 17.Chen J, Lin H, Hu M. Absorption and metabolism of genistein and its five isoflavone analogs in the human intestinal Caco-2 model. Cancer Chemother Pharmacol. 2005;55:159–169. doi: 10.1007/s00280-004-0842-x. [DOI] [PubMed] [Google Scholar]
- 18.Hu M, Chen J, Lin H. Metabolism of flavonoids via enteric recycling: mechanistic studies of disposition of apigenin in the Caco-2 cell culture model. J Pharmacol Exp Ther. 2003;307:314–321. doi: 10.1124/jpet.103.053496. [DOI] [PubMed] [Google Scholar]
- 19.Jia X, Chen J, Lin H, Hu M. Disposition of flavonoids via enteric recycling: enzyme-transporter coupling affects metabolism of biochanin A and formononetin and excretion of their phase II conjugates. J Pharmacol Exp Ther. 2004;310:1103–1113. doi: 10.1124/jpet.104.068403. [DOI] [PubMed] [Google Scholar]
- 20.Chen J, Wang S, Jia X, Bajimaya S, Tam V, Hu M. Disposition of flavonoids via recycling: comparison of intestinal versus hepatic disposition. Drug Metab Dispos. 2005;33:1777–1784. doi: 10.1124/dmd.105.003673. [DOI] [PubMed] [Google Scholar]
- 21.Jeong EJ, Jia X, Hu M. Disposition of formononetin via enteric recycling: metabolism and excretion in mouse intestinal perfusion and Caco-2 cell models. Mol Pharm. 2005;2:319–328. doi: 10.1021/mp0498852. [DOI] [PubMed] [Google Scholar]
- 22.Wang SW, Chen J, Jia X, Tam VH, Hu M. Disposition of flavonoids via enteric recycling: structural effects and lack of correlations between in vitro and in situ metabolic properties. Drug Metab Dispos. 2006;34:1837–1848. doi: 10.1124/dmd.106.009910. [DOI] [PubMed] [Google Scholar]
- 23.Hu M, Sinko PJ, de Meere AL, Johnson DA, Amidon GL. Membrane permeability parameters for some amino acids and beta-lactam antibiotics: application of the boundary layer approach. J Theor Biol. 1988;131:107–114. doi: 10.1016/S0022-5193(88)80124-3. [DOI] [PubMed] [Google Scholar]
- 24.Hu M, Roland K, Ge L, Chen J, Li Y, Tyle P, et al. Determination of absorption characteristics of AG337, a novel thymidylate synthase inhibitor, using a perfused rat intestinal model. J Pharm Sci. 1998;87:886–890. doi: 10.1021/js970251e. [DOI] [PubMed] [Google Scholar]
- 25.Jeong EJ, Lin H, Hu M. Disposition mechanisms of raloxifene in the human intestinal Caco-2 model. J Pharmacol Exp Ther. 2004;310:376–385. doi: 10.1124/jpet.103.063925. [DOI] [PubMed] [Google Scholar]
- 26.Adachi Y, Suzuki H, Schinkel AH, Sugiyama Y. Role of breast cancer resistance protein (Bcrp1/Abcg2) in the extrusion of glucuronide and sulfate conjugates from enterocytes to intestinal lumen. Mol Pharmacol. 2005;67:923–928. doi: 10.1124/mol.104.007393. [DOI] [PubMed] [Google Scholar]
- 27.Ebert B, Seidel A, Lampen A. Identification of BCRP as transporter of benzo[a]pyrene conjugates metabolically formed in Caco-2 cells and its induction by Ah-receptor agonists. Carcinogenesis. 2005;26:1754–1763. doi: 10.1093/carcin/bgi139. [DOI] [PubMed] [Google Scholar]
- 28.Zhang S, Wang X, Sagawa K, Morris ME. Flavonoids chrysin and benzoflavone, potent breast cancer resistance protein inhibitors, have no significant effect on topotecan pharmacokinetics in rats or mdr1a/1b (-/-) mice. Drug Metab Dispos. 2005;33:341–348. doi: 10.1124/dmd.104.002501. [DOI] [PubMed] [Google Scholar]
- 29.Bobrowska-Hagerstrand M, Wrobel A, Rychlik B, Ohman I, Hagerstrand H. Flow cytometric monitoring of multidrug drug resistance protein 1 (MRP1/ABCC1) -mediated transport of 2′, 7′-bis-(3-carboxypropyl)-5-(and-6)- carboxyfluorescein (BCPCF) into human erythrocyte membrane inside-out vesicles. Mol Membr Biol. 2007;24:485–495. doi: 10.1080/09687680701383069. [DOI] [PubMed] [Google Scholar]
- 30.Zhang L, Lin G, Kovacs B, Jani M, Krajcsi P, Zuo Z. Mechanistic study on the intestinal absorption and disposition of baicalein. Eur J Pharm Sci. 2007;31:221–231. doi: 10.1016/j.ejps.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 31.Abbott BL. ABCG2 (BCRP): a cytoprotectant in normal and malignant stem cells. Clin Adv Hematol Oncol. 2006;4:63–72. [PubMed] [Google Scholar]
- 32.Vander Borght S, Libbrecht L, Katoonizadeh A, van Pelt J, Cassiman D, Nevens F, et al. Breast cancer resistance protein (BCRP/ABCG2) is expressed by progenitor cells/reactive ductules and hepatocytes and its expression pattern is influenced by disease etiology and species type: possible functional consequences. J Histochem Cytochem. 2006;54:1051–1059. doi: 10.1369/jhc.5A6912.2006. [DOI] [PubMed] [Google Scholar]
- 33.Cheng AL, Hsu CH, Lin JK, Hsu MM, Ho YF, Shen TS, et al. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res. 2001;21:2895–2900. [PubMed] [Google Scholar]
- 34.Shelnutt SR, Cimino CO, Wiggins PA, Ronis MJ, Badger TM. Pharmacokinetics of the glucuronide and sulfate conjugates of genistein and daidzein in men and women after consumption of a soy beverage. Am J Clin Nutr. 2002;76(3):588–594. doi: 10.1093/ajcn/76.3.588. [DOI] [PubMed] [Google Scholar]
- 35.Gu L, House SE, Prior RL, Fang N, Ronis MJ, Clarkson TB, et al. Metabolic phenotype of isoflavones differ among female rats, pigs, monkeys, and women. J Nutr. 2006;136(5):1215–1221. doi: 10.1093/jn/136.5.1215. [DOI] [PubMed] [Google Scholar]