Abstract
Most attempts to develop in vitro models of the blood–brain barrier (BBB) have resulted in models with low transendothelial electrical resistances (TEER), as compared to the native endothelium. The aim of the present study was to investigate the impact of culture pH and buffer concentration on paracellular tightness of an established in vitro model of the BBB consisting of bovine brain capillary endothelial cells (BCEC) co-cultured with rat astrocytes. BCEC and rat astrocytes were isolated and co-cultured using astrocyte-conditioned media with cAMP increasing agonists and dexamethasone. The co-culture had average TEER values from 261 ± 26 Ω cm2 to 760 ± 46 Ω cm2 dependent on BCEC isolation batches. Furthermore, mRNA of occludin, claudin-1, claudin-5, JAM-1, and ZO-1 were detected. Increased buffer concentration by addition of HEPES, MOPS, or TES to the media during differentiation increased the TEER up to 1,638 ± 256 Ω cm2 independent of the type of buffer. This correlated with increased expression of claudin-5, while expression of the other tight junction proteins remained unchanged. Thus, we show for the first time that increased buffer capacity of the medium during differentiation significantly increases tightness of the BCEC/astrocyte in vitro BBB model. This regulation may be mediated by increased claudin-5 expression. The observations have practical implications for generating tighter BBB cell culture models, and may also have physiological implications, if similar sensitivity to pH-changes can be demonstrated in vivo.
Key words: blood–brain barrier, buffer capacity, drug delivery, in vitro model, tight junction regulation
Introduction
The small capillaries in the brain constitute the “blood–brain barrier” (BBB) (1). The BBB regulates transport of nutrients and acts as a barrier for uptake of drug compounds from the circulation. Complex tight junctions between the endothelial cells limit paracellular permeability, and thus restrict passive diffusion of small hydrophilic drug compounds (2,3). Furthermore, the endothelial cells express a number of efflux-transporters in the apical membrane as well as a number of enzymes, which contribute to the barrier properties (4,5). The tightness and complexity of the BBB represent a major challenge for delivery of drugs into the brain (6). A number of research groups have developed in vitro models based on BBB-endothelial cells (for review see Deli et al. (7)). Deli et al. highlighted the inability of many of these in vitro models to develop and uphold transendothelial electrical resistance (TEER) values resembling the in vivo BBB tightness. Most models displayed TEER values in the range of 100–800 Ω cm2 and only a few were able to resemble the estimated tightness of the in vivo BBB of approximately 1,900 Ω cm2 (2,7).
Previous studies have shown significant effects of culture pH on endothelial cell growth, with the pH optimum varying between cell types (8,9). Moreover studies have indicated that endothelial paracellular tightness could be affected by pH fluctuations. It has been shown that lung endothelial cells cultured atop gold electrodes in pulsed CO2 incubators experienced changes in cell impedance best explained by decreased paracellular tightness in response to small pH fluctuations (10). A possibly related phenomenon has been observed in epithelial cells. Dickinson et al. showed a 40% increase in TEER across primary alveolar epithelial cells, when the cells were cultured in Dulbecco's Modified Eagles Medium (DMEM) containing HEPES compared to DMEM without (11). The authors speculated that this could be a direct effect of HEPES or a result of improved buffer capacity in the medium, which led to a more stable pH during culture.
We hypothesized that the establishment and maintenance of endothelial cell tight junctions could be sensitive to small pH fluctuations in the medium. The aim of the present study was to investigate the influence of the culture medium buffer type, buffer concentration and pH on tight junction establishment in an in vitro BBB model consisting of a co-culture of bovine brain capillary endothelial cells (BCEC) and rat astrocytes. When total buffer concentration was increased by addition of HEPES, MOPS, or TES to the standard culture media during the last 3 days of culturing, barrier tightness rose significantly as measured by TEER and permeability of mannitol. Using PCR and western blotting, we showed that the increase in TEER correlated with an increase in mRNA levels and protein expression of the tight junction protein claudin-5. Our results thus indicate that barrier tightness is increased with increasing buffer capacity of the medium, possibly via an increased expression of claudin-5.
Materials and Methods
Materials
Rabbit-anti hClaudin-5 and rabbit-anti hGAPDH was from Abcam (Cambridge, UK). Total RNA isolation reagent was from ABgene (Epsom, United Kingdom). RO-20-1724 was from Calbiochem (San-Diego, USA). All primers were from DNA Technology (Århus, Denmark). Powdered Dulbecco's Modified Eagles Medium was from Gibco (Breda, Netherlands). Mouse anti hTransferrin Receptor antibody and Superscript™ III First-Strand Synthesis SuperMix for qRT-PCR were from Invitrogen (Taastrup, Denmark). Midori green DNA stain was from Kem-En-Tech A/S (Taastrup, Denmark). Alexa 488-phalloidin, goat-anti-mouse IgG (H + L)–peroxidase conjugated, goat-anti-rabbit IgG–Alexa 488 F(AB')2, goat-anti-rabbit IgG (H + L)—Peroxidase conjugated, propidium iodide, RNAse and rabbit-anti-human Occludin were from Molecular Probes (Leiden, The Netherlands). Fetal bovine serum (FBS) was from PAA-Laboratories (Pasching, Austria). 13C Mannitol and Ultima Gold Scintillation fluid were from Perkin Elmer (Hvidovre, Denmark). HotStarTaq Plus DNA Polymerase was from Qiagen (Copenhagen, Denmark). Fibronectin was from Roche Diagnostics (Hvidovre, Denmark). Rabbit-anti-rat GFAP (H50): sc-9065, rabbit-anti-human VWF (H-300): sc-14014 and rabbit-anti-human MDR-1 (C-19): sc-1517 were from Santa Cruz Biotechnology (Heidelberg, Germany). Collagenase type III, Trypsin TRL and DNAse 1 were from Worthington (Lakewood, USA). All other chemicals were from SIGMA-ALDRICH (Steinheim, Germany).
Culture Media
The composition of the culture media is outlined in Table I.
Table I.
Overview of the Composition of the Culture Media
| Abbreviation | Buffer/Media | Content |
|---|---|---|
| DMEM | Complete Dulbecco’s Modified Eagle’s Medium | DMEM-AQ |
| FBS 10% | ||
| Non-essential amino acids (×100) 10 ml/l | ||
| penicillin/streptomycin solution 100 U/ml/100 μg/ml | ||
| DMEM + HEPES | Dulbecco’s Modified Eagle’s Medium with HEPES | Powdered DMEM 13.3 g/l |
| HEPES 11.9 g/l (50 mM) | ||
| Non-essential amino acids (×100) 10 ml/l | ||
| Penicillin/streptomycin solution 100 U/ml/100 μg/ml, l-gluthamine 2 mM | ||
| ACM | Astrocyte-conditioned medium | DMEM removed from cultured astrocytes after 2 days of culture |
| GM- | Growth medium− | DMEM |
| Heparin 125 μg/ml | ||
| GM+ | Growth medium+ | DMEM:ACM = 1:1 |
| Heparin 125 μg/ml | ||
| DM | Differentiation medium | DMEM |
| 8-(4-CPT)cAMP 312.5 μM | ||
| Dexamethasone 0.5 μM | ||
| RO-20-1724 17.5 μM | ||
| DM + HEPES | Differentiation medium with HEPES | powdered DMEM 13.3 g/l |
| HEPES 11.9 g/l (50 mM) | ||
| non-essential amino acids (x100) 10 ml/l | ||
| penicillin/streptomycin solution 100 U/ml/100 μg/ml | ||
| l-Gluthamine 2 mM | ||
| FBS 10% | ||
| 8-(4-CPT)cAMP 312.5 μM | ||
| Dexamethasone 0.5 μM | ||
| RO-20-1724 17.5 μM | ||
| DM + MOPS | Differentiation medium with MOPS | Powdered DMEM 13.3 g/l |
| MOPS 10.5 g/l (50 mM) | ||
| penicillin/streptomycin solution 100 U/ml/100 μg/ml | ||
| Non-essential amino acids (–×100) 10 ml/l | ||
| l-Gluthamine 2 mM | ||
| FBS 10% | ||
| 8-(4-CPT)cAMP 312.5 μM | ||
| Dexamethasone 0.5 μM | ||
| RO-20-1724 17.5 μM | ||
| DM + TES | Differentiation medium with TES | Powdered DMEM 13.3 g/l |
| TES 11.5 g/l (50 mM) | ||
| penicillin/streptomycin solution 100 U/ml/100 μg/ml | ||
| Non-essential amino acids (×100) 10 ml/l | ||
| l-Gluthamine 2 mM. | ||
| FBS 10% | ||
| 8-(4-CPT)cAMP 312.5 μM | ||
| Dexamethasone 0.5 μM | ||
| RO-20-1724 17.5 μM |
HEPES 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid, MOPS 3-(N-morpholino)propanesulfonic acid, TES N-Tris(hydroxymethyl)methyl-2-aminoethanesulfonic Acid, cAMP cyclic Adenosine monophosphate, FBS Foetal Bovine Serum
Isolation of Primary Bovine Brain Endothelial Cells and Rat Astrocytes
The isolations of BCEC and astrocytes, as well as the preparation of co-cultures, were performed basically according to the methods of Gaillard et al. with some modifications (12). Briefly, bovine brains were acquired from calves (age 12–32 months) from the local slaughterhouse (The Danish Meat Trade College, Roskilde, Denmark) and transported in ice-cold PBS. Meninges were removed, and the gray matter was isolated, homogenized in a Dounce tissue grinder (Wheaton Science Products, Millville, USA) and filtered through 150 μm mesh filters (Merrem & La Porte, Zaltbommel, The Netherlands). The trapped capillaries were resuspended in DMEM and digested for 1 h in an enzyme mix of DNAse I (170 U/ml), Collagenase type III (200 U/ml) and trypsin TRL (90 U/ml). The suspension was filtered through 200 μm mesh filters and resuspended in FBS:DMSO (9:1). The suspension was split in ten aliquots and stored in liquid nitrogen.
Astrocytes were isolated from newborn Sprague–Dawley rats (Taconic, Ejby, Denmark). The pups were sacrificed and the cerebral cortices were isolated, homogenized and incubated in trypsin–EDTA in DMEM + HEPES. The suspension was filtered through 120 and 45 μm mesh filters, and the filtrate was seeded in T75 flasks (one flask for every two pups) and cultured until confluence (37°C, 10% CO2). Upon confluence, the flasks were shaken overnight at room temperature and subsequently cultured to confluence again. The confluent astrocytes were passaged to poly-d-lysine-coated flasks (split ratio 1:3) and cultured, during which time the medium was collected as astrocyte-conditioned medium (ACM) three times a week. After 2 weeks the astrocytes were passaged with trypsin–EDTA, resuspended in FBS:DMSO (9:1) and frozen in aliquots (two vials per flask). The total yield from the astrocyte isolation was approximately 21 million cells and 500 ml ACM, which was enough for approximately 200 Transwell filter inserts (area = 1.12 cm2) (Corning, New York, USA).
Cell Culture for the Co-culture Model
T75 flasks were coated with collagen type IV and fibronectin. Frozen bovine brain microvessels were thawed, seeded in the flasks and cultured for 5–7 days (37°C, 10% CO2) in growth medium (GM)+.
Three days prior to confluence, Transwell filter inserts (area = 1.12 cm2) were coated with collagen type IV and fibronectin, and astrocytes were seeded at the bottom of the filter inserts, 130,000 cells/filter insert. The astrocytes were cultured until the passage of the endothelial cells (37°C, 10% CO2).
When the endothelial cells had reached 60–80% confluence, they were trypsinized and seeded at a density of 90,000 cells/filter insert and cultured in the co-culture system in GM−. After 3 days the medium was changed to differentiation medium (DM), DM + HEPES, DM + MOPS or DM + TES (see Table I for overview of media) and cultured for further 3 days.
Characterization of Blood–Brain Barrier Properties
TEER across the filter inserts with the BBB co-culture was measured using a Millicell-ERS device (Millipore, Massachusetts, USA) equipped with an Endohm 12 cup electrode chamber (World Precision Instruments, Sarasota, Florida).
The isolated cells were investigated with immunocytochemical staining against multi-drug resistance protein member 1 (MDR-1), glial fibrillary acidic protein (GFAP), von Willebrands factor (VWF), occludin, claudin-5 and fibrous actin. The cells were fixed on the filter inserts with 3% paraformaldehyde, treated with 0.1% Triton X-100 and blocked in PBS supplemented with 2% bovine serum albumin. The filter inserts were removed, treated with RNAse and incubated with the relevant antibody overnight. All preparations except stainings for fibrous actin were incubated with goat-anti-rabbit igG coupled with Alexa 488 and subsequently counterstained with propidium iodide before confocal laser scanning microscopy (CLSM) examination with a Zeiss LSM 510 laser confocal microscope (Carl Zeiss, Jena, Germany).
Functional integrity of the monolayers was investigated by addition of 14C mannitol (specific activity 56.5 mCi/mmol) to the culture medium to a final concentration of 1 μCi/ml. Transcellular transport was followed by withdrawing receiver samples after 15, 30, 60, 90, 120, and 150 min and donor samples after 5 and 150 min. Samples were added Ultima Gold scintillation fluid and counted in a Tri-Carb 2100 TR Liquid Scintillation Analyzer (Packard Instrument Company, Meriden, Connecticut, USA). Steady-state fluxes, permeability values were subsequently calculated.
pH measurements of the media during the culture period and transcellular transport experiments were performed using a PHM 240 PH/ION meter equipped with a PHC2401-8 combined electrode (Meterlab—Radiometer Analytical, Lyon, France).
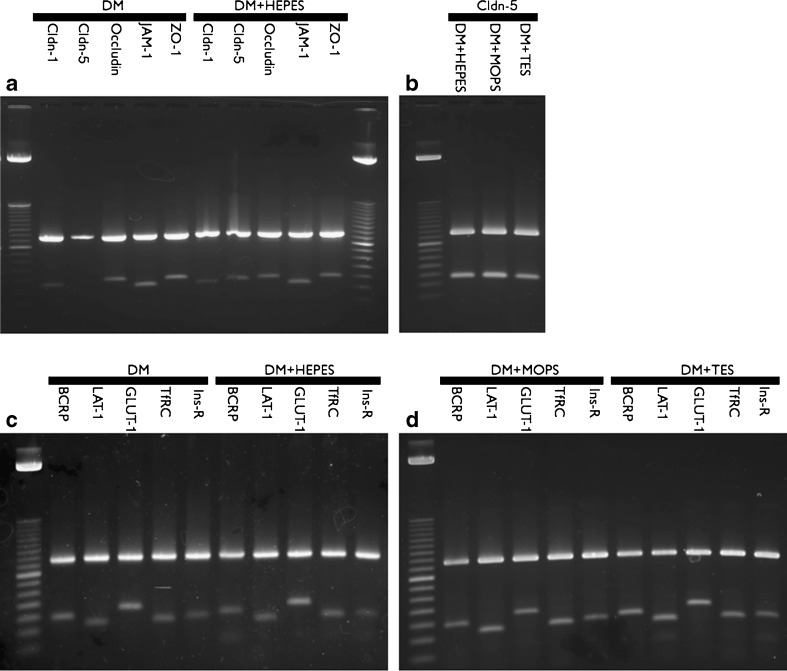
Total RNA was isolated from co-cultures (day 6 after seeding) using Total RNA Isolation Reagent according to the manufacturers protocol (ABgene, Epsom, United Kingdom). The isolated RNA was treated with DNAse I Amplification grade according to manufacturer's protocol (SIGMA-ALDRICH, Steinheim, Germany) prior to reverse transcription with Superscript™ III First-Strand Synthesis SuperMix for qRT-PCR according to manufacturers protocol (Invitrogen, Taastrup, Denmark). cDNA concentrations were determined by UV spectrophotometry and PCR reactions were carried out with approximately 1 μg cDNA/reaction using HotStarTaq Plus DNA Polymerase according to manufacturers protocol (Qiagen, Copenhagen, Denmark). PCR was run for 36 cycles with 60 s annealing, 60 s extension, and 30 s denaturation. Applied primers are shown in Table II. PCR products were run in 1.5% agarose gels and visualized using Midori Green DNA stain (5 μl/100 ml gel) and a fluorchemQ image station (Alpha Innotech/Cell Biosciences, Santa Clara, CA, USA). Expression was quantified by densiometric measurements and standardized against ß-actin expression using AlphaViewQ software version 3.0.3.0.
Table II.
Overview of Applied Primers
| Primers | |||
|---|---|---|---|
| Target protein | Forward strand | Reverse strand | Product size |
| BCRP | CCAGGCGTTCATTCAAAAAT | GCTCTGTTCTGGATTCCTGC | 139 |
| Claudin-1 | CCGTTGGCATGAAGTGTATG | CCATGCTGTGGCAACTAAAA | 122 |
| Claudin-5 | CAGAAGTACGAGCTGGGAGC | TACTTCACCGGGAAGCTGAA | 134 |
| GLUT-1 | CCCCAGAAGGTGATTGAAGA | GCCGAAACGGTTAACAAAAA | 168 |
| Ins-R | AAAGAGGCCCCTTACCAGAA | CCAGGGTGGTTCTGTGACTT | 128 |
| JAM-1 | TGCTGACCTGCTCAGAGAGA | GGAAGAGTTGCTGAAGGCAC | 113 |
| LAT-1 | TAGCCAATCTGGATCCCAAG | TCAAGTAATTCCATCCCCCA | 111 |
| Occludin | CCGGAAGATGAAATTCTCCA | GTTCCATAGCCTCTGTCCCA | 143 |
| TfRC | TTTAGTCTGGCTCGGCAAGT | CGGTTTTGCGATACTGGTTT | 120 |
| ZO-1 | CGACCAGATCCTCAGGGTAA | GGATTCTACGATGCGACGAT | 148 |
| ß-actin | AGGCTGTGCTGTCCCTGTAT | AGGTAGTTTCGTGAATGCCG | 426 |
BCRP breast cancer resistance protein, GLUT-1 glucose transporter member 1, Ins-R insulin receptor, JAM-1 junction adhesion molecule 1, LAT-1 large neutral amino acid transporter member 1, TfRC transferring receptor, ZO-1 zonula occludens 1
Alkaline phosphatase activity was tested on filter inserts after treatment with lysis buffer (15 mM Tris–HCl [pH = 7.5], 250 mM sucrose, 60 mM KCl, 15 mM NaCl, 15 mM MgCl2, 1 mM CaCl2, 0.6% NP-40, 5% glycerol) for 10 min and subsequent centrifugation. Protein content was determined by Bradford Assay, and 1 μg protein was diluted to 20 μl. The alkaline phosphatase reaction was initiated by addition of 200 μl assay buffer (0.1 M glycine [pH = 10.3], 1 mM ZnCl2, 1 mM MgCl2) with 1 mg/ml p-nitrophenyl phosphate. The assay was run at 37°C for 25 min and the absorbance at 414 nm was measured and compared to a p-nitrophenol standard curve. The activity was defined as U/mg protein, 1 unit was equivalent to formation of 1 μg p-nitrophenol per minute.
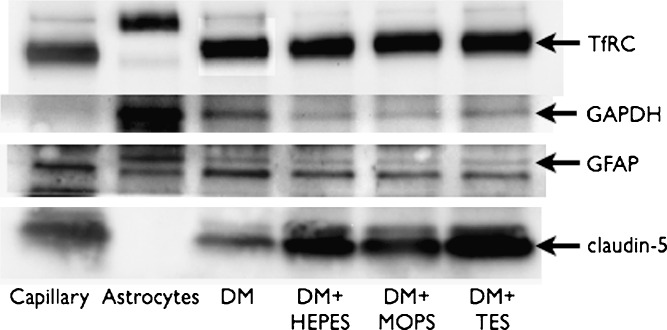
Western blot analysis was performed on protein extracts from co-cultures (day 6 after seeding). Cells were lysed in RIPA buffer (0.01 M Tris–HCl [pH 8.0], 0.14 M NaCl, 1% Triton X-100, 0.1% Na-deoxycholate, 0.1% SDS, 10 μg/ml proteinase inhibitors, 1 mM PMSF and 10 mM Na-pyrophosphate) for 20 min on ice and subsequently centrifuged (18,000×g, 10 min, 4°C). The protein concentration was determined by the Bradford assay. Prior to SDS-PAGE, 2 μg of total protein from the different cell lysates were mixed with 5× Laemli-SDS-sample buffer and 0.1 M DTT and heated at 95°C for 10 min. SDS-PAGE was run on 4–15% gradient gels. Proteins were visualized via chemiluminescence (ECL-Plus, Amersham Biosciences) using horseradish peroxidase (HP)-conjugated secondary antibodies and exposure to ECL Hyperfilms (Amersham Biosciences). The blots were visualized in a fluorchemQ image station.
Data Analysis
TEER values were standardized by subtracting the resistance across empty filter inserts and multiplying by the cross-sectional area.
The transcellular transport data was plotted with total amount of nanomoles transported in each well against time. The fluxes were calculated as the slope of the straight lines at steady-state divided by the cross-sectional area of transport. Apparent permeability values were calculated from the steady-state fluxes:
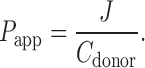 |
1 |
where Papp is the apparent permeability, J is the observed steady-state flux and Cdonor is the added concentration to the donor compartment.
Donor samples were taken at the beginning and end of each experiment to ensure that sink conditions were applicable (less than 10% of total amount transported). Standardized TEER values and permeability coefficients were compared using a student’s t test or one-sided ANOVA followed by Newman–Keuls test to investigate for significance between the individual populations (α = 0.05).
Results
Growth Capacity of Endothelial Cells from Bovine Brain Capillaries was Dependent on Animal Age
Isolations of endothelial cells from seven individual bovine brains were performed. The endothelial cells were characterized with respect to growth rate and barrier integrity (Table III). The total yield of endothelial cells after 7 days in culture varied from 2–20 × 106 endothelial cells per brain. Three of these batches yielded less than 10 × 106 cells per brain, or contained more than 5% pericytes as judged by visual inspection, and were discarded due to failure to produce sufficiently tight monolayers. The discarded cultures all originated from animals with an age of 32 months or older, whereas the cultures yielding high cell numbers and low pericyte contamination originated from animals with an age of 15 months or younger. This indicated that brains from younger animals had the largest growth capacity.
Table III.
Overview of Isolation Outcomes of the Different Batches. Impurities were Defined as any Cell Growth that did not Display Endothelial Cell Morphology. Transendothelial Electrical Resistance (TEER) Values are Average Values of 6–12 Filter Inserts (n = 6–12) from 1 to 5 Different Experiments (N = 1–5)
| Isolations of bovine brain capillary endothelial cells. | ||||
|---|---|---|---|---|
| Batch | Age of calf (months) | Impurities (%) | Yield upon passage (million cells) | TEER day 6 in DM (Mean ± SD, Ω ⋅ cm2) |
| A | Unknown | 0–2 | 2 | 261 ± 26 |
| B | 15 | 1–3 | 1.8 | 337 ± 62 |
| C | 12 | 0–2 | 2 | 505 ± 110 |
| D | 32 | 10 | 0.45 | – |
| E | 14 | 0–2 | 1.1 | 760 ± 46 |
| F | 30 | 1–5 | 0.65 | – |
| G | 30 | 5–10 | 0.2–0.3 | – |
DM differentiation medium
Bovine Endothelial Cells Developed Tight Monolayers when Co-cultured with Rat Astrocytes
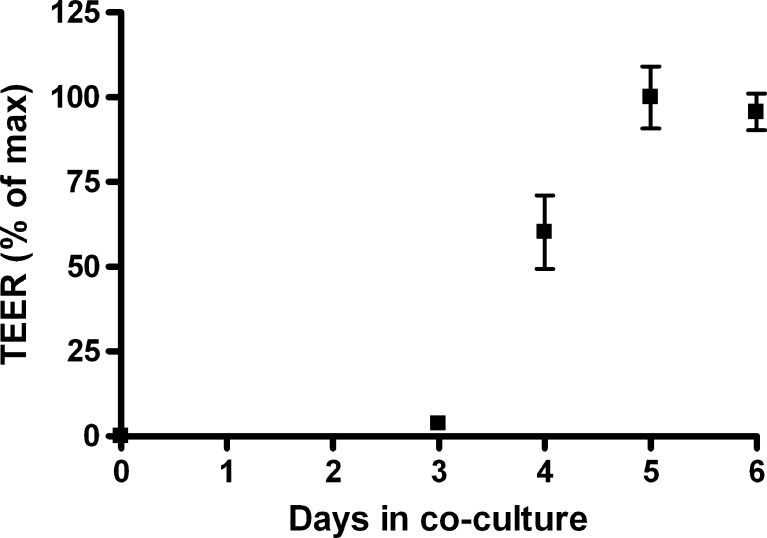
Endothelial cells were co-cultured with astrocytes on permeable filter supports. The development of TEER across the monolayers was followed from the day of seeding the endothelial cells (Fig. 1). The TEER remained low for the first 3 days of growth. DM was added on day 3 (after the TEER measurement), which led to a marked increase in monolayer resistance. A plateau was reached at day 5 and 6, which indicated that the endothelial cells were fully differentiated concerning establishment of tight junctions. Maximum TEER averages on day 6 varied between isolations from 261 ± 26 to 760 ± 46 Ω·cm2 under standard culture conditions (DM). Maximal TEER values varied considerably between endothelial cell isolation batches, whereas TEER was stable within batches with relative small standard deviations (Table III).
Fig. 1.
Development in TEER across the co-culture model from the day of seeding. The graph displays average TEER values standardized against the maximal average TEER (n = 12) with standard deviation
Endothelial Cells Grown in Co-culture, Displayed Endothelial Cell Morphology and Expressed Von Willebrands Factor, Occludin, MDR-1 and Alkaline Phosphatase Activity
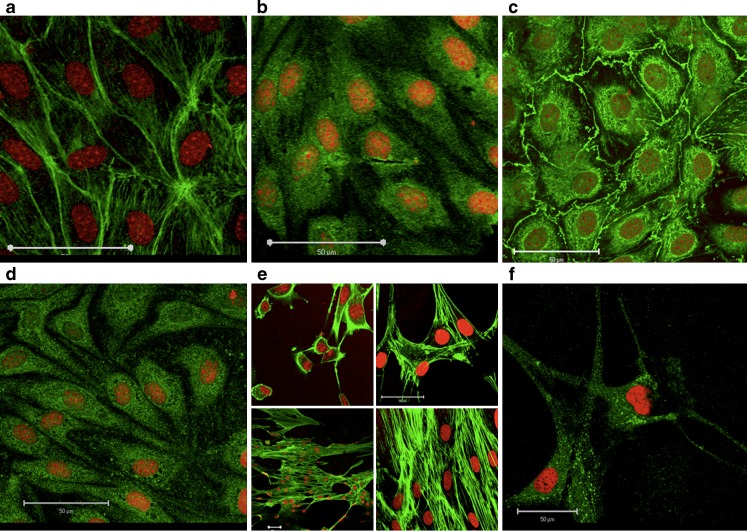
Endothelial cells, co-cultured with astrocytes for 6 days under standard culture conditions were characterized by immunostaining with respect to cell morphology. The cells in the monolayers were rhombic-shaped when non-confluent and spindle-shaped with localization of fibrous actin at the cell contact regions when confluent (Fig. 2a). The cells had a length of 40–60 μm (Fig. 2a) and a thickness ranging from 0.7 ± 0.2 μm at the cytosol to 2.4 ± 1.3 μm at the nuclei, as judged from confocal XYZ-stacks. The cells expressed the endothelial specific VWF (Fig. 2b), as well as the tight junction associated protein occludin (Fig. 2c) and the efflux transporter MDR-1 (Fig. 2d). The cells thus displayed characteristics of blood–brain barrier endothelial cell morphology and protein expression. The cortical cells isolated from rat brains gradually grew to cover the bottom of the filter inserts, and they displayed a star-shaped morphology (Fig. 2e) and GFAP (Fig. 2f), thus supporting that the cells yielded from the rat brain isolation indeed were astrocytes.
Fig. 2.
Immunolocalization of a Actin, b Von Willebrand's Factor, c Occludin, d Multidrug resistance protein member 1 (green) in endothelial cells 6 days after seeding in co-culture. Astrocytes stained for e Actin (green) 1, 3, 5, and 6 days after seeding and f Glial fibrillary acidic protein (green) 3 days after seeding. The nuclei were visualized by propidium iodide staining (red). Bars 50 μm
The co-cultures displayed alkaline phosphatase activity of 1.27 ± 0.18 U/mg protein.
Increased Buffer Concentration of Culture Media Caused an Increase in TEER and a Decrease in Transendothelial Mannitol Flux
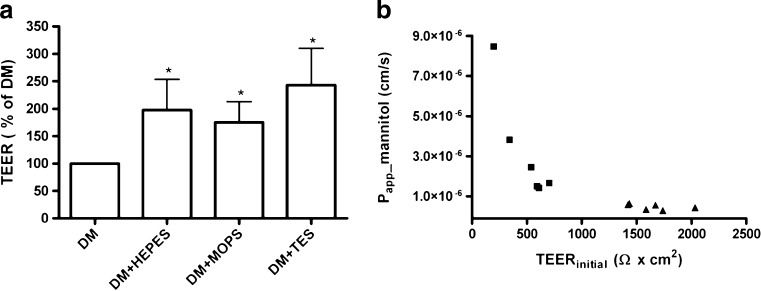
The effect of buffer composition on barrier integrity (Fig. 3a) was investigated by increasing the overall buffer concentration in the DM with HEPES, MOPS, or TES. Endothelial/astrocyte co-cultures were thus grown for 3 days in GM- followed by 3 days in buffered DM. The TEER values obtained from cells cultured in DM + HEPES, MOPS, and TES were normalized against values from cells cultured in DM in order to compare results between batches from separate isolations. Increased buffer concentration caused TEER values to increase with magnitudes ranging from 40 to 100% with effects as high as 300% observed in some batches. The TEER values across cells cultured in DM + HEPES, MOPS, and TES ranged from 375 ± 77 Ω·cm2 to 1,638 ± 256 Ω·cm2. The addition of HEPES, MOPS, or TES all resulted in significantly increased TEER compared to the standard medium (P < 0.05), while there were no significant differences between the individual buffer effects. Transepithelial apical-basolateral fluxes of radiolabelled mannitol were measured and Papp was calculated (Eq. 1). The Papp of mannitol decreased from 3.20 ± 2.7 × 10−6 cm·s−1 in DM to 0.483 ± 0.14 · 10−6 cm s−1 in DM + HEPES (Fig. 3b). The mannitol permeability and the TEER showed an inverse relationship, indicative of a clear correlation between the two parameters. The relationship appeared to be of an inverse exponential type, which in practical terms means that above a certain TEER (500–600 Ω cm2), further increases in TEER caused only minor decreases in mannitol permeability.
Fig. 3.
Influence of culture medium on TEER. a TEER values across endothelial cells after 6 days in co-culture. Data are presented as mean values from 6 different co-culture setups (N = 6) each containing 3–6 filter inserts with the different media (n = 3–6). Error bars represent standard deviations of the averages from the different cultivations. DM Differentiation medium. b Apparent mannitol permeability across the endothelial cells after 6 days in co-culture. Data are single determinations from 6 filter inserts cultured in differentiation medium (filled square) and 6 filter inserts cultured in differentiation medium + HEPES (filled triangle)
The Increased TEER was Caused Both by Changes of the pH in Culture Media and by the Increased Buffer Concentration
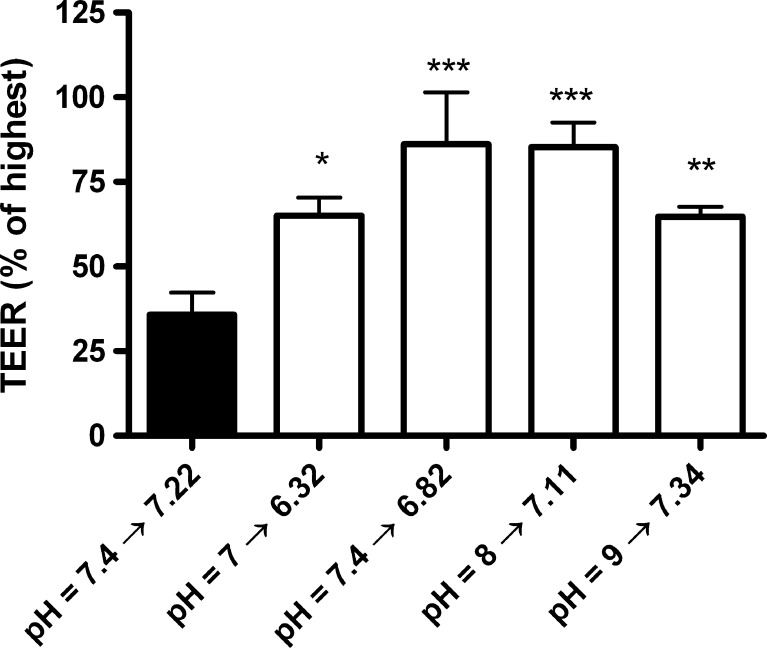
All culture media were initially buffered to pH 7.4 but pCO2 in the incubator caused an acidification to pH 7.2 in DM and 6.8 in DM + HEPES, MOPS, and TES. In order to investigate whether the lower pH of the media caused the increase in TEER, monolayers were cultured in DM + HEPES, which was initially titrated to pH values of 6, 7, 7.4, 8, and 9. TEER and pH were measured after 6 days of co-culture (Fig. 4). pH values had undergone acidification to pH = 5.30, 6.32, 6.82, 7.11, and 7.34, respectively. TEER was significantly increased in all HEPES buffered media as compared to the control, except in pH 5.3 (data not shown), where the TEER values did not increase above those measured across an empty filter insert (P ranging from <0.05 to <0.001). This indicates that the buffering effect itself, and not the absolute pH value during growth, caused the majority of the increase in monolayer tightness. The monolayers cultured at pH values 7.11 and 6.82 reached significantly higher TEER values than all other conditions (P < 0.05) but did not differ significantly between each other. Likewise, culture pH values of 6.32 and 7.34 reached similar TEER values. Altogether, Fig. 4 indicates that the differences in TEER observed in Fig. 3a were caused by a combination of a weakly acidic culture pH and an increased buffer concentration, where the increased buffer concentration was the most important factor.
Fig. 4.
Influence of pH on TEER. TEER values as percentage of highest achieved TEER in the batch of endothelial cells after 6 days in co-culture cultured in regular differentiation medium (black column) or differentiation medium + HEPES set to initial pH values of 7, 7.4, 8 and 9 (white columns). During culture the pH values changed to 6.32, 6.82, 7.11 and 7.34, respectively. Data are pooled from 2 different co-culture setups (N = 2) with three to four filter inserts per condition (n = 3–4) with standard deviations. *P < 0.05, **P < 0.01, ***P < 0.001 as compared to the differentiation medium control
Endothelial Cells Grown in HEPES, MOPS, or TES-Buffered Media Showed a Selective Up-Regulation of the Tight Junction Protein Claudin-5
The expression of mRNA transcript of the tight junction proteins claudin-1, claudin-5, occludin, JAM-1 and ZO-1 (see Table II for primers) was examined in control- and HEPES buffered media. The cells cultured in DM showed equal expressions of JAM-1, ZO-1, occludin and claudin-1 as compared to cells cultured in DM + HEPES. However, claudin-5 expression was significantly lower in cells cultured in DM than in cells cultured in DM + HEPES, MOPS, and TES (Fig. 5a and b). This indicated that the increased buffer concentration somehow led to an increased expression of claudin-5. mRNA levels of the endogenous transporters breast cancer resistance protein (BCRP), large neutral amino acid transporter member 1 (LAT-1) and glucose transporter 1 (GLUT-1) as well as the transferrin receptor (TfRC) and insulin receptor (Ins-R) were subsequently examined in all media (Fig. 5c and d). The expression was unaltered between the DM medium and the DM + HEPES, MOPS, and TES media of all the investigated transporters and receptors. The up-regulation of claudin-5 on the protein level was confirmed by western blot analysis (Fig. 6). Claudin-5 was highly expressed in co-cultures grown in DM + HEPES, MOPS, or TES media, whereas expression was significantly lower in cells cultured in DM. Moreover, the western blot confirmed an unaltered expression of TfRC between the culture media, which confirmed the observations on the mRNA levels (Fig. 5).
Fig. 5.
RT-PCR products of primers run on cDNA isolated from endothelial cells/astrocytes after 6 days in co-culture. a Co-culture in differentiation medium (lanes 2–6) or differentiation medium + HEPES (lanes 7–11). b Co-culture in differentiation medium + HEPES, MOPS and TES. c Co-culture in differentiation medium (lanes 2–6) or differentiation medium + HEPES (lanes 7–11). d: Co-culture in differentiation medium + MOPS (lanes 2–6) or differentiation medium + TES (lane 7–11). Upper bands ß-actin, lower bands experimental product. Cldn claudin, Ladder 50 bp
Fig. 6.
Western blot on 2 μg protein extracts from capillary fragments (lane 1), Astrocytes (lane 2) and endothelial cells/astrocytes after 6 days in co-culture in differentiation medium (lane 3) and differentiation medium + HEPES (lane 4), MOPS (lane 5) and TES (lane 6)
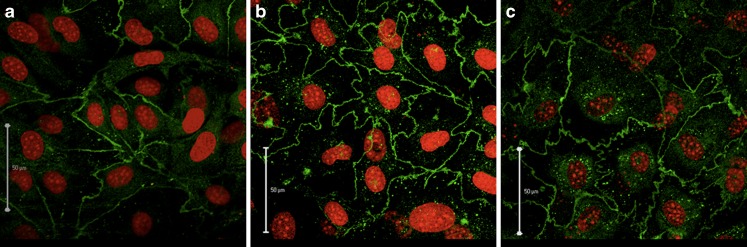
Localization of claudin-5 was investigated in cells cultured in the different media (Fig. 7) using immunocytochemistry and CLSM, as described in methods. Claudin-5 was localized mainly along the cell borders irrespective of the culture media used. However, in DM + HEPES and DM + TES more zipper-like structures were observed at the cell borders as compared to the control cells (Fig. 7). This structure resulted in an increase in tight junction contact zones from 169 ± 39 μm in DM to 272 ± 50 μm in DM + HEPES and 276 ± 26 μm in DM + TES (Fig. 7).
Fig. 7.
Endothelial cells stained with an antibody against claudin-5 (green) and propidium iodide (green) after 6 days in co-culture. a Differentiation medium, b differentiation medium + HEPES, c differentiation medium + TES, Bars 50 μm
Transendothelial Resistance and Claudin-5-mRNA Transcript Levels Correlated, which Indicates a Close Coupling Between Paracellular Tightness and Claudin 5 Expression
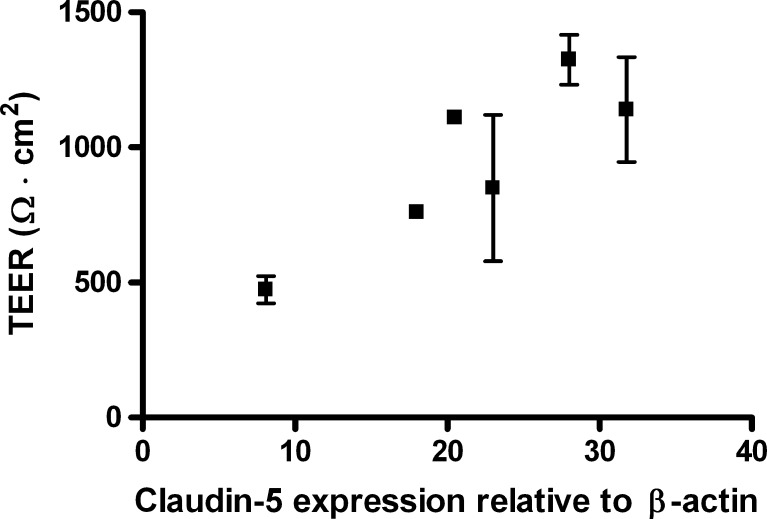
mRNA expression levels in the BBB model was examined by densiometric measurements of band intensities from the PCR products in Fig. 5a + b as well as four other independent determinations and quantifying the claudin-5 intensity against ß-actin. A correlation between TEER and claudin-5 expression was observed (Fig. 8), which suggests a clear role of this tight junction protein in the observed BBB tightening.
Fig. 8.
TEER values as a function of claudin-5 expression. Total RNA was isolated from endothelial cells cultured in differentiation medium and differentiation medium + HEPES, differentiation medium + MOPS or differentiation medium + TES. Data are presented as mean values (n = 3) with standard deviations
Discussion
The present study consisted of a characterization of an in vitro blood–brain barrier model, i.e. BCEC co-cultured with rat astrocytes, and an investigation of the influence of media buffer capacity and culture pH on barrier tightness of the endothelial monolayer.
Increased Buffer Concentration of the Culture Media During Growth, Caused an Increased in Monolayer Tightness
Dickinson et al. previously observed increased TEER values across alveolar epithelial cells when cultured in HEPES buffered medium as compared to medium only buffered by bicarbonate/CO2, which was speculated to be caused by better pH control (11). Moreover, Lo et al. previously showed how small fluctuations in pCO2 in incubators caused an apparent loss of paracellular tightness in pulmonary endothelial cells (10). Based on this, our hypothesis was that the establishment and maintenance of endothelial cell tight junctions could be sensitive to small pH fluctuations in the media.
The addition of 50 mM of HEPES, TES, or MOPS to the culture medium during growth led to increases in average TEER from 40% up to 300%. This correlated with a decreased mannitol permeability, which confirmed a functional tightening of the junctions towards small molecules. Permeability values of small compounds like sodium fluorescein or sucrose in the ranges of 1–6 × 10−6 cm/s have been reported (13–17). In the present study, we obtained values ranging from 0.48 (TEER average of 1,640 Ω cm2) to 3.2 × 10−6 cm/s (TEER average of 630 Ω cm2), and, to our knowledge, only two studies have reported equally low permeability values (18,19) These values were obtained from porcine endothelial cultures, which have generally been known to produce high TEER values. The permeability data reported here are thus among the lowest reported for the bovine endothelial model (7).
The Main Effect of the Additionally Buffered Media was Most Likely Due to Protection from pH fluctuations
The observed effects of the HEPES, MOPS, and TES-buffered DM on the tightness of the endothelial cells, could in theory be due to (1) a change in culture media pH caused by acidification of the media when placed in the CO2 incubators, (2) an effect of the buffer concentration itself, by protecting cells from fluctuations in pH caused by cell metabolism or variations in pCO2 in the incubator, or (3) a direct action of the buffer compounds on the co-culture.
Absolute pH values of the culture media during growth seemed to influence paracellular tightness. The effect of absolute pH was not alone enough to account for the differences between the culture media though, as all HEPES buffered media within pH range of 6.32–7.33 developed significantly higher TEER values than the DM control. A significantly higher TEER was observed at pH 6.82 and 7.11, as compared to more acidic or alkaline media (pH = 6.32 and 7.33). In contrast, previous studies have shown disruption of the BBB with following protein leakage when exposed to low pH values induced by increased pCO2 (20,21). In one study, the pH was lowered to 7.04, but it was shown that a metabolic acidosis resulting in the same pH value did not prompt albumin leakage (20). In another study, protein leakage was only observed at pH values of 6.2 or below, while pH 7.4 and pH 6.6 caused no visible leakage (21). Although these studies have shown negative effects of acidosis in vivo, they cannot be compared directly to our findings, as the pH values of investigation were lower than ours. Extracellular pH values in the ranges comparable to our study have been shown to interfere with second messenger pathways in endothelial cells, as impaired Ca2+ release and nitric oxide- and PGI2 production following bradykinin stimulation has been shown at extracellular pH values of 6.4 and 6.9 compared to control pH 7.4 (22). Hence, caution must be taken when interpreting data from a model cultured at non-physiological pH values.
It is difficult to conclude whether the apparent effects of absolute pH values observed in this study were indeed caused by the culture pH. The pKa value of HEPES of 7.31 at 37°C caused the overall buffer capacity of the media to differ even though the buffer concentration was unchanged. Hereby the buffer capacity of the media cultured at pH 6.32 would be significantly lower than the other situations, whereby the effect of pH cannot be isolated. It would be expected that the medium at pH 7.34 had the highest buffer capacity, whereby cells cultured at this pH should have developed the highest TEER values, if buffer capacity was the sole determining factor. HEPES-, MOPS-, and TES-buffered DM all increased TEER in equal magnitudes. At 37°C, their pKa values are 7.31, 7.098, and 7.16, respectively (23). Hereby, the buffer capacity of the media differed slightly, which could be expected to affect the TEER values. All media would still be expected to possess a buffer capacity significantly higher than the regular DM, and as CO2 fluctuations are presumed to be within the range of 0.5% (i.e. 9.5–10.5%) (10), the buffer capacity of the HEPES-, MOPS-, and TES-buffered DM should all be sufficient to stabilize the culture pH.
The apparent effect of the DM + HEPES, MOPS, and TES buffer could also be caused by a decrease in HCO3− concentration. The regular DM contained approximately 44 mM HCO3−/CO2 while the other media contained approximately 3.4 mM, as calculated from equilibrium constants between dissolved CO2 and the pCO2 of 10% in the incubator. This may have contributed to the observed effects, although Dickinson et al. observed similar tightening effects of HEPES in culture medium, where HCO3− was also present in a concentration of 44 mM (11).
HEPES, MOPS, and TES have all been found to exert a wide range of different adverse effects in different cell culture systems (24–27). Luo et al. showed that ATP production increased with 70% in MDCK-MDR-1 cells grown in culture medium with 20 mM HEPES for 6–7 days compared to the same cell line in medium without HEPES. This caused an increase in MDR-1 efflux activity, which could be a relevant effect in our model (26). Care should be taken when interpreting data from artificially buffered cell cultures, as the buffer compounds may exert an influence, independent of the buffering effect. However, since the three investigated buffers elicited responses of comparable nature and magnitudes and no regulation in expression levels of investigated transporters and receptors were observed, it seems unlikely that the increase in TEER and the increased claudin-5 expression is due to some unknown effect of the buffer compounds, rather than the buffer effect itself.
Hence, it seems that increased buffer concentration in the culture media increased paracellular tightness across a BBB-endothelial/astrocytic co-culture by a combination of stabilizing culture pH and inducing a slightly more acidic overall pH value, where the main effect was due to protection from culture pH fluctuations.
The Increase in Monolayer Tightness Correlated with an Increase in Claudin-5 Expression
Claudin-5 is an important part of the tight junction complexes at the BBB (28–30). Expression of claudin-5 has been shown to increase tightness of tight junction complexes, especially towards small molecules (29,31,32). Koto et al. and Ishizaki et al. have both shown correlation between increased claudin-5 expression and increased TEER values. In support, Nitta and co-workers previously showed that claudin-5 knock-out mice displayed a leaky BBB concerning a 443D primary amine-reactive biotinylation reagent, whereas no serum albumin leakage, and hence no general BBB breakdown, was observed. However another study found that cAMP-dependent phosphorylation of claudin-5 led to decreased TEER in the leaky rat lung endothelial cell line transfected with wild-type mouse-claudin-5. This led to the conclusion that different tight junction proteins probably are involved in the known cAMP-induced tightening of endothelial cells (33). Furthermore Liebner et al. have previously suggested an important role of claudin-1 in the regulation of vascular permeability, as they observed very low claudin-1 expression in leaky brain microvessels removed from human suffering from glioblastoma multiforme (34). This made it interesting to investigate, which tight junction proteins could be involved in the observed tightening of our model.
Occludin, ZO-1, JAM-1 and claudin-1 expressions were not significantly altered in the different culture media. However, a significant up-regulation of claudin-5 on both the mRNA and protein level was observed in the media with increased buffer capacity. This up-regulation correlated with an increase in TEER values and a decrease in apparent permeability of mannitol. Furthermore, the increased buffer capacity appears to induce an increase in zipper-like junction zones as well as an increase in total junction zone length, as observed with claudin-5 immunolabelling. This correlated with the higher TEER values and claudin-5 expression. The significance of this has yet to be investigated.
The findings of Nitta et al. correlate well with our findings, where the endothelial cells maintained tight junction expression under all conditions but the junctions with the low claudin-5 level displayed the highest mannitol permeability and lowest TEER. Moreover, claudin-5 transfection into CaCo2 cells has been shown to increase TEER with a corresponding decrease in mannitol permeability (35). On the contrary, claudin-5 has been shown to increase TEER in MDCK-II cells without an accompanying decrease in mannitol flux, which could be attributed towards a selectivity towards restricting movement of positively charged compounds (35,36). An explanation could be that the effect of claudin-5 on restricting paracellular passage is dependent on the cell culture system because differences in tight junction composition lead to different heterophilic interaction opportunities for claudin-5 (for review, see (37)).
The studies that founded our initial hypothesis did not investigate the mechanisms behind the changes they observed in paracellular tightness (10,11), but it is possible that the same mechanism was involved in their observations. Claudin-5 expression has been shown both in pulmonary endothelial cells and in isolated rat alveolar type II epithelial cells (38,39). It therefore seems plausible that the increased claudin-5 expression is, at least in part, the cause of the observed increase in barrier tightness. The linkage between fluctuations in growth media pH and claudin-5 expression is not known at the moment and must be addressed in future studies.
The Established Model Displayed Classic Blood–Brain Barrier Characteristics
The endothelial cells cultured in combination with astrocytes, i.e., co-cultures, developed into tight monolayers with occludin and claudin-5 expression along the cell borders, alkaline phosphatase activity, MDR-1, BCRP, LAT-1, GLUT-1, TfRC and Ins-R expression and TEER values in the range 300–730 Ω cm2, which are comparable to other bovine blood–brain barrier models (7,40).
This study has demonstrated a basic characterization of our in vitro BBB model, as well as an improved tight junction expression. Regardless, a thorough characterization is still needed to estimate the usability of the model for screening purposes. Previous studies have shown poor in vivo/in vitro correlations regarding BBB permeability in a wide range of cellular models (41). This was partly caused by poor recognition of influx transporter substrates in BBB models, which to a wide extent was attributed to paracellular leakage leading to failure to identify active influx. Poor transporter expression has been supported by other reports showing an incomplete transporter expression pattern in in vitro BBB models (42,43). These studies have been performed in model systems different from ours. Lyck et al. performed their study in a non-contact co-culture of murine-derived cells, while Calbria et al. performed their study in a porcine endothelial cell single culture. It has previously been shown, that the cell–cell contact between astrocytes and endothelial cells is important for the maintenance of BBB characteristics (16,19). Altogether this stresses the need to thoroughly characterize our model regarding transporter expression and permeability of a range of model compounds.
Conclusions
For the first time, it is shown that increased buffer concentration can increase paracellular tightness of an in vitro blood–brain barrier model. This may be utilized to improve barrier properties of in vitro blood–brain barrier models, and may also have a physiological impact. It has been shown numerous times that both acidosis and alkalosis have the potential to disrupt BBB integrity. This study extends these examinations and shows that even small fluctuations within physiologically relevant pH values may have an influence on BBB selectivity towards paracellular permeation through a regulation of claudin-5 expression. The mechanism whereby claudin-5 expression is regulated remains unclear and will be the subject of future studies.
Acknowledgments
The authors would like to thank Carlsberg foundation, the Predicting Drug Absorption Consortium and the Novo Scholarship Programme for financial support. Furthermore, we would like to thank Assoc. Professor—Ph.D. A.G. de Boer for generous help and collaboration during the start up of the co-culture system as well as senior laboratory technicians Heidi Nielsen, Maria D. Læssøe Pedersen and Bettina Dinitzen for their expert technical assistance.
References
- 1.Reese TS, Karnovsky MJ. Fine structural localization of a blood–brain barrier to exogenous peroxidase. J Cell Biol. 1967;34(1):207–17. doi: 10.1083/jcb.34.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crone C, Olesen SP. Electrical resistance of brain microvascular endothelium. Brain Res. 1982;241(1):49–55. doi: 10.1016/0006-8993(82)91227-6. [DOI] [PubMed] [Google Scholar]
- 3.Brightman MW, Reese TS. Junctions between intimately apposed cell membranes in the vertebrate brain. J Cell Biol. 1969;40(3):648–77. doi: 10.1083/jcb.40.3.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Boer AG, Gaillard PJ, Breimer DD. The transference of results between blood–brain barrier cell culture systems. Eur J Pharm Sci. 1999;8(1):1–4. doi: 10.1016/S0928-0987(99)00003-2. [DOI] [PubMed] [Google Scholar]
- 5.el-Bacha RS, Minn A. Drug metabolizing enzymes in cerebrovascular endothelial cells afford a metabolic protection to the brain. Cell Mol Biol (Noisy-le-grand) 1999;45(1):15–23. [PubMed] [Google Scholar]
- 6.Cecchelli R, Berezowski V, Lundquist S, Culot M, Renftel M, Dehouck MP, et al. Modelling of the blood–brain barrier in drug discovery and development. Nat Rev Drug Discov. 2007;6(8):650–61. doi: 10.1038/nrd2368. [DOI] [PubMed] [Google Scholar]
- 7.Deli MA, Abraham CS, Kataoka Y, Niwa M. Permeability studies on in vitro blood–brain barrier models: physiology, pathology, and pharmacology. Cell Mol Neurobiol. 2005;25(1):59–127. doi: 10.1007/s10571-004-1377-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taylor AC. Responses of cells to pH changes in the medium. J Cell Biol. 1962;15:201–9. doi: 10.1083/jcb.15.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mackenzie CG, Mackenzie JB, Beck P. The effect of pH on growth, protein synthesis, and lipid-rich particles of cultured mammalian cells. J Biophys Biochem Cytol. 1961;9:141–56. doi: 10.1083/jcb.9.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lo CM, Keese CR, Giaever I. pH changes in pulsed CO2 incubators cause periodic changes in cell morphology. Exp Cell Res. 1994;213(2):391–7. doi: 10.1006/excr.1994.1214. [DOI] [PubMed] [Google Scholar]
- 11.Dickinson PA, Evans JP, Farr SJ, Kellaway IW, Appelqvist TP, Hann AC, et al. Putrescine uptake by alveolar epithelial cell monolayers exhibiting differing transepithelial electrical resistances. J Pharm Sci. 1996;85(10):1112–6. doi: 10.1021/js9504898. [DOI] [PubMed] [Google Scholar]
- 12.Gaillard PJ, de Boer AG. 2B-Trans technology: targeted drug delivery across the blood–brain barrier. Methods Mol Biol. 2008;437:161–75. doi: 10.1007/978-1-59745-210-6_8. [DOI] [PubMed] [Google Scholar]
- 13.Boveri M, Berezowski V, Price A, Slupek S, Lenfant AM, Benaud C, et al. Induction of blood–brain barrier properties in cultured brain capillary endothelial cells: comparison between primary glial cells and C6 cell line. Glia. 2005;51(3):187–98. doi: 10.1002/glia.20189. [DOI] [PubMed] [Google Scholar]
- 14.Culot M, Lundquist S, Vanuxeem D, Nion S, Landry C, Delplace Y, et al. An in vitro blood–brain barrier model for high throughput (HTS) toxicological screening. Toxicol In Vitro. 2008;22(3):799–811. doi: 10.1016/j.tiv.2007.12.016. [DOI] [PubMed] [Google Scholar]
- 15.Franke H, Galla HJ, Beuckmann CT. An improved low-permeability in vitro-model of the blood–brain barrier: transport studies on retinoids, sucrose, haloperidol, caffeine and mannitol. Brain Res. 1999;818(1):65–71. doi: 10.1016/S0006-8993(98)01282-7. [DOI] [PubMed] [Google Scholar]
- 16.Gaillard PJ, Voorwinden LH, Nielsen JL, Ivanov A, Atsumi R, Engman H, et al. Establishment and functional characterization of an in vitro model of the blood–brain barrier, comprising a co-culture of brain capillary endothelial cells and astrocytes. Eur J Pharm Sci. 2001;12(3):215–22. doi: 10.1016/S0928-0987(00)00123-8. [DOI] [PubMed] [Google Scholar]
- 17.Nakagawa S, Deli MA, Nakao S, Honda M, Hayashi K, Nakaoke R, et al. Pericytes from brain microvessels strengthen the barrier integrity in primary cultures of rat brain endothelial cells. Cell Mol Neurobiol. 2007;27(6):687–94. doi: 10.1007/s10571-007-9195-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoheisel D, Nitz T, Franke H, Wegener J, Hakvoort A, Tilling T, et al. Hydrocortisone reinforces the blood–brain properties in a serum free cell culture system. Biochem Biophys Res Commun. 1998;247(2):312–5. doi: 10.1006/bbrc.1997.8051. [DOI] [PubMed] [Google Scholar]
- 19.Malina KCK, Cooper I, Teichberg VI. Closing the gap between the in-vivo and in-vitro blood–brain barrier tightness. Brain Res. 2009;1284:12–21. doi: 10.1016/j.brainres.2009.05.072. [DOI] [PubMed] [Google Scholar]
- 20.Bratlid D, Cashore WJ, Oh W. Effect of acidosis on bilirubin deposition in rat brain. Pediatrics. 1984;73(4):431–4. [PubMed] [Google Scholar]
- 21.Nagy Z, Szabo M, Huttner I. Blood–brain barrier impairment by low pH buffer perfusion via the internal carotid artery in rat. Acta Neuropathol. 1985;68(2):160–3. doi: 10.1007/BF00688639. [DOI] [PubMed] [Google Scholar]
- 22.Asai M, Takeuchi K, Saotome M, Urushida T, Katoh H, Satoh H, et al. Extracellular acidosis suppresses endothelial function by inhibiting store-operated Ca2+ entry via non-selective cation channels. Cardiovasc Res. 2009;83(1):97–105. doi: 10.1093/cvr/cvp105. [DOI] [PubMed] [Google Scholar]
- 23.Gueffroy DE, editor. Buffers a guide for preparation and use of buffers in biological systems. San Diego: Calbiochem-Novabiochem Corporation; 1993.
- 24.Altura BM, Carella A, Altura BT. Adverse effects of Tris. HEPES and MOPS buffers on contractile responses of arterial and venous smooth muscle induced by prostaglandins. Prostaglandins Med. 1980;5(2):123–30. doi: 10.1016/0161-4630(80)90099-3. [DOI] [PubMed] [Google Scholar]
- 25.Lelong IH, Rebel G. pH drift of “physiological buffers” and culture media used for cell incubation during in vitro studies. J Pharmacol Toxicol Methods. 1998;39(4):203–10. doi: 10.1016/S1056-8719(98)00019-7. [DOI] [PubMed] [Google Scholar]
- 26.Luo S, Pal D, Shah SJ, Kwatra D, Paturi KD, Mitra AK. Effect of HEPES buffer on the uptake and transport of P-glycoprotein substrates and large neutral amino acids. Mol Pharm. Apr 5;7(2):412–20 [DOI] [PMC free article] [PubMed]
- 27.Poole CA, Reilly HC, Flint MH. The adverse effects of HEPES, TES, and BES zwitterion buffers on the ultrastructure of cultured chick embryo epiphyseal chondrocytes. In Vitro. 1982;18(9):755–65. doi: 10.1007/BF02796499. [DOI] [PubMed] [Google Scholar]
- 28.Morita K, Sasaki H, Furuse M, Tsukita S. Endothelial claudin: Claudin-5/TMVCF constitutes tight junction strands in endothelial cells. J Cell Biol. 1999;147(1):185–94. doi: 10.1083/jcb.147.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nitta T, Hata M, Gotoh S, Seo Y, Sasaki H, Hashimoto N, et al. Size-selective loosening of the blood–brain barrier in claudin-5-deficient mice. J Cell Biol. 2003;161(3):653–60. doi: 10.1083/jcb.200302070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen Z, Zandonatti M, Jakubowski D, Fox HS. Brain capillary endothelial cells express MBEC1, a protein that is related to the Clostridium perfringens enterotoxin receptors. Lab Investig. 1998;78(3):353–63. [PubMed] [Google Scholar]
- 31.Ishizaki T, Chiba H, Kojima T, Fujibe M, Soma T, Miyajima H, et al. Cyclic AMP induces phosphorylation of claudin-5 immunoprecipitates and expression of claudin-5 gene in blood–brain-barrier endothelial cells via protein kinase A-dependent and -independent pathways. Exp Cell Res. 2003;290(2):275–88. doi: 10.1016/S0014-4827(03)00354-9. [DOI] [PubMed] [Google Scholar]
- 32.Koto T, Takubo K, Ishida S, Shinoda H, Inoue M, Tsubota K, et al. Hypoxia disrupts the barrier function of neural blood vessels through changes in the expression of claudin-5 in endothelial cells. Am J Pathol. 2007;170(4):1389–97. doi: 10.2353/ajpath.2007.060693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Soma T, Chiba H, Kato-Mori Y, Wada T, Yamashita T, Kojima T, et al. Thr(207) of claudin-5 is involved in size-selective loosening of the endothelial barrier by cyclic AMP. Exp Cell Res. 2004;300(1):202–12. doi: 10.1016/j.yexcr.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 34.Liebner S, Fischmann A, Rascher G, Duffner F, Grote EH, Kalbacher H, et al. Claudin-1 and claudin-5 expression and tight junction morphology are altered in blood vessels of human glioblastoma multiforme. Acta Neuropathol. 2000;100(3):323–31. doi: 10.1007/s004010000180. [DOI] [PubMed] [Google Scholar]
- 35.Amasheh S, Schmidt T, Mahn M, Florian P, Mankertz J, Tavalali S, et al. Contribution of claudin-5 to barrier properties in tight junctions of epithelial cells. Cell Tissue Res. 2005;321(1):89–96. doi: 10.1007/s00441-005-1101-0. [DOI] [PubMed] [Google Scholar]
- 36.Wen H, Watry DD, Marcondes MC, Fox HS. Selective decrease in paracellular conductance of tight junctions: role of the first extracellular domain of claudin-5. Mol Cell Biol. 2004;24(19):8408–17. doi: 10.1128/MCB.24.19.8408-8417.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Krause G, Winkler L, Mueller SL, Haseloff RF, Piontek J, Blasig IE. Structure and function of claudins. Biochim Biophys Acta. 2008;1778(3):631–45. doi: 10.1016/j.bbamem.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 38.Favre CJ, Mancuso M, Maas K, McLean JW, Baluk P, McDonald DM. Expression of genes involved in vascular development and angiogenesis in endothelial cells of adult lung. Am J Physiol Heart Circ Physiol. 2003;285(5):H1917–38. doi: 10.1152/ajpheart.00983.2002. [DOI] [PubMed] [Google Scholar]
- 39.Wang F, Daugherty B, Keise LL, Wei Z, Foley JP, Savani RC, et al. Heterogeneity of claudin expression by alveolar epithelial cells. Am J Respir Cell Mol Biol. 2003;29(1):62–70. doi: 10.1165/rcmb.2002-0180OC. [DOI] [PubMed] [Google Scholar]
- 40.Gumbleton M, Audus KL. Progress and limitations in the use of in vitro cell cultures to serve as a permeability screen for the blood–brain barrier. J Pharm Sci. 2001;90(11):1681–98. doi: 10.1002/jps.1119. [DOI] [PubMed] [Google Scholar]
- 41.Garberg P, Ball M, Borg N, Cecchelli R, Fenart L, Hurst RD, et al. In vitro models for the blood–brain barrier. Toxicol In Vitro. 2005;19(3):299–334. doi: 10.1016/j.tiv.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 42.Calabria AR, Shusta EV. A genomic comparison of in vivo and in vitro brain microvascular endothelial cells. J Cereb Blood Flow Metab. 2008;28(1):135–48. doi: 10.1038/sj.jcbfm.9600518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lyck R, Ruderisch N, Moll AG, Steiner O, Cohen CD, Engelhardt B, et al. Culture-induced changes in blood–brain barrier transcriptome: implications for amino-acid transporters in vivo. J Cereb Blood Flow Metab. 2009;29(9):1491–502. doi: 10.1038/jcbfm.2009.72. [DOI] [PubMed] [Google Scholar]