Abstract
Insertion of a cutaneous microdialysis catheter into normal dermis has been shown to induce the production of IL1b, IL6 and IL8 in an innate response to minimal trauma. In the present study, skin biopsy for immunohistochemistry has been performed at the site of the microdialysis catheter to compare the findings with that of the microdialysis findings 24 h after insertion. Of the three named cytokines, concordance between the two investigated technologies was highest for IL8 (100%) followed by IL6 (70%) and IL1b (50%). For seven other pro-inflammatory and T cell-relevant cytokines studied, concordance ranged between 50% and 80%. The total number of positive (microdialysis or immunofluorescence) findings was similar between the two methodologies. Technical and biological phenomenon can explain the differences. We conclude that both methodologies illustrate important features of tissue biology and that a combination of the two methods in clinical research can provide the chronology of soluble mediator participation and the more classic, but also more invasive, biopsy-based methodology at a point which constitutes the end of the observation period. We conclude further that at the 24-h time period here studied, microdialysis catheters are still functional and thus capable of producing relevant data which can be corroborated and extended by the “end point biopsy”.
Key words: cutaneous microdialysis, cytokines, human, immunostaining, innate
INTRODUCTION
Cytokines, chemokines and growth factors are important proteins participating in many homeostatic and immune responses, both innate and adaptive (1–5). These key proteins regulate cellular functions via autocrine, paracrine and endocrine pathways, not the least in skin. Much of our knowledge about these molecules has been gained in a laboratory environment using isolated cells and cell lines and, more recently, genetically engineered models in mice or flies. In the translation of findings to the human (clinical) in vivo situation, the main methods of obtaining data have been peripheral blood, which summates effects at a whole organism level, and skin biopsy, which gives tissue-specific (skin) data on the presence of or gene induction for the production of a protein at the specific time point the biopsy was taken. Cutaneous microdialysis (CMD) enables continuous in vivo sampling of the interstitial fluid in the intact skin and is thus an attractive, newer and arguably less tissue destructive alternative for the measurement of cytokines actually present in the dermal extracellular environment over a period commensurate with the expected pathogenesis of the reaction under study (6–11). The present study is on normal skin and combines CMD with end point biopsy for immunohistochemistry of the actual microdialysis area to compare the findings of the two methodologies at a specific time point, 24 h.
Knowledge of the effect of microdialysis catheter insertion itself into the skin is important as a basis for the interpretation of data from skin, whether it be normal, experimentally provoked or diseased (10–16). The issues of tissue reactivity, levels of trauma and recovery (equilibration) times are central to any discussion of CMD. This is especially so in the interpretation of findings on cytokines and other biologically active molecules such as chemokines and growth factors (9,10,17–19), which patently have longer “equilibration” times than axon reflex-mediated microvascular events (11–13). Other central issues concern whether membranes retain their laboratory bench determined function in relation to passage of target molecules in vivo in the short- (e.g. recovery of a given molecule) and longer term (e.g. biofouling) and whether depletion of target molecules in the immediate area around the membrane occurs (20–24). This study complements previously published data on CMD findings after catheter insertion (18) and allows discussion of these questions within that time period, which includes axon reflex-mediated mechanisms, skin reactivity mechanisms and “biofouling”.
The objective of the present study was to use immunofluorescence staining technique to compare, in skin biopsies taken from the actual site of a microdialysis catheter membrane in normal forearm skin, the presence or absence of ten pro-inflammatory/lymphocyte regulatory cytokines which were measured in the microdialysate from the same site an hour prior to the biopsy, approximately 24 h after insertion of the catheter.
MATERIAL AND METHODS
Subjects
Ten healthy volunteers, four women and six men, with an age range of 27–55 years, were given verbal and written information of the procedure. The study was approved by the Regional Ethics Committee for Human Research at Linköping University no. 03–250.
Clinical Experimental Design
Subjects had participated in a previously published 24-h microdialysis study. The catheter (CMA71 Microdialysis AB, Stockholm, Sweden), a 10-mm polyethersulfone membrane with a molecular weight cutoff of 100 kDa, was primed for 1 h before insertion into the forearm to ensure perfusate flow with Ringer Dextran Braun 60 (Apoketsbolaget, Gothenburg Sweden), a colloid osmotic dextran solutionused in the clinic for intravenous infusion (a physiological Ringer buffer containing dextran where more than 90% of the dextran molecules have a molecular weight between 20,000 and 110,000). A site was chosen so as to avoid obvious veins as well as for convenience of fixation. The point of insertion was anaesthetised with a local anaesthetic (Xylocain ® 10 mg/ml Astra Läkemedel AB, Södertälje, Sweden), injected intradermally. An 18-gauge Venflon catheter (Viggo Products, Helsingborg, Sweden) was used as a guide, tunnelling in the deep dermis or subcutaneous tissue for the first 1.5 cm and then intradermally for the last centimetre in order to position the dialysis catheter membrane as superficially as possible. The catheter was inserted through the guide, the guide withdrawn and the catheter tubing taped in position together with a plastic frame designed to reduce torsion of the skin with pronation and supination of the forearm. Thus, the dialysis membrane at the tip of the catheter was situated intradermally 1.5–2.5 cm from the point of insertion. Dermascan A (Sonotron AB, Sweden) was used to measure the depth of the membrane and the thickness of the dermis. Once the catheter was in place, the pump (CMA 106, CMA Microdialysis AB) was started and the dialysate was collected at a flow rate of 0.3 μl/min. The pump and tubing was strapped to the forearm with supportative bandages to ensure free movement of the arm. The outlet tubing and micro-sampling tube (Elkay Products, Kemila, Stockholm, Sweden) was so positioned as to ensure ease of tube changing. Subjects were instructed to participate in normal activities, though not heavy work or sport. Twenty-four to 28 h after catheter insertion, the position of the membrane lying in the dermis was marked on the skin surface with a marker pen. The catheter was removed and after anaesthetisation with local anaesthetic a 4-mm punch biopsy was taken from the area where the membrane had lain within the skin, positioning the marking across the diameter of the circular biopsy. The biopsy, mounted in OCT Compound (Histolab, Gothenburg, Sweden), was positioned prior to freezing so that the skin marking (which was a projection of the probe placement on the skin surface) was perpendicular to the section plane. The tissue was snap-frozen in liquid nitrogen and placed in −70°C before processing.
Microdialysate Recovery Experiments
The recovery (the amount of cytokines diffusing across the 100-kDa membrane) was assessed under benchtop conditions as earlier described (25). The cytokines (IL-1b, 2, 4, 5, 6, 8, 10, GM-CSF, INFg and TNFa) were obtained from the Human cytokine 10-plex antibody kit (Biosource, Nivelles, Belgium). The membranes were perfused with Ringer Dextran 60 at a flow rate of 0.3 μl/min. The in vitro recovery for the measured cytokines was expressed as the median (n = 3). GM-CSF and IL1b gave the highest per cent recovery: 21.8% and 21.6%, respectively, IL5, IL6, IL8 and IFNg between 11.8% and 18.1%, and IL2, IL4, IL10 and TNFa showed recoveries of 5.3% or less.
Microdialysate Testing for Total Protein Content
The microdialysate aliquots taken throughout the experiment including the hour before biopsy were analysed for total protein content with the DC Protein Assay (BioRad, Stockholm, Sweden) requiring a sample volume of 5 μl (25).
Microdialysate Testing for Cytokine Content
The microdialysate taken during the hour before biopsy was frozen in −70°C before being analysed for cytokine content with the Human cytokine 10 plex kit (Biosource). The method used is based on multiplex bead technology (26). Briefly, polystyrene beads 5.6 μm in diameter contain a different ratio of red and infrared fluorescent dyes particular for each bead population, making 100 populations possible. Each bead population has been covalently coupled with an antibody directed against one cytokine, in this study IL1b, 2, 4, 5, 6, 8, 10, GM-CSF, IFNg or TNFa. All these ten different bead populations are mixed together with the sample. The bead–antibody–cytokine complexes are then mixed with the detection antibodies (conjugated with biotin) directed against the same cytokines bound to the bead–antibody complexes. Streptavidin conjugated to phycoerythrin is then added and the bead–antibody–cytokine–antibody complex is then analysed on a Luminex 100 IS instrument (Biosource) calibrated and validated with Luminex control beads (Luminex, Austin, TX USA). The Star Station acquisition programme (v2, Applied Cytometry Systems, Sheffield, UK) processes the fluorescence intensity of the beads and bound cytokine–antibody complexes. Standard curves are generated by a five-parameter algorithm in the analysis programme and plotted on a log–log scale. The concentrations of the samples are reported by the software. Results for the 1-h sample immediately prior to biopsy will be shown in this paper. Other time points in the microdialysis analysis are previously published (18). Figure 1 summarises these findings. The lower detection limits for the multiplex assay were (in pg/ml): IL1b, 5.9; IL2, 5.0; IL4, 5.0; IL5, 6.4; IL6, 4.1; IL8, 8.7; IL10, 4.6; GM-CSF, 6.9; INFg, 4.0; and TNFa, 3.8.
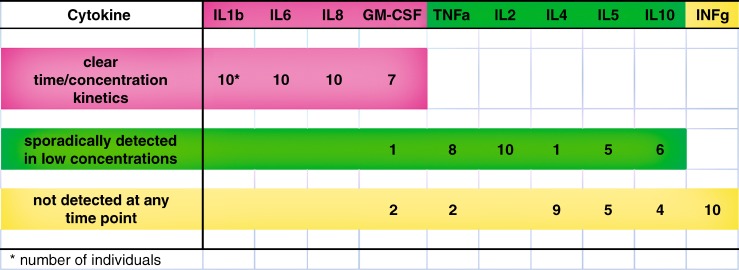
Fig. 1.
Summary of the findings in all cutaneous microdialysis samples in ten healthy individuals for each of the ten cytokines over 24 h. The findings for each cytokine are the number of individuals: showing kinetics (rising and falling) of cytokine concentrations over time; for whom only a few samples had detectable concentrations; not having detectable cytokine concentrations in any sample
Immunofluorescence Staining for Demonstration of Cytokines
The biopsies were analysed for the same cytokines as those tested for in the microdialysate. The primary antibodies used were monoclonal mouse anti-human IL1b, IL2, IL4, IL5, IL6, IL10, GM-CSF, IFNg, TNFa and a polyclonal goat anti-human IL8 (RnD Systems, Oxon, UK), all extensively tested for immunohistochemical use on formalin-fixed cryostat sections. Biotinylated goat anti-mouse immunoglobulin G (IgG) and rabbit anti-goat IgG (DakoCytomation, Stockholm Sweden) were used as secondary antibodies. To enhance the immunostaining signal of the cytokines, streptavidin conjugated with Alexa Fluor™ 546 (Molecular Probes, Lieden, the Netherlands) was used as the detection fluorophore. All antibodies were titrated to give maximal immunofluorescence with a low background staining. The staining procedure was as follows: the biopsy was sectioned on a Leitz CM3050 cryostat (Göteborg, Sweden) at a thickness of 10 μm. Two to three sections were placed in each well of an eight-well microscope slide (Novakemi, Stockholm, Sweden) coated with 0.1% poly-l-lysine (Sigma, Stockholm, Sweden) (27). The sections were air-dried and placed in −70°C for at least 1 h. All slides were identified with the biopsy and glass number following sectioning. After fixation in 4% paraformaldehyde for 15 min, the sections were blocked with 5% normal goat serum (Immunkemi, Stockhom, Sweden) or 5% normal rabbit serum (DakoCytomation) diluted in 0.1% saponin/phosphate-buffered saline (VWR, Stockholm, Sweden). This was followed by incubation with streptavidin biotin blocking reagent from Vector (Immunkemi). The primary antibodies directed against the cytokines IL1b, IL2, IL4, IL5, IL6, IL8, IL10, GM-CSF, IFNg and TNFa and controls were then applied to the sections in a predetermined and documented pattern. After incubation overnight, the slides were washed and the sections were incubated with the secondary antibodies, biotinylated goat anti-mouse immunoglobulin (Ig) or biotinylated rabbit anti-goat Ig. Incubation at room temperature was followed by washing. Alexafluor™546 conjugated streptavidin was applied to the slides and incubated once more. After washing, slides were mounted in Anti Fade from Molecular Probes, their identity was covered and renumbered in a randomly chosen fashion, and stored in the dark at 4°C until analysis. The code was broken after all slides had been evaluated. Assessment of the immunofluorescent staining was performed by examining the whole section. Any red fluorescence found within the epidermis or dermis localised in a morphologically recognisable cellular structure was considered positive. The amount of positive cells per viewfield was evaluated semiquantitatively as absent (“0”), “+” (<5 cells per view field), “++” (>5 cells >20 per view field) or “+++” (>20 cells per field) and noted together with the number of the slide and well in the experimental protocol. All sections were evaluated by one experienced investigator (FS). Negative control of the staining procedure was done by substituting the primary antibody with an isotype and concentration-matched non-immune Ig (DAKOCytomation, Copenhagen, Denmark). Phosphate-buffered saline was also substituted for the primary and secondary antibodies. A tonsil from a patient with recurrent tonsillitis was used as a positive control tissue for the cytokine antibodies (28,29) and titrationexperiments. Findings seen in the dermis of normal (non-CMD) skin were mostly negative as reported in the literature (30–32).
Instrumentation
The immunofluorescence staining was examined with a confocal laser scanning microscope, LSCM Nikon Eclipse (Nikon, Stockholm, Sweden) equipped with a 20/0.75 NA (air/oil) plan-apochromat objective. The excitation source for the subjective semiquantitative assessment of the staining was a 100-W mercury arc lamp using epi-illumination. For laser scanning and image acquisition, a 488-nm argon (Ar) laser and a 546-nm helium neon (HeNe) laser were used. The intensity of the two laser signals were monitored to give an optimal signal without contributing excessively to the spillover/background noise and photobleaching of the preparation. The software EZ.C2 2000 (Teknoptics, Stockholm, Sweden) controlled the confocal microscope, compensated for the refractive index and calculated the area of the sectional plane. The images were viewed as 512 × 512 pixels per sectional plane and 0.34 × 0.34 μm per pixel.
RESULTS
Subjects tolerated the procedures well.
Microdialysate Analysis
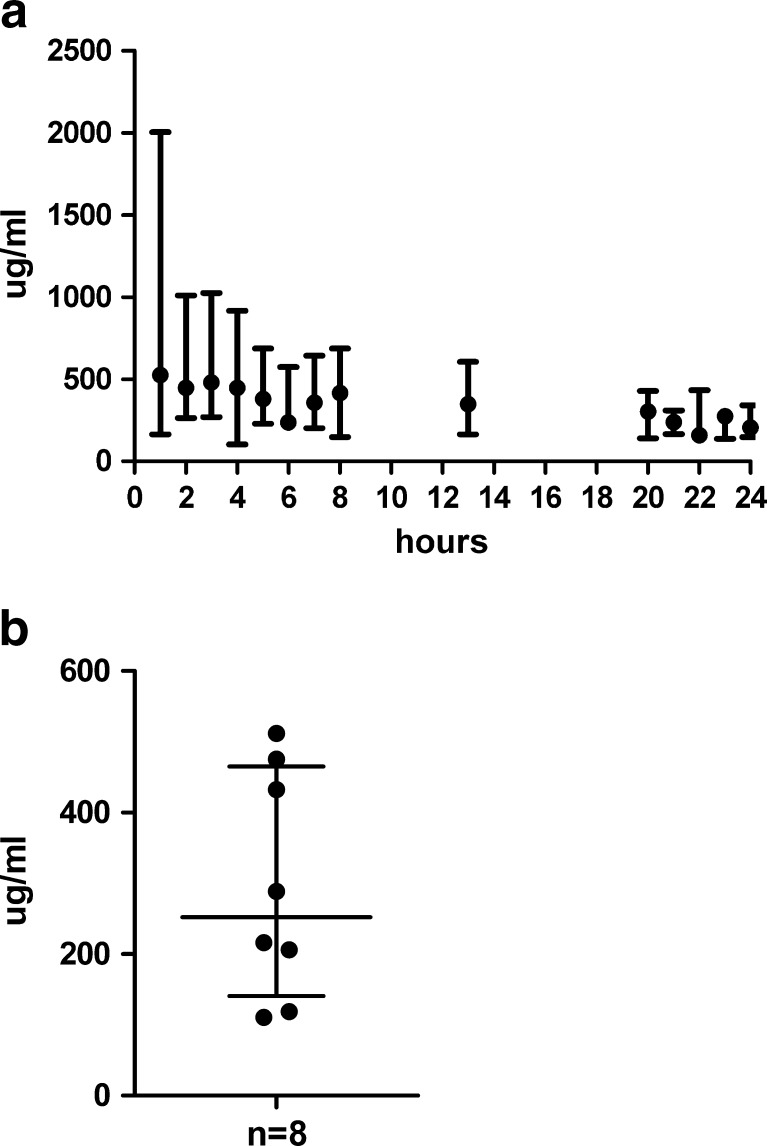
The presence of total protein in the dialysate confirms that the membrane is positioned in tissue and functioning. The levels are variable, but much higher (hundreds of micrograms per millilitre) than the levels of cytokines measured (picograms per millilitre). Total protein levels at 24 h are lower than during insertion trauma, but still at around 50% of initial values (Fig. 2a). The individual value for total protein in the sample immediately prior to biopsy was available in eight subjects (Fig. 2b).
Fig. 2.
a Median and range of total protein concentration (in μg/ml) in cutaneous microdialysis samples from ten individuals. The samples were collected hourly during the first 8 h, thereafter one sample collected during the following 5 h, one sample collected during the night and then again hourly collection the next morning until 24 h after catheter insertion. b Total protein concentration in (μg/ml) analysed in the cutaneous microdialysis sample collected the hour before biopsy, 24 h after catheter insertion. The figure shows the median and interquartile range for eight of the ten individuals
Detailed findings on the 24-h chronology of cytokine production are available in a previous publication (18) and are summarised in regard to their detection or not at an individual subject level in Fig. 1. The cytokine concentrations reported are not corrected for blood flow or in vitro recovery and thus do not reflect the actual tissue concentration. Table I tabulates the results for CMD and immunofluorescence (IF). Individual cytokine suspension array values in the hour prior to biopsy can be seen in Table I in column “M”. At this time point, a range of findings was seen from positivity in every individual (IL6 and IL8) to values failing to reach, in any subject, the lowest standard point of the analysis (IFNg). IL1b and IL2 were above the lowest standard point in six subjects. GM-CSF was above the lowest standard in four subjects, with the remaining cytokines (IL4, IL5, IL10, TNFa) being above the lowest standard in one to three subjects. The total protein levels (not shown in Table I) showed no correlation to the cytokine concentrations.
Table I.
Findings of Ten Cytokines in Ten Individuals 24 h after Insertion of a Cutaneous Microdialysis Catheter Membrane
| Subjects | IL1b | IL6 | IL8 | GM-CSF | TNFa | IL2 | IL4 | IL5 | IL10 | INFg | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IF | M | IF | M | IF | M | IF | M | IF | M | IF | M | IF | M | IF | M | IF | M | IF | M | |
| 1 | 0 | 17.7 | ++ | 141.3 | ++ | 134 | 0 | 14.5 | +++ | 8.2 | + | 7.9 | 0 | 10.6 | ND | 10.5 | 0 | 0 | 0 | 0 |
| 2 | 0 | 0 | 0 | 40.9 | ++ | 23.3 | 0 | 0 | + | 0 | 0 | 0 | 0 | 0 | ND | 0 | 0 | 0 | 0 | 0 |
| 3 | +++ | 22.8 | ++ | 820.7 | ++ | 504.2 | + | 20.9 | 0 | 8.2 | + | 0 | 0 | 0 | ND | 8 | 0 | 6 | 0 | 0 |
| 4 | +++ | 0 | +++ | 24.7 | ++ | 252.0 | +++ | 7.7 | ++ | 0 | +++ | 12.6 | ++ | 0 | ND | 0 | 0 | 0 | 0 | 0 |
| 5 | ++ | 12.2 | ++ | 116.8 | ++ | 154.9 | ND | 7.7 | 0 | 8.2 | ND | 0 | ND | 0 | ND | 0 | ND | 0 | + | 0 |
| 6 | 0 | 0 | + | 436.8 | ++ | 778.6 | + | 0 | 0 | 0 | ++ | 14.7 | 0 | 0 | 0 | 0 | +++ | 0 | + | 0 |
| 7 | 0 | 27.4 | + | 702.6 | ++ | 296.8 | 0 | 0 | + | 0 | 0 | 5.5 | 0 | 0 | + | 0 | 0 | 0 | 0 | 0 |
| 8 | 0 | 19.7 | 0 | 699.5 | ++ | 982.1 | 0 | 0 | 0 | 0 | 0 | 5.5 | 0 | 0 | 0 | 0 | 0 | 0 | ++ | 0 |
| 9 | 0 | 0 | 0 | 25.2 | ++ | 1040.3 | 0 | 0 | 0 | 0 | 0 | 5.5 | 0 | 0 | 0 | 0 | + | 0 | 0 | 0 |
| 10 | 0 | 6.2 | +++ | 225.8 | ++ | 220.2 | 0 | 0 | ++ | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | + | 0 |
| Analysable IF and Ma | 10 | 10 | 10 | 10 | 10 | 10 | 9 | 10 | 10 | 10 | 9 | 10 | 9 | 10 | 5 | 10 | 9 | 10 | 10 | 10 |
| Total positive IF and Mb | 3 | 6 | 7 | 10 | 10 | 10 | 3 | 4 | 6 | 3 | 4 | 6 | 1 | 1 | 1 | 2 | 2 | 1 | 4 | 0 |
| Analysable pair comparisonsc | 10 | 10 | 10 | 9 | 10 | 9 | 9 | 5 | 9 | 10 | ||||||||||
| Total individual concordance (%)d | 50 | 70 | 100 | 77 | 50 | 55 | 77 | 80 | 66 | 60 | ||||||||||
IF staining was performed on skin biopsies taken from the site of the catheter membrane. A semiquantitative scale was chosen: 0 = no cellular staining, + = 5 cells or less stained, ++ = between 5 and 20 cells, +++ = more than 20 cells. Three to four viewfields 37 × 104 μm2 were evaluated at ×200 magnification. ND = technical obstructions. The cytokine concentrations in the microdialysis (M) samples (in pg/ml) are from samples collected the hour before biopsy. Concentrations that did not exceed the lowest standard concentration are noted as 0
aNumber of evaluated subjects
bIF = Number of positive immunofluorescence findings. M = Number of subjects who had cytokine levels above the lowest standard concentration in the last microdialysis sample
cNumber of analysable observations of both immunofluorescence and cytokine concentrations in the same individual
dPer cent concordance of the methods; number of evaluated positive or negative findings in agreement between IF + M divided by the number of paired comparisions × 100
Immunofluorescence Demonstration of Cytokines
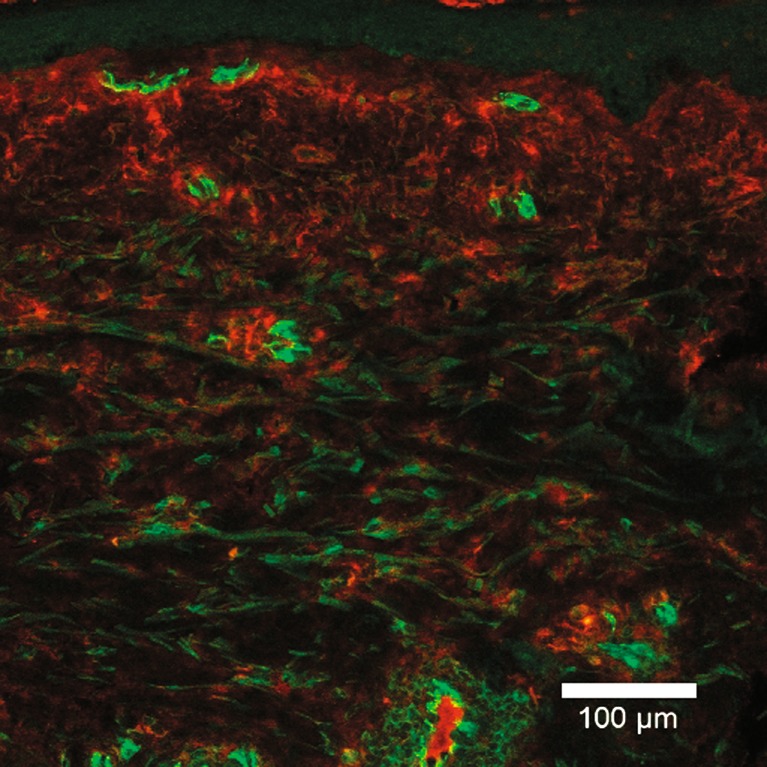
Column “IF” in Table I shows the outcome of the semiquantitative assessment of fluorescence for the ten cytokines in the “end point” (24-h) biopsies of the ten subjects (column IF). The cellular localisation of the cytokines was the criterion for inclusion as positive, and this was seen to varying degrees (0, +, ++, +++). IL8 was seen in all subjects, IL6 was seen in 70% of the subjects, and TNFa in 50%. IL1b, IL2, GM-CSF and IFNg were seen in 30–40% of the subjects; IL4, IL5 and IL10 were seen least often, in 10–20% of the subjects. For five cytokines in one patient, no assessment could be performed due to a technical problem (noted as ND). IL5 was applied at the wrong concentration in five individuals and excluded from the evaluation (noted as ND). Figure 3 illustrates the IF findings in one subject, with a red stain showing the presence of IL6 and a green stain depicting a cell surface marker for endothelial cells.
Fig. 3.
A ×20 micrograph of an immunofluorescence stained section of a skin biopsy taken from the site where a microdialysis catheter membrane had lain for 24 h. The epidermis is uppermost. The red colour shows the location of the antibody directed against the cytokine IL6. The green colour shows the location of the antibody directed against a membrane antigen found on endothelial cells
In order to facilitate an interpretation and comparison of IF and M findings, Table I also introduces and summates various concepts: the number of observations available for the assessment for IF and M (Analysable IF and M); the positive findings of the two methods at the present sensitivity thresholds for the individual cytokines (Total positive IF and M); the number of individual subjects for whom both IF and M were available (Analysable pair comparisons); and the number of concordant paired findings, whether positive or negative (% total individual concordance).
The total number of positive findings (IF and M) was approximately the same for IF (41 positive) and M (43 positive). Viewed from the aspect of the individual cytokine in the whole group, the fluorescence technique gave a larger number of positives than the microdialysis technique for IFNg, TNFa and IL10 (four more biopsies, two more biopsies and one more biopsy, respectively). For IL4 and IL8, the number of positives was the same for the two techniques (the individual concordance levels were 77% and 100%, respectively). For IL1b, IL2, IL5 and GM-CSF, the number of positives was greater for microdialysis. By counting the number of paired findings that were the same, concordance at an individual subject level was found to be highest for IL8 (100%) and lowest for TNFa (50%), with the remaining cytokines distributed between these two values.
DISCUSSION
This comparison of microdialysis (CMD) and IF findings is based on data at one point in time, 24 h. The twinning of microdialysis and skin biopsy analysis in this study, though supportive of the assertion that catheter insertion trauma induces IL1b, IL6 and IL8, did not show complete concordance of findings at an individual level. This is not necessarily surprising, and a consideration of the possible sources of the lack of total concordance can illustrate facts and concepts about the methodologies and their use, the nature of the tissue biology involved, as well as ways in which future studies can use the techniques as complementary to each other in experimental protocols which are designed to facilitate interpretation of data.
Cytokines are proteins produced by cells and act in autocrine, paracrine and endocrine pathways “cell to cell” (1–3). Therefore, the criterion for analysis of the immunohistological findings was intracellular and membrane-bound presence of the cytokine. Demonstration of such in a biopsy constitutes good evidence for the involvement of that cytokine in the tissue process under study. In many studies, IF findings are presented as “stand-alone” findings. The most notable IF findings in the present study were the demonstration of IL1b, IL6 and IL8 reactivity in the dermis in the vicinity of the minimal wound caused by catheter insertion. CMD detects cytokines in the extracellular space of a tissue. The technique’s chief advantage is that observations can be made over the course of a reaction rather than at one point in time. If the 24-h sample were used as a “stand-alone” finding, the most notable feature here would be the finding that the pro-inflammatory cytokines IL1b, IL6 and IL8, though lower than in the first 12 h of the catheter insertion, are still elevated, underlining the long recovery time of the skin’s reactivity to insertion trauma in regard to cytokines.
Detailed comparison of CMD and IF showed that the two techniques had comparable numbers of “positives” at a group level but varying concordance of findings at an individual level. For both CMD and IF, false positivity is unlikely since the techniques have high specificity and since technical routines for the techniques were followed rigorously. Thus, in the comparison of differing results, the emphasis can be on reasons for “false negativity” on the part of one technique compared to the other.
If a particular cytokine is not detected with CMD, there are alternative explanations to be considered apart from the actual non-involvement of the cytokine:
The analytical technique may not be sufficiently sensitive to detect the low levels of cytokines.
For better sensitivity, the volume of the sample could be increased or the perfusion speed reduced. Also high-sensitivity bead arrays would allow these cytokines to be detected at sub-picogram concentrations (33).
-
2.
All secreted cytokines are bound to their cognate membrane receptors.
The CMD only samples secreted unbound protein in the extracellular space. Thus, histological techniques are required for the detection of cytokines bound to cellular membrane surfaces and intracellular stores.
-
3.
There may be sufficient release of cytokines into the extracellular space, but the cytokine is consumed by regulatory processes.
Cytokine binding is a complex issue. Several cytokines such as IL1, IL2, IL4, IL6, TNFa and IFNg have soluble binding proteins which function as physiological regulators of cytokine activities. The expression of most cytokines is tightly regulated at many levels and influenced by a balance of signals (34). Interpretation of findings will need to be done on a case-by-case basis based on a detailed knowledge of the cytokine in question.
-
4.
The cytokine is available in extracellular space but the microdialysis membrane or some other component of the catheter system is impermeable for, incompatible with or binds the cytokine.
Characterisation of the interaction of macromolecules with membranes and other catheter components is necessary in this area. As we and others have found, passage of cytokines through the PES membrane is not only dependent upon the molecular weight but is also far more complex and includes the actual structure, charge and other molecule characteristics of the proteins (17,18,25,35). Much of this work is of common interest for all researchers using microdialysis. Use of a commercially available probe, with the regulatory and quality control procedures involved in the individual components and manufacturing, is an advantage in this regard.
When IF findings are negative for a particular cytokine (i.e. no evidence at a cellular cytoplasm or membrane level), there are a number of possible explanations other than the lack of actual participation of the cytokine at this particular time point or that cellular factors released by the insertion trauma do not activate de novo production:
The cytokine in preformed vesicles has been released at an earlier time point and metabolised.
Cellular production of the cytokine is downregulated because the surface receptor of the target cell is internalised, shedded or transmodulated (36–39).
The cytokine may have been blocked from binding to its surface receptor by a receptor antagonist (34).
The actual demonstration of the required morphology was limited by the small number of cells available for assessment in the histological slide. This is particularly problematic in the assessment of blood-derived cells in normal skin since they are present in low numbers (15).
In the here presented findings, IF implicated IFNg, TNFa and IL10 in the tissue reaction more often than the microdialysis findings. The total individual concordance for these same cytokines was 60%, 50% and 66%, respectively. In these cases, it can be speculated that poor sensitivity of the analysis of the microdialysate might be an explanation. Though IL4, IL5 and IL8 were implicated in the same number of subjects for each technology, the individual concordance levels were 77%, 80% and 100%, respectively. Thus, “better” performance was distributed between the two technologies. IL1b and IL6 were positive less often in the biopsy than in the microdialysate, with an individual concordance of 50% and 70%. In these cases, (cellular production may be downregulated but) residual cytokines might still be present in the extracellular space from cellular activity/production at an earlier stage in the reaction chronology and thus detectable at CMD. Alternatively, receptor blocking by an antagonist may have occurred in the IF. We conclude that information from the two technologies is complementary and that availability of both result sets may be an advantage in the interpretation of findings from an experimental situation.
Given the general confirmation of the CMD findings by the IF findings, we also conclude that CMD findings on cytokines in the period up to around 24 h after catheter insertion are legitimately accessible to analysis despite issues under discussion as to the tissue reactivity to the catheter and events after insertion which may compromise catheter function (19,22,40). Initial histamine release and axon reflex-mediated hyperaemia can be accommodated for by the observance of an “equilibration period” in the study protocol (11,12). The “equilibration period” for IL1b, IL6 and IL8 (and of course other as yet not studied molecules involved in the process) needs to be of the order 12–24 h to allow for the return of cytokine levels to a level from which possibly significant elevations can be discerned. Blood flow is the classical variable in microdialysis, especially for small molecules for which modelling/normalisation of data needs to be considered. For larger molecules like cytokines, blood flow is probably less important, although the question is as yet not fully elucidated. After the short-term changes (17), deposition of proteinaceous material on the catheter membrane referred to as “biofouling” (19,22,40) can be suspected of causing changes in catheter membrane performance. Such deposition is reported to occur within the 24-h time frame of this experiment (40), but our findings indicate that they do not necessarily preclude an attempt at interpretation of findings. Other tissue reactivity forms such as encapsulation and foreign body responses may occur in catheters present in the tissue for longer periods (days/weeks) (41,42). In every experimental situation, matters such as these need consideration on a case-by-case basis in regard to, e.g. the specific membrane, the perfusate, the tissue and the length of the experiment (18).
Tissue reactivity to insertion trauma can be considered at another more positive level in microdialysis studies. We have suggested (18) that the skin reaction to the mechanical trauma of catheter insertion can be seen as an innate immune response of inherent protective and regenerative value. As such, the reactivity which introduces challenges in the performance and interpretation of CMD also introduces a potential “bonus” in that reactivity may be individually variable and thus describe the “phenotype” of the subject under study. Age may be an example of a relatively benign source of reduction in reactivity to various non-microbial inductions of innate reactivity (e.g. UVB, irritants). Even common disease entities like psoriasis and atopic eczema are widely considered to reflect varying innate reactivity levels (43–47). A more uncommon example of variant reactivity to minimal trauma in disease is the pathergy reaction to minimal trauma seen in Behçet’s disease and some other inflammatory diseases (48). Whilst insertion of a microdialysis catheter is more complicated than necessary for the directed study of reactivity to minimal trauma (49), it should be noted that CMD can be viewed as involving a similar minimal skin trauma—but with dermal reactivity isolated from epidermal reactivity. The point we wish to make is that the insertion trauma of the catheter insertion is not merely a troublesome event needing consideration in the design of the research protocol, but also a potential built in provocation of innate functional reactivity in the individual skin under study. This can enhance the experimental design by better classification of the participating subject’s phenotype and, in some scenarios centering on innate immunity, actually be a part of the research scenario.
The 24-h period studied in this paper is an earlier period than the 3- to 10-day time points which gave rise to the discussion of biofouling and encapsulation, which might question the reliability or the relevance of microdialysis data gathered particularly on cytokines, chemokines and growth factors. We have demonstrated that total protein levels in microdialysates are still elevated at 24 h, which we believe can be interpreted as evidence for the functionality of the membrane. The relatively good agreement at an individual subject level between CMD and IF findings supports this contention. Differences when seen should be considered, in the first instance, as the possible result of different methodological problems and then as the result of actual differences in tissue biology suiting one methodology more than the other. The present experimental protocol (chronological microdialysis study followed by end point biopsy at around 24 h) is attractive in human research since it has the potential to give the following points of information in a studied individual: presence of extracellular cytokines in the skin prior at the time of catheter insertion; the nature and degree of the innate response to minimal trauma which the catheter insertion constitutes; the presence and level of cytokines in the recovery phase of the insertion trauma response; the presence and level of cytokines “post-equilibration”; and actual presence of cytokine at a cellular level at “end point” biopsy at 24 h. Interpretation of cytokine data at a later time than 24 h may well require greater consideration of biofouling, encapsulation and other longer term consequences of the microdialysis catheter insertion into the tissue.
CONCLUSION
Histology has, for centuries, been the methodological workhorse in extending knowledge on tissue biology in health and disease. Particularly, IF has extended our detailed knowledge of tissue processes at a particular point in time. CMD has only around two decades of history. Tracking change is the strong point of microdialysis technique. It can demonstrate dynamic processes in a tissue over a period of time and is less invasive than repeated biopsies. Insertion of a microdialysis catheter, though well tolerated, is still a minimally invasive event, the effects of which need to be accommodated for in protocols if experimental results particularly on cytokines are to be interpretable. The study of the period up to 24 h after catheter insertion in normal (uninvolved) skin shows that IL6, IL8 and IL1b concentrations, though lower than at earlier time points, are still elevated. This means that in studies of provoked or diseased skin, higher levels than those reported here would need to be seen to infer participation of a particular cytokine in a studied tissue process at a particular time point. At the same time, seven other here studied cytokines show no or relatively low levels at the 24-h time point—deducing involvement in a provoked or diseased tissue process would thus be easier for these cytokines. For other inflammatory or homeostatic mediators not here studied, the chronology of the effects of catheter insertion into normal skin would need to be established. In future translational and clinical studies of the complex areas of homeostatic, reactive or pathological tissue processes, the combination of microdialysis and “end point” biopsy can be predicted to have the ability to produce complementary data sets relevant for the understanding of disease processes at an individual and group level.
Acknowledgements
The authors would like to express their appreciation to Emil Axelsson for stimulating discussions and advice concerning confocal microscopy and Karin Davidsson for discussion in the preparation of the final text. This work has been supported by the Swedish Psoriasis Foundation.
References
- 1.Clark R, Kupper T. Old meets new: the interaction between innate and adaptive immunity. J Invest Dermatol. 2005;125:629–37. doi: 10.1111/j.0022-202X.2005.23856.x. [DOI] [PubMed] [Google Scholar]
- 2.Janeway C, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 3.Janeway C, Travers P, Walport M, Shlomchik M, editors. Immunobiology 5. New York: Garland; 2001.
- 4.Mariathasan S, Monack D. Inflammasome adaptors and sensors: intracellular regulators of infection and inflammation. Nat Rev Immunol. 2007;7:31–40. doi: 10.1038/nri1997. [DOI] [PubMed] [Google Scholar]
- 5.Yamasaki K, Muot J, Taylor K, Cogen A, Audish D, Bertin J, et al. NLRP3/cryopyrin is necessary for interleukin-1b (IL-1b) release in response to hyaluronan, an endogenous trigger of inflammation in response to injury. J Biol Chem. 2009;284:12762–71. doi: 10.1074/jbc.M806084200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anderson C, Svensson C, Sjögren F, Andersson T, Wårdell K. Human in vivo microdialysis technique can be used to measure cytokines in contact reactions. Curr Probl Dermatol. 1995;23:121–30. doi: 10.1159/000424307. [DOI] [PubMed] [Google Scholar]
- 7.Angst M, Clark J, Carvalho B, Tingle M, Schmelz M, Yeomans D. Cytokine profile in human skin in response to experimental inflammation, noxious stimulation, and adminsitration of a COX-inhibitor: a microdialysis study. Pain. 2008;139:15–27. doi: 10.1016/j.pain.2008.02.028. [DOI] [PubMed] [Google Scholar]
- 8.Averbeck B, Beilharz S, Bauer M, Gebhardt C, Hochleitner K, Kauer F, et al. In situ profiling and quantification of cytokines released during ultraviolet B induced inflammation by combining dermal microdialysis and protein microarrays. Exp Dermatol. 2006;15:447–54. doi: 10.1111/j.0906-6705.2006.00429.x. [DOI] [PubMed] [Google Scholar]
- 9.Clough G, Jackson C, Lee J, Jamal S, Church M. What can microdialysis tell us about the temporal and spatial generation of cytokines in allergen induced responses in human skin in vivo. J Invest Dermatol. 2007;234:443. doi: 10.1038/sj.jid.5700930. [DOI] [PubMed] [Google Scholar]
- 10.Sjögren F, Svensson C, Anderson C. Technical prerequisites for in vivo microdialysis determination of interleukin-6 in human dermis. Br J Dermatol. 2002;146:375–82. [PubMed] [Google Scholar]
- 11.Anderson C. Cutaneous microdialysis: is it worth the sweat? J Invest Dermatol. 2006;126:1207–9. doi: 10.1038/sj.jid.5700221. [DOI] [PubMed] [Google Scholar]
- 12.Anderson C, Andersson T, Andersson R. In-vivo microdialysis estimation of histamine in human skin. Skin Pharmacol. 1992;5:177–83. doi: 10.1159/000211035. [DOI] [PubMed] [Google Scholar]
- 13.Anderson C, Andersson T, Wårdell K. Changes in skin circulation after insertion of a microdialysis probe visualized by laser Doppler perfusion imaging. J Invest Dermatol. 1994;102:807–11. doi: 10.1111/1523-1747.ep12378630. [DOI] [PubMed] [Google Scholar]
- 14.Andersson T, Svensson C, Anderson C. The effect of probe depth on histamine levels in human in vivo cutaneous microdialysis. Skin Res Technol. 1996;2:23–26. doi: 10.1111/j.1600-0846.1996.tb00050.x. [DOI] [PubMed] [Google Scholar]
- 15.Groth L, Serup J. Cutaneous microdialysis in man: effects of needle insertion trauma and anaesthesia on skin perfusion, erythema and skin thickness. Acta Derm Venereol. 1998;78:5–9. doi: 10.1080/000155598441855. [DOI] [PubMed] [Google Scholar]
- 16.Krogstad A, Jansson P, Gisslèn P, Lönnroth P. Microdialysis methodology for the measurment of dermal interstitial fluid in humans. Br J Dermatol. 1996;134:1005–12. doi: 10.1111/j.1365-2133.1996.tb07934.x. [DOI] [PubMed] [Google Scholar]
- 17.Bungay P, Newton-Vinson P, Garris P, Justice J. Microdialysis of dopamine interpreted with quantitative model incorporating probe implantation trauma. J Neurochem. 2003;86:932–46. doi: 10.1046/j.1471-4159.2003.01904.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clough G. Microdialysis of large molecules. AAPS J. 2005;7:E686–E92. doi: 10.1208/aapsj070369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sjögren F, Anderson C. Sterile trauma to normal human dermis invariably induces IL1ß, IL6 and IL8 in an innate response to “danger”. Act Dermatol Venereol. 2009;89:459–65. doi: 10.2340/00015555-0683. [DOI] [PubMed] [Google Scholar]
- 20.Stenken J, Church M, Gill C, Clough G. How minimally invasive is microdialysis sampling? A cautionary note for cytokine collection in human skin and other clinical studies. AAPS J. 2010;12:73–8. doi: 10.1208/s12248-009-9163-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bungay P, Morrison P, Dedrick R. Steady-state theory for quantitative microdialysis of solutes and water in vivo and in vitro. Life Sci. 1990;46:105–19. doi: 10.1016/0024-3205(90)90043-Q. [DOI] [PubMed] [Google Scholar]
- 22.Elmquist W, Sawchuk R. Application of microdialysis in pharmacokinetic studies. Pharm Res. 1997;14:267–88. doi: 10.1023/A:1012081501464. [DOI] [PubMed] [Google Scholar]
- 23.Helmy A, Carpenter K, Skeppar J, Kirkpatrick P, Pickard J, Hutchinson P. Microdialysis of cytokines: methodological considerations, scanning electron microscopy and determination of relative recovery. J Neurotrauma. 2009;26:549–61. doi: 10.1089/neu.2008.0719. [DOI] [PubMed] [Google Scholar]
- 24.Sun H, Bungay P, Elmquist W. Effect of capillary efflux transport inhibition on the determination of probe recovery during in vivo microdialysis in the brain. J Pharmacol Exp Ther. 2001;297:991–1000. [PubMed] [Google Scholar]
- 25.Tang A, Bungay P, Gonzales R. Characterization of probe and issue factors that influence interpretation of quantitative microdialysis experiments for dopamine. J Neurosci Methods. 2003;126:1–11. doi: 10.1016/S0165-0270(03)00003-7. [DOI] [PubMed] [Google Scholar]
- 26.Sjögren F. Dermal cell trafficking: from microscopy to microdialysis. Medical dissertation, Linköping; 2005
- 27.Fulwyler M, McHugh T. Flow microsphere immunoassay for the quantitative and simultaneous detection of multiple soluble analytes. Methods Cell Biol. 1990;33:613–29. doi: 10.1016/S0091-679X(08)60556-7. [DOI] [PubMed] [Google Scholar]
- 28.Huang W, Gibson S, Facer P, Gu J, Polak J. Improved section adhesion for immunocytochemistry using high molecular weight polymers of l-lysine as a slide coating. Histochemistry. 1983;77:275–79. doi: 10.1007/BF00506570. [DOI] [PubMed] [Google Scholar]
- 29.Andersson J, Abrams J, Björk L, Funa M, Litton M, Ågren K, et al. Concomitant in vivo production of 19 different cytokines in human tonsils. Immunol. 1994;83:16–24. [PMC free article] [PubMed] [Google Scholar]
- 30.Sander B, Andersson J, Andersson U. Assessment of cytokines by immunofluorescence and the paraformaldehyde-saponin procedure. Immunol Rev. 1991;119:65–93. doi: 10.1111/j.1600-065X.1991.tb00578.x. [DOI] [PubMed] [Google Scholar]
- 31.Nickoloff B, Karabin G, Barker J, Griffiths C, Sarma V, Mitra R, et al. Cellular localization of interleukin-8 and its inducer, tumor necrosis factor-alpha in psoriasis. Am J Pathol. 1991;138:129–40. [PMC free article] [PubMed] [Google Scholar]
- 32.Paquet P, Piérard G. Interleukin-6 and the skin. Inter Arch Allergy Immunol. 1996;109:308–17. doi: 10.1159/000237257. [DOI] [PubMed] [Google Scholar]
- 33.Uyemura K, Yamamura M, Fivenson D, Modlin R, Nickoloff B. The cytokine network in lesional and lesion-free psoriatic skin is characterized by a T-helper type 1 cell-mediated response. J Invest Dermatol. 1993;101:701–5. doi: 10.1111/1523-1747.ep12371679. [DOI] [PubMed] [Google Scholar]
- 34.Gu Y, Zeleniuch-Jacquotte A, Linkov F, Koenig K, Liu M, Velikokhatnaya L, et al. Reproducibilty of serum cytokines and growth factors. Cytokine. 2009;45:44–49. doi: 10.1016/j.cyto.2008.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ibelgaufts H. Cytokines online pathfinder encyclopaedia. 2003. www.copewithcytokines.de.
- 36.Ao X, Wang X, Lennartz M, Loegering D, Stenken J. Multiplexed cytokine detection in microliter microdialysis samples obtained from activated cutured macrophages. J Pharm Biomed Anal. 2006;40:915–21. doi: 10.1016/j.jpba.2005.07.044. [DOI] [PubMed] [Google Scholar]
- 37.Gasson J. Molecular physiology of granulocyte–macrophage colony-stimulating factor. Blood. 1991;77:1131–45. [PubMed] [Google Scholar]
- 38.Heinrich P, Behrmann I, Haan H, Muller-Newen G, Schaper F. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem J. 2003;374:1–20. doi: 10.1042/BJ20030407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.LeRoy C, Wrana J. Clathrin- and non-clathrin-mediated endocytic regulation of cell signalling. Nat Rev Molecular Biol. 2005;6:112–26. doi: 10.1038/nrm1571. [DOI] [PubMed] [Google Scholar]
- 40.Weber A, Wasiliew P, Kracht M. Interleukin-1 (IL1) pathway. Sci Signaling. 2010;3:1–8. doi: 10.1126/scisignal.3105cm1. [DOI] [PubMed] [Google Scholar]
- 41.Wisniewski N, Klitzman B, Miller B, Reichert W. Decreased analyte transport through implanted membranes: differentiation of biofouling from tissue effects. J Biomed Mater Res. 2001;57:513–21. doi: 10.1002/1097-4636(20011215)57:4<513::AID-JBM1197>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 42.Anderson J, Rodriguez A, Chang D. Foreign body reaction to biomaterials. Sem Immunol. 2008;20:86–100. doi: 10.1016/j.smim.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Clark H, Barbari T, Stump K, Rao G. Histologic evaluation of the inflammatory response around implanted hollow fiber membranes. J Biomed Mater Res. 2000;52:183–92. doi: 10.1002/1097-4636(200010)52:1<183::AID-JBM24>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 44.Bos J. Psoriasis, innate immunity and gene pools. J Am Acad Dermatol. 2007;56:468–71. doi: 10.1016/j.jaad.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 45.Gaspari A. Innate and adaptive immunity and the pathophysiology of psoriasis. J Am Acad Dermatol. 2006;54:S67–80. doi: 10.1016/j.jaad.2005.10.057. [DOI] [PubMed] [Google Scholar]
- 46.Lowes M, Lew W, Krueger J. Current concepts in the immunopathogenesis of psoriasis. Dermatol Clin. 2004;22:349–69. doi: 10.1016/j.det.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 47.Nickoloff B. Skin innate immune system in psoriasis: friend or foe. J Clin Invest. 1999;104:1161–64. doi: 10.1172/JCI8633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Varol A, Seifert O, Anderson C. The skin pathergy test: innately useful? AODR. 2010;302:155–68. doi: 10.1007/s00403-009-1008-9. [DOI] [PubMed] [Google Scholar]
- 49.Varol A, Anderson C. A minimally invasive human in vivo cutaneous wound model for the evaluation of normal wound healing status and topical wound therapy. AODR. 2010;302:383–93. doi: 10.1007/s00403-010-1043-6. [DOI] [PubMed] [Google Scholar]