Abstract
In this study, we examined the effect of various factors on gene delivery efficiency of tail vein injection of plasmid DNA into rats. We measured the level of reporter gene expression in the internal organs including the lung, heart, spleen, kidney, and liver as function of injection volume, injection time, and DNA dose. Persistency of reporter gene expression in transfected animals was also examined. We demonstrated that plasmid delivery to rats by the tail vein is effective as long as the volume of injected DNA solution is adjusted to 7–8% of body weight with an injection time of less than 10 s. With the exception of a short-term increase in serum concentration of alanine aminotransferase and transient irregularity in cardiac function during and soon after the injection, the procedure is well tolerated. Lac Z staining of the liver from transfected animals showed approximately 5–10% positive cells. Persistency test for transgene expression in animals using plasmid carrying cDNA of human alpha 1 antitrypsin gene driven by chicken beta actin gene promoter with CMV enhancers showed peak level of transgene product 1 day after the injection followed by a gradual decline with time. Peak level was regained by a second injection performed on day 38 after the first injection. These results show that tail vein injection is an effective means for introducing plasmid DNA into liver cells in rats. We believe that this procedure will be extremely useful for gene function studies in the context of whole animal in rats.
Key words: gene delivery, gene therapy, hydrodynamic gene delivery, nonviral vectors, siRNA delivery
INTRODUCTION
Compared to mice that are more commonly used as an animal model for monogenic diseases, rats have been an excellent animal model for complex diseases including diabetes, cardio disease, obesity, and hypertension (1). Understanding the molecular basis of these human diseases and elucidating the physiological function of the genes involved have become a rate-limiting step in the post-genome era. To define the physiological function of a genomic sequence, one will have to analyze the changes in the context of whole animals by overexpressing or depleting the product of the candidate gene. A crucial element for gene function study is the development of a safe and effective method to introduce gene-coding plasmids or siRNA to cells in vivo.
Both viral and nonviral approaches have been explored for gene delivery in the past (2,3). Among the methods developed so far, the procedure of hydrodynamic gene delivery has received significant attention in recent years because of its simplicity, convenience, safety, and effectiveness for in vivo gene delivery (4,5). It was demonstrated in mice that both macromolecules such as DNA, RNA, and proteins and small molecules can be delivered to hepatocytes through a simple injection into the tail vein (6,7). In gene therapy studies, hydrodynamic delivery of plasmid DNA has generated gene product at the therapeutic level and resulted in complete cure of diseases (8–11). By combining the technique of image-guided catheter insertion with a computer-controlled injection device (12), we have demonstrated successful gene delivery to cells in the liver (13) and skeletal muscle (14) in pigs, suggesting that site-specific gene delivery can be achieved in large animals and it is feasible to apply hydrodynamic gene delivery to treatment of human diseases.
Despite its wide use in mice for gene therapy studies (6,7) and its potential for human gene therapy, only a limited number of reports have appeared in the literature applying hydrodynamic delivery to gene transfer in rats. Among many possible reasons responsible for lack of common use of the procedure for gene transfer studies in rats, lack of a systematic study to provide the detailed experimental condition and lack of thorough evaluation on the overall impact of the procedure on rats are two most likely reasons. To fill such a gap, we have taken a systematic approach to characterize various parameters affecting hydrodynamic gene delivery efficiency and the level of transgene expression using Sprague Dawley rats as a model. We demonstrated that the volume of DNA solution injected, rate of injection, and DNA dose are critical parameters that determine gene delivery efficiency and the overall level of transgene expression in rats.
MATERIAL AND METHODS
Materials
Plasmids of pCMV-Luc containing firefly luciferase cDNA driven by CMV promoter, pCB-hAAT containing cDNA of human alpha 1 antitrypsin gene driven by chicken beta actin promoter with CMV enhancers, and pCMV-Lac Z containing β-galactosidase gene under the control of CMV promoter were purified by the method of CsCl ethidium bromide gradient centrifugation and kept in saline at −20°C until use. The purity of the plasmid preparations was checked by absorbency at 260 and 280 nm and 1% agarose gel electrophoresis. The kits for luciferase assay were purchased from Promega. The reagents used for histochemical analysis of β-galactosidase gene expression were from Sigma. Sprague Dawley rats (female, 60–420 g) were purchased from Charles River.
In Vivo Gene Delivery and Assessment of Reporter Gene Expression
All animal experiments were conducted in full compliance with regulation and approved by the Institutional Animal Care and Use Committee at the University of Pittsburgh, Pittsburgh, Pennsylvania. Rats were injected with plasmid DNA in saline at the indicated volume, injection time, and concentration of plasmid DNA via the tail vein. Appropriate size syringes and 19–23 gauge needle were used depending on the injection volume and animal weight. Unless otherwise mentioned, animals were sacrificed 9 h post-injection of plasmid DNA. Different organs including the lung, heart, spleen, kidney, and liver were dissected from animals, and small pieces of tissue (~200 mg) were cut from each organ and placed into a test tube containing 1 ml of lysis buffer (0.1 M Tris–HCl, 2 mM EDTA, and 0.1% Triton X-100, pH 7.8). Tissue samples were homogenized for 30 s with the Tissue Tearor at maximal speed, and the tissue homogenates were centrifuged in a microcentrifuge for 10 min at 13,000×g at 4°C. Protein concentration of the supernatant was determined by using Coomassie Blue Plus Protein assay kit (Bio-Rad, CA). For luciferase assay of the liver extract, the supernatant was further diluted tenfold using HEPES buffer. Ten microliters of supernatant of lung, heart, spleen, and kidney homogenates and diluted liver extract were mixed with 100 μl of luciferase assay reagent, and the luciferase activity was measured in a luminometer (AutoLumant LB 953, EG&G, Salem, MA) for 10 s. Luciferase activity obtained as the relative light units (RLU) was converted to luciferase protein amount using standard curve established using reagents and procedure from Analytic Luminescence Laboratory (ALL, San Diego, CA). The amount of luciferase protein was calculated using the equation derived from the standard curve in which luciferase protein (pg) = 7.98 × 10–5 RLU + 0.093 (R2 = 0.9999).
X-gal Staining
The level of Lac Z gene expression in the internal organs including the lung, heart, spleen, kidney, and liver was determined by X-gal staining method according to previous study (4). In brief, animals were sacrificed 9 h after hydrodynamic injection of pCMV-Lac Z plasmid (10 μg/ml); organs were removed and frozen immediately on dry ice. Tissue sections (10 μm thick) were made using a cryostat and fixed with 1% glutaraldehyde solution. Slides were stained for 4 h at room temperature in 400 μg/ml X-gal solution and counterstained with eosin.
Electrocardiogram
The electrocardiogram (ECG) was examined by the BIOPAC 100C ECG detection system (Santa Barbara, CA), with the Monopol needle electrodes (EL452, 15 mm TP) inserted subcutaneously into the chest area of anesthetized rats according to the user’s manual. The ECG waves were recorded and processed using the Acqcknowledge software before, during, and after hydrodynamic injection.
Microscopic Analysis
Animals with hydrodynamic treatment were anesthetized 5 min after the procedure, and a midsection was made to expose the peritoneal cavity and the internal organs. Twenty milliliters of glutaraldehyde solution (2.5%) in phosphate-buffered saline (PBS) was infused through the inferior vena cava (IVC) of treated and untreated (control) animals, and the liver was removed immediately after. Small pieces of liver samples were made and kept in the same fixative solution overnight for scanning electron microscopy (SEM) or 3 days for transmission electron microscopy (TEM). For light microscopic examination, thin sections were made, heated onto glass slides, stained with filtered 0.5% aqueous toluidine blue, and rinsed with water. For SEM, fixed liver samples were further processed according to Suda et al. (15). The samples were sputter-coated with 3.5 nm gold/palladium and viewed under a JEOL JSM-6330F scanning electron microscope, and the images were taken with the interactive Quartz PCI image system.
Determination of Serum Concentration of ALT
Blood samples were collected from each rat at 1, 3, 24, 48, 72, and 120 h after hydrodynamic injection. The IDEXX VetTest Chemistry analyzer (Westbrook, ME) was utilized for automated analysis of serum alanine transaminase (ALT) level.
Determination of Serum Concentration of hAAT by ELISA
hAAT concentration was determined by enzyme-linked immunosorbent assay (ELISA) according to previously established procedure (16). In brief, serum samples were collected at appropriate time and diluted serially with 1% BSA in PBS–Tween buffer before a standard ELISA was performed. A rabbit anti-hAAT polyclonal antibody was used for plate coating, and the biotinylated goat anti-hAAT polyclonal antibody was used as the second antibody for binding to hAAT that bound to the well. Streptavidin horseradish peroxidase conjugates and 3,3′,5,5′-tetramethylbenzidine were employed for quantitative measurement at 450 nm in an ELISA reader. The hAAT concentration was calculated based on the standard curve established using pure hAAT. The concentration range for the standard curve was 1–32 ng/ml.
RESULTS
Effect of Hydrodynamic Parameters on Gene Delivery Efficiency
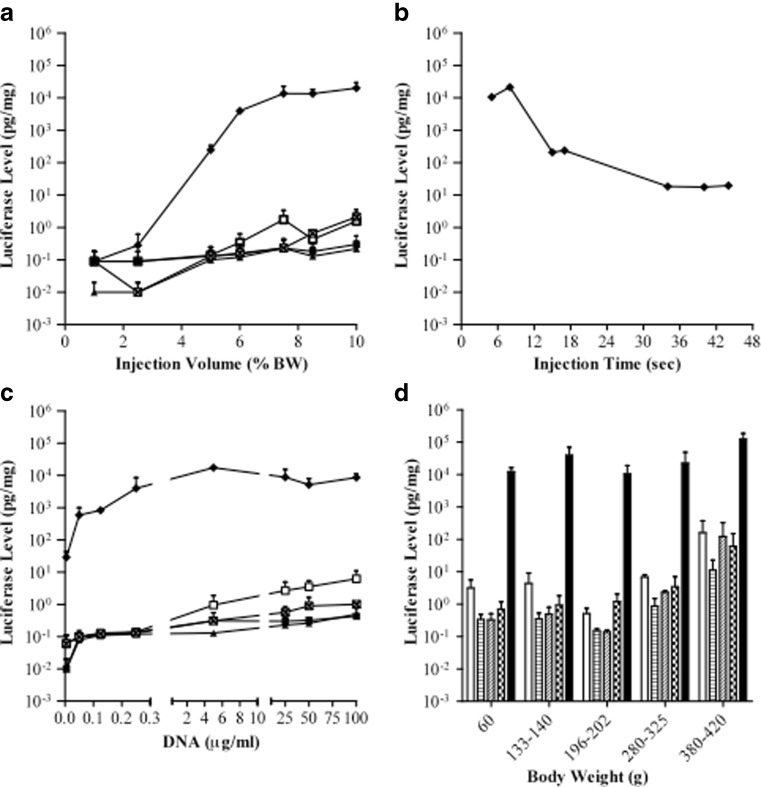
The relationships between the gene delivery efficiency and injection volume, injection time at a fixed volume, and DNA amount were established using luciferase gene as a reporter. Animals (60–80 g) were injected with pCMV-Luc plasmids in saline from the tail vein and sacrificed 9 h later for assessment of luciferase gene expression in various organs including the lung, heart, spleen, kidney, and liver. The effect of injection volume on gene delivery efficiency is shown in Fig. 1a. The level of luciferase gene expression in all organs examined increases when increasing injection volume, and the maximal gene expression was obtained at the volume equal to 7–8% of the body weight (BW). The highest level of luciferase gene expression was seen in the liver at 15 ng/mg of extracted proteins, 10,000-fold higher than that in other organs.
Fig. 1.
Parameters affecting the level of reporter gene expression. Three groups of rat were intravenously injected from the tail vein with pCMV-Luc plasmid DNA at a various injection volume, b injection time with fixed volume at 7.5% BW, and c different concentration of plasmid DNA (volume equal to 7.5% BW, injection time, 7 s). DNA concentration for experiments in a, b, and d was 10 μg/ml. Symbols used in a–c are lung □, heart ■, spleen ▲, kidney  , and liver ♦ The effect of animal weight on the level of reporter gene expression (d) was examined using injection volume of 7.5% BW and injection time of 7–10 s. Luciferase protein in the lung (
, and liver ♦ The effect of animal weight on the level of reporter gene expression (d) was examined using injection volume of 7.5% BW and injection time of 7–10 s. Luciferase protein in the lung ( ), heart (
), heart ( ), spleen (
), spleen ( ), kidney (
), kidney ( ), and liver (
), and liver ( ) was determined 9 h post-DNA injection. Error bar represents the SD of mean from three animals
) was determined 9 h post-DNA injection. Error bar represents the SD of mean from three animals
The effect of injection time on gene delivery efficiency to the liver was examined in 60–80 g rats using injection volume equal to 7.5% BW, and the results are shown in Fig. 1b. A rapid injection (5 s) generated 21 ng of luciferase protein per milligram of liver proteins, 1,200-fold higher than an injection in 40 s. These results suggest that an injection of 7.5% BW in 8 s or less is optimal for gene delivery to rat liver via the tail vein.
Dose–response curve was established using the injection volume of 7.5% BW in 7 s. It is evident in Fig. 1c that significant level of reporter gene expression was achieved at DNA concentration of 0.05 μg/ml. The maximal luciferase gene expression was achieved at about 5 μg/ml or greater. No further increase in luciferase gene expression was seen with DNA concentration greater than 5 μg/ml, suggesting that the transcription/translation machinery in transfected liver cells reached saturation for reporter gene expression.
The same procedure was performed to examine whether the optimal parameters differ in animals with different body weights ranging from 60 to 420 g. With an injection volume fixed at 7.5% BW and injection time of 7–10 s, hydrodynamic injection of pCMV-Luc plasmids (10 μg/ml) resulted in similar level of luciferase gene expression in the liver among the groups of animals with different body weights (Fig. 1d). These results suggest that the optimal parameters derived from Fig. 1a–c are generally applicable to rats of different ages.
Histochemical Study of Reporter Gene Expression in Different Organs
The relative number of transfected cells in different organs was examined by employing histochemical analysis using Lac Z gene as the reporter. Nine hours after transfection using optimal condition (injection volume, 7.5% BW; injection time, 7 s; DNA concentration, 10 μg/ml), animals were sacrificed, organs collected, and tissue sections made and stained with X-gal solution. Photographs in Fig. 2 show a few Lac-Z-positive cells in the lung (arrowheads), none in the spleen, heart, and kidney, and approximately 5–10% positive cells in the liver.
Fig. 2.
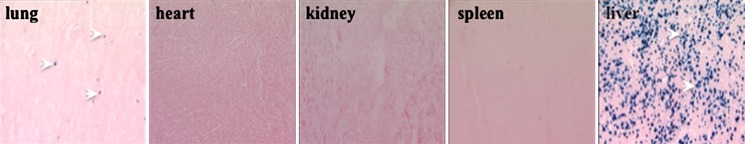
Histochemical analysis of β-galactosidase gene expression in different organs. pCMV-Lac Z plasmid DNA was injected into each rat via the tail vein using conditions employed in Fig. 1d. β-Galactosidase gene expression was assayed 9 h post-injection. Blue dots represent the cells transfected with β-galactosidase gene (magnification, ×50)
Impact of Hydrodynamic Injection on Cardiac Function
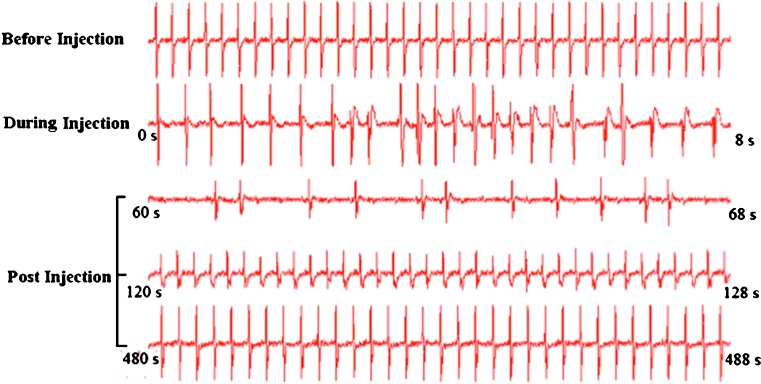
Impact of rapid tail vein injection of DNA solution on cardiac activity of the animals was evaluated by monitoring ECG before, during, and soon after the procedure. Figure 3 clearly shows immediate impact on cardiac function upon injection. Decrease in heart rate (HR) and weak QRS wave were seen during the injection. HR decreased to almost 25% of normal rate 1 min after the injection. However, ECG showed recovery at 2 min, and almost normal ECG was seen 8 min after the injection. These results suggest that the impact of hydrodynamic injection on cardiac function is transient.
Fig. 3.
Impact of hydrodynamic injection on cardiac function. ECG was recorded in an anesthetized animal using Biopack 100C system. Eight-second fragments of spectrum recorded before, during, and after hydrodynamic injection are shown
Assessment of Hydrodynamic Impact on Liver Structure and Tissue Damage
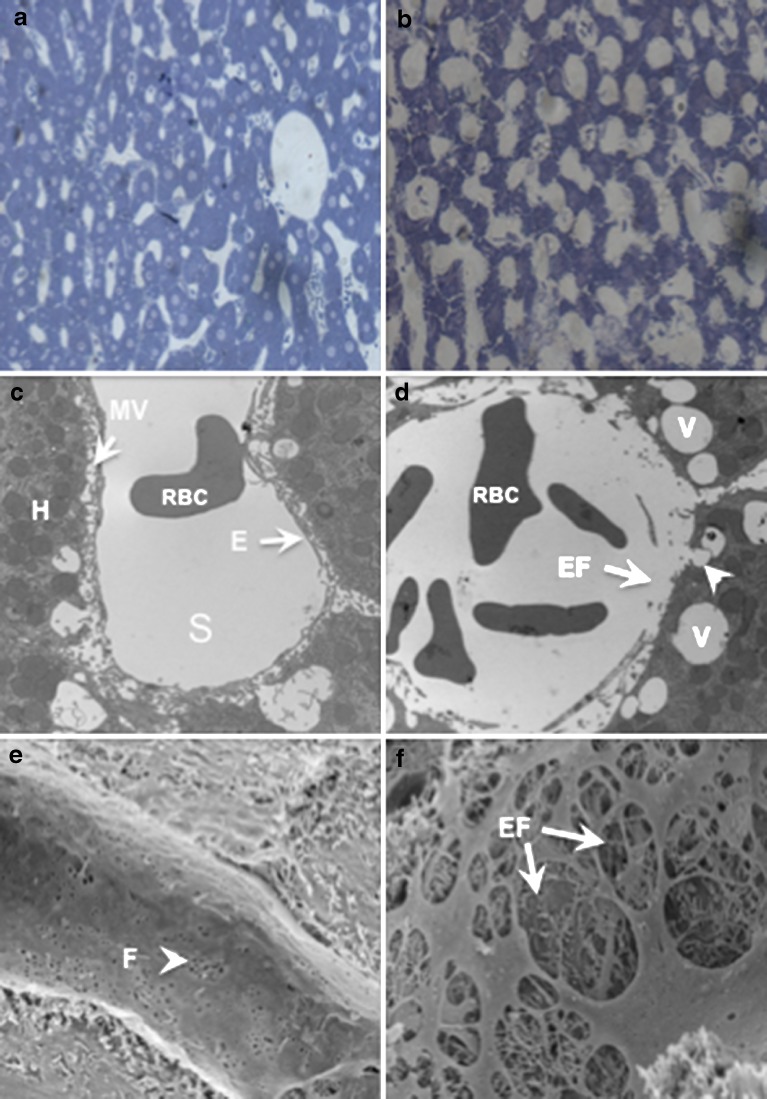
Microscopy and serum biochemistry tests were performed to assess the impact of the procedure on the liver. Toluidine blue staining showed fully expanded blood vessels in hydrodynamically treated animal (Fig. 4b) compared to those in control animal (Fig. 4a). Further examinations were performed using electron microscopy. TEM shows a perfect lining of a sinusoid by endothelia cells in untreated animal (Fig. 4c). However, image from hydrodynamically treated animal (Fig. 4d) showed disruption of the endothelium and enlargement of the fenestrae. In addition, large number of vesicle-like structures was seen. These vesicle-like structures appear to be initiated from the lumen side of plasma membrane of hepatocytes as a half-closed vesicle is seen (arrowhead in Fig. 4d). SEM reveals perfect lining of endothelium with fenestrae readily seen (Fig. 4e). However, enlargement of fenestrae (endothelial disruption) can been seen in hydrodynamically treated animals (Fig. 4f). These changes recovered in a few days after the injection (data not shown). These results suggest that hydrodynamic injection induced structural changes in endothelium of the sinusoids and hepatocytes of the liver
Fig. 4.
Microscopic examination for the impact of hydrodynamic injection on rat liver. Micrographs a and b show the liver structure from a control and hydrodynamically treated rat under a light microscope. Micrograph c shows the TEM of liver sinusoid (S) with endothelia lining (E) and hepatocyte (H) with microvilla (MV) facing the lumen. Micrograph d shows enlarged fenestrae (EF with arrow) and intracellular vesicles (v) near (arrow) and inside the hepatocytes after hydrodynamic treatment. Micrograph e shows sinusoidal structure of the control liver without hydrodynamic injection (F fenestrae). Micrograph f shows the enlarged fenestrae (EF) in the sinusoid of the liver which has undergone hydrodynamic injection. Hydrodynamic treatment involves injection of volume equal to 7.5% BW in 7–10 s. Liver perfusion with fixative was performed 5 min after the injection. Liver sections were prepared and examined according to procedure described in “MATERIALS AND METHODS” section. Magnification, ×100 for a and b; ×10,000 for c, d, e, and f
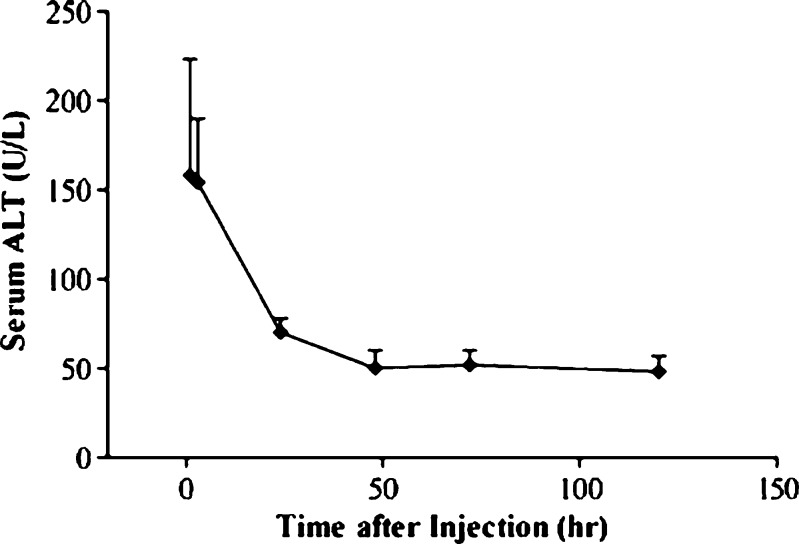
The potential liver damage of the hydrodynamic delivery on animals was assessed. Figure 5 shows elevation of ALT concentration in serum, peaking at 150–200 U/l in 1 day and declining thereafter. The ALT level returned to normal range in 3 days after the injection. These results suggest that hydrodynamic procedure induced transient liver damage.
Fig. 5.
Effect of hydrodynamic gene delivery on serum concentration of alanine aminotransferase. Each animal was injected with pCMV-Luc DNA (10 μg/ml) at the volume equal to 7.5% BW in 7 s. Blood samples were collected from the tail vein before and at the desirable time after the injection. ALT concentration in the blood was determined using the IDEXX VetTest Chemistry Analyzer. Data represent mean (SD) (n = 5)
Persistence of Transgene Expression
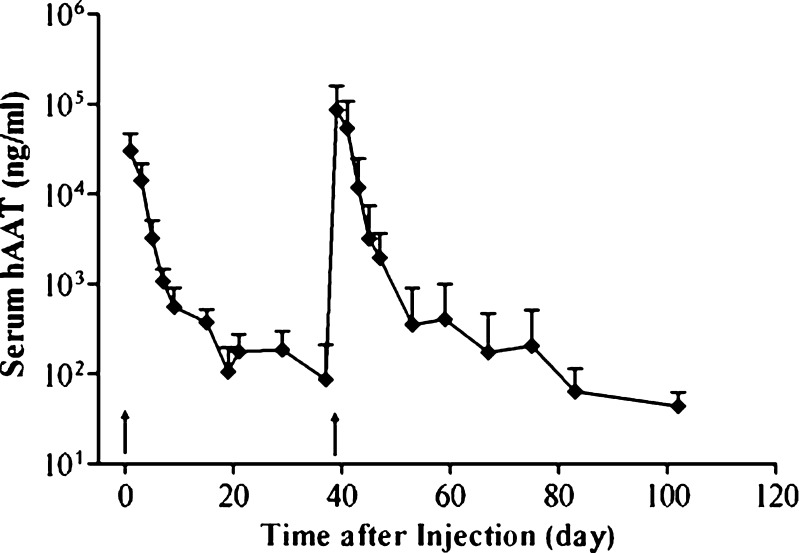
Time course was established to monitor reporter gene expression using human alpha-antitrypsin gene as a reporter. Figure 6 shows peak level of hAAT (30 μg/ml) in blood 1 day after the injection followed by a quick and then a slower decline. The fast decline phase occurred between 1 and 20 days, and the slower phase took place after 10 days. A repeat injection boosted the hAAT level to the peak level, and the pattern of hAAT expression resulting from the second injection was no different from that of the first injection. These results suggest that the procedure can be repeatedly performed on the same animals.
Fig. 6.
Persistence of hAAT gene expression and the effect of repeat injection. Animals were injected with pCB-hAAT (10 μg/ml) in a volume of 7.5% BW in 7 s. Blood samples were collected at different times, and serum concentration of hAAT was determined. The same condition was used for the second injection on day 38 after the first injection. Arrows indicate the time when the injection was performed. Error bars represent SD (n = 5)
DISCUSSION
We demonstrated in this study that hydrodynamic tail vein injection of plasmids is an effective method for gene transfer to cells in rats. The pattern of reporter gene expression achieved and the parameters affecting the delivery efficiency are very similar to those in mice (4,5). For maximal gene expression, one will need to use an injection volume of 7–8% BW and an injection time of 5–10 s. These optimal parameters are slightly different from a previous study using an infusion pump for injection which reported 10% BW as a preferred volume and an injection time of 15 s (17). DNA concentration at about 5 μg/ml or greater is preferred for maximal gene expression. Technically, we have found that animals with a body weight of 60–150 g are preferred because a hand injection of the volume of more than 15 ml is hard to manage. In addition, it is easier to see the tail vein for needle insertion in younger rats.
Large volume of DNA solution and rapid injection are two critical parameters for hydrodynamic gene delivery with the liver exhibiting the highest level of reporter gene expression. Mechanistically, when a large volume of DNA solution is forced into the tail vein that links to IVC, the largest vein that carries the venous blood from the rear part of a rat into the right atrium of the heart, the injected DNA solution causes congestion as the volume in the IVC exceeds the cardiac capacity. Consequently, the DNA solution enters the liver from the hepatic vein in retrograde, enlarges the liver fenestrae, and permeabilizes the cell membrane, and plasmid DNA enters the hepatocytes (18). Other than transient and recoverable shocks to the liver and heart, animals endure the procedure well.
Two alternative procedures for gene delivery to rat liver have been previously explored. Zhang et al. reported a procedure of anterograde regional injection through the portal vein (19). Gene expression was seen in the liver with a direct injection of 3-ml DNA solution into the right lobe. The drawback of this procedure is that it requires a surgical procedure to expose the liver for needle insertion into the portal vein. Obstruction of the outflow to the IVC during the injection was also necessary (19). Another approach that has been explored in the past involves intraportal injection of hypertonic DNA solution to shrink endothelium and increase endothelial permeability (20,21). While gene transfer to the liver was seen, the gene product level achieved was significantly lower compared to the procedure described in this report.
Pattern of reporter gene expression with time between rats and mice (16) is similar. Gene product level reached peak soon after the injection and declined with time. The consentaneous explanation for a rapid decline of gene product level is gene silencing mediated by methylation of CpG motives in the plasmid (22,23). It has been reported that CpG removal from the plasmid prolonged gene expression in mice (22,23). We and others have shown that plasmid DNA in the transfected mouse liver stays in episomal form without integration into genome (16,22,23). Data shown in Fig. 6 suggest that gene expression level can be controlled by repeat injection.
Over 99% of gene expression following a hydrodynamic tail vein delivery of plasmid DNA occurs in the liver. It is commonly believed that such an unbalanced distribution of gene expression among all the internal organs is due to the unique structure of the liver sinusoids (15). Lack of base membrane in liver sinusoids makes the endothelium the only barrier for DNA to reach the parenchyma cells. At cellular level, the fenestrae of the liver sinusoids have a pore size of 100–150 nm (24) that can be enlarged by the hydrodynamic pressure, allowing plasmid DNA to pass through the endothelium and reach the hepatocytes. It is important to point out that the transfection efficiency of 5–10% obtained here in rat liver (Fig. 2) is significantly lower than 30–40% reported previously in mice (4). While additional study is needed to investigate the mechanism underlying the difference in transfection efficiency, it is possible that the difference in liver size, sinusoid structure, composition of plasma membrane of the hepatocytes, and/or the transcription and translation efficiency between rats and mice has significant influence over the amount of gene product generated.
In summary, the significant increase in genomic resources in recent years provides a great opportunity and immediate need to link genes to their function. Defining the physiological functions of a genome sequence requires an analysis in the context of whole animal using a gain- or loss-of-function approach, and appropriate gene transfer procedures are in urgent need. We demonstrated here that the hydrodynamic tail vein injection produces a high level of transgene expression in rats. Although a significant impact of the procedure on cardiac function and liver structure was observed, animals tolerate the procedure well. Such a gene transfer procedure will be invaluable for studies requiring overexpression or reduction of a gene product through gene or siRNA transfer in vivo. Applying this procedure to over 500 well-characterized rat strains (http://rgd.mcw.edu/strains/), most of which were developed as models for complex and common diseases, one can now evaluate the therapeutic potential of a gene in rat model carrying the disease. Similarly, the impact of elevated or reduced gene expression through gene or siRNA transfer on physiological function or pathophysiology can also be evaluated in those well-characterized rat models by a simple injection of DNA or RNA into the tail vein.
Acknowledgement
The study was supported in part by grants from NIH (R01EB007357, RO1EB007357S, and RO1HL098295).
References
- 1.Mashimo T, Serikawa T. Rat resources in biomedical research. Curr Pharm Biotechnol. 2009;10:214–220. doi: 10.2174/138920109787315105. [DOI] [PubMed] [Google Scholar]
- 2.Gao X, Kim K-S, Liu D. Nonviral gene delivery: what we know and what is next. AAPS J. 2007;9:E92–E104. doi: 10.1208/aapsj0901009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bouard D, Alazard-Dany D, Cosset FL. Viral vectors: from virology to transgene expression. Br J Pharmacol. 2009;157:153–165. doi: 10.1038/bjp.2008.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu F, Song Y, Liu D. Hydrodynamics-based transfection in animals by systemic administration of plasmid DNA. Gene Ther. 1999;6:1258–1266. doi: 10.1038/sj.gt.3300947. [DOI] [PubMed] [Google Scholar]
- 5.Zhang G, Budker V, Wolff JA. High levels of foreign gene expression in hepatocytes after tail vein injections of naked plasmid DNA. Hum Gene Ther. 1999;10:1735–1737. doi: 10.1089/10430349950017734. [DOI] [PubMed] [Google Scholar]
- 6.Suda T, Liu D. Hydrodynamic gene delivery: its principles and applications. Mol Ther. 2007;15:2063–2069. doi: 10.1038/sj.mt.6300314. [DOI] [PubMed] [Google Scholar]
- 7.Herweijer H, Wolff JA. Gene therapy progress and prospects: hydrodynamic gene delivery. Gene Ther. 2007;14:99–107. doi: 10.1038/sj.gt.3302891. [DOI] [PubMed] [Google Scholar]
- 8.Miao CH, Thompson AR, Loeb K, Ye X. Long-term and therapeutic-level hepatic gene expression of human factor IX after naked plasmid transfer in vivo. Mol Ther. 2001;3:947–957. doi: 10.1006/mthe.2001.0333. [DOI] [PubMed] [Google Scholar]
- 9.Ye X, Loeb KR, Stafford DW, Thompson AR, Miao CH. Complete and sustained phenotypic correction of hemophilia B in mice following hepatic gene transfer of a high-expressing human factor IX plasmid. J Thromb Haemost. 2003;1:103–111. doi: 10.1046/j.1538-7836.2003.00024.x. [DOI] [PubMed] [Google Scholar]
- 10.Chen L, Woo SL. Complete and persistent phenotypic correction of phenylketonuria in mice by site-specific genome integration of murine phenylalanine hydroxylase cDNA. Proc Natl Acad Sci USA. 2005;102:15581–15586. doi: 10.1073/pnas.0503877102. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 11.Montini E, Held PK, Noll M, Morcinek N, Al Dhalimy M, Finegold M, et al. In vivo correction of murine tyrosinemia type I by DNA-mediated transposition. Mol Ther. 2002;6:759–769. doi: 10.1006/mthe.2002.0812. [DOI] [PubMed] [Google Scholar]
- 12.Suda T, Suda K, Liu D. Computer-assisted hydrodynamic gene delivery. Mol Ther. 2008;16:1098–1104. doi: 10.1038/mt.2008.66. [DOI] [PubMed] [Google Scholar]
- 13.Kamimura K, Suda T, Xu W, Zhang G, Liu D. Image-guided, lobe-specific hydrodynamic gene delivery to swine liver. Mol Ther. 2009;17:491–499. doi: 10.1038/mt.2008.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kamimura K, Zhang G, Liu D. Image-guided, intravascular hydrodynamic gene delivery to skeletal muscle in pigs. Mol Ther. 2010;18:93–100. doi: 10.1038/mt.2009.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suda T, Gao X, Stolz DB, Liu D. Structural impact of hydrodynamic injection on mouse liver. Gene Ther. 2007;14:129–37. doi: 10.1038/sj.gt.3302865. [DOI] [PubMed] [Google Scholar]
- 16.Zhang G, Song YK, Liu D. Long-term expression of human alpha1-antitrypsin gene in mouse liver achieved by intravenous administration of plasmid DNA using a hydrodynamics-based procedure. Gene Ther. 2000;7:1344–1349. doi: 10.1038/sj.gt.3301229. [DOI] [PubMed] [Google Scholar]
- 17.Maruyama H, Higuchi N, Nishikawa Y, Kameda S, Iino N, Kazama JJ, et al. High-level expression of naked DNA delivered to rat liver via tail vein injection. J Gene Med. 2002;4:333–341. doi: 10.1002/jgm.281. [DOI] [PubMed] [Google Scholar]
- 18.Zhang G, Gao X, Song YK, Vollmer R, Stolz DB, Gasiorowski JZ, et al. Hydroporation as the mechanism of hydrodynamic delivery. Gene Ther. 2004;11:675–682. doi: 10.1038/sj.gt.3302210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang X, Dong X, Sawyer GJ, Collins L, Fabre JW. Regional hydrodynamic gene delivery to the rat liver with physiological volumes of DNA solution. J Gene Med. 2004;6:693–703. doi: 10.1002/jgm.595. [DOI] [PubMed] [Google Scholar]
- 20.Budker V, Zhang G, Knechtle S, Wolff JA. Naked DNA delivered intraportally expresses efficiently in hepatocytes. Gene Ther. 1996;3:593–98. [PubMed] [Google Scholar]
- 21.Zhang G, Vargo D, Budker V, Armstrong N, Knechtle S, Wolff JA. Expression of naked plasmid DNA injected into the afferent and efferent vessels of rodent and dog livers. Hum Gene Ther. 1997;8:1763–72. doi: 10.1089/hum.1997.8.15-1763. [DOI] [PubMed] [Google Scholar]
- 22.Chen ZY, He CY, Ehrhardt A, Kay MA. Minicircle DNA vectors devoid of bacterial DNA result in persistent and high-level transgene expression in vivo. Mol Ther. 2003;8:495–500. doi: 10.1016/S1525-0016(03)00168-0. [DOI] [PubMed] [Google Scholar]
- 23.Hodges BL, Taylor KM, Joseph MF, Bourgeois SA, Scheule RK. Long-term transgene expression from plasmid DNA gene therapy vectors is negatively affected by CpG dinucleotides. Mol Ther. 2004;10:269–278. doi: 10.1016/j.ymthe.2004.04.018. [DOI] [PubMed] [Google Scholar]
- 24.DeLeve LD, Wang X, McCuskey MK, McCuskey RS. Rat liver endothelial cells isolated by anti-CD31 immunomagnetic separation lack fenestrae and sieve plates. Am J Physiol Gastrointest Liver Physiol. 2006;291:1187–1189. doi: 10.1152/ajpgi.00229.2006. [DOI] [PubMed] [Google Scholar]