Abstract
Most bioactive peptides are generated by proteolytic cleavage of large precursor proteins followed by storage in secretory vesicles from where they are released upon cell stimulation. Examples of such bioactive peptides include peptide neurotransmitters, classical neuropeptides, and peptide hormones. In the last decade, it has become apparent that the breakdown of cytosolic proteins can generate peptides that have biological activity. A case in point and the focus of this review are hemoglobin-derived peptides. In vertebrates, hemoglobin (Hb) consists of a tetramer of two α- and two β-globin chains each containing a prosthetic heme group, and is primarily involved in oxygen delivery to tissues and in redox reactions (Schechter Blood 112:3927–3938, 2008). The presence of α- and/or β-globin chain in tissues besides red blood cells including rodent and human brain and peripheral tissues (Liu et al. Proc Natl Acad Sci USA 96:6643–6647, 1999; Newton et al. J Biol Chem 281:5668–5676, 2006; Wride et al. Mol Vis 9:360–396, 2003; Setton-Avruj Exp Neurol 203:568–578, 2007; Ohyagi et al. Brain Res 635:323–327, 1994; Schelshorn et al. J Cereb Blood Flow Metab 29:585–595, 2009; Richter et al. J Comp Neurol 515:538–547, 2009) suggests that globins and/or derived peptidic fragments might play additional physiological functions in different tissues. In support of this hypothesis, a number of Hb-derived peptides have been identified and shown to have diverse functions (Ivanov et al. Biopoly 43:171–188, 1997; Karelin et al. Neurochem Res 24:1117–1124, 1999). Modern mass spectrometric analyses have helped in the identification of additional Hb peptides (Newton et al. J Biol Chem 281:5668–5676, 2006; Setton-Avruj Exp Neurol 203:568–578, 2007; Gomes et al. FASEB J 23:3020–3029, 2009); the molecular targets for these are only recently beginning to be revealed. Here, we review the status of the Hb peptide field and highlight recent reports on the identification of a molecular target for a novel set of Hb peptides, hemopressins, and the implication of these peptides to normal cell function and disease. The potential therapeutic applications for these Hb-derived hemopressin peptides will also be discussed.
Key words: endocannabinoid, hemoglobin, hemopressin, hemorphin
INTRODUCTION
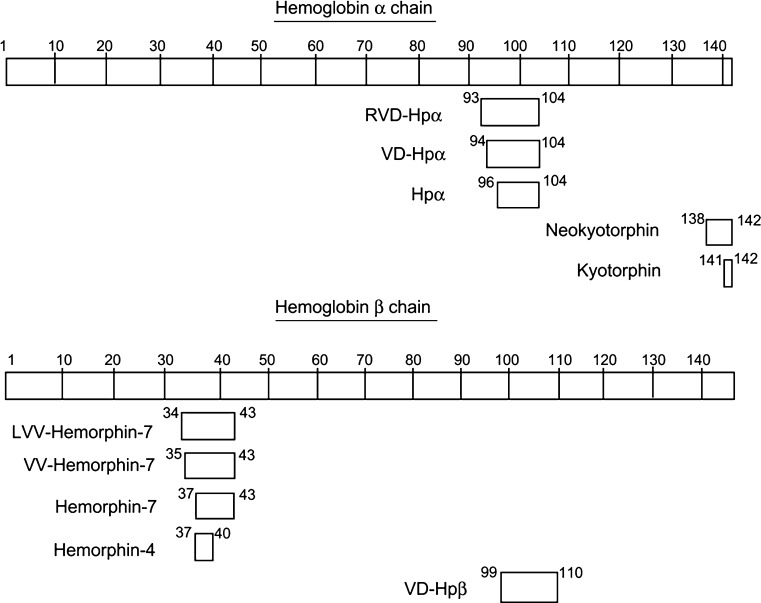
Early efforts in the 1980s to identify endogenous opioid peptides led to the characterization of Hb-derived peptides that have opiate-like activity (1,2). These were short 4-8 amino acid peptides derived from the β-globin chain that were named hemorphins (2) and neokyotorphin (1). In addition to their activity at opioid receptors, these peptides have been implicated in several biologic processes as described below. In the mid-2000, using an enzyme substrate capture assay, the presence of a peptide derived from the Hbα chain, termed “hemopressin” in rodent brain hot acid extracts was reported (3,4). This peptide was later shown to function as a CB1 cannabinoid receptor antagonist (5). Recent mass spectrometric analysis revealed the presence of N-terminal extensions of hemopressin, representing endogenous hemopressins, named RVD-hemopressin (RVD-Hpα) and VD-hemopressin (VD-Hpα) (6). A peptide derived from the Hbβ chain that exhibited sequence similarity to hemopressin was also identified and, named VD-Hpβ (6). These longer hemopressin peptides were found to exhibit agonistic activity in contrast to the original hemopressin that acts as an antagonist at cannabinoid receptors (6). The bioactive peptides derived from Hb are summarized in Table I and a schematic showing where these peptides are present in Hbα or Hbβ chain in shown in Fig. 1. In the following sections, we describe these non-classical peptides, the probable mechanisms involved in their generation, and implications in signaling and disease states.
Table I.
Bioactive Peptides Derived from Hemoglobin
| Peptide name | Peptide sequence | Biological targets | Biological functions | References | |
|---|---|---|---|---|---|
| Hpα138-142 | Neokyotorphin | TSKYR | Unknown | Non-opioid analgesic; thermoregulation; protection from seizures;modulation of vagal influence on cardiac rhythm; antibacterial; proliferation of adipocytes and cancer cells | (1,42–47,49–52) |
| Hpα141-142 | Kyotorphin | YR | Unknown | Non-opioid analgesic | (58) |
| Hpα96-104 | Hemopressin | PVNFKFLSH | CB1 cannabinoid receptors | Induces hypotension; non-opioid anticociceptive; anti-hyperalgesic; reduces food intake | (3,61,63,66) |
| Hpα93-104 | RVD-Hpα | RVDPVNFKFLSH | CB1 cannabinoid receptors | Unknown | (6) |
| Hpα93-104 | VD-Hpα | VDPVNFKFLSH | CB1 cannabinoid receptors | Unknown | (6) |
| Hpβ37-40 | hemorphin-4 | YPWT | Opioid receptors | Antinociception | (7) |
| Hpβ37-43 | hemorphin-7 | YPWTQRF | Opioid receptors | Antinociception; anti-inflammatory | (7,9) |
| Hpβ35-43 | VV-hemorphin-7 | VVYPWTQRF | Opioid and bombesin 3 receptors | Antinociception | (7,27) |
| Hpβ34-43 | LVV-hemorphin-7 | LVVYPWTQRF | Opioid, angiotensin IV and bombesin 3 receptors; angiotensin converting enzyme | Antinociception; blood pressure regulation; learning and memory; Potentiation of cholinergic transmission | (7,10,11,15–19,24–27) |
| Hpβ99-110 | VD-Hpβ | VDPENFRLLCNM | CB1 and CB2 cannabinoid receptors | Unknown | (6) |
Fig. 1.
Schematic showing positions in Hbα and Hbβ chains of the various bioactive peptides described in this review. Hb hemoglobin, Hp hemopressin
HEMOGLOBIN-DERIVED PEPTIDES
Hemorphins
Hemorphins are short peptides derived from the N-terminal region of Hbβ sharing a central tetrapeptide core, Tyr-Pro-Trp-Thr (7). Both N- and C-terminal extensions of this peptide have been isolated from human and bovine tissues (7). Hemorphins have been shown to inhibit electrically induced contractions in the guinea pig ileum bioassay (GPI bioassay) that could be blocked by the opioid receptor antagonist, naloxone, thereby strongly supporting the notion that they exert their effects at opioid receptors (2,7). Like opioids, hemorphins can induce dose-dependent antinociception in the tail-flick assay that is reversed by naloxone (8). Interestingly, some hemorphins may function as partial agonists of opioid receptors since they exhibit antagonistic effects under certain conditions. For example, hemorphin-4 was found to act as an antagonist of the selective opioid receptor agonist, DAMGO, in GPI bioassays when the ileum preparations were treated with agents that decreased receptor number (such as the alkylating agent, β-chloronaltrexamine) (9) and in morphine-tolerant animals that are thought to have reduced receptor reserve (9).
Among the hemorphins, the functional activity of LVV-hemorphin 7, a ten-residue peptide (LVVYPWTQRF) derived from either Hbβ,γ,δ or ε chain, has been extensively studied. In addition to exhibiting opiate-like effects LVV-hemorphin 7 was identified as an endogenous high-affinity ligand of the putative angiotensin IV receptor, AT4 (10,11). However, further characterization of this putative receptor revealed the protein to be analogous to insulin-regulated aminopeptidase (IRAP), a type II integral membrane protein (12,13) whose catalytic activity is inhibited by angiotensin IV and LVV-hemorphin 7 (14). LVV-hemorphin 7 has been implicated in a number of physiological processes consistent with the idea that by inhibiting IRAP and/or other peptidases such as angiotensin-converting enzyme this peptide could protect a variety of biologically active peptides from proteolysis. A role for LVV-hemorphin 7 in blood pressure regulation is suggested by several studies. For example, an intraperitoneal injection of LVV-hemorphin 7 was found to cause a significant decrease in blood pressure and heart rate in conscious spontaneously hypertensive rats (15). It was also found to potentiate the hypotensive effect of bradykinin in anesthetized rats (16). Finally, the ability of LVV-hemorphin 7 to inhibit angiotensin-converting enzyme, a component of the rennin-angiotensin system, is consistent with a major role in regulation of blood pressure (17). A number of studies have suggested that LVV-hemorphin 7 could play a role in learning and memory. For example, intracerebral administration of LVV-hemorphin 7 was found to lead to enhanced spatial learning in rats (18) and to attenuate the effects of scopolamine-induced learning deficits in fear conditioning and spatial learning tests (18,19). It is thought that by inhibiting IRAP activity, LVV-hemorphin 7 protects substrates of IRAP known to play a role in learning and memory such as vasopressin and oxytocin among others (20–23). It is also likely that LVV-hemorphin 7 directly acts on other targets. This is supported by a study showing that LVV-hemorphin 7 can potentiate depolarisation-induced release of acetylcholine from hippocampal slices (24) thereby potentiating cholinergic transmission and enhancing cognition. It has also been suggested that IRAP ligands could enhance spatial memory by potentiating hippocampal neuronal glucose uptake. Data supporting this hypothesis is contradictory since one study showed that LVV-hemorphin 7 potentiated activity-elicited glucose uptake in neuronal hippocampal cells from wild-type mice but not IRAP-knockout animals (25) while another study used in vivo microdyalysis to show that LVV-hemorphin 7 enhancement of spatial working memory did not cause increases in hippocampal glucose uptake or blood flow (26). In addition to functioning as a ligand of opioid and angiotensin AT4 (IRAP) receptors, LVV-hemorphin 7 as well as a shorter peptide, VV-hemorphin 7 has been identified as low-affinity agonists of the human bombesin 3 receptor (27). Taken together, these studies suggest that LVV-hemorphin 7 modulates several important physiological processes not only by blocking IRAP activity but also by additional mechanisms including binding to distinct receptor types.
Given that LVV-hemorphin 7 is involved in a number of physiological processes, a question arises as to the relative abundance of hemorphins in the body and their regulation during disease. Several studies have reported changes in different hemorphin peptide levels under different physiologic and pathological conditions. A study examining the effect of exercise on endogenous peptides reported increased levels of immunoreactive LVV-hemorphin 7 in blood (28). Another study reported low circulating levels of VV-hemorphin 7 in sera from human diabetic subjects (29). In the case of Alzheimer’s disease (AD), quantitative MALDI-TOF mass spectrometry detected increased levels of LVV-hemorphin 6 (but not hemorphin 7) in temporal neocortex of AD brains compared to normal controls (30). This suggests that cerebral amyloid angiopathy associated with neurodegenerative disease and aging could lead to vascular abnormalities leading to increased hemorphin levels. Taken together, these studies suggest that hemorphin peptide levels are regulated in vivo.
A number of hemorphin peptides have been identified in vivo and since hemorphins mediate several physiological responses and their levels are modulated in physiological or pathophysiological conditions raises the question as to how they are generated and what factors control their levels. Studies show that hemorphins can be generated from Hb in vitro through the actions of a variety of cytosolic, secreted, and lysosomal proteases (31–36). Enzymes such as prolyl oligopeptidase (37), angiotensin-converting enzyme (38,39), cathepsin B (36), endopeptidase 24.15 (3), neurolysin (3), dipeptidyl peptidase IV (40), and aminopeptidase M (39) have been found to be involved in the degradation of hemorphins. Interestingly, in diabetic patients, low-circulating levels of VV-hemorphin 7 were accompanied by increased cathepsin D activity (putative biosynthetic enzyme) and a decrease in dipeptidyl peptidase IV activity (putative biodegrading enzyme) (29). Given that cathepsin D is a lysosomal enzyme with acidic pH optima and that Hb (the hemorphin precursor) is a cytosolic protein with neutral pH optima raises the question about the cellular compartment where Hb is processed to hemorphins by cathepsin D. Further studies are required to elucidate the enzymes responsible for the in vivo regulation of hemorphin levels. Also, since LVV-hemorphin 7 has been thought to be an endogenous ligand of AT4/IRAP, studies are needed to address how and where LVV-hemorphin binds to AT4/IRAP and whether additional targets for LVV-hemorphin exist given that subcellular localization studies using electron microscopy reveal that AT4/IRAP are localized to neurosecretory vesicles as well as endoplasmic reticulum, trans Golgi network, and endosomes (41).
Neokyotorphin
Neokyotorphin (Thr-Ser-Lys-Tyr-Arg) was originally isolated from bovine brain by gel filtration and cation exchange chromatography (1,42). This peptide is derived from the C-terminal region of Hbα and early studies revealed that it exhibits analgesic activity similar to Leu-enkephalin, an endogenous opioid peptide derived from the classic neuropeptide precursor proenkephalin (1,42). The analgesic effects of neokyotorphin are mediated by a non-opioid mechanism since they are not blocked by the opioid receptor antagonist, naloxone (43). In addition, neokyotorphin inhibits the Ca+2-dependent and depolarization-evoked release of 3H-GABA from crude synaptosomes indicating that inhibition of GABA in the brain could be involved in neokyotorphin-induced analgesia (43).
Like hemorphins, neokyotorphin has been implicated in modulating a diverse set of functions ranging from thermoregulation (44), protection from seizures in an animal model of epilepsy (45), modulation of vagal influence on cardiac rhythm (46), regulation of antibacterial activity (47), modulation of brain function in hibernating ground squirrels (48), and proliferation of adipocytes (49) and cancer cells (50–52). However, the molecular target/(s) for this peptide have not yet been identified. In this context, neokyotorphin has been shown to inhibit the activities of aminopeptidase, dipeptidyl aminopeptidase, and angiotensin-converting enzyme which could lead to an increase in the half-life of Met-enkephalin and/or other peptides by preventing their degradation by dipeptidyl aminopeptidase (53).
Very little information is available about how neokyotorphin is generated from Hb or how it is degraded. In vitro studies have implicated pepsin (54,55) and cathepsin D (56) in the generation of neokyotorphin from Hb. Neokyotorphin can be hydrolysed by partially purified angiotensin-converting enzyme (57) to generate kyotorphin (Tyr-Arg) an analgesic dipeptide that releases Met-enkephalin from brain and spinal cord by depolarizing enkephalinergic neurons (58). Given that neokyotorphin has been implicated in several physiological roles, further studies are required to not only identify its molecular target/(s) but also to characterize the enzymes responsible for its generation in vivo from Hb as well as those responsible for its degradation.
Hemopressin
Hemopressin (PVNFKFLSH) was first identified as a peptide substrate for a series of metallopeptidases (thimet oligopeptidase (EP24.15), neurolysin (EP24.16), and angiotensin-converting enzyme) using an approach employing the catalytic site-inactive mutant EP24.15 or EP24.16 to capture endogenous peptides that bind to the enzymes (3). This led to the identification of a number of peptides derived from intracellular proteins (3) including Hb fragments such as LVV-hemorphin 7 (33,59), VV-hemorphin 7 (60), shorter N- and C-terminally truncated forms from Hbβ (3) and Hp from the Hbα1 chain (3). Examination of the pharmacological properties of the latter peptide, Hp, showed that it could induce potent hypotension in anesthetized rats (3) and transient hypotension following intravenous or intra-arterial administration into mice, rats, or rabbits (61). Since the hypotensive effects of Hp were not accompanied by changes in cardiac output, a function as a vasodilator to regulate local blood flow through the release of nitric oxide was proposed (62). In addition, Hp was found to exhibit antinociceptive effects in an inflammatory pain model (63). In this model, paw pressure is used as a mechanical stimulus to directly activate the nociceptors of C and Aδ fibers, resulting in a motor response that leads to paw withdrawal (64). In this model, Hp inhibited the hyperalgesia induced by either carrageenan or bradykinin administration (63). These effects were not inhibited by naloxone, indicating a nonopioid receptor-mediated analgesic effect (63). Two fragments of Hp (PVNFKF and PVNFKFL) were as effective as Hp in exerting an antihyperalgesic action whereas shorter fragments (PVNFK and PVNF) were inactive (63). Hp did not impair motor activity or alter pentobarbital-induced sleeping time, indicating the absence of sedative or motor abnormalities that could account for its antinociceptive action (5). The effects of Hp on carrageenan-induced heperalgesia were independent of route of administration (oral, local, or intrathecal) (5,63) raising the possibility that Hp could be developed as a potential therapeutic drug for the treatment of pain.
In order to identify the molecular target of Hp we used previously generated conformation-sensitive antibodies to a variety of G protein-coupled receptors including opioid and cannabinoid receptors (65). These antibodies were raised to an epitope in the N-terminal region that was proximal to putative glycosylation sites (65). These conformation-sensitive antibodies exhibit increased recognition of agonist-treated receptors and decreased recognition of antagonist-treated receptors in an enzyme-linked immunosorbent assay and could therefore be used to screen for receptor-specific ligands (5,65). Using these antibodies, we found Hp to selectively bind to CB1 cannabinoid but not to CB2 cannabinoid or to μ or δ opioid, α2A, or β2 adrenergic receptors (5). We found that Hp exhibits antagonist/inverse agonist activity at CB1 cannabinoid receptors; it is able to block both the agonist induced as well as the constitutive activity of this receptor to the same extent as its well-characterized antagonist, rimonabant (SR141716) (5).
A recent study examining the effect of Hp on feeding behavior provided additional support for CB1 cannabinoid receptors as the molecular target for Hp (66). The study found that central (intracerebroventricular) or systemic (intraperitoneal) administration of Hp into rats, mice or obese ob/ob mice caused a dose-dependent decrease in night-time food intake without causing obvious side-effects (66). This Hp-mediated decrease in food intake was not observed in mice lacking CB1 receptors (66). In addition, Hp also blocked CB1 agonist-mediated increase in food intake in wild-type mice (66). These observations suggest that Hp could serve as a scaffold for the generation of a novel class of drugs for the treatment of obesity.
Although Hp was first isolated from rat brain extracts, questions regarding its origin arose since cleavage at the aspartic acid-proline bond (such as that found in Hb) is know to be susceptible to acid extraction conditions (used in studies reporting the identification of Hp, 3). To test this, we extracted brain peptides by an alternative method that did not involve acid extraction; this led to the identification of N-terminally extended peptides RVDPVNFKFLSH and VDPVNFKFLSH. Treatment of these extended peptides with acid (under conditions used for acid extraction) led to the generation of Hp consistent with the idea that Hp is generated from longer Hps and that the latter represent endogenous Hb-derived peptides (their characterization is described below).
Extended Hemopressin Peptides
Peptidomics studies exploring the repertoire of endogenous peptides in mouse brain extracts detected the presence of RVDPVNFKFLSH and VDPVNFKFLSH in different brain regions (6). These peptides termed RVD-Hpα and VD-Hpα, respectively, represent N-terminally extended forms of Hp derived from Hbα chain. We also identified a Hpβ peptide, VDPENFRLLCNM; since it had sequence similarity to Hp it was termed VD-Hpβ (6). Characterization of the longer Hp peptides indicates that in contrast to Hp, they exhibit agonistic activity at cannabinoid receptors (6). Since previously identified endogenous ligands of CB1 receptors, anandamide, and 2-arachidonoylglycerol, are derived from lipids, longer Hps represent the first identified peptide agonists, “peptide endocannabinoids”, that selectively activate CB1 receptors. In the following sections, we describe the similarities and differences in the functional activities of these peptides in comparison to classical non-peptidic endocannabinoid ligands.
RVD-Hpα and VD-Hpα
Receptor activity studies in heterologous cells expressing recombinant receptors or in cells expressing endogenous receptors demonstrate that RVD-Hpα and VD-Hpα behave as specific agonists of CB1 cannabinoid receptors and to a lesser extent of CB2 cannabinoid receptors but not of either μ, δ opioid, α2A, β2 adrenergic, and AT1 angiotensin receptors (6). These Hb-derived peptides could selectively bind CB1 receptors with nanomolar affinity although they induced a lower maximal displacement of radiolabeled agonist binding than the classical CB1 receptor antagonist, SR141716 (6). Examination of various functional properties of RVD-Hpα and VD-Hpα showed that signaling by these peptides could be blocked by SR141716 in cells expressing CB1 but not CB2 cannabinoid or GPR55 receptors (6). Interestingly, comparison of the time course of signaling by longer Hps to that of the classic CB1 ligand, Hu-210, showed differences in temporal dynamics. The longer Hps exhibited peak activity at 30 min compared to the peak activity of Hu-210 (5 min) (6). This data, together with the differences in sensitivity to pertussis toxin (6) suggests that stimulation of CB1 receptors by longer Hps leads to activation of a signaling pathway distinct from that activated by classical cannabinoid ligands. We explored this possibility by examining the dynamics of Ca+2 release in Neuro 2A cells that endogenously express CB1 receptors (as well as in HEK-293 cells expressing recombinant receptors). Treatment with longer Hps leads to a sustained increase in Ca+2 release that is faster and more robust compared to that seen with the endocannabinoid 2-AG, or the classical agonist Hu-210 (6). The longer Hp-mediated Ca+2 release in the Neuro 2A cells is seen in the absence of extracellular calcium indicating that the Ca+2 release is from intracellular stores (6). These results are exciting and indicate that peptide endocannabinoids activate signal transduction pathways distinct from that seen with lipidic endocannabiniods such as 2-AG. The differential signaling activated by peptide and non-peptide endocannabinoids of the CB1 receptor is likely to increase its repertoire of signaling and significantly affect modulation of CB1 response under physiologic and pathophysiologic conditions. Thus, these peptide agonists could be developed as tools to improve our understanding of CB1 receptor function and serve as scaffolds for the development of potential therapeutic drugs to treat pathologies in which CB1 receptors have been implicated.
VD-Hpβ
Our mass spectrometric analysis of mouse brain peptidomics also detected a peptide derived from Hbβ chain, VD-Hpβ. This peptide behaves as an agonist of both CB1 and CB2 receptors (6). In contrast to longer Hps derived from Hbα chain (RVD-Hpα and VD-Hpα), agonistic activity of VD-Hpβ was only partially blocked by pretreatment of CB1 receptors with the selective antagonist, SR141716 (6). These studies suggest that VD-Hpβ could have multiple molecular targets (in addition to CB1 receptors) and/or could function as an allosteric modulator of cannabinoid receptors.
Hemopressin; Oligomerization and Solubility
We and others have found that synthetic Hp exhibits variability in activity in in vitro and in vivo assays. There are several examples in the literature where bioactive peptides show large variability among different experiments/laboratories, and this has been generally associated with peptide solubility and/or oligomerization properties. A case in point is Aβ1-42 peptide that is generated from the amyloid precursor protein and has been implicated in Alzheimer’s disease. Studies show that Aβ1-42 can exist as monomers or oligomers (67) and that while synthetic Aβ1-42 monomers promote survival and protect mature neurons from excitotoxic death (68), self-association of these monomers into oligomers causes neuronal cell death (69–71). Another study showed that at lower concentrations Aβ peptides exhibited neurotrophic effects while at higher concentrations they exhibited neurotoxic effects (72). This was attributed to increased aggregation of Aβ peptides at higher concentrations as supported by experiments showing that Aβ1-42 made in DMSO and stored at –20°C exhibited increased oligomerization with time of storage (73). Treatment of PC12 cells with the older Aβ samples that contained higher levels of oligomeric Aβ peptides led to decreased cell viability thereby supporting the idea that oligomerization was toxic to the cells (73).
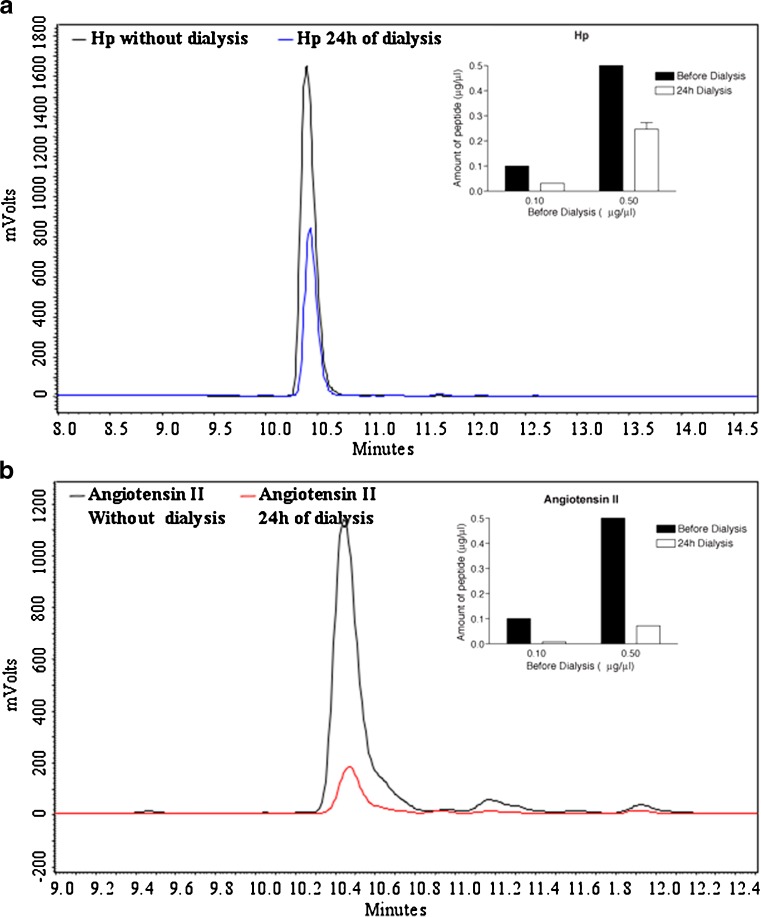
We tested whether a similar mechanism could explain the variability in Hp activity. For this, we examined the relative level of Hp (MW~1 kDa) remaining in a 2 kDa cut-off dialysis cassette following 24 h dialysis against PBS and compared it with that of angiotensin II, a peptide of similar molecular weight (~1 kDa) that does not undergo significant aggregation. We used either 0.1 or 0.5 mg/ml of these peptides for dialysis and the amount of peptide retained in the dialysis cassette was determined by subjecting aliquots (20 μl) to HPLC analysis. The area under the curve was used to calculate the amount of retained peptide using a Hp or angiotensin II standard curve (0-0.5 mg/ml). We also determined the amount of peptide adsorbed to the dialysis membrane and find that <5% of either Hp or angiotensin II is adsorbed. We find that a higher proportion of Hp is retained in the dialysis cassette following 24 h dialysis with higher concentrations of Hp (0.5 mg/ml) compared to lower concentrations (0.1 mg/ml; Fig. 2). In addition, the levels of Hp retained in the dialysis cassette are higher than those of angiotensin II, a peptide with a similar molecular weight. This suggests that at higher concentrations Hp can dimerize/oligomerize to form complexes that are retained in the dialysis cassette. The tendency of Hp to form aggregates could at least in part explain the variability among different experiments and batches of Hp. Conditions to help protect Hp from oligomerization could facilitate studies examining the functional role of this peptide at CB1 cannabinoid receptors.
Fig. 2.
Comparison of the amount of Hp and Angiotensin II present in 2 kDa dialysis bags. 1 ml Hp a or angiotensin II b solution (0.1 or 0.5 mg/ml) was placed in a dialysis bag of 2 kDa cutoff (Slide-A-Lyzer Dialysis Cassettes, Pierce) and subjected to dialysis against PBS for 24 h. Aliquots (20 μl) of the 24 h dialysate were subjected to HPLC analysis. The amount of Hp or angiotensin II in the bag before and after dialysis was calculated from the area under the curve and from a standard curved obtained by HPLC analysis of Hp or angiotensin II (0-0.5 mg/ml). There was <5% peptide associated with the dialysis cassette itself. a The area under the curve for Hp (0.5 mg/ml) before dialysis was 15758985 and after 24 h dialysis was 7014526. b The area under the curve for angiotensin II (0.5 mg/ml) before dialysis was 10389021 and after 24 h dialysis was 1490275. Insets in a and b show the amount of hemopressin or angiotensin II peptide in the dialysis bag before and 24 h after dialysis. Data shows that compared to angiotensin II more Hp is retained in the dialysis bag suggesting that there is increased aggregation of Hp. Data is the mean ± SE of three experiments
Tissue Distribution of α- and β-Globin Chains
As described above several peptides derived from Hbα- and β-globin chains have been implicated in a diverse array of physiological activities. The question that arises is whether these peptides are generated from the breakdown of Hb or if they are selectively processed in situ in specific tissues. Data collected in the last decade using a variety of techniques ranging from immunohistochemical studies to microarray, mass spectrometry, and RT-PCR studies show that cell types other then erythrocytes can produce Hbα- and/or β-chains providing support to the idea that Hb-derived peptides are generated under specific conditions/tissues and not due to breakdown of Hb from erythrocytes. These studies are described below and summarized in Table II.
Table II.
Distribution of α and β Hemoglobin Chains in Non-Erythrocyte Cells
| Hb chain | Cell type | Probable biological function | References |
|---|---|---|---|
| α and β | Type II alveolar cells | Unknown | (74,75) |
| α and β | Lens | Gene sharing; iron homeostasis; oxygen transporters/oxygen “sink”; denucleation of lens fiber cells | (76) |
| α and β | Mesangial cells of kidney glomeruli | Scavenging reactive oxygen species | (78) |
| α and β | Α9 neurons of mesocorticolimbic pathway; | Oxygen homeostasis, oxidative phosphorylation; GPCR activation | (79) |
| Neurons in striatum, SNC, cerebral cortex and hippocampus; | (80) | ||
| Dopaminergic neurons; | (69,79–81) | ||
| Cortical pyramidal neurons; | (80,81) | ||
| Striatal GABAergic projection neurons; | (80,81) | ||
| Ventral midbrain neurons | (79) | ||
| α and β | Sciatic nerve myelin | Sciatic nerve function and pathology | (83) |
| α1 and β | Oligodendrocyte precursors | Oligodendrocyte differentiation | (79) |
| βminor | Macrophage | Unknown | (77) |
GPCR, G-protein coupled receptor; GABA, gamma amino butyric acid; SNC; substancia nigra pars compacta
Alveolar Cells
Gene profiling of freshly isolated type I and type II alveolar epithelial cells using a 10 K rat gene DNA microarray found that two of the genes with highest fold change between type II and type I cells were for Hbα- and β-chains (74). RT-PCR analysis showed that the α- and β-globin chain mRNAs were highly expressed in Type II but not detectable in Type I alveolar epithelial cells (74). In addition, trans-differentiation of Type II into Type I cells led to a decrease in globin chain mRNA with increasing trans-differentiation (74). Quantitative RT-PCR detected the presence of α- and β-globin chains in cell lines, including primary type II alveolar epithelial cells that express genes characteristic of pulmonary epithelial cells such as surfactant protein B (75). Erythroid specific genes such as erythrocyte anion exchanger (AE1) and band 3 protein (75) were not detected indicating that detection of transcripts for globin genes was not due to erythrocyte contamination (75). The presence of Hbα- and β-chains in type II epithelial alveolar cells was also shown by double staining a lung cell mixture with anti-Hb and anti-LB-180 antibodies (marker for type II epithelial alveolar cells) as well as by immunostaining perfused rat lung tissue (to remove red blood cells prior to fixation and sectioning) which revealed the presence of Hb in the corners of alveoli occupied by type II cells (74). Tandem mass spectrometric analysis of a tryptic digest of proteins from primary cultures of rat type II epithelial alveolar cells identified peptides derived from Hbα- and β-chains (75). Taken together, these studies indicate that α- and β-globin are specifically present in type II epithelial alveolar cells. However, further studies are required to ascertain the role of globins and their peptides in normal lung function.
Lens
Comparison of genes expressed in the lens with non-lens tissues using cDNA microarrays detected the presence of several Hbα- and β-isoforms in the newborn, 7-day-old and adult mouse lens (76). Semiquantitative RT-PCR using primers specific for each Hb isoform confirmed their presence in mouse lens (76). Further studies are required to elucidate the role of globin chains in the lens which could range from gene sharing (a property whereby the lens recruits stress proteins and metabolic enzymes to form crystallins), lens iron homeostasis, oxygen transporters/oxygen “sink” to maintain the normally low oxygen levels characteristic of the lens or a pro-apoptotic role to promote denucleation of lens fiber cells (76).
Macrophages
RT-PCR using primers specific for the Hbβ subunit detected the presence of a fragment of the size predicted for the mRNA substrate (~150 bp) in macrophages treated with interferron-γ and lipopolysaccharide but not in untreated cells (77). Sequencing of the fragment identified it as a βminor hemoglobin transcript (77). Western blot analysis with rabbit antisera to mouse Hb detected its presence in macrophage RAW264.6 cells stimulated with interferron-γ and lipopolysaccharide (77). The induction of the βminor globin mRNA and protein required a 3.5-24 h treatment with interferron-γ and lipopolysaccharide (77). Further studies are needed to determine the role of globin derived peptides in macrophage function.
Mesangial Cells
Microarray analysis and proteomics of perfused rat kidneys (to avoid blood contamination) detected the presence of α- and β-globin genes which were transiently up-regulated during chronic hypoxia (78). Proteomic studies found that the β-globin protein was up-regulated by ~6.4-fold under hypoxic conditions (78). RT-PCR detected the presence of α- and β-globin mRNA but not the presence of the mRNA for AE1, a gene that is abundant in erythrocytes, in perfused isolated rat kidney glomeruli (78) suggesting that globin gene expression was not due to erythroid cell contamination. The restricted expression of globin genes to kidney glomeruli was confirmed by RT-PCR of different nephron compartments isolated by manual dissection or by laser capture microdissection (78). Immunoblotting studies using polyclonal antibodies to Hb detected its presence in lysates from glomeruli isolated form saline perfused kidneys (78). In situ hybridization using antisense RNA probes specific for rat α- and β-globin showed that these genes are expressed in the mesangial region of kidney glomeruli (78). This was supported by colocalization of staining for Hb with OX-7, a marker of mesangial cells (78) and by detection of α- and β-globin in primary cultures of rat mesangial cells (78). Interestingly, stimuli associated with chronic hypoxia such as low oxygen levels, treatment with angiotensin II or hydrogen peroxide led to up-regulation of α- and β-globin mRNA levels in cultured primary rat mesangial cells (78). In addition, overexpression of both the α- and β-globin genes in these cells led to reduction of reactive oxygen species and improved mesangial cell viability under hypoxic conditions (78) suggesting that the globin gene products may play a role in the scavenging of reactive oxygen species in rat kidney mesangial cells.
Neuronal Cells
Several studies detected the presence of Hbα- and β-chains in the brain. For example, laser caption microdissection followed by cDNA microarrays or a nanoscale version of the cap analysis of gene expression (nanoCAGE) detected Hbα- and β-chain transcripts in A9 neurons of the nigrostriatal pathway (79). This was validated by in situ hybridization which detected co-localization of antisense signals for α- and β-globin chains in the cytosol of tyrosine-hydroxylase (TH)-positive A9 and to a lesser extent, A10 neurons of the mesocorticolimbic pathway (79). Quantitative PCR analysis of brain sections obtained by laser caption microdissection from mice that selectively express green fluorescent protein (GFP) in cathecolaminergic cells under the control of the TH gene promotor showed that TH-positive A9 neurons expressed twice the amount of α- and β-globin chain transcripts as A10 neurons (79). Microarray studies of TH-positive neurons detected the presence of mRNA for α- and β-globin chains in the rat substancia nigra pars compacta (SNC), as well as mouse SNC and striatum (80). mRNAs for erythroid markers (GATA1 and Eraf) were not detected indicating that the presence of globin chains was not due to blood contamination. In situ hybridization with globin RNA probes detected Hb mRNAs in neurons of all layers of the rat cerebral cortex, hippocampus and SNC (80). RT-PCR and real time qPCR of neurons isolated by laser caption microdissection detected the presence of α- and β-globin chains in primary cortical cultures of Wistar rats and in rat nigral domaminergic neurons, cortical pyramidal neurons, and striatal GABAergic projection neurons (80,81). Taken together, these results indicate that Hbα- and β-chains are expressed in discrete brain regions indicating that they may have a physiologic role in neuronal brain function.
Immunohistochemical studies also support the presence of α- and β-globin chains in discrete brain cell populations. Immunohistochemical studies using anti-Hb antibodies that did not exhibit cross-reactivity with atypical globins normally expressed in the brain detected Hb immunoreactivity in ~65% A9 neurons, ~3% A10 neurons in the substantia nigra, and ~73% of hippocampal and cortical astrocytes and in ~99% of mature oligodendrocytes as well as in primary cultures of mouse ventral midbrain, cortex, and hippocampus (79). The presence of Hb in select neuronal, astrocytic, and oligodendrocytic populations was elegantly demonstrated by RT-PCR analysis of mesolimbic dopaminergic neurons, astrocytes, and oligodendrocytes obtained from brains of mice expressing either TH-GFP, GFAP-GFP, or CNP-GFP (markers for dopaminergic neurons, astrocytes, and oligodendrocytes, respectively) by fluorescence-activated cell sorting to minimize endothelial, microglial, and red blood cell contamination of the preparation (79). This pattern of Hb expression was found to be conserved in mice of different genetic backgrounds, in rats, and in human post-mortem brains (79). However, another study detected α-globin staining in neurons of the rat cerebral cortex, cerebellum, hippocampus, and striatum but not in astrocytes or oligodendrocytes (81). Strongest signals were detected along dendrites and axons of individual neurons and in subcortical fiber tracts (81). Interestingly, a study using polyclonal antibodies to either rat or human α-or β-chains detected the presence of α-globin mostly in the cell body and nucleus while β-chains where also detected in cellular processes (80). A strong α-chain staining was observed in cortex, basal ganglia, hippocampus, and hypothalamus and β-chain staining in cortical and thalamic dendrites, hippocampal cells, and processes and substantia nigra pars reticulata of rat and human brain (80). The use of more selective antibodies to Hbα-or β-chains in combination with markers for specific cell populations or cytosolic, axonal, and dendritic markers would help in elucidating the brain cell populations that express these proteins as well as where in the cell are the α- and β-globin chains located which would provide a clue to their physiological role in the brain.
Studies examining regulation of Hb expression have reported dynamic changes and provided clues to the function of Hb-derived peptides in the brain. Hb immunoreactivity was detected as early as postnatal day 6 (79). Overexpresion of mouse globin chains in a dopaminergic cell line affected genes involved in oxygen homeostasis and oxidative phosphorylation. Changes were also observed in genes involved in oxidative stress, iron metabolism, and nitric oxide synthesis (79). In addition, elevated levels of α-globin mRNA were observed in erythropoietin transgenic mice (81). Administration of pimonidazole, a marker that detects oxygen levels below 10 mmHg, indicated a reciprocal relationship between Hbα levels and hypoxia suggesting that cells expressing α-globin had higher oxygen content (81). Furthermore, treatment of rats with rotenone, an inhibitor of the complex I of the mitochondrial respiratory chain, led to a decrease in Hbα- and β-chains but not in neuroglobin or cytoglobin in nigral dopaminergic neurons, cortical pyramidal, and striatal GABAergic projection neurons (80). Taken together, these studies show that Hbα- and β-chains exhibit discrete cellular and subcellular localization in the brain and that these proteins or peptides generated from their processing could play a role in diverse brain functions ranging from oxygen homeostasis, oxidative phosphorylation to activation of distinct signaling pathways.
Oligodendrocytes
Affymetrix rat genomic U34 chips used to quantitatively examine gene expression changes during differentiation of oligodendrocyte precursor cells to oligodendrocytes found that Hbβ was among the top 50 genes most down-regulated during oligodendrocyte differentiation (82). In addition, both Hbα1 and β genes were among the top oligodendrocyte precursor cell-specific expressed genes (82). Since there is a change in the expression of Hb genes during oligodendrocyte differentiation, further studies are required not only to elucidate their role in oligodendrocyte differentiation but also to determine the physiological role of globin chains and derived peptides in mature oligodendrocytes.
Sciatic Nerve
Real-time RT-PCR detected the presence of mRNA for α-gobin in isolated sciatic nerves as well as the proximal and distal stumps of ligated sciatic nerves (83). The levels of α-globin mRNA were found to be very low in Schwann cells isolated from sciatic nerves (83). Mass spectrometric analysis of tryptic peptides obtained from sciatic nerve myelin detected the presence of four peptides derived from the α-globin sequence and nine derived from the β-globin sequence (83). Further studies are required to determine the forms of bioactive peptides derived from Hb that are present and regulated in this non-erythroid tissue, and to what extent these proteins and derived peptides play a role in normal function and pathology.
Perspectives
The understanding of classical neurotransmission suggests that signaling molecules are stored in vesicles to be released upon cell stimulation (84). We propose that Hps modulate neurotransmission via a “non-classical” modality. Because Hb is a well-established cytosolic protein, generation of Hb-derived bioactive peptides such as hemorphins and Hps raise questions of how these peptides are formed and how they can reach their target GPCRs.
In erythrocytes, Hb is degraded by the proteasome after its ubiquitination or oxidation (85,86). The proteasome is a large proteolytic complex ubiquitously distributed among mammalian cells including neuronal cells (87). Therefore, one possibility is that in neurons, Hb could be a cytosolic substrate of the proteasome. The proteasome is known to generate peptides ranging from two to 20 amino acids (88); the size of the Hb-derived peptides match that of peptides generated by the proteasome. Additional studies are needed to demonstrate that the Hb peptides such as hemorphins, neokyotorphin, and Hps can be generated by the proteasomal degradation of hemoglobin.
Another important question is if/how these peptides are released by regulated secretion and, if so, how are they sequestered. Although most secreted proteins and neuropeptide precursors have a signal peptide sequence that drives their entry into the secretory pathway, the unconventional secretion of cytoplasmic proteins and bioactive peptides without entering the secretory pathway is also well known (89). One possible mechanism for the unconventional secretion of cytosolic bioactive peptides are specialized ATP-binding cassette (ABC) transporters, which have been well characterized to carry antigenic peptides from the cytosol into the endoplasmic reticulum as well as to function in the shuttling of peptides across the plasma membrane (90–92). Therefore, it is possible to envision that cytosolic peptides could be released either directly from the cytosol or enter the secretory pathway through the endoplasmic reticulum using the ABC transporters and then be released by conventional secretion. Further investigations exploring this exciting new perspective would substantially add to our current understanding of cell signaling.
Finally, Hb-derived peptides due to their multiple roles in a variety of diseases have great potential to be attractive candidates to be developed as therapeutic agents. The endocannabinoid system (consisting of the receptors and endogenous ligands) has been implicated in many pathophysiological processes including Parkinson’s disease, Alzheimer’s disease, depression, inflammation, neuropathic pain, and obesity. This suggests that compounds that modulate cannabinoid receptors are good targets for development of drugs that could be useful in the treatment of such diseases. In this context, the finding that Hps exhibit antinociceptive and antihyperalgesic activity (3,5,6) and that Hp can inhibit food intake (66) suggests the possibility that these peptides can be developed as a new class of drugs for the treatment of neuropathic pain and obesity.
Acknowledgments
Supported by NIH grants DA019521 and GM071558 to LAD; FAPESP grants 04/04933-2 to ESF and 04/14258-0 to ASH and CNPq (to ESF).
Contributor Information
Andrea S. Heimann, Email: andrea@proteimaxnet.com.br
Lakshmi A. Devi, Email: lakshmi.devi@mssm.edu
References
- 1.Fukui K, Shiomi H, Takagi H, Hayashi K, Kiso Y, Kitagawa K. Isolation from bovine brain of a novel analgesic peptapeptide, neo-kyotorphin, containing the Tyr-Arg (kyotorphin) unit. Neuropharmacology. 1983;22:191–6. doi: 10.1016/0028-3908(83)90008-4. [DOI] [PubMed] [Google Scholar]
- 2.Brantl V, Gramsch C, Lottspeich F, Mertz R, Jaeger KH, Herz A. Novel opioid peptides derived from hemoglobin: hemorphins. Eur J Pharmacol. 1986;125:309–10. doi: 10.1016/0014-2999(86)90044-0. [DOI] [PubMed] [Google Scholar]
- 3.Rioli V, Gozzo FC, Heimann AS, Linardi A, Krieger JE, Shida CS, Almeida PC, Hyslop S, Eberlin MN, Ferro ES. Novel natural peptide substrates for endopeptidase 24.15, neurolysin, and angiotensin-converting enzyme. J Biol Chem. 2003;278:8547–55. doi: 10.1074/jbc.M212030200. [DOI] [PubMed] [Google Scholar]
- 4.Dale CS, Pagano Rde L, Rioli V. Hemopressin: a novel bioactive peptide derived from the alpha1-chain of hemoglobin. Mem Inst Oswaldo Cruz. 2005;100:105–6. doi: 10.1590/S0074-02762005000900017. [DOI] [PubMed] [Google Scholar]
- 5.Heimann AS, Gomes I, Dale CS, Pagano RL, Gupta A, de Souza LL, Luchessi AD, Castro LM, Giorgi R, Rioli V, Ferro ES, Devi LA. Hemopressin is an inverse agonist of CB1 cannabinoid receptors. Proc Natl Acad Sci USA. 2007;104:20588–93. doi: 10.1073/pnas.0706980105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gomes I, Grushko JS, Golebiewska U, Hoogendoorn S, Gupta A, Heimann AS, Ferro ES, Scarlata S, Fricker LD, Devi LA. Novel endogenous peptide agonists of cannabinoid receptors. FASEB J. 2009;23(9):3020–9. doi: 10.1096/fj.09-132142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nyberg F, Sanderson K, Glämsta EL. The hemorphins: a new class of opioid peptides derived from the blood protein hemoglobin. Biopolymers. 1997;43:147–56. doi: 10.1002/(SICI)1097-0282(1997)43:2<147::AID-BIP8>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 8.Davis TP, Gillespie TJ, Porreca F. Peptide fragments derived from the beta-chain of hemoglobin (hemorphins) are centrally active in vivo. Peptides. 1989;10:747–51. doi: 10.1016/0196-9781(89)90107-1. [DOI] [PubMed] [Google Scholar]
- 9.Zadina JE, Kastin AJ, Kersh D, Wyatt A. Tyr-MIF-1 and hemorphin can act as opiate agonists as well as antagonists in the guinea pig ileum. Life Sci. 1992;51:869–85. doi: 10.1016/0024-3205(92)90615-V. [DOI] [PubMed] [Google Scholar]
- 10.Moeller I, Lew RA, Mendelsohn FA, Smith AI, Brennan ME, Tetaz TJ, Chai SY. The globin fragment LVV-hemorphin-7 is an endogenous ligand for the AT4 receptor in the brain. J Neurochem. 1997;68:2530–7. doi: 10.1046/j.1471-4159.1997.68062530.x. [DOI] [PubMed] [Google Scholar]
- 11.Moeller I, Albiston AL, Lew RA, Mendelsohn FA, Chai SY. A globin fragment, LVV-hemorphin-7, induces [3H]thymidine incorporation in a neuronal cell line via the AT4 receptor. J Neurochem. 1999;73:301–8. doi: 10.1046/j.1471-4159.1999.0730301.x. [DOI] [PubMed] [Google Scholar]
- 12.Albiston AL, McDowall SG, Matsacos D, Sim P, Clune E, Mustafa T, Lee J, Mendelsohn FA, Simpson RJ, Connolly LM, Chai SY. Evidence that the angiotensin IV (AT(4)) receptor is the enzyme insulin-regulated aminopeptidase. J Biol Chem. 2001;276:48623–6. doi: 10.1074/jbc.C100512200. [DOI] [PubMed] [Google Scholar]
- 13.Chai SY, Fernando R, Peck G, Ye SY, Mendelsohn FA, Jenkins TA, Albiston AL. The angiotensin IV/AT4 receptor. Cell Mol Life Sci. 2004;61:2728–37. doi: 10.1007/s00018-004-4246-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lew RA, Mustafa T, Ye S, McDowall SG, Chai SY, Albiston AL. Angiotensin AT4 ligands are potent, competitive inhibitors of insulin regulated aminopeptidase (IRAP) J Neurochem. 2003;86:344–50. doi: 10.1046/j.1471-4159.2003.01852.x. [DOI] [PubMed] [Google Scholar]
- 15.Cejka J, Zelezná B, Velek J, Zicha J, Kunes J. LVV-hemorphin-7 lowers blood pressure in spontaneously hypertensive rats: radiotelemetry study. Physiol Res. 2004;53:603–7. [PubMed] [Google Scholar]
- 16.Ianzer D, Konno K, Xavier CH, Stöcklin R, Santos RA, de Camargo AC, Pimenta DC. Hemorphin and hemorphin-like peptides isolated from dog pancreas and sheep brain are able to potentiate bradykinin activity in vivo. Peptides. 2006;27:2957–66. doi: 10.1016/j.peptides.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 17.Fruitier-Arnaudin I, Cohen M, Bordenave S, Sannier F, Piot JM. Comparative effects of angiotensin IV and two hemorphins on angiotensin-converting enzyme activity. Peptides. 2002;23:1465–70. doi: 10.1016/S0196-9781(02)00083-9. [DOI] [PubMed] [Google Scholar]
- 18.Lee J, Albiston AL, Allen AM, Mendelsohn FA, Ping SE, Barrett GL, Murphy M, Morris MJ, McDowall SG, Chai SY. Effect of I.C.V. injection of AT4 receptor ligands, NLE1-angiotensin IV and LVV-hemorphin 7, on spatial learning in rats. Neuroscience. 2004;124:341–9. doi: 10.1016/j.neuroscience.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 19.Albiston AL, Pederson ES, Burns P, Purcell B, Wright JW, Harding JW, Mendelsohn FA, Weisinger RS, Chai SY. Attenuation of scopolamine-induced learning deficits by LVV-hemorphin-7 in rats in the passive avoidance and water maze paradigms. Behav Brain Res. 2004;154:239–43. doi: 10.1016/j.bbr.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 20.Herbst JJ, Ross SA, Scott HM, Bobin SA, Morris NJ, Lienhard GE, Keller SR. Insulin stimulates cell surface aminopeptidase activity toward vasopressin in adipocytes. Am J Physiol. 1997;272:E600–6. doi: 10.1152/ajpendo.1997.272.4.E600. [DOI] [PubMed] [Google Scholar]
- 21.Matsumoto H, Nagasaka T, Hattori A, Rogi T, Tsuruoka N, Mizutani S, Tsujimoto M. Expression of placental leucine aminopeptidase/oxytocinase in neuronal cells and its action on neuronal peptides. Eur J Biochem. 2001;268:3259–66. doi: 10.1046/j.1432-1327.2001.02221.x. [DOI] [PubMed] [Google Scholar]
- 22.Kovacs GL, De Wied D. Peptidergic modulation of learning and memory processes. Pharmacol Rev. 1994;46:269–91. [PubMed] [Google Scholar]
- 23.Engelmann M, Wotjak CT, Neumann I, Ludwig M, Landgraf R. (1996) Behavioural consequences of intracerebral vasopressin and oxytocin: focus on learning and memory. Neurosci Biobehav Rev. 1996;20:341–58. doi: 10.1016/0149-7634(95)00059-3. [DOI] [PubMed] [Google Scholar]
- 24.Lee J, Chai SY, Mendelsohn FA, Morris MJ, Allen AM. Potentiation of cholinergic transmission in the rat hippocampus by angiotensin IV and LVV-hemorphin-7. Neuropharmacology. 2001;40:618–23. doi: 10.1016/S0028-3908(00)00188-X. [DOI] [PubMed] [Google Scholar]
- 25.Fernando RN, Albiston AL, Chai SY. The insulin-regulated aminopeptidase IRAP is colocalised with GLUT4 in the mouse hippocampus-potential role in modulation of glucose uptake in neurones? Eur J Neurosci. 2008;28:588–98. doi: 10.1111/j.1460-9568.2008.06347.x. [DOI] [PubMed] [Google Scholar]
- 26.De Bundel D, Smolders I, Yang R, Albiston AL, Michotte Y, Chai SY. Angiotensin IV and LVV-haemorphin 7 enhance spatial working memory in rats: effects on hippocampal glucose levels and blood flow. Neurobiol Learn Mem. 2009;92:19–26. doi: 10.1016/j.nlm.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 27.Lammerich HP, Busmann A, Kutzleb C, Wendland M, Seiler P, Berger C, Eickelmann P, Meyer M, Forssmann WG, Maronde E. Identification and functional characterization of hemorphins VV-H-7 and LVV-H-7 as low-affinity agonists for the orphan bombesin receptor subtype 3. Br J Pharmacol. 2003;138:1431–40. doi: 10.1038/sj.bjp.0705177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Collinder E, Nyberg F, Sanderson-Nydahl K, Gottlieb-Vedi M, Lindholm A. The opioid haemorphin-7 in horses during low-speed and high-speed treadmill exercise to fatigue. J Vet Med A Physiol Pathol Clin Med. 2005;52:162–5. doi: 10.1111/j.1439-0442.2005.00712.x. [DOI] [PubMed] [Google Scholar]
- 29.Feron D, Begu-Le Corroller A, Piot JM, Frelicot C, Vialettes B, Fruitier-Arnaudin I. Significant lower VVH7-like immunoreactivity serum level in diabetic patients: evidence for independence from metabolic control and three key enzymes in hemorphin metabolism, cathepsin D, ACE and DPP-IV. Peptides. 2009;30:256–61. doi: 10.1016/j.peptides.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 30.Poljak A, McLean CA, Sachdev P, Brodaty H, Smythe GA. Quantification of hemorphins in Alzheimer's disease brains. J Neurosci Res. 2004;75:704–14. doi: 10.1002/jnr.20020. [DOI] [PubMed] [Google Scholar]
- 31.Fruitier I, Garreau I, Lacroix A, Cupo A, Piot JM. Proteolytic degradation of hemoglobin by endogenous lysosomal proteases gives rise to bioactive peptides: hemorphins. FEBS Lett. 1999;447:81–6. doi: 10.1016/S0014-5793(99)00271-9. [DOI] [PubMed] [Google Scholar]
- 32.Choisnard L, Durand D, Vercaigne-Marko D, Nedjar-Arroume N, Dhulster P, Guillochon D. A simple method for the two-step preparation of two pure haemorphins from a total haemoglobin peptic hydrolysate by conventional low-pressure chromatographies. Biotechnol Appl Biochem. 2001;34:173–81. doi: 10.1042/BA20010039. [DOI] [PubMed] [Google Scholar]
- 33.Piot JM, Zhao Q, Guillochon D, Ricart G, Thomas D. Isolation and characterization of two opioid peptides from a bovine hemoglobin peptic hydrolysate. Biochem Biophys Res Commun. 1992;89:101–10. doi: 10.1016/0006-291X(92)91531-T. [DOI] [PubMed] [Google Scholar]
- 34.Jinsmaa Y, Yoshikawa M. Release of hemorphin-5 from human hemoglobin by pancreatic elastase. Biosci Biotechnol Biochem. 2002;66:1130–2. doi: 10.1271/bbb.66.1130. [DOI] [PubMed] [Google Scholar]
- 35.Garreau I, Cucumel K, Dagouassat N, Zhao Q, Cupo A, Piot JM. Hemorphin peptides are released from hemoglobin by cathepsin D. radioimmunoassay against the C-part of V-V-hemorphin-7: an alternative assay for the cathepsin D activity. Peptides. 1997;18:293–300. doi: 10.1016/S0196-9781(96)00284-7. [DOI] [PubMed] [Google Scholar]
- 36.Fruitier I, Garreau I, Piot JM. Cathepsin D is a good candidate for the specific release of a stable hemorphin from hemoglobin in vivo: VV-hemorphin-7. Biochem Biophys Res Commun. 1998;246:719–24. doi: 10.1006/bbrc.1998.8614. [DOI] [PubMed] [Google Scholar]
- 37.Fruitier-Arnaudin I, Cohen M, Coitoux C, Piot JM. In vitro metabolism of LVV-hemorphin-7 by renal cytosol and purified prolyl endopeptidase. Peptides. 2003;24:1201–6. doi: 10.1016/j.peptides.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 38.Murillo L, Piot JM, Coitoux C, Fruitier-Arnaudin I. Brain processing of hemorphin-7 peptides in various subcellular fractions from rats. Peptides. 2006;27:3331–40. doi: 10.1016/j.peptides.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 39.John H, Schulz S, Forssmann WG. Comparative in vitro degradation of the human hemorphin LVV-H7 in mammalian plasma analysed by capillary zone electrophoresis and mass spectrometry. Biopharm Drug Dispos. 2007;28:73–85. doi: 10.1002/bdd.533. [DOI] [PubMed] [Google Scholar]
- 40.Cohen M, Fruitier-Arnaudin I, Piot JM. Hemorphins: substrates and/or inhibitors of dipeptidyl peptidase IV. Hemorphins N-terminus sequence influence on the interaction between hemorphins and DPPIV. Biochimie. 2004;86:31–7. doi: 10.1016/j.biochi.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 41.Fernando RN, Luff SE, Albiston AL, Chai SY. Sub-cellular localization of insulin-regulated membrane aminopeptidase, IRAP to vesicles in neurons. J Neurochem. 2007;102:967–76. doi: 10.1111/j.1471-4159.2007.04659.x. [DOI] [PubMed] [Google Scholar]
- 42.Kiso Y, Kitagawa K, Kawai N, Akita T, Takagi H, Amano H, Fukui K. Neo-kyotorphin (Thr-Ser-Lys-Tyr-Arg), a new analgesic peptide. FEBS Lett. 1983;155:281–4. doi: 10.1016/0014-5793(82)80621-2. [DOI] [PubMed] [Google Scholar]
- 43.Ueda H, Ge M, Satoh M, Takagi H. Non-opioid analgesia of the neuropeptide, neo-kyotorphin and possible mediation by inhibition of GABA release in the mouse brain. Peptides. 1987;8:905–9. doi: 10.1016/0196-9781(87)90079-9. [DOI] [PubMed] [Google Scholar]
- 44.Kolaeva SH, Lee TF, Wang LC, Paproski SM. Effect of intracerebroventricular injection of neokyotorphin on the thermoregulatory responses in rats. Brain Res Bull. 1990;25:407–10. doi: 10.1016/0361-9230(90)90228-R. [DOI] [PubMed] [Google Scholar]
- 45.Godlevsky LS, Shandra AA, Mikhaleva II, Vastyanov RS, Mazarati AM. Seizure-protecting effects of kyotorphin and related peptides in an animal model of epilepsy. Brain Res Bull. 1995;37:223–6. doi: 10.1016/0361-9230(94)00274-5. [DOI] [PubMed] [Google Scholar]
- 46.Pokrovsky VM, Osadchiy OE. Regulatory peptides as modulators of vagal influence on cardiac rhythm. Can J Physiol Pharmacol. 1995;73:1235–45. doi: 10.1139/y95-175. [DOI] [PubMed] [Google Scholar]
- 47.Nedjar-Arroume N, Dubois-Delval V, Miloudi K, Daoud R, Krier F, Kouach M, Briand G, Guillochon D. Isolation and characterization of four antibacterial peptides from bovine hemoglobin. Peptides. 2006;27:2082–9. doi: 10.1016/j.peptides.2006.03.033. [DOI] [PubMed] [Google Scholar]
- 48.Popova IY, Vinogradova OS, Kokoz YM, Ziganshin RKh, Ivanov VT. Neuropeptide modulation of evoked responses of neurons in the medial septal region of hibernating ground squirrels in conditions of chronic isolation of the medial septal region from preoptic-hypothalamic structures. Neurosci Behav Physiol. 2003;33:521–8. doi: 10.1023/A:1023475503848. [DOI] [PubMed] [Google Scholar]
- 49.Bronnikov GE, Kolaeva SG, Dolgacheva LP, Kramarova LI. Kyotorphin suppresses proliferation and Ca2 + signaling in brown preadipocytes. Bull Exp Biol Med. 2006;141:223–5. doi: 10.1007/s10517-006-0133-0. [DOI] [PubMed] [Google Scholar]
- 50.Blishchenko EY, Kalinina OA, Sazonova OV, Khaidukov SV, Egorova NS, Surovoy AY, Philippova MM, Vass AA, Karelin AA, Ivanov VT. Endogenous fragment of hemoglobin, neokyotorphin, as cell growth factor. Peptides. 2001;22:1999–2008. doi: 10.1016/S0196-9781(01)00565-4. [DOI] [PubMed] [Google Scholar]
- 51.Sazonova OV, Blishchenko EY, Kalinina OA, Egorova NS, Surovoy AY, Philippova MM, Karelin AA, Ivanov VT. Proliferative activity of neokyotorphin-related hemoglobin fragments in cell cultures. Protein Pept Lett. 2003;10:386–95. doi: 10.2174/0929866033478780. [DOI] [PubMed] [Google Scholar]
- 52.Sazonova OV, Blishchenko EY, Tolmazova AG, Khachin DP, Leontiev KV, Karelin AA, Ivanov VT. Stimulation of fibroblast proliferation by neokyotorphin requires Ca influx and activation of PKA, CaMK II and MAPK/ERK. FEBS J. 2007;274:474–84. doi: 10.1111/j.1742-4658.2006.05594.x. [DOI] [PubMed] [Google Scholar]
- 53.Hazato T, Kase R, Ueda H, Takagi H, Katayama T. Inhibitory effects of the analgesic neuropeptides kyotorphin and neo-kyotorphin on enkephalin-degrading enzymes from monkey brain. Biochem Int. 1986;12:379–83. [PubMed] [Google Scholar]
- 54.Ticu EL, Vercaigne-Marko D, Huma A, Artenie V, Toma O, Guillochon D. A kinetic study of bovine haemoglobin hydrolysis by pepsin immobilized on a functionalized alumina to prepare hydrolysates containing bioactive peptides. Biotechnol Appl Biochem. 2004;39:199–208. doi: 10.1042/BA20030131. [DOI] [PubMed] [Google Scholar]
- 55.Lignot B, Froidevaux R, Nedjar-Arroume N, Guillochon D. Solvent effect on kinetics of appearance of neokyotorphin, VV-haemorphin-4 and a bradykinin-potentiating peptide in the course of peptic hydrolysis of bovine haemoglobin. Biotechnol Appl Biochem. 1999;30:201–7. [PubMed] [Google Scholar]
- 56.Zhao Q, Piot JM. Neokyotorphin formation and quantitative evolution following human hemoglobin hydrolysis with cathepsin D. Peptides. 1998;19:759–66. doi: 10.1016/S0196-9781(98)00002-3. [DOI] [PubMed] [Google Scholar]
- 57.Kase R, Sekine R, Katayama T, Takagi H, Hazato T. Hydrolysis of neo-kyotorphin (Thr-Ser-Lys-Tyr-Arg) and [Met]enkephalin-Arg6-Phe7 by angiotensin-converting enzyme from monkey brain. Biochem Pharmacol. 1986;35:4499–503. doi: 10.1016/0006-2952(86)90770-7. [DOI] [PubMed] [Google Scholar]
- 58.Shiomi H, Kuraishi Y, Ueda H, Harada Y, Amano H, Takagi H. Mechanism of kyotorphin-induced release of Met-enkephalin from guinea pig striatum and spinal cord. Brain Res. 1981;221:161–9. doi: 10.1016/0006-8993(81)91070-2. [DOI] [PubMed] [Google Scholar]
- 59.Moisan S, Harvey N, Beaudry G, Forzani P, Burhop KE, Drapeau G, Rioux F. Structural requirements and mechanism of the pressor activity of Leu-Vall_Val-hemorphin-7, a fragment of hemoglobin beta-chain in rats. Peptides. 1998;19:119–31. doi: 10.1016/S0196-9781(97)00273-8. [DOI] [PubMed] [Google Scholar]
- 60.Dagouassat N, Garreau I, Zhao Q, Sannier F, Piot JM. Kinetic of in vitro generation of some hemorphins: early release of LVV-hemorphin-7, precursor of VV-hemorphin-7. Neuropeptides. 1996;30:1–5. doi: 10.1016/S0143-4179(96)90047-5. [DOI] [PubMed] [Google Scholar]
- 61.Blais PA, Cote J, Morin J, Larouche A, Gendron G, Fortier A, Regoli D, Neugebauer W, Gobeil F., Jr Hypotensive effects of hemopressin and bradykinin in rabbits, rats and mice. A comparative study. Peptides. 2005;26:1317–22. doi: 10.1016/j.peptides.2005.03.026. [DOI] [PubMed] [Google Scholar]
- 62.Lippton H, Lin B, Gumusel B, Witriol N, Wasserman A, Knight M. Hemopressin, a hemoglobin fragment, dilates the rat systemic vascular bed through the release of nitric oxide. Peptides. 2006;27:2284–8. doi: 10.1016/j.peptides.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 63.Dale CS, de Lima Pagano R, Rioli V, Hyslop S, Giorgi R, Ferro ES. Antinociceptive action of hemopressin in experimental hyperalgesia. Peptides. 2005;26:431–6. doi: 10.1016/j.peptides.2004.10.026. [DOI] [PubMed] [Google Scholar]
- 64.Randall LO, Selitto JJ. A method for measurement of analgesic activity on inflamed tissue. Arch Int Pharmacodyn Thér. 1957;111:409–19. [PubMed] [Google Scholar]
- 65.Gupta A, Décaillot FM, Gomes I, Tkalych O, Heimann AS, Ferro ES, Devi LA. Conformation state-sensitive antibodies to G-protein-coupled receptors. J Biol Chem. 2007;282:5116–24. doi: 10.1074/jbc.M609254200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dodd GT, Mancini G, Lutz B, Luckman SM. The peptide hemopressin acts through CB1 cannabinoid receptors to reduce food intake in rats and mice. J Neurosci. 2010;30:7369–76. doi: 10.1523/JNEUROSCI.5455-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shoji M. Cerebrospinal fluid Abeta40 and Abeta42: natural course and clinical usefulness. Front Biosci. 2002;7:d997–1006. doi: 10.2741/shoji. [DOI] [PubMed] [Google Scholar]
- 68.Giuffrida ML, Caraci F, Pignataro B, Cataldo S, De Bona P, Bruno V, Molinaro G, Pappalardo G, Messina A, Palmigiano A, Garozzo D, Nicoletti F, Rizzarelli E, Copani A. Beta-amyloid monomers are neuroprotective. J Neurosci. 2009;29:10582–7. doi: 10.1523/JNEUROSCI.1736-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Walsh DM, Klyubin I, Fadeeva JV, Cullen WK, Anwyl R, Wolfe MS, Rowan MJ, Selkoe DJ. Naturally secreted oligomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo. Nature. 2002;416:535–9. doi: 10.1038/416535a. [DOI] [PubMed] [Google Scholar]
- 70.Shankar GM, Li S, Mehta TH, Garcia-Munoz A, Shepardson NE, Smith I, Brett FM, Farrell MA, Rowan MJ, Lemere CA, Regan CM, Walsh DM, Sabatini BL, Selkoe DJ. Amyloid-beta protein dimers isolated directly from Alzheimer's brains impair synaptic plasticity and memory. Nat Med. 2008;14:837–42. doi: 10.1038/nm1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lambert MP, Barlow AK, Chromy BA, Edwards C, Freed R, Liosatos M, Morgan TE, Rozovsky I, Trommer B, Viola KL, Wals P, Zhang C, Finch CE, Krafft GA, Klein WL. Diffusible, nonfibrillar ligands derived from Abeta1-42 are potent central nervous system neurotoxins. Proc Natl Acad Sci USA. 1998;95:6448–53. doi: 10.1073/pnas.95.11.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Arevalo MA, Roldan PM, Chacón PJ, Rodríguez-Tebar A. Amyloid beta serves as an NGF-like neurotrophic factor or acts as a NGF antagonist depending on its concentration. J Neurochem. 2009;111:1425–33. doi: 10.1111/j.1471-4159.2009.06412.x. [DOI] [PubMed] [Google Scholar]
- 73.Watson D, Castaño E, Kokjohn TA, Kuo YM, Lyubchenko Y, Pinsky D, Connolly ES, Jr, Esh C, Luehrs DC, Stine WB, Rowse LM, Emmerling MR, Roher AE. Physicochemical characteristics of soluble oligomeric Abeta and their pathologic role in Alzheimer's disease. Neurol Res. 2005;27:869–81. doi: 10.1179/016164105X49436. [DOI] [PubMed] [Google Scholar]
- 74.Bhaskaran M, Chen H, Chen Z, Liu L. Hemoglobin is expressed in alveolar epithelial type II cells. Biochem Biophys Res Commun. 2005;333:1348–52. doi: 10.1016/j.bbrc.2005.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Newton DA, Rao KM, Dluhy RA, Baatz JE. Hemoglobin is expressed by alveolar epithelial cells. J Biol Chem. 2006;281:5668–76. doi: 10.1074/jbc.M509314200. [DOI] [PubMed] [Google Scholar]
- 76.Wride MA, Mansergh FC, Adams S, Everitt R, Minnema SE, Rancourt DE, Evans MJ. Expression profiling and gene discovery in the mouse lens. Mol Vis. 2003;9:360–96. [PubMed] [Google Scholar]
- 77.Liu L, Zeng M, Stamler JS. Hemoglobin induction in mouse macrophages. Proc Natl Acad Sci USA. 1999;96:6643–7. doi: 10.1073/pnas.96.12.6643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nishi H, Inagi R, Kato H, Tanemoto M, Kojima I, Son D, Fujita T, Nangaku M. Hemoglobin is expressed by mesangial cells and reduces oxidant stress. J Am Soc Nephrol. 2008;19:1500–8. doi: 10.1681/ASN.2007101085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Biagioli M, Pinto M, Cesselli D, Zaninello M, Lazarevic D, Roncaglia P, Simone R, Vlachouli C, Plessy C, Bertin N, Beltrami A, Kobayashi K, Gallo V, Santoro C, Ferrer I, Rivella S, Beltrami CA, Carninci P, Raviola E, Gustincich S. Unexpected expression of alpha- and beta-globin in mesencephalic dopaminergic neurons and glial cells. Proc Natl Acad Sci USA. 2009;106:15454–9. doi: 10.1073/pnas.0813216106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Richter F, Meurers BH, Zhu C, Medvedeva VP, Chesselet MF. Neurons express hemoglobin alpha- and beta chains in rat and human brains. J Comp Neurol. 2009;515:538–47. doi: 10.1002/cne.22062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Schelshorn DW, Schneider A, Kuschinsky W, Weber D, Krüger C, Dittgen T, Bürgers HF, Sabouri F, Gassler N, Bach A, Maurer MH. Expression of hemoglobin in rodent neurons. J Cereb Blood Flow Metab. 2009;29:585–95. doi: 10.1038/jcbfm.2008.152. [DOI] [PubMed] [Google Scholar]
- 82.Dugas JC, Tai YC, Speed TP, Ngai J. Barres BA Functional genomic analysis of oligodendrocyte differentiation. J Neurosci. 2006;26:10967–83. doi: 10.1523/JNEUROSCI.2572-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Setton-Avruj CP, Musolino PL, Salis C, Allo M, Bizzozero O, Villar MJ, Soto EF, Pasquini JM. Presence of alpha-globin mRNA and migration of bone marrow cells after sciatic nerve injury suggests their participation in the degeneration/regeneration process. Exp Neurol. 2007;203:568–78. doi: 10.1016/j.expneurol.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 84.Hook V, Funkelstein L, Lu D, Bark S, Wegrzyn J, Hwang SR. Proteases for processing proneuropeptides into peptide neurotransmitters and hormones. Annu Rev Pharmacol Toxicol. 2008;48:393–423. doi: 10.1146/annurev.pharmtox.48.113006.094812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wenzel T, Baumeister W. Thermoplasma acidophilum proteasomes degrade partially unfolded and ubiquitin-associated proteins. FEBS Lett. 1993;326:215–8. doi: 10.1016/0014-5793(93)81793-Y. [DOI] [PubMed] [Google Scholar]
- 86.Pacifici RE, Kono Y, Davies KJ. Hydrophobicity as the signal for selective degradation of hydroxyl radical-modified hemoglobin by the multicatalytic proteinase complex, proteasome. J Biol Chem. 1993;268:15405–11. [PubMed] [Google Scholar]
- 87.Tai HC, Schuman EM. Ubiquitin, the proteasome and protein degradation in neuronal function and dysfunction. Nat Rev Neurosci. 2008;9:826–38. doi: 10.1038/nrn2499. [DOI] [PubMed] [Google Scholar]
- 88.Kisselev AF, Akopian TN, Woo KM, Goldberg AL. The sizes of peptides generated from protein by mammalian 26 and 20S proteasomes. Implications for understanding the degradative mechanism and antigen presentation. J Biol Chem. 1999;274:3363–71. doi: 10.1074/jbc.274.6.3363. [DOI] [PubMed] [Google Scholar]
- 89.Nickel W, Rabouille C. Mechanisms of regulated unconventional protein secretion. Nat Rev Mol Cell Biol. 2009;10:148–55. doi: 10.1038/nrm2617. [DOI] [PubMed] [Google Scholar]
- 90.Borst P, Elferink RO. Mammalian ABC transporters in health and disease. Annu Rev Biochem. 2002;71:537–92. doi: 10.1146/annurev.biochem.71.102301.093055. [DOI] [PubMed] [Google Scholar]
- 91.Procko E, Gaudet R. Antigen processing and presentation: TAPping into ABC transporters. Curr Opin Immunol. 2009;21:84–91. doi: 10.1016/j.coi.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 92.Procko E, O'Mara ML, Bennett WF, Tieleman DP, Gaudet R. The mechanism of ABC transporters: general lessons from structural and functional studies of an antigenic peptide transporter. FASEB J. 2009;23:1287–302. doi: 10.1096/fj.08-121855. [DOI] [PubMed] [Google Scholar]