Abstract
Hepatitis B vaccination typically requires a multi-dose administration protocol over a course of 3–6 months. Aiming at developing a single-shot formulation for hepatitis B vaccine (hepatitis B surface antigen (HBsAg)), a novel vaccine delivery system, the composite microspheres of alginate–chitosan–poly(lactic-co-glycolic acid) (PLGA), was synthesized by a two-step preparation. The composite microspheres showed distinct advantages over the conventional PLGA microspheres in aspects of the high loading capacity and the elimination of lyophilizing process. The loading capacity of the composite microspheres was about seven times higher than those in the conventional PLGA microspheres, due to the protein-friendly microenvironment created by the hydrophilic alginate–chitosan cores of the composite microspheres. This vaccine delivery system was shown to be able to induce robust immune responses by single injection and display no significant difference in HBsAg-specific antibody levels compared to the double-injection method.
Key words: alginate, chitosan, microsphere, polylactic acid, vaccine delivery
INTRODUCTION
Hepatitis B Virus (HBV) infection is a global public health problem, especially in developing countries that harbor about 90% of the total number of the HBV-infected patients (1). It has been well established that chronic HBV infection leads to chronic hepatitis, liver cirrhosis, and hepatocellular carcinoma (2). Approximately 30% of the world’s population has been infected with HBV, and 350 million are HBV persistent carriers (3). However, no effective therapy against HBV infection is clinically available so far. Therefore, preventive vaccination still remains as the most cost-effective solution for HBV-related disease control.
Currently, the immunization course for hepatitis B vaccine requires three injections at 0, 1, and 6 months to elicit effectively protective responses. However, the completion rates of these standard hepatitis B immunization protocols among adolescents only reach 11% due to the poor compliance of the multi-dose protocol (4). This problem is more severe in developing countries which generally lack permanent clinics and healthcare professionals to implement this protocol at appropriate periods. This, in turn, exacerbates the already poor vaccination coverage in such areas. Development of single-dose vaccine formulations may alleviate this problem by eliminating of the need for follow-up visits to the clinics. However, it is a formidable challenge to develop a single-dose of hepatitis B surface antigen (HBsAg) vaccine with programmed-release profiles, which can auto-boost an immune response in a manner comparable to three injections of a common aluminum-formulated vaccine. Poly(lactic-co-glycolic acid) (PLGA)-based systems are promising candidate for vaccines in that PLGA microspheres may simplify vaccination programs, enhance efficiency (5,6), and increase the immunogenicity of the encapsulated antigen (7). However, if applied as a single-shot vaccine delivery system, several common problems need to be solved for PLGA-based drug carriers (8). First, the encapsulation processes of vaccine into PLGA microspheres usually compromise the activity of proteins. Secondly, the low loading efficiencies of protein drugs are normally seen in PLGA microspheres, presumably owing to the incompatibility between the hydrophilic nature of protein and the hydrophobic PLGA polymer. Thirdly, a high burst effect may drain off the payload and leave an insufficient dose behind. Thus, it has been reported that after a single injection of vaccine, an insufficient subsequent immunity was observed despite an appropriate burst release of antigen as the first dose (9), which indicated that subsequent release of the vaccine, acting as a booster, also played an important role in eliciting adequate immunity.
A novel type of composite microspheres of alginate–chitosan–PLGA, developed in our laboratory, was demonstrated to possess advantages of improving the stability of encapsulated proteins and increasing the subsequent release drug amount (10). We reasoned that these composite microspheres could be developed into a novel vaccine delivery system. Therefore, we investigated the feasibility of the immune effects of this type of composite microspheres for HBsAg in this presented study.
MATERIALS AND METHODS
Chitosan (≥80% deacetylation, MW 80 kDa) was purchased from Yuhuan Oceanic Biochemistry Co. Ltd., China. Alginate (viscosity 6 mPa s for 1% at 25°C) and polyvinyl alcohol (PVA; average MW 30–70 kDa) were obtained from Shanghai Chemical Reagent Company of Chinese Medicine. PLGA (50:50) and PLGA (70:30) (MW 50 kDa) were procured from Shandong Medical Instrumental Institute, China. Polyoxyethylene sorbitan trioleate (Tween 80), sorbitan trioleate (Span 80), and all other reagents were analytical grade and purchased from Huadong Medical Co., China. Recombinant HBsAg secreted by yeast (185 μg/ml) and hepatitis B vaccine with aluminum hydroxide as the adjuvant (1 mg/ml) were all provided by Beijing Institute of Biological Products. Bicinchoninic acid (BCA) protein assay kits were procured from Beyotime Co., China. Diagnostic kit for antibody to hepatitis B surface antigen (enzyme-linked immunosorbent assay (ELISA)) was produced by Shanghai Kehua Company; goat anti-mouse IgG conjugated horse radish peroxidase (HRP), mixed IgA, IgG, and IgM were produced by KPL, USA; goat anti-mouse conjugated HRP IgG1 and IgG2a were produced by South Biotech, USA.
Conventional and Composite PLGA Microspheres of HBsAg
Conventional and composite PLGA microspheres of HBsAg were fabricated according to our previously reported method (10). The key steps of preparation are briefly described as follows:
For HBsAg-PLGA microspheres, a w/o primary emulsion was prepared by mixing 200 µl of HBsAg aqueous solution (185 µg/ml) and 4 ml of 7.5% PLGA methylene chloride solution. This primary emulsion was added into 40 ml of 1.0% PVA and stirred at 12,000 rpm with a mechanical stirrer (FJ-200 homogenizer, Shanghai Biaoben Model Factory, China) for the preparation of a w/o/w emulsion. After removal of the organic solvent, the HBsAg-loaded PLGA microspheres were obtained.
For the alginate–chitosan microcapsules, 10 ml of the internal aqueous phase containing HBsAg solution (185 µg/ml), 2% alginate solution, and distilled water at the volume ratio of 3:5:2 was used to prepare an emulsion with 20 ml of isooctane containing 5% Span 80 and stirred at 16,000 rpm. A subsequent addition of 1.5 ml Tween 80 was used as secondary emulsifier and then 4 ml of 8% calcium chloride as a cross-linker. The resulting microspheres were further solidified by addition of 20 ml of isopropyl alcohol. The microspheres were incubated with chitosan solution (0.5%, pH 4) to form alginate–chitosan complex membranes. The obtained alginate–chitosan microcapsules were used for the preparation of the composite microspheres.
For the alginate–chitosan–PLGA composite microspheres, the alginate–chitosan microcapsules were suspended in 3 ml of acetonitrile containing PLGA (50:50, 0.24 g/ml; or 70:30, 0.3 g/ml) with the ratio of microspheres to PLGA in weight between 1 to 9 and 1 to 12. The suspension was added into the soybean oil containing 6.25% Span 80 with a stirring speed of 1,000 rpm and then 12,000 rpm. After washing with petroleum ether and complete volatilization at 37°C, the resulting HBsAg-loaded composite microspheres were collected.
Morphology Observation
Characterization of the PLGA microspheres and PLGA composite microspheres was carried out with optical microscopy and/or scanning electron microscopy (SEM, XL30, PHILIPS). The size of the alginate–chitosan microcapsules was measured with a particle size analyzer (Zetasizer 3000 HAS, Malvern Instrument, England). To acquire a clear morphologic image of the alginate–chitosan–PLGA composite microspheres, large composite microspheres of superoxide dismutase (SOD), a model protein drug, were prepared and observed under SEM.
Determination of the Loading/Entrapment Efficiency of HBsAg
HBsAg entrapped in either of these microspheres was extracted by the method we previously reported (10). The quantitative HBsAg in the extracts was then determined by Micro-BCA method. The loading capacity and entrapment efficiency were calculated according to the following formulas:
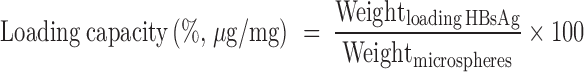 |
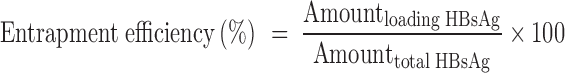 |
Determination of the Residual Organic Solvents in Microspheres
The sample solutions were prepared by dissolving 200 mg of microspheres in 10 ml of N,N-dimethylformamide with sonication. The residual acetonitrile and methylene chloride in these microspheres were analyzed using headspace gas chromatography (HGC). HGC conditions are described as follows: chromatographic column, HP-5 capillary column (Agilent, 30 m × 0.25 mm); operation temperatures, column (50°C), flame ionization detector (220°C) injector (240°C); carrier gas, nitrogen with flow rate 1.0 ml/min; the split ratio, 20:1; the vial of liquid sample was kept at 90°C (Agilent 7694E) for 10 min and injected 1.0 ml into GC column. All the residual amounts of acetonitrile and dichloromethane were compared with the allowed limits allowed in International Conference on Harmonization.
Immunization Protocol of Mice
All the animal experiments were approved by the Animal Care and Use Committee of Zhejiang University. Female Balb/C mice (6–8 weeks, 20–22 g) were obtained from the Animal Center of Zhejiang University School of Medicine (Hangzhou, China). The mice were maintained on a normal diet throughout the study, and 35 of mice were randomly divided into five groups (Table I). All the microspheres were dispersed by sterile saline. Each mouse was injected subcutaneously in the back with 0.25 ml of solution or suspension. Blood samples were collected from the tail vein at 0, 1, 2, and 3 months. The antibody levels in serum were determined by a quantitative ELISA method. The antibody levels of immunized mice, including the mixed anti-HBsAg antibodies (IgA + IgM + IgG) and antibody subtypes (IgG, IgG1, and IgG2a), were expressed as the logarithmic geometric mean of the antibody titer (log10GMT).
Table I.
Immunization Study in Mice (n = 7)
| Groups | Formulation | Immunization at |
|---|---|---|
| A | HBsAg–aluminum in saline (control) | Month 0 and 1 |
| B | HBsAg–PLGA microspheres | Month 0 |
| C | Composite HBsAg–PLGA microspheres | Month 0 |
| D | A mixture of IFN-α-2a (2,500 IU) and composite HBsAg–PLGA microspheres | Month 0 |
| E | A mixture of equal dose of HBsAg–aluminum and composite HBsAg–PLGA microspheres | Month 0 |
Each mouse was injected subcutaneously in the back with 0.25 ml of HBsAg solution or microsphere suspensions containing a total of 10 µg HBsAg; the PLGA composite or non-composite microspheres containing equal amounts of PLGA (50:50) and PLGA (70:30)
ELISA Procedure
The assay was processed according to the protocol provided by the ELISA kit. In brief, microwell strips were coated with 100 μl of 1 μg/ml of HBsAg per well and incubated overnight at 4°C, followed by a thorough wash. The serum samples, in a series of double dilution, were added to the microwells and incubated for 30 min at 37°C. After washing, the microwells were incubated with 50 μl/well of the diluted goat anti-mouse antibody HRP conjugate (1:3,000 for IgG or the mixture of IgA + IgM + IgG; 1:6,000 for IgG1 or IgG2a) for 30 min at 37°C. The microwells were washed again, and then the color developer A and B were added. After incubation for 15 min at 37°C, the reaction was stopped by adding the stop solution and immediately read at 405 nm using an ELISA spectrophotometer (BioRad).
The antibody titers were calculated by ODsample/ODnegative ≥ 2.1, and serum sample (1:100 dilution) from non-immunization mice was used as negative control.
Statistical Analysis
Results were presented as mean ± SD, and the data were analyzed statistically using Student’s t test for comparison between any two groups. Differences with p < 0.05 were considered to be significant.
RESULTS
Characteristics of Alginate–Chitosan–PLGA Composite Microspheres
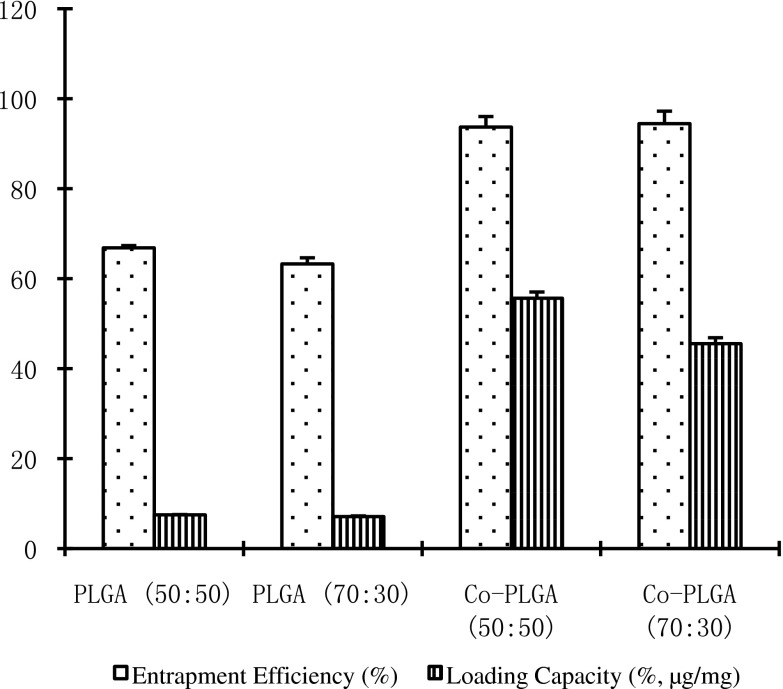
The alginate–chitosan microcapsules about 300 nm in size were prepared and then entrapped into PLGA to form the composite microspheres with a size less than 5 μm, and the PLGA microspheres were prepared with the same size. The entrapment efficiencies and loading efficiencies of HBsAg in PLGA (50:50), PLGA (70:30), composite PLGA (50:50), and composite PLGA (70:30) microspheres were presented in Fig. 1.
Fig. 1.
The entrapment efficiencies and loading capacities of HBsAg in conventional and composite PLGA microspheres
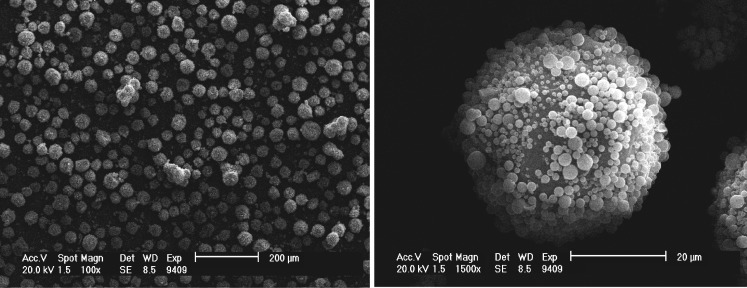
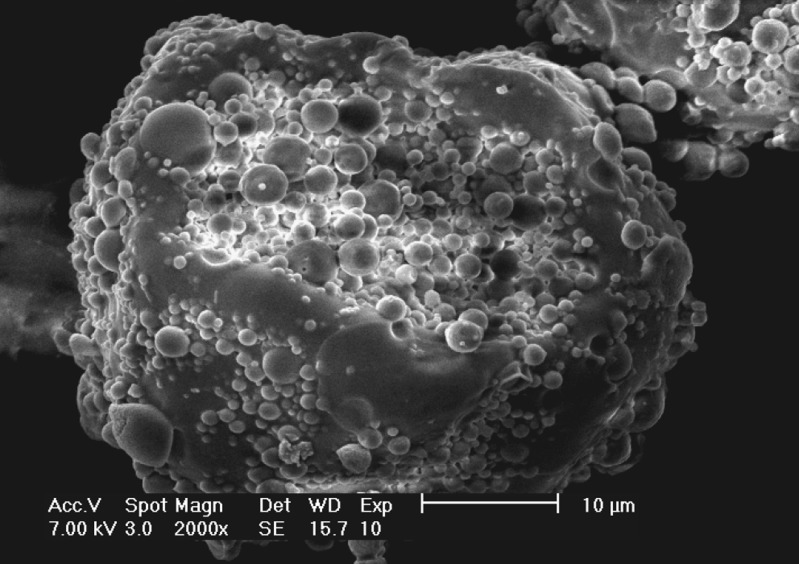
Large composite microspheres with the model drug were prepared, and the morphology was observed by SEM (Fig. 2). Observed from the cross-section of the large composite microspheres (Fig. 3), many small spherical microcapsules scattered in PLGA to form whole composite microspheres.
Fig. 2.
The scanning electron micrographs of large alginate–chitosan–PLGA composite microspheres of SOD, a model protein drug
Fig. 3.
SEM of the cross-section of large alginate–chitosan–PLGA composite microspheres of SOD as a model drug
Determination of Solvent Residual Amount in the Microspheres
The peak areas of acetonitrile and dichloromethane in samples of the composite PLGA microspheres and the conventional PLGA microspheres were determined as described. The maximum concentrations of acetonitrile and methylene chloride in the tested composite and conventional PLGA (50:50) microspheres were 5.6 and 37.8 μg/g, respectively.
Evaluation of the Antibody Levels
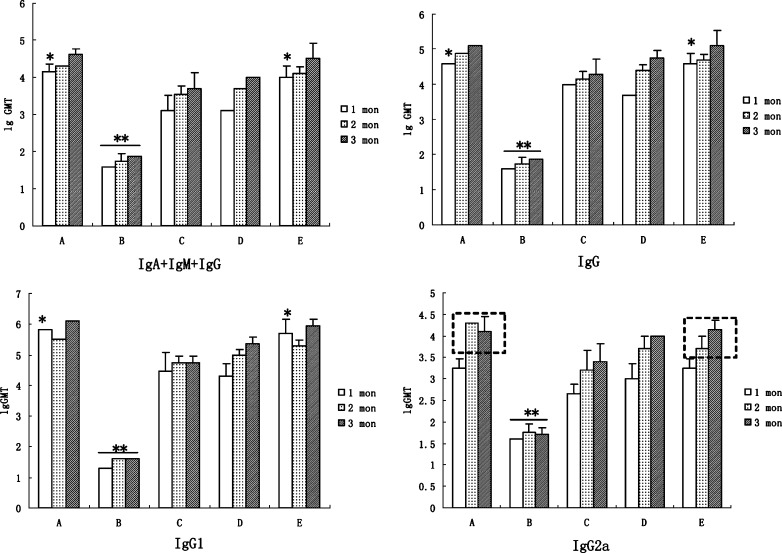
The antibody levels of immunized mice were shown in Fig. 4. No statistical difference had been found between each of the single-injection groups of the composite PLGA microspheres, i.e., C, D, or E, and the control group (A) of double injection of HBsAg–aluminum (p > 0.05). Among them, the antibody titers of groups A and E rapidly increased to relatively high levels at the 1-month mark and displayed statistical differences from those of the other groups (p < 0.05). However, the antibody titers of PLGA non-composite microspheres were significantly lower than those of their corresponding counterparts (p < 0.01) at all sampling time points. In the antibody subgroups, the levels of IgG2a induced by alginate–chitosan–PLGA composite microspheres were gradually ascending, while those in the control group displayed a decreasing profile (Fig. 4, IgG2a section, box with dotted line).
Fig. 4.
Logarithmic geometric mean of total antibody titer (lgGMT) obtained from Balb/C mice after immunizations at 0-, 1-, 2-, and 3-month intervals (the total dose of HBsAg was 10 μg/mouse). A, double injections of aluminum–HBsAg vaccine at 0 and 1 month; B, equal amount of microspheres of PLGA (50:50) and PLGA (70:30); C, the equal amount of composite microspheres of PLGA (50:50) and PLGA (70:30); D, C and IFN of 2,500 IU; E, mixture of aluminum–HBsAg vaccine (5 μg) and composite microspheres of PLGA (50:50; 2.5 μg) and PLGA (70:30; 2.5 μg). Note: *p < 0.05, **p < 0.01
DISCUSSION
These PLGA composite microspheres possess several distinct advantages over other conventional PLGA non-composite counterparts. First, high loading efficiencies of HBsAg seen in the composite PLGA (50:50) and composite PLGA (70:30) microspheres were 7.4 and 6.4 times over those non-composite PLGA (50:50) and PLGA (70:30) counterparts. The high encapsulation efficiency of HBsAg in the PLGA composite microspheres was apparently due to their hydrophilic alginate–chitosan cores. Secondly, the presented preparation method not only avoids the lyophilizing process, which potentially causes the activity loss of the entrapped protein, but also provides a protein-friendly microenvironment created by the pre-forming hydrophilic alginate–chitosan cores. Since the bottleneck for the application of PLGA microspheres as a vaccine delivery system is the instability of entrapped protein antigen (11,12), our method offers a promising solution to this problem.
The in vitro release profiles of the non-composite PLGA microspheres, alginate–chitosan microcapsules, and the alginate–chitosan–PLGA composite microspheres have been previously reported by our group. In short, the burst release of model drug in 24 h from alginate–chitosan–PLGA (50:50) composite microspheres was 38.9% in phosphate buffer solution (PBS) and 14.0% in saline (10), lower than 96.8% in PBS or 45.5% in saline from alginate–chitosan microcapsules (13) and >50% in PBS or saline from conventional PLGA microspheres (10). The release from composite microspheres was more complete, 71% at 13 weeks vs 66% from PLGA microspheres (10). Since similar efficacies were achieved by both the two-dose and three-dose injection vaccination protocols (14–16), in this study the control group was given two-dose injection of the HBsAg-aluminum vaccine. In addition, although IFN-α-2a was reported as an efficient immuno-adjuvant in DNA vaccines (17), in our experiments IFN-α-2a did not show any significant immune enhancement.
Due to the benefits of the subcutaneous route of vaccine administration, e.g., better immune effects than that of the intramuscular injection (18,19), the subcutaneous injection was selected as the administration route. The in vivo fate and uptake pathways of PLGA microspheres following subcutaneous injection were reported (20). A mixture of HBsAg-loaded PLGA microspheres with different release behaviors possessed a potential advantage as a single-dose vaccine (21). Therefore, a mixture of PLGA composite microspheres containing equal amounts of PLGA (50:50) and PLGA (70:30) was used in the experiment above and the same procedure to the PLGA non-composite microspheres.
It should be pointed out that local adverse reactions were observed in this study. Bumps appeared around the subcutaneous injection sites of mice after the administration of microsphere suspension samples, but no bumps were seen with the injection of the HBsAg solution sample. These bumps vanished gradually within 6 weeks. However, skin rashes were not found in these areas. The materials (alginate, chitosan, PLGA) are known for their biocompatibility and biodegradability and have been widely applied in drug delivery and tissue engineering (22,23). The composite microspheres would raise little safety concern on the vaccination application. Therefore, bumps may simply result from the deposit of the injected microspheres.
The antibody levels elicited by the single injection of composite microspheres were high and comparable to that after double injection of HBsAg-aluminum. Interestingly, the antibody titers of groups A and E rapidly increased to relatively high levels after 1 month. Another positive observation was that the levels of IgG2a induced by PLGA composite microspheres were gradually ascending, while those in the control group displayed a decreasing profile. Serum IgG is the primary indicator of protection induced by preventive vaccines. The HBsAg composite microspheres induced significantly higher levels of both IgG1 and IgG2a compared to HBsAg–PLGA microspheres. Since Th1-type immune responses are indicated by the IgG2a production which is associated with the cellular immunity, a significant benefit of the composite microspheres may be derived from their effect on eliciting more comprehensive immunities. The superiority in eliciting higher and more comprehensive immune responses of the PLGA composite microspheres over the non-composite microspheres is presumably due to the presence of hydrophilic cores as well as the reduced burst effect, thus allowing better release of HBsAg from the composite microspheres.
CONCLUSION
A novel composite microspheres of alginate–chitosan–poly(lactic-co-glycolic acid) of hepatitis B surface antigen as single -injection vaccines is shown to improve on the low entrapment efficiency of the conventional HBsAg–PLGA microspheres and to maintain the higher antibody levels achieved by the double injections of HBsAg-aluminum vaccine and single dose of PLGA microspheres of HBsAg. This system holds some promise for improving PLGA-based protein delivery and achieving single-shot vaccination.
Acknowledgments
The project is supported by the Zhejiang Provincial Natural Science Foundation of China (No. Y2080185).
References
- 1.Akbar SM, Hiasa Y, Mishiro S, Onji M. Treatment of hepatitis B virus-infected patients: utility of therapeutic recommendations in developing countries. Expert Opin Pharmacother. 2009;10(10):1605–14. doi: 10.1517/14656560903005579. [DOI] [PubMed] [Google Scholar]
- 2.Hann HW, Hann RS, Maddrey WC. Hepatitis B virus infection in 6,130 unvaccinated Korean–Americans surveyed between 1988 and 1990. Am J Gastroenterol. 2007;102(4):767–72. doi: 10.1111/j.1572-0241.2007.01060.x. [DOI] [PubMed] [Google Scholar]
- 3.Zeng Z, Guan L, An P, Sun S, O’Brien SJ, Winkler CA. A population-based study to investigate host genetic factors associated with hepatitis B infection and pathogenesis in the Chinese population. BMC Infect Dis. 2008;8:1. doi: 10.1186/1471-2334-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heron L, Selnikova O, Moiseieva A, Van-Damme P, van-der-Wielen M, Levie K, et al. Immunogenicity, reactogenicity and safety of two-dose versus three-dose (standard care) hepatitis B immunisation of healthy adolescents aged 11–15 years: a randomised controlled trial. Vaccine. 2000;25(15):2817–22. doi: 10.1016/j.vaccine.2006.12.021. [DOI] [PubMed] [Google Scholar]
- 5.Waeckerle-Men Y, Allmen EU, Gander B, Scandella E, Schlosser E, Schmidtke G, et al. Encapsulation of proteins and peptides into biodegradable poly(d,l-lactide-co-glycolide) microspheres prolongs and enhances antigen presentation by human dendritic cells. Vaccine. 2006;24(11):1847–57. doi: 10.1016/j.vaccine.2005.10.032. [DOI] [PubMed] [Google Scholar]
- 6.Quintilio W, Takata CS, Sant’Anna OA, da-Costa MH, Raw I. Evaluation of a diphtheria and tetanus PLGA microencapsulated vaccine formulation without stabilizers. Curr Drug Deliv. 2009;6(3):297–304. doi: 10.2174/156720109788680886. [DOI] [PubMed] [Google Scholar]
- 7.Cui C, Stevens VC, Schwendeman SP. Injectable polymer microspheres enhance immunogenicity of a contraceptive peptide vaccine. Vaccine. 2007;25(3):500–9. doi: 10.1016/j.vaccine.2006.07.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu FH, Zhang Q. Recent advances in the preparation progress of protein/peptide drug loaded PLA/PLGA microspheres. Yao Xue Xue Bao. 2007;42(1):1–7. [PubMed] [Google Scholar]
- 9.Duncan G, Jess TJ, Mohamed F, Price NC, Kelly SM, van-der-Walle CF. The influence of protein solubilisation, conformation and size on the burst release from poly(lactide-co-glycolide) microspheres. J Control Release. 2005;110(1):34–48. doi: 10.1016/j.jconrel.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 10.Zheng CH, Gao JQ, Zhang YP, Liang WQ. A protein delivery system: biodegradable alginate–chitosan–poly(lactic-co-glycolic acid) composite microspheres. Biochem Biophys Res Commun. 2004;323:1321–7. doi: 10.1016/j.bbrc.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 11.Jaganathan KS, Singh P, Prabakaran D, Mishra V, Vyas SP. Development of a single-dose stabilized poly(d,l-lactic-co-glycolic acid) microspheres-based vaccine against hepatitis B. J Pharm Pharmacol. 2004;56(10):1243–50. doi: 10.1211/0022357044418. [DOI] [PubMed] [Google Scholar]
- 12.Jiang W, Schwendeman SP. Stabilization of tetanus toxoid encapsulated in PLGA microspheres. Mol Pharm. 2008;5(5):808–17. doi: 10.1021/mp800027f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zheng CH, Gao JQ, Liang WQ, Yu HY, Zhang YL. Optimization and characterization of chitosan-coated alginate microcapsules containing albumin. Pharmazie. 2005;60(6):434–8. [PubMed] [Google Scholar]
- 14.van der Sande MAB, Mendy M, Waight P, Doherty C, McConkey SJ, Hall AJ, et al. Similar long-term vaccine efficacy of two versus three doses of HBV vaccine in early life. Vaccine. 2007;25(8):1509–12. doi: 10.1016/j.vaccine.2006.10.023. [DOI] [PubMed] [Google Scholar]
- 15.But DY, Lai CL, Lim WL, Fung J, Wong DK, Yuen MF. Twenty-two years follow-up of a prospective randomized trial of hepatitis B vaccines without booster dose in children: final report. Vaccine. 2008;26(51):6587–91. doi: 10.1016/j.vaccine.2008.09.034. [DOI] [PubMed] [Google Scholar]
- 16.Jain AK, Goyal AK, Gupta PN, Khatri K, Mishra N, Mehta A, et al. Synthesis, characterization and evaluation of novel triblock copolymer based nanoparticles for vaccine delivery against hepatitis B. J Control Release. 2009;136:161–9. doi: 10.1016/j.jconrel.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 17.Long JE, Huang LN, Qin ZQ, Wang WY, Qu D. IFN-gamma increases efficiency of DNA vaccine in protecting ducks against infection. World J Gastroenterol. 2005;11(32):4967–73. doi: 10.3748/wjg.v11.i32.4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Micozkadioglu H, Zumrutdal A, Torun D, Sezer S, Ozdemir FN, Haberal M. Low dose intradermal vaccination is superior to high dose intramuscular vaccination for hepatitis B in unresponsive hemodialysis patients. Ren Fail. 2007;29(3):285–8. doi: 10.1080/08860220601166263. [DOI] [PubMed] [Google Scholar]
- 19.Bramwell VW, Eyles JE, Somavarapu S, Alpar HO. Liposome/DNA complexes coated with biodegradable PLA improve immune responses to plasmid encoding hepatitis B surface antigen. Immunology. 2002;106:412–8. doi: 10.1046/j.1365-2567.2002.01448.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peyre M, Fleck R, Hockley D, Gander B, Sesardic D. In vivo uptake of an experimental microencapsulated diphtheria vaccine following sub-cutaneous immunisation. Vaccine. 2004;22(19):2430–7. doi: 10.1016/j.vaccine.2003.11.068. [DOI] [PubMed] [Google Scholar]
- 21.Feng L, Zhou XJ, Qi XR. Preparation, release and immunogenicity evaluation of HBsAg–PLGA microspheres. Beijing Da Xue Xue Bao. 2005;37(5):527–31. [PubMed] [Google Scholar]
- 22.Li Z, Leung M, Hopper R, Ellenbogen R, Zhang M. Feeder-free self-renewal of human embryonic stem cells in 3D porous natural polymer scaffolds. Biomaterials. 2010;31(3):404–12. doi: 10.1016/j.biomaterials.2009.09.070. [DOI] [PubMed] [Google Scholar]
- 23.Mundargi RC, Babu VR, Rangaswamy V, Patel P, Aminabhavi TM. Nano/micro technologies for delivering macromolecular therapeutics using poly(d,l-lactide-co-glycolide) and its derivatives. J Control Release. 2008;125(3):193–209. doi: 10.1016/j.jconrel.2007.09.013. [DOI] [PubMed] [Google Scholar]